Abstract
Common bunt, caused by Tilletia tritici (Bjerk.) G. Wint. in Rabenh. and T. laevis Kϋhn in Rabenh., is an economically important disease of wheat (Triticum aestivum L.) worldwide. The hexaploid wheat cultivar ‘McKenzie’ possesses effective resistance against known races of common bunt in western Canada. Understanding the nature of this resistance through DNA marker mapping will be useful for breeding. Disease reaction to T. tritici race T19 and T. laevis race L16 from field tests was analysed with simple sequence repeat markers for 174 doubled haploid lines using interval mapping. Markers Xgwm573 and Xwmc17, located on wheat chromosome 7B, were strongly associated with the resistance. The quantitative trait locus was designated QCbt.spa-7B.1. The markers were validated on two other populations in which ‘McKenzie’ was in the parentage. The markers will be useful in breeding as a tool for selecting for common bunt resistance from ‘McKenzie’ and will assist in identifying the resistance among potential new sources.
Résumé
La carie du blé, causée par Tilletia tritici (Bjerk.) G. Wint. in Rabenh. et T. laevis Kϋhn in Rabenh., est une maladie du blé (Triricum aestivum L.) de grande importance économique, et ce, à la grandeur de la planète. Le cultivar hexaploïde de blé ‘McKenzie’ possède une bonne résistance aux races de la carie dans l'Ouest canadien. La compréhension de ce type de résistance, grâce à la cartographie des marqueurs d'ADN, s'avérera utile lors d'améliorations génétiques. La réaction à la maladie causée en champ par les races T19 de T. tritici et L16 de T. laevis a été analysée avec des marqueurs des répétitions de séquences simples pour 174 lignées double haploïde par cartographie par intervalle. Les marqueurs Xgwm573 et Xwmc17, situés sur le chromosome 7B du blé, étaient fortement associés à la résistance. Le locus à caractère quantitatif a été répertorié en tant que QCbt.spa-7B.1. Les marqueurs ont été confirmés dans deux autres populations chez lesquelles on trouvait des liens de parenté avec le cultivar ‘McKenzie’. Les marqueurs s'avéreront utiles lors d'améliorations génétiques en tant qu'outils visant la résistance à la carie du blé à partir de ‘McKenzie’, et contribueront à déterminer la résistance chez de nouvelles sources possibles.
Introduction
Common bunt, caused by Tilletia tritici (Bjerk.) G. Wint. in Rabenh. and T. laevis Kϋhn in Rabenh. lowers grain yield and reduces grain quality of wheat (Triticum spp.) in many regions worldwide (Saari et al., Citation1996; Bonman et al., Citation2006). Common bunt has been controlled largely by resistance in registered cultivars on the Canadian prairies. In recent years, major resistance genes such as Bt10 and Bt8 have been utilized (Gaudet et al., Citation1993; DePauw et al., Citation1998; Knox et al., Citation2007; McCallum & DePauw, Citation2008; Hiebert et al., Citation2011), and are characterized by both high penetrance and expressivity, limiting bunt in both incidence and severity (Gaudet & Puchalski, Citation1989b ). Vulnerability of major genes through intense selection pressure on the pathogen is always a concern (Gaudet et al., Citation1993; Wang et al., Citation2009).
Resistance to common bunt in hexaploid wheat (Triticum aestivum L.) on the Canadian prairies is characterized by moderate penetrance and expressivity. Cultivars such as ‘Neepawa’ (Campbell, Citation1970) and ‘Katepwa’ (Campbell & Czarnecki, Citation1987) covered large areas of the prairies for many consecutive years without the appearance of major bunt epidemics (Gaudet & Puchalski, Citation1989b ). These forms of resistance, which under intense disease pressure in artificially inoculated nurseries display incidence levels of up to 35%, are suitable to keep common bunt at trace levels under field conditions. The resistance has remained stable against shifts in virulence in the pathogen (Gaudet & Puchalski, Citation1989b ). Some cultivars, such as ‘Laura’ (DePauw et al., Citation1988), are considered susceptible from the perspective that high infection levels of bunt are observed in artificial nurseries. However, there are cultivars which are more susceptible than ‘Laura’, indicating ‘Laura’ has genetic factors that resist bunt incidence (Gaudet et al., Citation1993). Conversely, cultivars such as ‘Columbus’ (Campbell & Czarnecki, Citation1981) and ‘McKenzie’ (Graf et al., Citation2003) have highly expressive and penetrant resistance phenotypes similar to resistance of cultivars with major genes such as ‘AC Cadillac’ (DePauw et al., Citation1998), ‘Snowhite476’ (Knox et al., Citation2007) and others. Empirical observation in breeding programmes that use cultivars such as ‘Columbus’ and ‘McKenzie’ indicates the parental type is not always easily recovered, implying more than one gene is required for full expression of resistance.
Multigenic resistance to common bunt has been reported (McKenzie, Citation1964), but identification of the specific genes is lacking. Fofana et al. (Citation2008), Wang et al. (Citation2009) and Galaev et al. (Citation2006) have identified quantitative trait loci (QTL) on chromosomes 1B and 7A linked to resistant loci while Menzies et al. (Citation2006) and Hiebert et al. (Citation2011) have identified the location of Bt10 on chromosome 6D. Based on a random amplified polymorphic DNA marker developed by Demeke et al. (Citation1996), Laroche et al. (Citation2000) developed a more specific marker for Bt10.
Identifying resistant loci and the location of genes followed by the development of effective DNA markers will assist breeders to develop resistant cultivars and ensure long-lasting control of common bunt. The objective of this study was to better understand the genetic factors involved in common bunt resistance derived from the cultivar ‘McKenzie’ using DNA markers.
