Abstract
This article reviews the recent progress of research on fusarium head blight (FHB) of wheat. It addresses the broad areas of strategies for disease management, biological control, the pathogen (Fusarium graminearum (teleomorph Gibberella zeae)), mycotoxins, the effects of dwarfing genes on FHB severity, quantitative trait loci (QTLs) and new perspectives. Where there are recent reviews on this subject, we have deliberately examined the subsequent literature to provide an update on research. With few resistant cultivars available even now, the main tools to manage the disease remain rotation, varietal selection, disease forecasting and fungicides. A few biocontrol organisms are being considered for commercial application. The pathogen's sexual life cycle has been investigated in depth, and with its complete genome sequence known, the pathways and genes controlling the sexual development and ascospore release of F. graminearum are being explored. The 3-acetyldeoxynivalenol chemotype of F. graminearum has increased in prevalence in Canada with attendant risks of higher DON levels in cereal grain. Stringent limits on allowable levels of Fusarium mycotoxins in the food/feed chain have been enacted in Europe and the USA, but regulations for Canada are only at the discussion stage with the Canadian Food Inspection Agency. Efforts to develop FHB-resistant lines proceed apace, as these can be selected in most wheat populations despite the adverse effects of dwarfing genes on FHB severity. While more quantitative trait loci (QTLs) for disease resistance continue to be identified and mapped, new resistant cultivars remain disappointingly few. We present some encouraging early results from an alternative approach based on epigenetics.
Résumé
Cet article passe en revue les récents progrès obtenus en recherche sur la brûlure de l’épi (BE) chez le blé. Il aborde les grands domaines relatifs aux stratégies de gestion de la maladie, à la lutte biologique, à l'agent pathogène (Fusarium graminearum [téléomorphe Gibberella zeae]), aux mycotoxines, aux effets des gènes nanifiants sur la gravité de la BE, aux locus à caractère quantitatif (QTL) et aux nouvelles perspectives. Nous avons délibérément examiné la littérature subséquente aux revues récentes sur le sujet, le cas échéant, afin de présenter une mise à jour sur la recherche. Avec le peu de cultivars résistants dont nous disposons même actuellement, les principaux outils de gestion de la maladie demeurent la rotation des cultures, la sélection variétale, la prévision de la maladie et les fongicides. Quelques organismes de lutte biologique sont actuellement évalués en vue d'applications commerciales. Le cycle sexuel de l'agent pathogène a été étudié en profondeur et, connaissant sa séquence génomique complète, les voies et les gènes contrôlant le développement sexuel et la libération des ascospores de F. graminearum sont actuellement explorés. La fréquence du chimiotype 3-acétyldéoxynivalénol de F. graminearum a augmenté au Canada, ce qui implique des risques importants de taux plus élevés de DON dans le grain des céréales. Des limites plus exigeantes ont été imposées sur la teneur en mycotoxines de Fusarium dans la chaîne agricole et alimentaire en Europe et aux États-Unis, mais, au Canada, la réglementation n'en est qu'au stade de la discussion avec l'Agence canadienne d'inspection des aliments. Les efforts pour développer des lignées résistantes à la BE progressent rapidement étant donné que celles-ci peuvent être sélectionnées dans la majorité de populations de blé en dépit des effets négatifs des gènes nanifiants sur la gravité de la BE. Pendant que plus de QTL relatifs à la résistance à la maladie sont identifiés et cartographiés, le nombre de nouveaux cultivars résistant demeure, hélas, décevant. Nous présentons également quelques résultats encourageants issus d'une approche alternative basée sur l’épigénétique.
Introduction
Fusarium head blight (FHB) is arguably the most serious disease affecting wheat crops throughout the cereal-growing regions of the world. In Canada, the epidemics of 1980 in eastern Canada and that of 1993 in Manitoba spurred research into finding effective genetic resistance and management strategies. However, in FHB-endemic regions, it remains the case that few cultivars with even moderate resistance to FHB are available and the disease continues to inflict notable losses on cereal producers.
As a pathogen that survives the harshest winters on stubble, Fusarium graminearum (Schwabe) (teleomorph Gibberella zeae (Schwein.)) Petch can attack cultivated wheat in ways that challenge our best efforts to control, or even mitigate, the losses FHB imposes on yield, quality and food safety. While certain cultural practices or timely applications of fungicides may reduce disease severity and, consequently, inoculum levels and losses from FHB (Dill-Macky, Citation2008; McMullen et al., 2008; Gilbert & Tekauz, Citation2011), these control measures cannot be consistently relied on. Improving host genetic resistance is therefore seen as essential to achieving meaningful control (Anderson, Citation2007; Bonin & Kolb, Citation2009). Unfortunately, the comparatively few, well-characterized, sources of resistance are in backgrounds with poor agronomic and quality traits (Comeau et al., Citation2010). Moreover, such resistance as is available appears best explained by the combined action of multiple genes interacting in complex ways, making their exploitation more difficult (Miedaner & Korzun, Citation2012). For durum wheat, the challenge is even more daunting as there is little demonstrated variation of FHB resistance in the tetraploid germplasm pool (Somers et al., Citation2006), leaving breeders little to work with.
The fruits of the substantially augmented American efforts to first monitor, and then control FHB have recently appeared in a review authored by the leaders of the US Wheat and Barley Scab Initiative (USWBSI) (McMullen et al., 2012). We aim here, in this review, to distil the findings described in recent literature that illuminate the current understanding of how to best control FHB by exploiting its relevant points of vulnerability in the disease triangle. First, there are strategies for manipulating the environment through expedients such as cultural practices. Second, the pathogen may be attacked with fungicides and biocontrol agents. Moreover, the recently expanded understanding of the details of the pathogen's life cycle and how it produces and exploits mycotoxins provides points for intervention that might substantially reduce the harm caused by FHB. Finally, and this is usually considered as the most robust and reliable strategy, wheat hosts can be given stronger defences by introgressing genetic determinants of disease resistance. A more radical perspective now also aims to strengthen hosts’ defences by inducing them to express more effectively the defence genes they already have.
Management
General considerations
Several recent reviews have covered the most effective strategies for managing FHB (Yuen & Schoneweis, Citation2007; Gilbert & Tekauz, Citation2011; Wegulo et al., Citation2011; McMullen et al., 2012). There is agreement that no single strategy is effective against FHB. Combinations of variety selection, assessment of disease risk, fungicide application and cultural practices, including type of tillage and rotation, all help to control the disease. McMullen et al. (Citation2012) provide a thorough review of the utility and limitations of disease-forecasting models as an aid to making more cost-effective decisions about using fungicides. Guo et al. (Citation2010) demonstrated that F. graminearum inoculum increased where wheat or canola were grown in producer's fields in the previous two years. Fields in which zero or minimum tillage was practised had similar inoculum levels, but both conservation types of tillage produced significantly more inoculum than conventional tillage. In a North Dakota durum wheat study, the lowest disease severity, lowest DON, and highest yields were reported for plots sown to a moderately resistant cultivar, planted into canola rather than wheat stubble, and protected with an application of fungicide at flowering (McMullen et al., 2008). The same factors were quantified in a review that arrived at similar conclusions at what constituted best practice: choose a variety with the best level of resistance to FHB, plough under residues, avoid maize–wheat rotations, and apply an azole fungicide (other than fenbuconazole) (Beyer et al., Citation2006).
Choice of fungicides
When FHB re-emerged in North America during the 1990s, the fungicides then available were ineffective in controlling the disease (Milus, Citation1994). Although fungicides of the triazole group were available and registered for use on wheat (Jones, Citation2000), it was only after numerous empirical studies that effective combinations and modes of application of these fungicides were identified (Paul et al., Citation2008). Prothioconazole, both alone and in combination with tebuconazole, and metconazole, were more effective than either tebuconazole alone or propiconazole (Hollingsworth et al., Citation2006). To distil the essential and robust findings of more than 100 uniform fungicide trials conducted over 11 years from 14 different American states, a meta-analysis that accommodated multivariate random effects was conducted (Paul et al., Citation2008); it showed that the triazoles prothioconazole, metconazole and tebuconazole plus prothioconazole were indeed effective in suppressing FHB disease and controlling mycotoxins. A further analysis of a combination of 40 trials showed that in controlling disease, the beneficial effects of using resistant cultivars and applying effective fungicides were additive (Willyerd et al., Citation2012). In this case, the fungicide trials selected all used prothioconazole plus tebuconazole (Willyerd et al., Citation2012). In addition, a 5-year study showed that applying fungicides containing prothioconazole at the beginning of anthesis greatly suppressed FHB disease. As a consequence, grain yields rose and DON content in wheat kernels fell considerably, although treatment was more effective in common wheat than durum wheat (Haidukowski, Citation2012).
Another class of fungicides, the strobilurins, were tested at the end of the 1990s for efficacy against F. graminearum. Results varied, ranging from no effect on yield or DON accumulation (Milus & Weight, Citation1998; Haidukowski et al., 2005), to an increase (albeit not significant) in DON accumulation over the non-treated control (Hollingsworth & Motteberg, Citation2004). An examination of four field trials in the UK (two of which relied on natural inoculum, whereas the other two were artificially inoculated with grain infested with a mixture of Microdochium nivale var. majus (Wollenw.) Glynn & S. G. Edwards, comb. nov., F. culmorum (Wm. G. Sm.) Sacc., and F. avenaceum (Fr.) Sacc.), revealed that while azoxystrobin controlled M. nivale var. majus, it exerted little effect on the Fusarium spp. Conversely, tebuconazole controlled the Fusarium spp., but showed little control of M. nivale (Simpson et al., Citation2001). Applying azoxystrobin two days after inoculation with the fungal pathogens increased DON accumulation per unit of pathogen (Simpson et al., Citation2001). This conclusion was contradicted shortly afterward by another UK group who measured the amount of trichothecene-producing Fusarium present in harvested grain using competitive PCR based on primers derived from the trichodiene synthase gene Tri5 (Pirgozliev et al., Citation2002). They found a strong relationship between the amount of Fusarium present and the DON concentrations, and concluded that there was no evidence that fungicide applications per se increased DON accumulation. However, they also noted that azoxystrobin was significantly less effective against Fusarium than the triazole fungicide used for comparison, metconazole. A more recent study examined the expression of Tri5 in wheat infected by F. graminearum, with or without application of azoxystrobin; neither Tri5 expression nor DON levels differed significantly between the sets of fungicide-treated and control plants (Hallen-Adams et al., Citation2011). The highest Tri5 expression was at the infection front in asymptomatic kernels, confirming the role of DON as a virulence factor and explaining the disconcerting fact that healthy-appearing harvested grain could contain high levels of DON (Hallen-Adams et al., Citation2011).
Fungicide application, timing and DON accumulation
It is evident that the timing of application of strobilurin fungicides may affect DON accumulation. When strobilurin fungicides were applied after pathogen inoculation, or in plots exposed to natural inoculum, DON accumulation rose (Simpson et al., Citation2001; Hollingsworth & Motteberg, Citation2004; Blandino et al., 2009), whereas applying fungicides (both azoles and strobilurins) before inoculating wheat with Fusarium pathogens had little effect on DON accumulation (Pirgozliev et al., Citation2002; Haidukowski et al., 2005). Haidukowski et al. (Citation2005) concluded that this was either because the treatment was preventative or because the DON-decreasing effect of tebuconazole was greater than the DON-increasing effect of azoxystrobin. There is other evidence to support the early application of a strobilurin fungicide (for improved agronomic traits) during stem elongation, and an azole fungicide application for FHB control at anthesis to obtain the best results (Blandino et al., 2009). Upon applying such a strategy, Blandino et al. (Citation2009) reported delays in flag leaf senescence (+27%), improved FHB control (+11%), higher yield (+32%) and reduced DON contamination (−45%). The two fungicides have different modes of action, with azole fungicides inhibiting ergosterol biosynthesis in fungi, and the stobilurins inhibiting mitochondrial electron transport by binding the Qo site of cytochrome bc1 complex (Audenaert, Citation2010).
Multiple fungicide applications may or may not provide additional control over disease development and DON accumulation. Using inoculum in the form of Fusarium-infested oat grain at GS 30, the efficacy of prothioconazole applied at GS 31, 39 or 65, on FHB disease and DON accumulation in winter wheat was shown to increase with later timing of application, but the best results came from plots that had received all three applications (Edwards & Godley, Citation2010). Control of FHB after fungicide application at GS 31, 39 and 65 was 50, 58 and 83%, respectively, but with all three applications, disease control reached 97%. A similar effect was seen in reducing DON accumulation. Applying fungicides at GS 31, 39 and 65 reduced DON accumulation by 27, 49 and 57%, respectively, and by 83% with all three applications (Edwards & Godley, Citation2010). However, another winter wheat study reported that a single application at GS 59 of a mix of chemicals that included an azole produced lower, but not statistically different, levels of DON compared with untreated plots. By contrast, grain from plots receiving two and three applications (GS 31 + 59, GS 31 +37 +59) had higher DON levels (Giraud et al., Citation2011). A Japanese study recommended later applications at 20 days after anthesis, in order to reduce Fusarium-damaged kernels (FDK) and mycotoxin contamination in harvested grain (Yoshida et al., Citation2012). However, it is unlikely that producers in Canada would adopt a strategy with such a short pre-harvest interval (R.P. Roberts, Pest Management Regulatory Agency Infoserv, personal communication).
Crop rotation
In addition to cultivar selection and fungicide application, cropping history and the type of tillage affect FHB disease severity and DON accumulation. In some regions, a wheat–maize rotation is still practised, which increases the risk of FHB disease and higher DON content in grain (Oldenburg et al., Citation2007; Vogelgsang et al., Citation2011). Even in such a rotation, the resistance level of the selected wheat variety had the greatest effect on FHB. The risk of FHB was further reduced when residues of maize were treated with a field shredder followed by a rotary tiller, a forage harvester or a forestry mulcher. A fourth treatment using the field shredder, plus the other three all significantly reduced DON content in grain, although not consistently, to levels below the maximum of 1.25 ppm permitted in Europe (Vogelgsang et al., Citation2011). There has also been work to elucidate the effect of building up soil microbial populations that are antagonistic to Fusarium spp. (Perez et al., Citation2008).
Effects of changing climate
Other influences on FHB development studied recently include the potential effects on crops and pathogens of climate change (Chakraborty & Newton, Citation2011). The disease is driven by the environment. Warm humid weather or rain at anthesis, when the wheat crop is most susceptible, favours disease development, resulting in white to pink, shrivelled FDK, loss of yield, altered end-use quality, mycotoxin accumulation in the grain, and poor germination (Gilbert & Tekauz, Citation2000). The main pathogen in North America is F. graminearum, and annual disease surveys reveal increased percentages of isolations of this species from wheat from around 75% in the mid-1990s, to more than 95% since 1998 (Gilbert et al., Citation1996, Citation1999). Fusarium graminearum is favoured by warmer climates, whereas F. culmorum, the other principal DON-producing species, occurs in cooler climates. In the last decade, F. graminearum has become more prevalent in parts of Europe including the Netherlands (Waalwijk et al., Citation2003), England and Wales (Jennings et al., Citation2004; West et al., Citation2012) and Germany (Miedaner et al., Citation2008).
The effects of climate change may be manifold, but a single factor may be responsible for increased FHB disease. Corn/maize is now grown in southern England due to a warmer and longer growing season. As temperatures are predicted to rise an additional 2 °C in winter and 4 °C in summer (West et al., Citation2012), crops would then flower and mature an estimated 2 weeks earlier by 2050 (Semenov, Citation2009). An increase in maize production may very well provide more inoculum of F. graminearum, exacerbating the existing problems with FHB. In Canada, the disease re-emerged in the early 1990s in the eastern Prairies, and after two decades, is still considered the most important pathogen on cereals (Gilbert & Tekauz, Citation2011). A contributing factor may have been the change in precipitation patterns that were observed for the years in which the disease was first seen. Compared with the 30-year norms, the month with highest precipitation has changed from June to July, the month in which anthesis occurs in spring wheat on the Prairies. Similarly, in Brazil, FHB outbreaks have occurred with increasing frequency during the same period (Del Ponte et al., 2009). Del Ponte et al. (Citation2009) assessed the effects of climate variability over a 30-year period at Passo Fundo, Brazil using a crop-disease model. They suggest FHB epidemics in Brazil now occur with greater frequency because of increased rainfall linked to warmer sea surface temperatures in the Pacific Ocean.
Biocontrol
General considerations
Fungicides are not ideal means to control pathogenic fungi. Results of field fungicide trials are often inconsistent (Heier et al., Citation2005; Hollingsworth et al., Citation2006) and effective controls are desired that harm the environment less than do chemicals. These considerations have prompted the search among a wide array of organisms to identify antagonists of Fusarium spp. Bacteria predominate among such antagonists, but fungi and yeasts have also been identified. One conclusion of many studies is that biocontrol agents (BCAs) may most effectively be used as part of an integrated management programme to reduce, rather than completely replace, the chemical load on the environment (Palazzini et al., Citation2007; Zhang et al., 2007; Xue et al., Citation2009).
Fungal antagonists
Some of the difficulties associated with screening, detecting and identifying effective BCAs include the large numbers that need to be screened initially to determine which have the potential to restrict or prevent a pathogen's growth. Moreover, the isolates that work well under laboratory conditions need to be tested in the field. Luongo et al. (Citation2005) examined isolates of an array of genera obtained from varied sources of necrotic plant tissue to identify and select those that could suppress saprophytic colonization and sporulation of toxigenic Fusarium spp. on wheat straw (F. graminearum, F. culmorum) and on corn stalks (F. graminearum, F. culmorum, F. proliferatum (Matsushima) Nirenberg and F. verticillioides (Sacc.) Nirenberg). Of 135 isolates screened, 11 isolates of Clonostachys rosea (Link: Fries) Schroers, Samuels, Seifert & W. Gams, consistently suppressed sporulation of F. graminearum and F. culmorum on wheat straw and of all four Fusarium spp. on cornstalks under laboratory conditions. However, when these and other antagonists, including Cladosporium cladosporioides (Fr.) de Vries and F. equiseti (Corda) Sacc., were tested under field conditions, results were inconsistent. This may have been due to the effects of different micro-climates on the antagonist–pathogen interactions, and points to the intimate knowledge required of the ability of a successful BCA to adapt to fluctuating environmental conditions (Palazzini et al., Citation2009). Two of the C. rosea isolates, Cr016 and Cr1457, tested by Luongo et al. (Citation2005) were further tested on wheat stubble in Argentina (Palazzini et al., Citation2012). Of the two, Cr1457 was more effective than Cr016, but its biocontrol effect was observed only against F. graminearum, and not against other Fusarium spp. including F. verticillioides and F. avenaceum. In the same study, isolates of Trichoderma harzianum Rifai and Trichoderma viride Pers.: Fries were weaker antagonists of F. culmorum and F. graminearum than C. rosea and other Fusarium spp. (Luongo et al., Citation2005). Other researchers also report differences in biocontrol effectiveness among isolates of the same species. Inch & Gilbert (Citation2007) found that only four of 10 T. harzianum isolates reduced perithecial production on wheat straw to a level similar to that of isolate T-22 (Plant ShieldTM). Moreover, Luongo et al. (Citation2005) found no correlations between isolates that were effective against F. culmorum and F. graminearum on wheat, and the same antagonist isolates tested against different Fusarium pathogens on maize residues.