Materials and methods
Genetic materials for marker discovery
A doubled haploid (DH) population of 338 lines was developed from the F1 of the cross ‘McKenzie’/BW711 using the maize pollen method (Knox et al., Citation2000). ‘McKenzie’ is a doubled haploid hard red spring wheat cultivar produced from the F1 hybrid of the cross ‘Columbus’/‘Amidon’ (Graf et al., Citation2003). ‘McKenzie’ displays resistance to common bunt. BW711 was developed from the backcross ‘Pacific’*3//Seln 70-3524/8*‘Neepawa’. BW711 is a high-yielding hard red spring wheat cultivar with the Bt10 bunt resistance gene.
Genetic materials for marker validation
Two additional doubled haploid populations were used for the validation of molecular markers: ‘McKenzie’/ND744 and ‘McKenzie’/ND3085. Both ND744 (Mergoum et al., Citation2005) and ND3085 (Garvin, Citation2003) have an intermediate resistance reaction to bunt. The ‘McKenzie’/ND744 population consisted of 200 lines randomly selected from 286 lines. The 286 lines were derived from a breeding population previously selected based on agronomic traits, such as height, maturity and straw strength, from a population of 511 DH lines grown in a seed increase nursery at Leeston, New Zealand (Oct. 2005).
The population of 116 lines from the ‘McKenzie’/ND3085 cross were selections that remained after testing 559 DH lines in a disease nursery for leaf and stem rust and common bunt, grown near Swift Current, Saskatchewan, in May 2005 and after selection for agronomic traits in a seed increase nursery in Leeston, New Zealand (Oct. 2005).
Phenotypic evaluations
Identical tests of the ‘McKenzie’/BW711 population were seeded in nurseries near Swift Current, SK and Lethbridge, AB in late April 2001. The random 338 DH lines were grown in unreplicated trials consisting of 3 m rows planted with 25 seeds per line. Prior to planting, seed was inoculated with T. laevis race L16. The seed was inoculated in a similar manner to that described by Wang et al. (Citation2009) with seed placed in cells of seeding cassettes along with 0.08 g of inoculum. The lids were applied to the cassettes and the trays were shaken for 15 s. Controls included eight replications each of parents ‘McKenzie’ and BW711, resistant cultivar ‘Columbus’ (7 entries of this line in 2001), intermediate cultivar ‘Neepawa’ and moderately susceptible cultivar ‘Laura’. Controls were randomized and placed every nine lines (Wang et al., 2009). Prior knowledge of the parents indicated that L16 was a suitable race to reveal segregation for resistance. A warm spring and drought conditions at the Lethbridge location resulted in a lower than normal expression of bunt. Incidence was estimated as a percentage of bunted spikes over total spikes in the row based on a visual assessment. At Swift Current, all lines in the test were rated twice, each time by a different person, and the final rating was the mean of the two observations. The test was repeated in 2002 near Swift Current only with 100 seeds planted per row.
The ‘McKenzie’/BW711 population along with the parents, controls and eight replications of susceptible control ‘Biggar’ (DePauw et al., Citation1991) were grown in a two replicate randomized complete block design near Swift Current in 2008 in order to enhance precision for marker determination (see for the list of controls). One hundred kernels per row were inoculated with a 1:1 T. laevis race L16 and T. tritici race T19 mixture. The two races cover the virulence spectrum of races identified in Western Canada and were chosen for this trial to ensure resistance to which markers were being developed was effective (Hoffmann & Metzger, Citation1976; Gaudet & Puchalski, Citation1989a ). Incidence ratings were as described previously, but as a single score by one person.
The breeding population ‘McKenzie’/ND3085 was evaluated in 2006. Controls seeded with the 116 DH lines included three replications each of ‘Biggar’, ‘AC Barrie’, ‘AC Cadillac’, ‘AC Elsa’, ‘Katepwa’ and ‘Neepawa’. Two hundred DH lines of the marker validation population ‘McKenzie’/ND744 plus 10 plots each of ‘McKenzie’, ND744, ‘Columbus’, ‘Neepawa’, ‘Biggar’ and ‘Laura’ were evaluated in 2007 and 2008. The tests were inoculated with the 1:1 race mixture of races L16 and T19 and seeded in randomized unreplicated tests near Swift Current. Incidence was recorded for each plot as previously described. Seeding rate was 200 seeds per row. All tests were seeded late April and rated late July to mid-August.
Genetic evaluations
Two seeds per line from the 338 lines of the ‘McKenzie’/BW711 population were evaluated with the FSD-RSA primer pair (Laroche et al., Citation2000) as markers for Bt10 because parent BW711 carries gene Bt10 which is dominantly epistatic to other genes for bunt resistance. A third seed was evaluated to generate two data points for each line when a sample did not amplify. The marker analysis identified 164 lines possessing Bt10. These lines were excluded from further genotyping.
Bulk segregant analysis was used to identify putative markers to bunt resistance loci derived from ‘McKenzie’ (Michelmore et al., Citation1991). From the 174 lines that the Bt10 marker indicated did not possess this resistance allele, seven lines with an incidence of 1% bunt in the Swift Current, 2002 ‘McKenzie’/BW711 trial were placed in resistant bulk 1. Seven lines with an incidence of 2% bunt in the same trial were placed in resistant bulk 2. Lines with 0% incidence were not used to further minimize the inclusion of the few lines that might possess Bt10 as a result of a crossover between the Bt10-marker and the resistant allele. Seven of the most susceptible lines over the 2001 and 2002 trials were chosen for the susceptible bulk. The DNA was isolated from seedlings of individual lines identified for the bulks. Equal amounts of DNA of lines within a bulk were pooled. The three bulks and parents ‘McKenzie’ and BW711 were evaluated with 613 microsatellite primer pairs [229 gwm (Röder et al., Citation1998), 346 wmc (357 on parents alone) (Gupta et al., Citation2002) and 38 barc (165 on parents alone) (Song et al., Citation2005)] for polymorphism between resistant and susceptible bulks and parents. An additional 140 microsatellite primers (129 barc and 11 wmc) were tested on the parents with only polymorphic markers tested on the bulks. Of the 753 primers tested on the parents, 228 were polymorphic on the parents, and about 10% were also polymorphic between the resistant and susceptible bulks. Markers polymorphic between resistant and susceptible bulks were evaluated on individual lines of the bulks and 18 promising markers were evaluated on up to 174 individual lines of the ‘McKenzie’/BW711 population.