Trichoderma spp. are among the most effective fungal BCAs for many plant pathogens (Inch & Gilbert, Citation2011), although, as stated above, several other fungal genera (e.g. Clonostachys and Cladosporium) have been found to be effective (Luongo et al., Citation2005). The more recent literature has elucidated the modes of action of BCAs. Expression of the Trichoderma chitinase genes ech42 and nag1, which contribute to biocontrol activity, was monitored on maize residues using goxA as a gene fusion reporter (Lutz et al., Citation2003). Deoxynivalenol (DON)-producing Fusarium spp. repressed nag1-gox, while strains which did not produce DON, or in which trichothecene biosynthesis was impaired because the tri5 gene was disrupted, did not affect chitinase gene expression of T. atroviride Rifai strain P1. The DON repression was specific for nag1-gox and had no effect on ech42, the second chitinase gene studied. Rather, the repression provided evidence that DON might play a role in the defence of F. graminearum against T. atroviride strain P1 (Lutz et al., Citation2003). Naef et al. (Citation2006) studied the effects of DON production in the defence of F. graminearum against T. atroviride in autoclaved post-harvest maize residues. They used a wild-type F. graminearum strain and derived from it a mutant in which DON production was prevented by interruption of the tri5 gene. They showed that biomasses of both the wild type and its mutant F. graminearum strains were reduced in the presence of T. atroviride by 15% and 16% for wild-type and DON minus strains, respectively. However, biomass of T. atroviride itself was reduced even further when co-inoculated, by 50% and 55%, with the wild-type and DON minus strains, respectively. DON was detected only in tissues inoculated with the wild-type F. graminearum and DON production per unit biomass of wild-type F. graminearum was significantly lower in joint culture with T. atroviride than in solitary culture. Fungal biomass of the DON-plus and DON-minus F. graminearum strains did not differ, refuting the earlier conclusions of Lutz et al. (Citation2003) that DON production provides the wild-type F. graminearum strain with a competitive advantage against T. atroviride.
Other species of Trichoderma, namely T. gamsii Samuels & Druzhinina, sp. nov. and T. volutinum, also reduced DON production by F. graminearum when co-inoculated on a rice substrate (Matarese et al., Citation2012). At 7 days, there were no differences among the strains. However, by 14 days, T. gamsii 6085 had reduced F. graminearum biomass by 90%, and DON levels by 92% of that produced when F. graminearum was grown alone. Two other Trichoderma strains, T. gamsii 6317 and T. volutinum 4837, reduced DON production in F. graminearum by 60–67%. Grown alone, all the chitinases of T. gamsii 6317 were expressed at low levels, but in the presence of F. graminearum, all were expressed strongly except for ech42. Other Trichoderma strains expressed a different set of chitinase genes (Matarese et al., Citation2012). These results support previously reported findings that different isolates respond differently to triggers or stimuli, a finding which can impede the search for effective BCAs.
As reported by Luongo et al. (Citation2005), Clonostachys spp. were found to be effective BCAs, and indeed more effective than Trichoderma. Another C. rosea strain (ACM941) has been studied extensively (Xue et al., Citation2009). It was able to reduce Gibberella zeae perithecial production on a variety of substrates. The data were sufficiently robust to establish that the reduced level of perithecial production did not differ from the reduction achieved by the fungicide tebuconazole. Sprayed onto heads of wheat at anthesis, 2 days before inoculation with F. graminearum, C. rosea significantly reduced the number of infected spikelets and FDK in greenhouse experiments. In the field, it reduced FHB index, FDK and DON content in kernels significantly. However, these effects were less than those achieved by tebuconazole. Nonetheless, C. rosea strain ACM941 is a promising BCA which might be used effectively in an integrated FHB management programme.
Yeasts as antagonists
Compared with fungi and bacteria, there are fewer yeast strains that have been identified as effective BCAs against Fusarium spp. Field tests of several Cryptococcus spp. did show decreased FHB disease severity by as much as 50–60% (Khan et al., Citation2004). The effects of the yeasts could be seen more clearly when deployed to protect a susceptible wheat cultivar, although the lowest levels of disease occurred when antagonists were applied to the moderately resistant wheat cultivar ‘Freedom’. The effects were not uniform from year to year. For example, the negative control treatment (wetting agent plus buffer) resulted in low disease severity in one year, whereas in other years disease severity was always equivalent or higher than the untreated control. The effectiveness of the yeasts depended on dose, the carbon:nitrogen (C:N) ratio of the medium on which they were grown, and the Fusarium resistance of the wheat cultivar used in the field. When tested on the FHB-susceptible winter wheat ‘Pioneer 2545’, one antagonist was effective at low concentrations of colony-forming units (CFU), while another was effective at high CFU concentrations (Khan et al., 2004).
In subsequent experiments, Cryptococcus flavescens strain OH182.9 was tested for its ability to reduce FHB disease under greenhouse conditions, both alone and in combination with chemical inducers including salicylic acid (SA), sodium salt of salicylic acid (NaSA), isonicotinic acid (INA) and DL-β-amino-n-butyric acid (BABA) (Zhang et al., Citation2007). When sprayed alone or in combination onto wheat heads 3 days before inoculation with F. graminearum, NaSA and INA at 10 mM concentration reduced FHB severity compared with non-treated controls, whereas none of the chemical inducers reduced FHB severity at 1 mM when applied in a similar inoculation regime. It was striking that the lower dose of all treatments (except SA) if applied earlier, i.e. at 10 days before inoculation, significantly reduced FHB compared with non-treated controls. This clearly pointed to induced systemic resistance as being, in part, responsible for reducing FHB disease. The most effective inducer of resistance to FHB at low concentrations appeared to be INA, and the yeast strain OH182.9 alone did not significantly reduce FHB beyond levels achieved by chemical inducers (Zhang et al., Citation2007). The authors conclude that improved control of FHB might be achieved using chemical inducers in combination with BCAs such as C. flavescens OH182.9.
Bacteria as antagonists
The most widely studied genera of bacteria for the purposes of biocontrol include Bacillus (Shali et al., Citation2010; Dunlap et al., Citation2011), Pseudomonas and Streptomyces (Palazzini et al., Citation2007). Palazzini et al. (Citation2007) also investigated the effectiveness of a Brevibacillus sp., and other species and genera including Paenibacillus polymixa (He et al., 2009), Nocardioides and Deosia (Sato et al., Citation2012) and Lysobacter enzymogenes (Jochum et al., Citation2006; Li et al., Citation2008) are other potential candidates.
Some of the molecules and compounds produced by Bacillus spp. both repress growth of DON-producing fungal species, and reduce DON accumulation in the cereal host (Wang et al., Citation2007; Chan et al., Citation2009). The fengycins, for example, are antimicrobial peptides and members of a prominent class of membrane-active lipopeptides. Ramarathnam et al. (Citation2007) screened Bacillus spp. for genes encoding peptide synthetase in the biosynthetic pathways that produce fengycin and bacillomycin D. It has been known for more than two decades that B. subtilis produces these compounds (Vanittanakom, Citation1986). Recent advances in mass spectrometry have provided more reliable methods for identifying these and other metabolites in microbial systems, making it easier to discover BCA candidates (Dunlap et al., Citation2011). Another tool for sensitively detecting such antimicrobial compounds is matrix-assisted laser desorption ionization-time of flight-mass spectrometry (MALDI-TOF-MS) which has been used to detect fengycin and bacillomycin D (Ramarathnam et al., 2007; Dunlap et al., Citation2011).
Metabolic bases of antagonism
Of all the antifungal metabolites produced by microbes, chitinases have received the most attention. Investigations of the antifungal properties of chitinases from species such as B. pumilis (Shali et al., Citation2010) and Pseudomonas fluorescens (Khan & Doohan, Citation2009a , Citation2009b ) have characterized their optimization and specificities. For example, B. pumilis strain SG2, which inhibits hyphal extension of F. graminearum, was found to produce two different chitinases. However, adding glucose as a carbon source, rather than colloidal chitin, to the culture medium, switched off the expression of these chitinases and abolished the inhibition of fungal growth by B. pumilis SG2 (Shali et al., Citation2010). Certain P. fluorescens strains succeeded in controlling FHB symptoms caused by F. culmorum if they were applied before inoculation rather than after (Khan & Doohan, Citation2009a ). However, a more effective reduction of disease severity and DON accumulation was achieved with chitosan, which elicits defence reactions such as phenylalanine ammonia-lyase and peroxidase activity (Khan & Doohan, 2009b). Chitosan was more effective than the bacterium, which in turn was more effective than crude chitin extracts from crab shells or crude chitosan-based formulations prepared from crab and egg shells (Khan & Doohan, Citation2009b ).
Lysobacter enzymogenes strain C3 is another bacterium that inhibits fungi by the lytic activity of the chitinases, glucanases and antibiotics it produces (Jochum et al., Citation2006). Induced systemic resistance, which has the advantage of not being constrained by the environmental factors that often limit BCAs’ survival, was investigated as one of the means by which L. enzymogenes exerts its potency. Strain C3 was most effective as a whole chitin broth mixture combining living cells of L. enzymogenes and the antifungal factors it excreted into the culture medium. This mixture suppressed disease development when applied to wheat plants one day before they were inoculated with conidia of F. graminearum whether added as a soil drench or as a foliar spray, while C3 living cells on their own failed to induce a systemic resistant response against FHB. Only the spikelets which had been sprayed benefited from the treatment with L. enzymogenes (Jochum et al., Citation2006). This agent was further characterized by describing a heat-stable antifungal factor (HSAF) isolated from C3 and identifying a hybrid polyketide synthetase gene which coded for all the HSAF compounds (Li et al., Citation2008). When C3 mutants were generated by disrupting specific domains of the polyketide synthase non-ribosomal peptide synthetase gene, the mutants ceased to produce the HSAF-associated compounds and simultaneously lost antifungal activity. The principal chemical compound of HSAF was identified as a polyketide antibiotic, dihydromaltophilin, which belongs to the family of antibiotics that includes maltophilin and zanthobaccins. The authors of this work argue that strain C3 could serve as a potential model for understanding the biosynthesis and activity of a whole family of antibiotics, allowing novel strains of BCAs to be identified and forming the basis for developing new fungicides (Li et al., Citation2008). Although HSAF on its own was very active against F. graminearum in vitro, C3 mutants lacking HSAF controlled FHB disease as effectively as wild-type strain C3. Thus the key element of the biocontrol exerted by C3 may have been the induction of systemic resistance (Li et al., Citation2008).
Biocontrol agents and DON accumulation
To control FHB effectively, it is as essential to reduce mycotoxin accumulation as it is to inhibit fungal growth. Thus recent work has begun to emphasize the efficacy of BCAs in reducing mycotoxin content as well as disease symptoms, as emphasized, for example, by He et al. (Citation2009). Two strains of Paenibacillus polymyxa were identified that reduced F. graminearum colonization by more than half and DON accumulation by as much as 85–89% while increasing 100 kernel weight (He et al., 2009).
Interesting differences were found between Gram-positive and Gram-negative strains of bacteria in their ability to use DON as a carbon source in the growing medium (Sato et al., Citation2012). Thirteen strains of aerobic bacteria were isolated from a variety of substrates. All were able to reduce DON concentrations from 100 ppm to less than 0.5 ppm. Nine of the strains belonged to the Gram-positive genus Nocardioides while the remaining four belonged to the Gram-negative genus Devosia. Gram-positive bacteria such as Nocardioides used DON as a carbon source, but bacteria of the Gram-negative genus Devosia did not. Moreover, Gram-positive bacteria needed to be pre-incubated in medium containing DON to degrade DON most effectively. The metabolites produced by the Gram-positive and Gram-negative strains in the DON-containing media also differed, suggesting that they degraded DON following different pathways (Sato et al., Citation2012).
In Argentina, two bacterial strains were also identified, Brevibacillus sp. strain BRC263 and Streptomyces sp. strain BRC87B, that were simultaneously able to reduce the severity of FHB disease and the accumulation of DON mycotoxin (Palazzini et al., Citation2007). Further research by this group aimed to optimize the efficacy of BCAs for field conditions. Among factors which might render the agents less effective is periodic lack of freely available water. In order to select populations well-adapted to osmotic stress, they chose their growth medium accordingly (Palazzini et al., Citation2009). They found B. subtilis strain RC218, which had been selected in a medium amended with NaCl to provide 0.97aw , gave the best control of FHB under greenhouse conditions. It follows that bacteria can survive better during the stages of formulation, processing, storage and application if they have been selected for physiological adaptation by osmotic treatments and accumulation of compatible solutes.
Making biocontrol agents field-ready
A BCA applied under field conditions cannot be effective if it fails to survive. Ensuring that the formulation preserves biomass, and promotes viability during stabilization, drying and rehydration are essential elements for success (Schisler et al., Citation2004). In the field, BCAs are exposed to the degrading effects of UV radiation. Accordingly, Schisler et al.’s (Citation2004) review highlights the importance of identifying compounds that will protect bacteria. Adding UV protectants, such as sodium salt of lignin or the optical brightener Blankophor™ BBH improved the survival of cells, not only of those exposed to 6 hours of UV light, but also the control cells which received no UV radiation (Schisler et al., Citation2004). The authors caution that unless the protocols for discovering, producing and stabilizing the agents are properly conceived, their potential as commercial BCAs will be compromised.
If a BCA is to be commercially successful, it must have a reasonable shelf life and freeze-drying is a common expedient to this end. However, the formulation of growth medium on which a BCA is reared does influence its subsequent amenability to freeze-drying. For example, media with different C:N ratios and additional carbon loading in a semi-defined complete liquid medium were studied for the yeast Cryptococcus nodaensis strain 182.9 (Zhang et al., Citation2005). Cells survived better, in general, in the medium with a higher C:N ratio (30:1) than in media with lower C:N ratios (11:1, 15:1) after freeze-drying. Moreover, cells harvested from the medium after 48 hours incubation, rather than 72 hours, also survived better. For the most part, when cells were harvested at 48 hours, they were more effective in reducing FHB, whereas after 72 hours, cells almost uniformly failed to exert biocontrol. All the experiments were conducted using a standard carbon loading of 14 g L−1, as earlier studies had shown cells survived better after freeze-drying if grown in this medium. Subsequent experiments tested carbon loading of 7, 21 and 28 g L−1 and again showed superior survival of cells after 48 hours as opposed to 72 hours. Cell survival decreased dramatically after 72 hours in medium with 14 g L−1 carbon loading, but there was a significant interaction between carbon loading and cultivation time for all sampling time points (Zhang et al., Citation2005).
Potential BCAs should be isolated from the plant part which will be their target application site. In the case of FHB we must consider the anthers, which lead to the stamens and developing kernels. In the 1970s, choline and betaine in flower tissues was reported to stimulate the growth of F. graminearum and promote infection (Strange et al., 1974). Nkongolo et al. (Citation1993) had questioned the importance of these compounds in disease expression, as anther extracts of the resistant cultivar ‘Nobeoka Bozu’ stimulated growth, while the converse was true for anther extracts from a susceptible wheat cultivar. It followed that anthers could not be the main factor determining relative susceptibility of wheat spikes to attack by F. graminearum. More recently, other studies, which re-investigated the findings of Strange et al. (Citation1974), failed to confirm their results (Engle et al., Citation2004). Working from the hypothesis that differences in bacterial metabolism accounted for the findings of Strange's group, Schisler et al. (Citation2006) isolated bacteria from wheat anthers and examined them for their ability to metabolize choline. Choline-metabolizing strains numbered 123 out of 738 strains, of which 31 reduced FHB disease severity by at least 25% and 17 by at least 50% in greenhouse tests. Five strains were selected for field testing and three were as effective as the fungicide Folicur® for disease control. The low percentage of strains identified, however, would preclude choline metabolism as a predictor of biocontrol effectiveness (Schisler et al., Citation2006).
Even if a candidate BCA is in a suitable formulation it will not be effective unless it is deposited properly. This may account for the lack of consistency of L. enzymogenes strain C3 treatments (Jochum et al., Citation2006). This strain C3 needs to be uniformly deposited on florets and failure to do so probably accounts for the inconsistency of the effects of C3 treatments in the field as opposed to laboratory or greenhouse conditions (Jochum et al., Citation2006). This limitation is addressed in an indirect approach in which crops are grown in such a way that indigenous populations of microflora, antagonistic to the survival of F. graminearum in residues, are increased (Perez et al., Citation2008). To accomplish this, the Perez group examined the effect of incorporating green manures into soil in both greenhouse and field studies. The relevant parameters that were monitored from planting until 3 or 6 months after incorporating green manures, were density of soil bacteria, streptomycetes, and both density and inhibitory activity of antagonists. In general, as densities of streptomycetes and of F. graminearum antagonists increased, F. graminearum survival was reduced, although results were not consistent across all trials (Perez et al., Citation2008).
The pathogen
Taxonomy and systematics
Advances in molecular techniques have enabled advances in our understanding of how pathogens and hosts interact. Several reviews provide the most current description of the progress research has made by applying high-throughput genomic and phenomic technologies (Trail, Citation2009; Xu & Nicholson, Citation2009; Kazan et al., Citation2012).
In the mid-1990s, when FHB was becoming recognized as the most serious crop disease threat, F. graminearum Group 1 was considered a separate species and named Fusarium pseudograminearum O’ Donnell & T. Aoki sp. nov. with the teleomorph Gibberella coronicola T., while Group 2 retained the name Fusarium graminearum Aoki & O'Donnell, sp. nov. (Aoki & O'Donnell, Citation1999). Comparing DNA sequences of various genetic loci has led to recent taxonomic changes to F. graminearum, as reviewed by Glenn (Citation2007). The research findings have led phylogeneticists to fragment F. graminearum from a single species, into several species belonging to the same clade (Aoki & O'Donnell, Citation1999; O'Donnell et al., 2000). Lineages within the original F. graminearum Group 2 were then identified and named as nine separate lineages (O'Donnell et al., 2000, 2004; Ward et al., Citation2002). Analysis of additional isolates of F. graminearum, and use of multilocus DNA sequence data from more genes, added two more species to the complex (Starkey et al., Citation2007), and even more species have been added to render the current number of 14 (O'Donnell et al., 2008; Yli-Mattila et al., Citation2009; Sarver et al., Citation2011). The lineage found in North America is lineage 7 and has been designated F. graminearum sensu stricto (s.s.). Differences exist between F. graminearum s.s and F. boothii O'Donnell, T. Aoki, Kistler et Geiser, sp. nov. (lineage 3) (Malihipour et al., Citation2012), and between F. graminearum s.s. and F. meridionale T. Aoki, Kistler, Geiser et O'Donnell, sp. nov. (lineage 2) (Spolti et al., Citation2012). They were less aggressive than F. graminearum s.s., and F. meridionale distinguished itself by producing fewer spores, and germinating and growing more slowly. However, experiments proving that nine of the new species including F. graminearum s.s. (lineage 7) reported by O'Donnell et al. (Citation2000) were inter-fertile with at least one of three female tester strains of F. graminearum lineage 7 (i.e. a single species at the biological level), call into question whether the changes identified at the phylogenetic species level are sufficient to confer full species status on the lineages (Jurgenson et al., Citation2002; Bowden et al., Citation2004; Leslie & Bowden, Citation2008). Leslie & Bowden (Citation2008) noted two substantive problems with the interpretation that had split F. graminearum into 14 species. First, the phylogenetic species concept is applied retrospectively and the possibility therefore cannot be excluded that the changes in DNA sequence represent incipient speciation. On the other hand, if over evolutionary time these species drift back together, the species concept, based on inter-fertility, would be validated. This leads to the second problem. It leaves the question open as to what meaning can be ascribed to 14 designations for what may be a single species. A consequence of such a splitting might be that regulators would feel compelled to impose barriers on grain movements based on species names, amounting to distinctions without a difference (Leslie & Bowden, Citation2008).