Thirty additional primer pairs [13 cfa, 3 cfd (Sourdille et al., Citation2003), 1 gwm, 13 wmc] were evaluated on the parents for polymorphism in the region found linked to the resistance. Those polymorphic were evaluated on individual lines of the bulks with six evaluated on up to 174 population lines. Eight more primer pairs in this region from the original 228 primers that were polymorphic on the parents, but not initially appearing polymorphic in the bulks (likely due to one or more lines with a cross-over between the marker and the gene) were also evaluated on the population. Primer sequence, chromosome location and annealing temperature were obtained from Röder et al. (Citation1998), Somers et al. (Citation2004) and the GrainGenes database (http://wheat.pw.usda.gov).
DNA was extracted from 1 to 5 seedlings per line using a modified cetyltrimethylammonium bromide (CTAB) method (Doyle & Doyle, Citation1987). When the plants reached the three or four leaf stage, 10 cm leaf segments from primary leaves were harvested for genomic DNA isolation. The concentration of DNA was quantified spectrophotometrically using a GeneQuant instrument to allow equalization of concentrations for PCR.
The PCR reactions were performed in 0.2-mL strip tubes containing 10, 15 or 25 μL reaction volumes consisting of Invitrogen PCR Reaction Buffer [50 mM KCl, 20 mM Tris–HCl (pH 8.4)], 1.5 mM MgCl2, 0.2 mM of each dNTP (Fermentas), 0.2 μM microsatellite primers, 0.07 U μL−1 of Taq DNA Polymerase (New England Biolabs) and 2 ng μL−1 of genomic DNA in Gibco nuclease free water. The DNA amplification was performed in a PTC-100 thermocycler (MJ Research, Inc.) at 94 °C for 3 min for initial denaturation, followed by 44 cycles of 94 °C for 1 min, annealing for 1 min (temperatures were dependent on the individual microsatellite primers), and 72 °C for 1 min, followed by a final extension at 72 °C for 10 min before cooling to 4 °C. Amplification products were resolved by electrophoresis in mixed 2% metaphor and 1% agarose LE gels at 4 V cm−1 in TBE (0.045 m TRIS, 0.045 m Borate and 0.001 m EDTA) buffer and stained with ethidium bromide (0.5 μg mL−1). The DNA banding patterns were visualized with UV light and recorded by a Kodak EDAS-290 digital camera imaging system. The 50 bp ladder from New England Biolabs Inc. (Cat. No. N3236L) was used for sizing amplicons.
Seventy-four microsatellite primer sets (18 barc, 2 cfa, 2 cfd, 21 gwm and 31 wmc) in the region of putative resistance loci were evaluated for polymorphism on the parents of ‘McKenzie’/ND744 using the protocols previously described. Fourteen primer sets were evaluated on 10 bunt-resistant and 10 bunt-susceptible lines from the population and nine of the 14 sets were evaluated on the 180 remaining lines of the population. The ‘McKenzie’/ND3085 parents were evaluated with 31 of the same primer sets (10 barc, 7 gwm, 14 wmc) to check for polymorphism and the 116 lines were evaluated with four primer sets that were polymorphic.
Statistical and genetic analysis
Bunt incidence from trials of each population in each of the years was used for QTL analysis except for the ‘McKenzie’/BW711 2008 Swift Current trial in which least square means were used. An analysis of variance (SAS Institute Inc., Citation2008) was performed on common bunt incidence from the 2008 field trial to determine if lines varied significantly from each other. The model was a randomized complete block design of two replicates with lines considered fixed and replicates random. The SAS GLM procedure generated least square means for bunt incidence from the 2008 test and t-groupings of lines using least significant differences (LSD). The frequency distributions of bunt incidence in each of the trials were plotted with class intervals at 5% incidence of bunt.
Student's t-test using the SAS Mixed procedure and simple interval mapping (SIM) using MQTL (Tinker & Mather, Citation1995) were applied to quantify the probability of association between markers and bunt resistance. A marker map for use with MQTL was produced using MAPMAKER software (Lander et al., Citation1987). No option for doubled haploids existed in this software so we used the recombinant inbred line switch. Student's t-test compared the mean bunt incidence of lines with the marker variant of the ‘McKenzie’ parent to the mean bunt incidence of lines with the marker variant of the second parent. Chi-square goodness-of-fit analysis was completed to test that marker molecular variants fit a 1 : 1 random segregation ratio. Molecular markers were considered significantly associated with the resistance reaction if P < 0.001 in at least one environment or if P < 0.01 in more than one environment (Lander & Kruglyak, Citation1995).
Markers found through analysis of the ‘McKenzie’/BW711 population were validated through QTL analysis as described above on the ‘McKenzie’/ND744 and ‘McKenzie’/ND3085 populations. Means, standard deviation and standard error of the mean of parents, controls and populations within each environment were calculated using Statistix 7 (Statistix, Citation2000).