Pathogen life cycle
The sexual cycle of F. graminearum has recently been described in detail (Trail & Common, Citation2000; Guenther & Trail, Citation2005; Trail, Citation2009). The fungus overwinters on F. graminearum-infested crop debris on the soil surface. In the spring, perithecia develop, from which ascospores are forcibly discharged as the primary inoculum (Trail et al., Citation2005b ). Guenther & Trail (Citation2005) found that perithecia developed on the internode tissues of the host, where they emerged only above the stomata, and on the nodes of wheat stems where they were associated with stomata and silica cells. The chromosome complement of the mycelium is haploid for most of the life cycle (Trail, Citation2009). A single mating type locus (MAT) with two idiomorphs, Mat 1-1 and Mat 1-2, regulates sexual development in this homothallic fungus. If one of the genes is deleted the mutant is self-sterile but still capable of out-crossing (Lee et al., Citation2003).
In the initial phase of sexual development hyphae with binucleate cells form; the nuclei are genetically identical as the fungus is homothallic. Dikaryotic hyphae growing in the chlorenchyma and substomatal cavities initiate development of perithecia (Trail et al., Citation2005a ). The dikaryotic stage allows for somatic hybridization and recombination between nuclei without meiosis. The trigger for induction of the dikaryotic stage is unknown (Trail et al., Citation2005a ). Before the perithecia form, dense mats of hyphae containing abundant lipid bodies develop. This sequestration may provide the fungus with the energy it needs for the next stages (Trail, Citation2009). The binucleate hyphae coil and form perithecial initials composed of undifferentiated uninucleate cells which, in conjunction with stomata and silica cells, are the overwintering structures (Trail & Common, Citation2000; Guenther & Trail, Citation2005).These cells enlarge and remain uninucleate except when they are dividing. Once the ascogenous cells develop, apical paraphyses begin to elongate and become attached to the hymenium. Within the immature ascus, a diploid nucleus forms and undergoes meiotic and mitotic division, giving rise to eight haploid ascospores. As the perithecia mature, the ascospores arrange themselves in a biseriate fashion in the unitunicate asci. The mature perithecia consist of three intergraded layers, the outer, middle and inner layers. The outer layer is composed of thick-walled cells, 2–3 cells deep that are tubular and highly vacuolated. The middle layer consists of flattened, highly granulated cells. The inner wall consists of round cells with thin walls, 2–5 cells deep (Trail & Common, Citation2000; Guenther & Trail, Citation2005).
Pathogen dispersal
The mechanism by which ascospores discharge has also been investigated over the last decade (Trail & Common, Citation2000; Trail et al., Citation2005b ; Hallen et al., Citation2007; Hallen & Trail, Citation2008). Ascospores are forcibly discharged through a small tear or slit, the ostiole, at the apex of the perithecium (Trail & Common, Citation2000). Rainfall induces the ascus wall to rupture. As the relative humidity (RH) increases, the osmotic pressure rises within the perithecium due to an influx of mannitol and ions of potassium and chloride, leading to the build-up of turgor pressure and the forcible discharge of the ascospores (Trail et al., 2002). Analyses revealed the presence of three major osmolytes in the epiplasmic fluid that accompanied the ascospores. The critical role of ionic movement was shown by the selective inhibition of K+ and Ca++ ion channels preventing ascospore release. The turgor pressure generated by ion fluxes and mannitol accumulation is so great that it launches ascospores at an estimated speed of 34.5 m s−1 with an acceleration of 870000 g, the fastest recorded acceleration in biological organisms (Trail et al., Citation2002, Citation2005b ).
By this increased focus on understanding the fundamental biology of the pathogen, the last decade has seen changes to some earlier assumptions. Fernando et al. (Citation1997) found wind-driven gradients in plots inoculated with Gibberella zeae ascospores which suggested a short dispersal distance, indeed just metres from a source of inoculum. These results suggested that ascospores would need to land on susceptible host tissue and germinate rapidly after discharge to infect successfully. However, the data gained from using trap plants located some kilometres from wheat or corn fields indicated that the pathogen's spores dispersed over long distances (Francl et al., Citation1999). Data to strengthen this interpretation were supplied by remote-piloted vehicles which sampled air in the planetary boundary layer between 50 m and 1 km above the surface of the earth (Maldonado-Ramirez et al., 2005; Schmale et al., Citation2006). To address the question of whether spore releases were diurnally limited (Inch et al., Citation2005), perithecia were placed in a wind tunnel under a regime designed to simulate constant rain and varying day and night lengths (Trail et al., 2002). Ascospores were discharged under light and dark conditions. The numbers released during the light periods were 8–30% greater than at night and reached maximum discharge under conditions of constant light and 92% RH (Trail et al., 2002).
If the spores dispersed over long distances, did they remain viable? Beyer & Verreet (Citation2005) examined how age of spores since time of dispersal and environmental factors affected germination. They observed that ascospores lost their ability to germinate within a few minutes if they were exposed to low RH. In the natural environment, therefore, viable spores should not be able to disperse over long distances where RH is less than 50%. To determine just how long ascospores remained viable under different humidity regimes, Gilbert et al. (Citation2008) subjected freshly discharged (within 24 hours) ascospores to three temperatures and three different RH levels over intervals of 4, 24 and 48 hours (Gilbert et al., 2008). Unexpectedly, while germination was usually highest at 90% RH and lowest at 60% RH, the still lower RH (30%) tested, had intermediate levels of germination. However, viable ascospores were found at all temperatures and relative humidity levels tested, and all exposure periods, indicating that some ascospores are sufficiently robust to remain viable after release from perithecia under most environmental conditions for extended periods of time.
Ward et al. (Citation2008) showed that 3-ADON populations in North America are more closely related to Italian F. graminearum populations than to sympatric 15-ADON populations, suggesting that the 3-ADON populations in North America are the result of a transcontinental introduction, the precise origin of which is presently unknown. Recently, Schmale et al. (Citation2012) reported the collection of F. graminearum strains from the mesoboundary layer of the atmosphere using autonomous unmanned aerial vehicles at heights ranging from 40 to 320 m above the earth's surface. Strains were collected in all seasons, including autumn and winter when locally produced spores would not be present. The authors propose that aerial transport barriers which separate mobile air masses might be responsible for the long-distance transport of fungal spores over tens to hundreds of kilometres (Schmale et al., Citation2012). The findings of this study are important. They provide proof that F. graminearum chemotypes (3-ADON, 15-ADON and nivalenol (NIV)) are transported together over long distances and might explain, in part, the change in the chemotype of Canadian F. graminearum populations from 15-ADON to 3-ADON.
Pathogen genetics and molecular basis of pathogenicity
The publication of the genome sequence for F. graminearum by the Broad Institute in 2003 (http://www.broadinstitute.org/annotation/genome/fusarium_group/ accessed 29 October 2012) has greatly stimulated research into gene function and the efforts to understand how the pathogen has evolved to occupy its niche (Trail, Citation2009; Kazan et al., Citation2012). When genes that exert putative influences in the pathogen's life cycle are disrupted or deleted in transformants of F. graminearum, their multiple effects on metabolism and pathogenesis are revealed. For example, 15 polyketide synthase (PKS) genes were identified in F. graminearum and functions assigned to five of them (Gaffoor et al., Citation2005). Mycelial growth, relative to colony area of the wild type, was equal for six of the isolates with mutant PKSs, greater for seven and smaller for two, and disrupting the genes neither affected pathogenicity on wheat nor sexual development in vitro. The five PKSs with known function were responsible for producing the mycotoxins zearalenone, aurofusarin and fusarin C, and the black pigment of the perithecium (Gaffoor et al., Citation2005). Intriguingly, the mature perithecia from wheat straw treated with the BCA Trichoderma harzianum produced less pigment and were therefore paler than those from control plates, thus accounting for one of the possible ways this BCA exerts control against F. graminearum (Inch & Gilbert, Citation2011).
The function of several velvet genes has now been characterized. Among other functions, they regulate production of aerial hyphae, production of conidia (either increased-FgVeA and FgVelB, or much reduced-FgVe1), and trichothecene biosynthesis, and therefore affect pathogenicity (Jiang, Citation2011b ; Lee et al., Citation2012; Merhej, Citation2012). The gene FgVe1 also modulates production of aurofusarin pigment (Merhej, Citation2012).
Other studies have characterized the role of a type 2 protein phosphatase (Jiang, Citation2011a ), a stress-activated protein kinase (Nguyen et al., Citation2012) and a gene for ergosterol biosynthesis (Liu, Citation2012). All studies conclude that the proteins exert an influence in several biological processes. For example, the protein phosphatase, FgPtc3, plays a role in cell wall integrity, lipid metabolism, DON production and virulence (Jiang, Citation2011a ), and the FgERG4 gene plays a crucial role not only in ergosterol biosynthesis, but also in vegetative differentiation and virulence (Liu, Citation2012). The F. graminearum orthologue of the Saccharomyces cerevisiae Meyen ex E.C. Hansen HOG-1 stress-activated protein kinase FgOS-2 is a central regulator during the life cycle of F. graminearum, playing a role in regulating secondary metabolism, sexual development, pathogenicity and reactive oxygen species-related signalling (Nguyen et al., Citation2012).
Hallen et al. (Citation2007), using an Affymetrix Gene Chip probe set consisting of 17830 predicted genes, were able to identify over 2000 genes which were expressed only during sexual development. Of these, 175 were orphan genes (only found in association with G. zeae) and 162 were ion transporter genes which included genes for MirA-type siderophores, chloride channels, P-type ATPases and potassium transporters. When G. zeae’s homologue of the CCHI gene for calcium signalling of S. cereviseae was disrupted, perithecia of the mutant strains failed to discharge ascospores and vegetative growth rate in general was slowed (Hallen & Trail, Citation2008). Adding CaCl2 to the growth medium at concentrations of 38.46 and 76.92 mM partially restored the mutants to the wild-type vegetative phenotype and also restored the ability to discharge spores (Hallen & Trail, Citation2008). Cch1, an L-type calcium ion channel and its subunit Mid1, a stretch-activated non-selective channel capable of being permeated by calcium, comprise the high-affinity calcium uptake system (HACS) (Cavinder et al., Citation2011). Mutants of G. zeae defective for either Mid1, or both Mid 1 and Cch1, grew more slowly in the vegetative stage, had abnormal ascospore morphology and discharged their ascospores less forcibly from perithecia. The double mutants were more severely affected than the Mid1 mutants. For the Cch1 mutants, adding CaCl2 restored vegetative morphology and forcible ascospore discharge. Mid1 mutants and the double mutants (Cch1+Mid1) also benefited from added CaCl2, albeit producing abnormal ascospores. Taken together, these results indicate that the Cch1 and Mid1 complex is involved in signalling ascospore discharge (Cavinder et al., Citation2011). Further work on components of the low-affinity calcium system (LACS) has revealed that mutants whose transmembrane protein is disrupted, also fail to produce mature perithecia (Cavinder & Trail, Citation2012). The intracellular siderophore, ferricrocin, is essential for intracellular iron sequestration, and both ascus and ascospore development; G. zeae mutant strains with a deleted NPS2 gene, which encodes a non-ribosomal peptide synthetase responsible for the biosynthesis of ferricrocin, produced perithecia, but the asci looked abnormal and lacked ascospores (Oide et al., Citation2007). For a more comprehensive review that covers research on the molecular aspects of the host–pathogen interaction, see Kazan et al. (Citation2012).
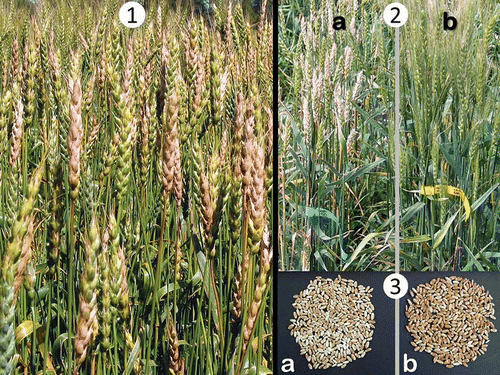
Fig. 1. Manitoba field of hard red spring wheat with fusarium head blight. (Photo A. Tekauz.)Fig. 2. ‘Roblin’ wheat in an inoculated fusarium head blight (FHB) nursery: a , ‘Roblin’ progenitor. b , Line derived from ‘Roblin’ showing epigenetically induced changes including awns and improved FHB reaction.Fig. 3. Grain from ‘Roblin’ wheat from inoculated fusarium head blight (FHB) nursery: a , Grain from ‘Roblin’ progenitor. b , Grain from line derived from ‘Roblin’ which was selected for improved FHB reaction among epigenetic variants.
Mycotoxins
Pathogen chemotypes
Fusarium graminearum produces several trichothecene mycotoxins, including nivalenol (NIV), DON and its acetylated forms 3-ADON or 15-ADON. In Canada, only DON and 3-ADON and 15-ADON have been found. Sampled populations have revealed a shift from isolates that produce DON and 15-ADON to those producing DON and 3-ADON (Ward et al., Citation2008). A higher percentage of isolates of F. graminearum collected in Manitoba between 1998 and 2004 were of the 3-ADON chemotype, whereas prior to 1998, the 15-ADON chemotype was considered the only significant cause of FHB in North America (Miller et al., Citation1991). Strains of F. graminearum were isolated over 4 consecutive years from the check cultivars and lines grown in a FHB nursery, artificially inoculated with an equal ratio of isolates producing either 3- or 15-ADON in addition to DON. To monitor the ratio of 3-ADON to 15-ADON chemotypes recovered, DNA was extracted and isolates identified to chemotype using PCR. In 2008, the ratio of 3-ADON to 15-ADON was 79:21. However, the ratio changed sharply to 55:45, 51:49 and 43:57 in 2009, 2010 and 2011, respectively. Under controlled conditions, the mean percentage of 3-ADON isolates recovered from the same six checks rose with increasing temperatures from 32% at 20 °C to 70% at 28 °C. However, an examination of weather variables over the 4 years of the field study showed no correlation between recovery of 3- and 15-ADON chemotypes and temperatures and precipitation (Gilbert et al., Citation2013). Although the ratio of recovered chemotypes in Manitoba has been closer to 1:1 in recent years, the percentage of 3-ADON isolates is high compared with other parts of North America (Gale et al., Citation2007; Schmale et al., Citation2011). The exception is Prince Edward Island where the proportion of the 3-ADON chemotype has been close to 100% for several years (Ward et al., Citation2008). It is of concern that the 3-ADON chemotype produced more DON than the 15-ADON chemotype in several recent studies. If this link should be consistently confirmed in future surveys and research, overall harvested grain might be affected (Gilbert et al., Citation2010; von der Ohe et al., Citation2010b ).
Mycotoxins in host–pathogen interactions
While the pathogen has evolved to evade the deleterious effects of its own toxins (e.g. toxins moving ahead of the pathogen and preparing an infection front; Hallen-Adams et al., 2011), the host too has an arsenal of defences which are activated upon challenge. Wheat produces antimicrobial compounds as part of its normal development (Glenn, Citation2007). They are the cyclic hydroxamic acids DIMBOA (2,4-dihydroxy-7-methoxy-2H-1,4-benzoxazin-3-one) and DIBOA (2,4-dihydroxy-2H-1,4-benzoxazin-3-one). Many insects, fungi and bacteria are deterred or inhibited by these compounds, yet certain Fusarium pathogens can actively convert them to harmless metabolites. Of 29 Fusarium spp. tested, however, F. graminearum was one of the most tolerant species. It is possible that this tolerance of antimicrobial compounds might explain, in part, F. graminearum’s competitive advantage. It has out-competed other species such as F. avenaceum and F. poae on wheat and barley in Manitoba and southeastern Saskatchewan (Gilbert & Tekauz, Citation2011). Likewise, in parts of Europe, F. culmorum and Microdochium nivale have been displaced by F. graminearum (Waalwijk et al., Citation2003; Jennings et al., Citation2004).
Mycotoxin production appears to be affected by inoculation with more than one species, or several strains of the same species. The species with the competitive advantage (F. graminearum) produced more DON when co-inoculated with F. culmorum, although nivalenol (NIV) production by F. culmorum was also increased. Thus combining inocula produced more toxin (Xu et al., Citation2007). A subsequent review by Xu & Nicholson (Citation2009) suggests that fungi with a competitive advantage do not gain a further advantage from the presence of a weaker competing fungus and total mycotoxin production in mixed inoculations may increase, decrease or remain the same, depending on the fungal species involved and the environmental conditions. They cite work in which four isolates of F. culmorum produced less mycotoxin when co-inoculated than when each isolate was inoculated singly (Xu & Nicholson, Citation2009). Müller et al. (Citation2012) came to a similar conclusion from their studies involving F. graminearum, F. culmorum and two Alternaria tenuissima ([Kunze ex Nees et T. Nees: Fries] Wiltshire) strains. In co-cultures, fungal biomass was up to 460% greater than that of individual cultures, but the method did not allow the pathogen responsible for the increase to be identified. Mycotoxin production was also affected; levels of the Alternaria toxins were depressed in co-cultures with Fusarium spp. While production of DON by F. culmorum was delayed for the first 8 days in co-culture with Alternaria, it increased strongly and exceeded that of individual cultures during the final days of incubation (days 8 to 12). Overall, the study showed that differences in fungal growth and mycotoxin production depended on the specific isolate and the length of incubation during co-culture of two mycotoxigenic pathogens (Müller et al., Citation2012).
Mycotoxins and safety of grain
Concerns over human food and animal feed safety have prompted the European Union (EU) to regulate the permissible levels of trichothecene mycotoxins (Anon., Citation2006). Several surveys have been published to determine to what extent food and feed are contaminated by mycotoxins, and whether or not the concentrations at which they are found fit within the EU regulatory standards (Edwards, Citation2009; Zinedine & Manes, Citation2009; Signorini et al., Citation2012). There are recent studies on the fate of Fusarium toxins after commercial processing which were conducted in order to identify the processing factors that might reduce contamination to levels that fit EU regulatory standards. However, different studies may not always arrive at the same conclusions (Lesnik et al., Citation2008; Scudamore et al., Citation2009; Bergamini et al., Citation2010). While DON is less acutely toxic than other trichothecenes, it is prevalent worldwide in crops used for food and feed, and thus needs to be considered an important issue of food safety (Placinta et al., Citation1999; Binder, Citation2007). In the EU, there is a move to ensure that testing practices including risk assessment, accreditation, guidelines on the analytical methods to use, participation in proficiency testing and use of common definitions/calculations for LOD, LOQ for repeatability, among others, are harmonized and effectively implemented among all members (Binder, Citation2007; Solfrizzo et al., Citation2009). New assays have been developed to rapidly test samples of cereals at the grain elevator. One recently developed offers a rapid (10 min per assay), on-site, lateral flow immunoassay for DON (Liu et al., Citation2012).