Table 1. Mean common bunt per cent incidence, number of observations in the mean, standard deviation and standard error of the mean for parents, controls and lines of the ‘McKenzie’/BW711 population for field trials at Swift Current and Lethbridge in 2001, 2002 and 2008
Results
Common bunt phenotype
Mean common bunt incidence of the ‘McKenzie’/BW711 population, available parents and controls included as indicators of different levels of resistance are presented in along with the standard deviation and standard error of each mean. demonstrates that the level of variation within a genotype is generally greater with increased susceptibility of the genotype, but the level of variation can change from year to year. Susceptible control cultivars ‘Laura’ and ‘Biggar’ indicated that bunt infection was very successful in trials near Swift Current in 2002 and 2008 (). Infection was slightly lower near Swift Current in 2001 and lowest near Lethbridge in 2001. The population mean trended the same across environments as the susceptible controls. For example, ‘Laura’ had the highest infections in 2002 and 2008 at Swift Current and the lowest at Lethbridge in 2001. The BW711 parent, which has Bt10, showed a slightly lower trend for common bunt incidence in all environments except Lethbridge in 2001 compared with ‘McKenzie’ which does not have Bt10. In 2008, six of the eight individual control test entries of BW711 expressed complete resistance (0% incidence) compared with two of eight ‘McKenzie’ test entries.
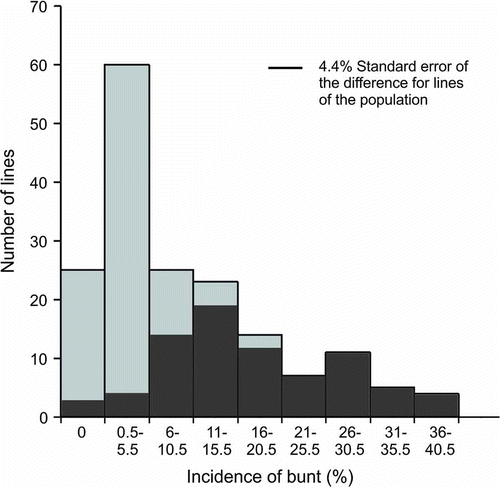
A skewed continuous distribution of common bunt incidence of ‘McKenzie’/BW711 (Bt10 lines removed) was obtained in plots of the frequency distribution for each environment with a preponderance of lines with resistance. The results from Swift Current 2008 are shown in ; the 2001 (range 0% to 40%) and 2002 (range 0% to 55%) distributions were similarly shaped. The distribution for Lethbridge 2001 was more compressed (range 0% to 35%). The population mean and standard deviation are further indicators of population response to environment (). The most susceptible lines in ‘McKenzie’/BW711 grown at Swift Current in 2008 had a significantly higher disease incidence than the more resistant lines based on the LSD (P < 0.05). ‘Biggar’ with a bunt incidence of 63.8% was significantly different from the moderately susceptible control ‘Laura’ with a bunt incidence of 50.6% (P < 0.05), which was significantly more susceptible than the most susceptible line of the ‘McKenzie’/BW711 population (Bt10 lines removed) with a bunt incidence of 40.0% (P < 0.05). In Swift Current 2002 and 2001, ‘Laura’ was similar to or more susceptible than the most susceptible line of the ‘McKenzie’/BW711 population.
Fig. 1. Frequency distribution of means for common bunt per cent incidence of the ‘McKenzie’/BW711 doubled haploid population (Bt10 lines removed) grown in the field near Swift Current, SK in 2008: Light grey columns with ‘McKenzie’ molecular variant for Xgwm573; dark grey columns with BW711 molecular variant for Xgwm573.
Marker discovery
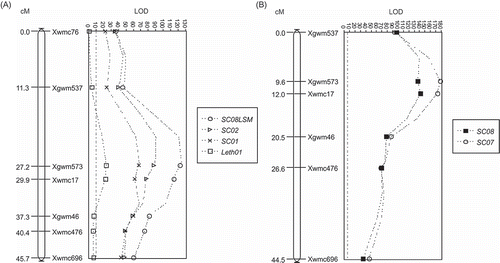
Thirty-two polymorphisms identified on parents and bulks from the ‘McKenzie’/BW711 population generated nine markers that were associated with bunt resistance according to preliminary analysis with Student's t-test at P < 0.0001, one marker at P < 0.001 and four others at P < 0.01. Mapmaker software placed seven of the 14 markers identified in the t-test in the same linkage group (). These markers have previously been mapped to chromosome 7B [Graingenes (http://wheat.pw.usda.gov/index.shtml)]. Application of simple interval mapping (SIM) using MQTL identified a major QTL on chromosome 7B (). The seven markers in were significantly trait related for the Swift Current 2001, 2002 and 2008 trials at the test statistic threshold generated by MQTL (P < 0.001). Only two markers, Xgwm573 and Xwmc17, located at the peak of the QTL were significantly associated with bunt resistance for the Lethbridge 2001 results when using the P < 0.001 threshold. In all environments the Xgwm573 showed the strongest association with bunt resistance.
Fig. 2. The QCbt.spa-7B.1 QTL for common bunt resistance identified in (A) ‘McKenzie’/BW711 population (Bt10 lines removed) using Simple Interval Mapping with MQTL with the least square means in 2008 (SC08LSM), 2001 (SC01) and 2002 (SC02) at Swift Current, and 2001 at Lethbridge (Leth01) and (B) validated in the ‘McKenzie’/ND744 population using Simple Interval Mapping with MQTL for Swift Current in 2007 (SC07) and 2008 (SC08). Values on each map provide cM distances between markers and significant markers are those with values (symbols) higher than the LOD threshold indicated in each chart by the straight line running from the top to the bottom of the chart.