Henry (Citation2006) has listed limits of allowable DON levels in feed for the USA, but human food contamination levels for the USA are not included. Meanwhile, the development of contemporary DON standards in Canada remains stalled (Hager, Citation2011). The latter article paints a disturbing picture about the lack of regulatory limits in Canadian cereals. Currently, the only limit in Canada, imposed some three decades ago, is 2 mg kg−1 body weight (2 ppm) for unprocessed soft white wheat and 1 mg kg−1 (1 ppm) for baby food. There are no guidelines for other classes of wheat or processed foods, and the permitted levels are higher than those imposed by the EU (75 mg kg−1 or 0.75 ppm for unprocessed cereals) (Hager, Citation2011). The Food Safety Action Plan (CFIA, Citation2010), tabled by the Canadian Food Inspection Agency, is a report of a targeted survey undertaken to provide baseline data for ochratoxin A and DON mycotoxin levels in Canadian foods. The limited survey (150 samples tested for DON) of consumer products that has been done, showed that more than half had detectable levels, of which the highest was 6.01 ppm in wheat. At a recent meeting of the Joint Expert Committee on Food Additives (JECFA72, Citation2011), the committee proposed a provisional maximum tolerable daily intake (PMTDI) of 1 μg kg−1 body mass (1 ppb) for DON and its acetylated derivatives, as 3-acetyl-deoxynivalenol (3-ADON) is converted to deoxynivalenol (DON) in vivo and therefore contributes to the total DON-induced toxicity. There is a fear such stringent measures would disadvantage Canadian producers and limit their market opportunities when conditions make it impossible for them to grow crops free of FHB damage. Under the Codex Alimentarius Commission, Canada has taken the lead for the working group on DON for the Codex Committee on Contaminants in Foods (CCCF). A first draft has been circulated to member countries which proposes 2 mg kg−1 (2 ppm) in raw cereals, but a limit of 1 mg kg−1 in foods derived from cereals intended for adult consumption and a limit of 0.5 mg kg−1 if intended for infants and young children up to 36 months (CCCF, Citation2011). While waiting for new DON standards to be finalized, Canadian millers have adopted a self-imposed standard of 1 ppm DON in flour which they achieve by blending different lots of wheat (Hager, Citation2011).
Recent developments in breeding for resistance, research on QTLs for FHB resistance, novel approaches
Association between plant height and fusarium head blight
The review of Buerstmayr et al. (Citation2009) of 52 QTL studies provides a base from which new developments can be described. In their overview, they conclude there is a need for further research and elucidation of how Rht dwarfing genes or plant height affect FHB development. The association between plant height and FHB resistance, while first reported by Couture (Citation1982), has since been confirmed for many wheat populations over the last three decades (Mesterhazy, Citation1995; Somers et al., Citation2003), and further supported by the observations of co-localization of QTLs for FHB resistance with QTLs for height (Gervais et al., Citation2003; Häberle et al., Citation2009b ). It has been suggested that the short stature in plants with Rht dwarfing genes renders them more susceptible to soilborne pathogens due, in part, to the proximity of inoculum arising from infested crop residues to the leaves and spikes (Miedaner & Voss, Citation2008). Three major dwarfing genes of Japanese origin have been deployed in more than half of the world's wheat cultivars. The ‘Norin 10’ dwarfing genes, Rht-B1b and Rht-D1b, located on chromosomes 4B and 4D, respectively, are gibberellic acid (GA)-insensitive. The third gene, Rht8c, located on chromosome 2D, is from ‘Aka komugi’ and is GA-sensitive. Several alleles have been identified for Rht genes. The wild-type Rht confers height and the dwarfing alleles Rht‘n’ confer a short, stiff-strawed stature. Rht8c is closely linked to the gene Ppd1 for photoperiod insensitivity. In regions with hot dry summers, Ppd1 shortens the plant's life cycle and promotes dwarfing, increased grain number per spikelet, higher grain fill and consequently higher yield before onset of summer desiccation. The Rht‘n’ dwarfing genes confer yield advantages when plants are not subjected to heat stress (Miedaner & Voss, Citation2008).
To separate the effects of Rht dwarfing genes from epidemiological effects, Miedaner & Voss (Citation2008) used two sets of near-isogenic lines (NILs) which had been developed from the winter wheat cultivars ‘Mercia’ and ‘Maris Huntsman’. Each set comprised plants with different dwarfing genes, either alone or in combination, while the parents both contained the wild-type Rht gene. The three check cultivars all carried the Rht-D1b dwarfing gene, but varied in response to FHB from 2 (resistant) to 7 (susceptible) on a scale of 1–9. The ‘Mercia’ NIL carrying the wild-type Rht allele was the most resistant at all locations in the test. Plants with any Rht gene experienced higher FHB disease levels in both years of the 2-year study, with Rht-D1b exerting the greatest effect. Although it reduced height to a similar extent as Rht-D1b, Rht8c exerted a smaller effect on FHB severity. Plants with Rht-B1b were less susceptible than those with Rht-D1b, and height reduction was less pronounced. Miedaner & Voss (Citation2008) concluded that as linkage with Ppd1 can be broken, Rht8c might be the most favourable option for providing short-statured plants with acceptable levels of FHB resistance in northern Europe.
Srinivasachary et al. (Citation2009) used the same NILs of ‘Mercia’ and ‘Maris Huntsman’ plus a doubled haploid (DH) population derived from ‘Soissons’ (Rht-B1b; Rht-D1a) × ‘Orvantis’ (Rht-B1a; Rht-D1b) in a study to determine if these two dwarfing alleles conferred similar effects on FHB susceptibility. ‘Soissons’ contributed a single major QTL which was linked to the Rht-D1 locus and conferred resistance to FHB. The Rht-B1b allele from ‘Soissons’ appeared to have little or no effect on FHB resistance. Researchers distinguish between different modes of FHB resistance since different genetic sources express different resistance phenotypes: Type I resistance is resistance to initial infection; type II, resistance to spread within the spike; and type III, resistance to toxin accumulation in the grain (Mesterhazy, Citation1995). When their effects in NILs of ‘Mercia’ and ‘Maris Huntsman’ were examined under high disease pressure, both alleles reduced type I resistance. However, Rht-D1b had no effect on type II resistance, while Rht-B1b significantly improved type II resistance (Srinivasachary et al., Citation2009).
Voss et al. (Citation2008) used progeny generated by crosses between winter wheat cultivars carrying either the Rht-D1b allele or the wild-type Rht-D1a allele to determine the influence that different alleles at the Rht-D1 locus exerted on FHB reaction. Within subpopulations, the presence of Rht-D1b reduced plant height and increased FHB severity, but disease ratings varied significantly in all subpopulations, indicating that moderately resistant genotypes with beneficial agronomic traits might be selected. The results from one cross – ‘Apache’/‘Biscay’ – were of particular interest because of the light they shed on the question of the effect exerted by height itself. The French variety ‘Apache’ contains the wild type allele Rht-D1a and ‘Biscay’ has the height-reducing allele Rht-D1b, yet ‘Apache’ was both shorter and less susceptible to FHB than ‘Biscay’. Consequently, plant height per se was not responsible for the total variance in FHB severity. In two other populations segregating for the same two alleles, the presence of Rht-D1b consistently resulted in higher mean FHB ratings compared with subpopulations with the wild-type allele (Voss et al., Citation2008). The latter study concluded that the results implied genetic linkage or pleiotropic physiological effects of Rht-D1b conferring susceptibility to FHB, or conversely, Rht-D1a conferring resistance.
In an attempt to combine data from different sources to reveal co-locations for plant height and FHB QTLs, Mao et al. (Citation2010) conducted a meta-analysis using data from 56 studies. Dwarfing genes affect FHB severity in different ways, and the mechanisms of the associations are complicated. However, the authors concluded that the negative effects of the dwarfing genes and the relationship between plant height and FHB resistance was confirmed by the meta-analysis. Coincident Meta-QTLs (MQTLs) were found on chromosomes 2D, 3A, 4B, 4D and 7A. All FHB QTLs originated from 16 resistance sources, including mainly ‘Sumai 3’ and ‘Wangshuibai’, but also from BW278, DH81 and ‘Ning 8026’. The data suggested that the different resistance sources contain the same FHB resistance, or that resistance genes are clustered in the consensus region, since the meta-analysis aggregated the FHB resistance from ‘Sumai 3’, ‘Wangshuibai’ and the winter wheat ‘Arina’. Four regions for FHB resistance were found on chromosomes 3B (two distinct regions for types II and III resistance), and 5A and 6B (two coincident regions for types I and II resistance), respectively. In some studies, the negative effects of the dwarfing genes were balanced by the presence of two major FHB QTLs (Lu et al., 2011). An F2-derived DH population of 171 lines was produced from the cross ‘Avle’/Line 685. ‘Avle’ is a FHB-susceptible Swedish spring wheat cultivar with the wild type allele Rht-D1a, while Line 685 is a winter wheat with resistance to FHB and the dwarfing allele Rht-D1b. The presence of the Rht-D1 locus explained 38% of the phenotypic variation and was the most important QTL for FHB severity under spray inoculation (testing type I resistance), but had no effect on disease severity after point inoculation (testing type II resistance). The action of the FHB resistance gene Fhb1 was evident after both spray and point inoculation, but was more important for type II resistance after point inoculation. Other QTLs detected included those on 5A and 2BL after spray inoculation and on 2D after point inoculation, which contributed to type I and type II resistance, respectively. A comparison of different combinations of alleles showed that Fhb1 plus the QTL 5A balanced the negative effects of Rht-D1b (Lu et al., 2011). However, as Miedaner & Voss (Citation2008) and Srinivasachary et al. (Citation2009) demonstrated, it is possible to achieve a desirable plant height with Rht-B1b, with a less negative effect on FHB severity than that conferred by Rht-D1b.