The two other QTL identified by single point analysis at the P < 0.0001 were Xbarc195 and Xgwm577, and at P < 0.001 was Xgwm111. The Xbarc195 marker mapped with Mapmaker in a linkage group with Xbarc121, Xbarc26 and Xwmc9 that most likely map to a group 7 chromosome, possibly 7A [Graingenes (http://wheat.pw.usda.gov/index.shtml)]. The markers Xbarc121, Xbarc26 and Xwmc9 were among the markers significant at the P < 0.01 level with single point analysis. Simple interval mapping with MQTL showed the highest probability between 0.1 and 0.05 with the peak at Xbarc195. The marker Xgwm577 (P < 0.0001) associated with chromosome 7B (Graingenes) was in a separate linkage group from the other 7B markers and did not generate a QTL with SIM. The Xgwm111 (P < 0.001), possibly on 7D (Graingenes), similarly mapped alone and did not appear as a significant QTL with SIM. The fourth marker reported at P < 0.01 with single point analysis was Xwmc695, which has been reported associated with a number of chromosomes (Graingenes).
The number of lines with the ‘McKenzie’ molecular variant did not differ from the number of lines with the BW711 molecular variant (Chi square test at P < 0.01) for five of the seven markers. Two of the seven markers, Xwmc696 and Xwmc76, did differ (P < 0.01) from a 1:1 segregation, but the smaller class (68 out of 174 lines) was large enough not to be a concern in distorting the outcome of the analysis.
The Xwmc76 marker produced a 250 bp fragment in ‘McKenzie’ and a 260 bp fragment in BW711. The Xgwm573 and Xwmc17 markers both produced a 225 bp fragment from BW711 and from ‘McKenzie’ a 200 bp fragment was produced for Xgwm573 and a null fragment for Xwmc17. In ‘McKenzie’, Xgwm46 produced a 150 bp fragment and a 175 bp fragment in BW711 while Xwmc476 produced a 210 bp fragment in ‘McKenzie’ and a 190 bp fragment in BW711. The Xwmc696 marker produced a 140 bp fragment in ‘McKenzie’ and a 150 bp fragment in BW711.
The means of the ‘McKenzie’/BW711 lines (Bt10 lines excluded) with the ‘McKenzie’ molecular variant for Xgwm573 and Xwmc17 microsatellite markers consistently had a lower bunt incidence than lines with the BW711 molecular variant in all environments. For example, the mean bunt incidence associated with the ‘McKenzie’ molecular variant of Xgwm573 at Swift Current in 2008 was 2.6%, whereas the mean bunt incidence associated with the BW711 molecular variant was significantly different at 17.6% (P < 0.0001). The distribution of the bunt scores associated with each molecular variant is shown in for Swift Current in 2008.
Table 2. Mean common bunt per cent incidence, number of observations in the mean, standard deviation and standard error of the mean for parents and controls and lines within the ‘McKenzie’/ND3085 population for field trials at Swift Current, 2006 and the ‘McKenzie’/ND744 population for field trials at Swift Current in 2007 and 2008
Marker validation
Bunt incidence in the validation populations ‘McKenzie’/ND744 and ‘McKenzie’/ND3085 was skewed similar to ‘McKenzie’/BW711. High infection was observed in the susceptible control ‘Biggar’ in all validation population trials, but was particularly high in 2006 (). The highest population mean incidence of bunt was in ‘McKenzie’/ND744 at Swift Current in 2008.
The parents of the ‘McKenzie’/ND744 population were polymorphic to six of the markers associated with the 7B QTL found in ‘McKenzie’/BW711. A Chi-square test indicated that Xgwm46, Xgwm537, Xgwm573, Xwmc17 and Xwmc476 fit a 1:1 segregation of the two molecular variants for each marker in the population. Although the Xwmc696 segregation did not fit a 1:1, the size of the smallest class was large enough not to distort the QTL analysis. Student's t-test (P < 0.0001) and simple interval mapping analyses indicated that each marker was associated with bunt resistance in both years of field testing ().
The ‘McKenzie’/ND3085 population was composed of lines selected previously showing no bunt incidence in a disease nursery where bunt was weakly expressed; nevertheless, bunted lines ranging up to 45% bunt incidence were observed. The parents were polymorphic to Xgwm573, Xwmc17 and Xwmc476 markers, with the ‘McKenzie’ type molecular variant in approximately 60% of the lines. Student's t-test indicated a very significant (P < 0.0001) association between each of the markers and bunt resistance. For example, the mean bunt incidence associated with the ‘McKenzie’ molecular variant of Xwmc17 was 1.9% compared with the ND3085 molecular variant of 16.4%.
Discussion
The high infection rates of the susceptible and moderately susceptible controls indicated there should be sufficient infection for common bunt in the lines of the populations, thus maximizing the differential necessary for good genetic characterization of resistance. The less than favourable conditions for common bunt at Lethbridge in 2001, as demonstrated by the controls, were consistent with a more compressed range of bunt incidence in the population, yet marker results were consistent with the other environments. The races L16 and T19 represent the virulence of bunt races on the Canadian Prairies (Hoffmann & Metzger, Citation1976; Gaudet & Puchalski, Citation1989a ). Using L16 and T19 allowed us to determine that the resistance tracked from ‘McKenzie’ is effective to the broad range of virulence found in races from the prairies. Multiple genes for bunt resistance are required to explain the results observed with the ‘McKenzie’/BW711 population. The probability of not observing the segregation of a moderately susceptible to susceptible line in a doubled haploid population the size of the ‘McKenzie’/BW711 population (174 lines) is remote. A population this size can discriminate the segregation of six genes at a significance level of P = 0.06. The likely explanation for the absence of moderately susceptible to susceptible lines in all environments is the presence of an incomplete resistance gene or genes shared in common between BW711 and ‘McKenzie’. The absence of the segregation of moderately susceptible to susceptible lines occurred in environments in which moderately susceptible to susceptible controls expressed high levels of bunt. Therefore, environment was unlikely a factor in the lack of moderately susceptible to susceptible lines of the population being observed.
‘Neepawa’ has an intermediate reaction to common bunt (Gaudet et al., Citation1993) and is a substantial component of the pedigree of both ‘McKenzie’ and BW711 (ICIS, http://www.icis.cgiar.org/icis/index.php/Main_Page). Given this common ancestor, which is a source of one or more bunt incomplete resistance genes, it is understandable that one or more genes could be found in common between BW711 and ‘McKenzie’. The expression of this background resistance is weak enough to allow expression of other resistance genes to be observed.
The continuous distribution of common bunt incidence in all environments required quantitative analysis to understand the nature of the segregation for resistance to bunt in the ‘McKenzie’/BW711 population. We identified a major QTL in the interval Xwmc76 to Xwmc696. Six of the markers associated with the QTL map to chromosome 7B (Somers et al., Citation2004) indicating the resistance is located on chromosome 7B. We designate the QTL QCbt.spa.-7B.1. Under conditions optimum for bunt infection, this QTL provided up to a 15% reduction in incidence of common bunt in the ‘McKenzie’/BW711 population. Because QCbt.spa.-7B.1 likely functions in a background with other genes for resistance, the level of penetrance of the gene on its own is not known.
One of the markers, Xwmc17, was previously reported on chromosome 7A (Somers et al., Citation2004), but it produces multiple fragments. The 225 bp fragment we characterized mapped to chromosome 7B, apparently priming a homeologous sequence to that reported by Somers et al. (Citation2004). The fact that QCbt.spa.-7B.1 showed up in all environments, including an environment (Lethbridge 2001) with weak bunt expression, indicates the QTL is stable for bunt resistance. The consistency and degree of association of the ‘McKenzie’ molecular variant for each marker with low bunt incidence indicates the bunt resistance is derived from the ‘McKenzie’ parent. The QTL was validated in populations ‘McKenzie’/ND744 and ‘McKenzie’/ND3085. Although seven markers were identified in the ‘McKenzie’/BW711 population, the amount of polymorphism varied from population to population, with only six markers polymorphic in ‘McKenzie’/ND744, and only three of the markers polymorphic in ‘McKenzie’/ND3085.
Minor factors segregating between ‘McKenzie’ and BW711 in addition to QCbt.spa.-7B.1, hypothesized based on the continuous nature of the bunt distribution, may relate to the QTL identified by single point analysis, but more research is needed to confirm the role of minor loci. For example, the effect on bunt incidence at the Xbarc195 locus being significant with single point analysis and weakly significant with SIM may identify a minor factor for resistance. The trait related amplicon generated by Xbarc195 appears to map to chromosome 7A, as do two other linked markers, Xwmc9 and Xbarc121 generating amplicons associated with the 7A chromosome (Somers et al., Citation2004). The Xgwm577 marker, although significant with single point analysis but not with SIM, was located to chromosome 7B (Somers et al., Citation2004), but mapped independent to QCbt.spa.-7B.1. The Xgwm111, significant with single point analysis, and previously located to chromosome 7D (Somers et al., Citation2004) may also indicate a minor factor.
The quantitative nature of bunt resistance segregation from ‘McKenzie’ was reinforced by the continuous distributions of bunt incidence in ‘McKenzie’/ND744 and ‘McKenzie’/ND3085. Once again the lack of segregation of highly susceptible lines in the validation populations indicated minor factors contributing to resistance were present in both parents of each validation population and did not segregate. For example, ‘McKenzie’ and ND3085 both have ‘Neepawa’ in their ancestry (ICIS, http://www.icis.cgiar.org/icis/index.php/Main_Page) from which they may have acquired common genes for common bunt field resistance.
The BW711 line possesses Bt10, and although the marker is close to the gene (Laroche et al., Citation2000), a few crossovers would be possible, such that a very few lines with Bt10 would have been included in the test population. To minimize this effect, after the Bt10 marker was used to remove lines possessing Bt10 from the analysis in which to identify resistance from ‘McKenzie’, lines with 0% incidence were excluded from the resistant bulks to further minimize the presence of lines that might possess Bt10. This approach appears to have been effective given no QTL for bunt resistance were identified on the chromosome 6D on which Bt10 resides (Menzies et al., Citation2006).
Although common bunt resistance genes have been located by marker or cytogenetic analysis to chromosomes 1B, 2B, 2D, 6D and 7A (Wang et al., Citation2009), no known reports indicate resistance on chromosome 7B of wheat. A QTL for Karnal bunt resistance was associated with Xgwm46 on chromosome 7B (Sukhwinder-Singh et al., Citation2003) putting it in the same region as the QCbt.spa-7B.1. Other QTL markers for common bunt resistance have been reported for chromosomes 1B and 7A (Fofana et al., Citation2008) and obviously differ from the QCbt.spa-7B.1 identified in ‘McKenzie’. Two QTL on chromosome 1B, Cbt.crc-1B.1 and Cbt.crc-1B.2 and one QTL on chromosome 7A, Cbt.crc-7A, were derived from cultivar ‘AC Domain’ which like ‘Neepawa’ has ‘Thatcher’ as a common ancestor (ICIS, http://www.icis.cgiar.org/icis/index.php/Main_Page).
The present work demonstrates that bunt resistance in ‘McKenzie’ must be controlled by multiple genetic factors of which one novel genetic factor was identified as a segregating QTL. Further work remains to identify other contributing factors. To better understand the resistance in ‘McKenzie’, it should be crossed with a completely susceptible parent. Such a study would demonstrate the penetrance of QCbt.spa-7B.1 in a completely susceptible background to determine if the gene at this locus is a major gene.
Although we were unable to confirm our hypothesis that ‘McKenzie’ bunt resistance was controlled by multiple genes with cumulative effects, we demonstrated that ‘McKenzie’ possesses more than one gene. ‘McKenzie’ possesses at least one minor gene that did not segregate and the major QTL QCbt.spa-7B.1. The QCbt.spa-7B.1 QTL has value in contributing bunt resistance and the markers we identified can be used to incorporate this gene into breeding lines. The QTL peak was nearest Xgwm573 and Xwmc17 in all three populations. However, based on consistency of amplification and ease of discrimination of the molecular variants, we recommend Xgwm537, Xwmc476 and Xgwm46 as preferred markers, with Xgwm46 being closer to the QTL peak.
Acknowledgements
We thank J. Neufeld, A. Banman, A. Balliet, C. Horbach, B. Meyer, J. Sauder, C. Barlow, T. Colenutt, M. Poppy, M. Dyck, J. Zhang and B. Neudorf for their technical assistance. We also gratefully acknowledge the support of the Wheat Producer Check-Off administered by the Western Grains Research Foundation matched with the Matching Investment Initiative and core funding of Agriculture and Agri-Food Canada.
References
- Bonman , J.M. , Bockelman , H.E. , Goates , B.J. , Obert , D.E. , McGuire , P.E. , Qualset , C.O. and Hijmans , R. 2006 . Geographic distribution of common and dwarf bunt resistance in landraces of . Triticum aestivum subsp. aestivum. Crop Sci. , 46 : 1622 – 1629 .
- Campbell , A.B. 1970 . Neepawa hard red spring wheat . Can. J. Plant Sci. , 50 : 752 – 753 .
- Campbell , A.B. and Czarnecki , E.M. 1981 . Columbus hard red spring wheat . Can. J. Plant Sci. , 61 : 147 – 148 .
- Campbell , A.B. and Czarnecki , E. 1987 . Katepwa hard red spring wheat . Can. J. Plant Sci. , 67 : 229 – 230 .
- Demeke , T. , Laroche , A. and Gaudet , D.A. 1996 . A DNA marker for the Bt-10 common bunt resistance gene in wheat . Genome , 39 : 51 – 55 .
- DePauw , R.M. , Townley-Smith , T.F. , McCaig , T.N. and Clarke , J.M. 1988 . Laura hard red spring wheat . Can. J. Plant Sci. , 68 : 203 – 206 .
- DePauw , R.M. , Preston , K.R. , Townley-Smith , T.F. , Hurd , E.A. , McCrystal , G.E. and Lendrum , C.W.B. 1991 . Biggar red spring wheat . Can. J. Plant Sci. , 71 : 519 – 522 .
- DePauw , R.M. , Thomas , J.B. , Knox , R.E. , Clarke , J.M. , Fernandez , M.R. , McCaig , T.N. and McLeod , J.G. 1998 . AC Cadillac hard red spring wheat . Can. J. Plant Sci. , 78 : 459 – 462 .
- Doyle , J.J. and Doyle , J.L. 1987 . A rapid DNA isolation procedure for small quantities of fresh leaf tissue . Phytochem. Bull. , 9 : 11 – 15 .
- Fofana , B. , Humphreys , D.G. , Cloutier , S. , McCartney , C.A. and Somers , D.J. 2008 . Mapping quantitative trait loci controlling common bunt resistance in a doubled haploid population derived from the spring wheat cross RL4452 × AC Domain . Mol. Breed. , 21 : 317 – 325 .
- Galaev , A.V. , Babayants , L.T. and Sivolap , Y.M. 2006 . DNA-markers for resistance to common bunt transferred from Aegilops cylindrica Host. to hexaploid wheat . Czech J. Genet. Plant Breed. , 42 : 62 – 65 .
- Garvin, D.F. (2003) Report of the 2002 Uniform Regional Scab Nursery for Spring Wheat Parents. 15 p. Available http://wheat.pw.usda.gov/ggpages/nursery/URSN_2002/URSN2002.pdf (http://wheat.pw.usda.gov/ggpages/nursery/URSN_2002/URSN2002.pdf)
- Gaudet , D.A. and Puchalski , B.L. 1989a . Races of common bunt of wheat in western Canada . Can. J. Plant Pathol. , 11 : 415 – 418 .
- Gaudet , D.A. and Puchalski , B.J. 1989b . Status of bunt resistance in western Canadian spring wheat and triticale . Can. J. Plant Sci. , 69 : 797 – 804 .
- Gaudet , D.A. , Puchalski , B.J. , Kozub , G.C. and Schaalje , G.B. 1993 . Susceptibility and resistance in Canadian spring wheat cultivars to common bunt (Tilletia tritici and T. Laevis) . Can. J. Plant Sci. , 73 : 1217 – 1224 .
- Graf , R.J. , Hucl , P. , Orshinsky , B.R. and Kartha , K.K. 2003 . Mckenzie hard red spring wheat . Can. J. Plant Sci. , 83 : 565 – 569 .
- Gupta , P.K. , Balyan , H.S. , Edwards , K.J. , Isaac , P. , Korzun , V. , Roder , M. , Gautier , M.F. , Joudrier , P. , Schlatter , A.R. and Dubcovsky , J. 2002 . Genetic mapping of 66 new microsatellite (SSR) loci in bread wheat . Theor. Appl. Genet. , 105 : 413 – 422 .
- Hiebert , C.W. , Fetch , T.G. , Zegeye , T. , Thomas , J.B. , Somers , D.J. , Humphreys , D.G. , McCallum , B.D. , Cloutier , S. , Singh , D. and Knott , D.R. 2011 . Genetics and mapping of seedling resistance to Ug99 stem rust in Canadian wheat cultivars ‘Peace’ and ‘AC Cadillac’ . Theor. Appl. Genet. , 122 : 143 – 149 .
- Hoffmann , J.A. and Metzger , R.J. 1976 . Current status of virulence genes and pathogenic races of the wheat bunt fungi in the northwestern USA . Phytopathology , 66 : 657 – 660 .
- Knox , R.E. , Clarke , J.M. and Depauw , R.M. 2000 . Dicamba and growth condition effects on doubled haploid production in durum wheat crossed with maize . Plant Breed. , 119 : 289 – 298 .
- Knox , R.E. , DePauw , R.M. , Clarke , F.R. , Clarke , J.M. , McCaig , T.N. and Fernandez , M.R. 2007 . Snowhite476 hard white spring wheat . Can. J. Plant Sci. , 87 : 521 – 526 .
- Lander , E. and Kruglyak , L. 1995 . Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results . Nat. Genet. , 11 : 241 – 247 .
- Lander , E.S. , Green , P. , Abrahamson , J. , Barlow , A. , Daly , M.J. , Lincoln , S.E. and Newburg , L. 1987 . Mapmaker: An interactive computer package for constructing primary genetic linkage maps of experimental and natural populations . Genomics , 1 : 174 – 181 .
- Laroche , A. , Demeke , T. , Gaudet , D.A. , Puchalski , B. , Frick , M. and McKenzie , R. 2000 . Development of a PCR marker for rapid identification of the Bt-10 gene for common bunt resistance in wheat . Genome , 43 : 217 – 223 .
- McCallum , B.D. and DePauw , R.M. 2008 . A review of wheat cultivars grown in the Canadian prairies . Can. J. Plant Sci. , 88 : 649 – 677 .
- McKenzie , H. 1964 . Inheritance of bunt reaction in a Redman X S-615 wheat cross . Can. J. Plant Sci. , 44 : 561 – 567 .
- Menzies , J.G. , Knox , R.E. , Popovic , Z. and Procunier , J.D. 2006 . Common bunt resistance gene Bt10 located on wheat chromosome 6D . Can. J. Plant Sci. , 86 : 1409 – 1412 .
- Mergoum , M. , Frohberg , R.C. , Miller , J.D. , Rasmussen , J.B. and Stack , R.W. 2005 . Registration of spring wheat germplasm ND 744 resistant to Fusarium head blight leaf and stem rusts . Crop Sci , 45 : 430 – 431 .
- Michelmore , R.W. , Paran , I. and Kesseli , R.V. 1991 . Identification of markers linked to disease-resistance genes by bulked segregant analysis: a rapid method to detect markers in specific genomic regions by using segregating populations . Proc. Natl. Acad. Sci. USA , 88 : 9828 – 9832 .
- Röder , M.S. , Korzun , V. , Wendehake , K. , Plaschke , J. , Tixier , M.H. , Leroy , P. and Ganal , M.W. 1998 . A microsatellite map of wheat . Genetics , 149 : 2007 – 2023 .
- Saari , E.E. , Mamluk , O.F. and Burnett , P.A. 1996 . “ Bunts and smuts of wheat ” . In Bunt and smut diseases of wheat: Concepts and methods of disease management , Edited by: Wilcoxson , R.D. and Saari , E.E. 12 – 25 . Mexico , D.F : CIMMYT .
- SAS Institute Inc . 2008 . SAS Software. Release Version 2.0.3 , NC , , USA : SAS Institute Inc. Cary .
- Somers , D.J. , Issac , P. and Edwards , K. 2004 . A high-density microsatellite consensus map for bread wheat (Triticum aestivum L.) . Theor. Appl. Genet. , 109 : 1105 – 1114 .
- Song , Q.J. , Shi , J.R. , Singh , S. , Fickus , E.W. , Costa , J.M. , Lewis , J. , Gill , B.S. , Ward , R. and Cregan , P.B. 2005 . Development and mapping of microsatellite (SSR) markers in wheat . Theor. Appl. Genet. , 110 : 550 – 560 .
- Sourdille , P. , Cadalen , T. , Guyomarc'h , H. , Snape , J.W. , Perretant , M.R. , Charmet , G. , Boeuf , C. , Bernard , S. and Bernard , M. 2003 . An update of the Courtot × Chinese Spring intervarietal molecular marker linkage map for the QTL detection of agronomic traits in wheat . Theor. Appl. Genet. , 106 : 530 – 538 .
- Statistix . 2000 . Statistix 7 User's Manual , FL : Analytical Software, Tallahassee .
- Theor. Appl. Genet , Sukhwinder-Singh, Brown-Guedira, G.L., Grewal, T.S., Dhaliwal, H.S., Nelson, J.C., Singh, H., & Gill, B.S. (2003). Mapping of a resistance gene effective against Karnal bunt pathogen of wheat. 106, 287–292.
- Tinker , N.A. and Mather , D.E. 1995 . MQTL: Software for simplified composite interval mapping of QTL in multiple environments . J. Agric. Genomics (formerly J. Quant. Trait Loci) , 1/2 : 2
- Wang , S. , Knox , R.E. , Depauw , R.M. , Clarke , F.R. , Clarke , J.M. and Thomas , J.B. 2009 . Markers to a common bunt resistance gene derived from ‘Blizzard’ wheat (Triticum aestivum L.) and mapped to chromosome arm 1BS . Theor. Appl. Genet. , 119 : 541 – 553 .