In contrast to these studies indicating the genetic effects of the dwarfing genes, other studies have adduced evidence that the effect of plant height per se on FHB resistance is morphological and positive. A recent study by Yan et al. (Citation2011) assessed these effects using NILs containing several distinct Rht dwarfing genes, but with dwarfed plants raised artificially to heights similar to those of isolines lacking Rht genes. Tall isolines consistently had lower FHB severity than their dwarf counterparts when grown and inoculated at their natural heights under controlled conditions. When the dwarf plants were raised to the height of their isolines which lacked dwarfing genes, these differences for the most part disappeared. In only one pair of isolines, comparing presence/absence of Rht-8+Rht-9, were the dwarfed isolines more diseased than their tall counterparts (Yan et al., 2011). Nine different Rht genes were present in the NILs and they were located in several different genomic regions. The authors point out that it would be unlikely for linkages to occur between each of the Rht loci and loci conferring FHB type I susceptibility.
Breeding for resistance – quantitative trait loci
In 2009, Buerstmayr et al. (Citation2009) reviewed the extent of what was then known of quantitative trait loci (QTLs) affecting FHB. Such QTLs had been found to occur on every wheat chromosome except 7D and numerous reports since then have expanded the known range of sources of FHB resistance. Even chromosome 7D has now been implicated in FHB resistance (Cativelli et al., Citation2011). Other landraces from China have been analysed since 2009, including ‘Haiyanzhong’ with a major QTL on 7D and minor ones on 6BS (2), 5AS and 1AS (Li et al., Citation2011), ‘Huangfangzhu’ with three minor QTLs in addition to three major QTLs on 3BS and 7AL (Li et al., Citation2012) and ‘Baishanyuehuang’ which is reported to have QTLs for type II resistance (Zhang et al., Citation2012b ). Related Triticum species such as Triticum macha Dekr. et Men. (Buerstmayr et al., 2011). T. timopheevii (Zhuk.) (Malihipour et al., Citation2008; Cao et al., Citation2009) and the wheatgrasses Thinopyrum elongatum (Host) D.R. Dewey (Wang et al., Citation2010) and Lophopyrum ponticum (Podp.) (Zhang et al., Citation2011) have also been investigated.
Novel QTLs from germplasm of Asian origin include one on chromosome 7A revealed in a ‘Chinese Spring’–‘Sumai 3’-7A disomic substitution line (Jayatilake et al., Citation2011). Two major QTLs mapped to 3BS and 7A. The 3BS QTL was considered to be the same as Fhb1 conferring type II resistance. The second, mapping to 7Ac close to the centromere, was derived from ‘Funo’, one of the parents of ‘Sumai 3’, and conferred type III resistance to DON accumulation. The effects were additive and together the two QTLs explained 56% of the variation. In a doubled haploid (DH) population derived from ‘Kukeihara 14’ and ‘Sumai 3’, Suzuki et al. (Citation2012) identified five known FHB resistance loci: 3BS, 5AS, 6BS, 2DL and 4BS. The 3BS and 5AS QTLs effectively reduced FHB damage but the others also influenced traits such as increased stem and spike length (4BS and 5AS), reduced kernel weight (2DL) and delayed heading (6BS), indicating the problems concerning linkage or pleiotropy when using ‘Sumai 3’. Similar findings were reported for FHB resistance QTLs introgressed into elite Canadian spring wheat germplasm from ‘Nyubai’, ‘Sumai 3’ and ‘Wuhan-1’(McCartney et al., 2007). While most loci were effective against FHB, the most effective, ‘Wuhan-1’ 4B, was also associated with an increased height of 9.3 cm and the ‘Sumai 3’ 5AS locus was associated with reduced grain protein. FHB resistance tended to improve when more resistance QTLs were introgressed (McCartney et al., 2007). There is also evidence that grain shattering may be linked to some of the FHB resistance QTLs in ‘Sumai 3’; one QTL for grain shattering was located just 1.5 cM away from a FHB QTL on 3B (Zhang et al., Citation2006).
Mapping populations have served not only to identify QTLs for resistance, but also those for susceptibility. For example, a gene for susceptibility was found on 2DS deriving from ‘Sumai 3’ (Basnet et al., Citation2012) a finding consistent with reports in the literature going back to the 1980s. The QTL mapping to 3BS in ‘Wangshuibai’ was also studied. Xiao et al. (Citation2011) showed that the loss of the 3BS fragment in ‘Wangshuibai’ resulted in increased susceptibility to FHB, suggesting that the QTL is the same as that found in ‘Sumai 3’. In a ‘Wuhan’/‘Nyubai’ population a QTL for fusarium seedling blight was identified on 5B which was different from the 5B QTL conferring resistance to FHB (Tamburic-Ilincic et al., Citation2009).
Research continues on the Chinese landrace ‘Wangshuibai’, and the expressed sequence tag (EST) project has added more than 500000 ESTs to the public domain. Naji et al. (Citation2008) mapped previously EST-derived STS markers localized to 3BS using an independently derived ‘Wangshuibai’/‘Seri82’ population to assess the stability of such markers. Using MAS, the authors also determined the effect of the genetic background in improving resistance to FHB in wheat. One of nine EST-derived STS markers, STS3B-138, was polymorphic between parental lines as well as between resistant and susceptible F3 plants, indicating that this marker could be used in MAS to enhance FHB resistance in breeding programmes. The 3BS locus is well-served by the marker Fhb1, but in general, it is advantageous for additional QTL markers to be identified, as it has been demonstrated that markers identified in a single mapping population may not automatically be informative for other populations (Xu & Crouch, Citation2008).
It is widely believed that Asian sources of FHB resistance will disrupt the genetic background of elite adapted winter wheat germplasm leading to reduced yields. Acting on this belief, most breeders are reluctant to use them (Häberle et al., Citation2009b ; Miedaner & Korzun, Citation2012). The search has continued, therefore, for FHB resistance not derived from Asian germplasm. Srinivasachary et al. (Citation2008) developed a spring wheat population from the cross RL4137 (a Canadian tall hard red spring wheat derived from ‘Frontana’) with the cultivar ‘Timgalen’. The QTLs identified for FHB resistance included 1B, 2B, 3A, 6A, 6B (6B conferring susceptibility from ‘Timgalen’), 7A and 7D, all with LOD scores above 3. The population was tested in more than one environment and the authors concluded that the disease scores were relatively stable. The 6A and 2B QTLs were associated with weight of infected spikelets. Two minor QTLs for DON were found on 2B and 7A. The 2B QTL explained up to 24% of the phenotypic variance and was derived from ‘Timgalen’. Unfortunately, ‘Timgalen’ carries a large introgressed segment in 2B from ‘T. timopheevii’. This reduces recombination, making it impractical to map precisely the RL4137 2B QTL in a ‘Timgalen’ background.
Buerstmayr et al. (Citation2011) provided evidence of an association between a major QTL for FHB resistance in T. macha at the Q locus. The 5A QTL explained 23% of the phenotypic variation associated with the wild-type allele ‘q’, but it was unclear if ‘q’ had a pleiotropic effect or was closely linked to 5A. The explanatory power of the population lay in the fact that each line was derived from a single BC2 plant, resulting in maximum diversity in the population and a high probability of recombination. Using advanced backcross (AB)-QTL analysis, the 5AL QTL also associated with plant height, spike length, spike density, threshability and glaucousness. The T. macha alleles conferred resistance, elongated lax spikes, height and reduced threshability; the authors concluded, however, that selected lines might still be useful for practical breeding purposes.
A map of chromosome 7e was constructed from two ‘Thatcher’–Lophopyrum ponticum substitution lines ‘K11463’ [7el1 (7D)] and ‘K2620’ [7el2 (7D)] (Zhang et al., 2011). The parents were screened with some 530 markers from wheat chromosome group 7 of which 118 were polymorphic. A map of 7E was constructed with 64 markers. A gene (named FhbLoP) mapped to the distal region of 7EL and conferred type II FHB resistance. Resistance gene Lr19 for leaf rust also mapped to the same location. The authors concluded that the construction of this map was a starting point for exploiting desired genes from 7E in cultivated species, for the closely linked markers would facilitate MAS for pyramiding both FhbLoP and Lr19 in wheat.
Little of the recent literature deals with improving FHB resistance in durum wheat. However, the research group at North Dakota State University has reported some progress. Chu et al. (Citation2011) identified and mapped two QTLs on chromosome arms 5AS and 5AL in accession PI 277012 explaining up to 20 and 32%, respectively, of the variation in FHB severity, and providing a simultaneous reduction in FDK and DON accumulation in grain. The experimental line PI 277012 has T. timopheevii in its background and was mistakenly described as T. dicoccum. However, based on plant morphology and cytogenetic analyses, it was confirmed as hexaploid (Chu et al., Citation2011). Nonetheless, the markers are currently being used for marker-assisted introgression into both durum and hard red spring wheat cultivars. Sources of resistance have been identified in other tetraploid accessions, although none appears to be comparable to that of ‘Sumai 3’. However, breeding efforts have yielded durum cultivars such as ‘Divide’, which has improved FHB resistance and is widely grown in North Dakota (Cai et al., Citation2011). Others have found T. timopheevii to be a source of FHB resistance. The registered germplasm TC 67 incorporates this source and shows better resistance in trials than the resistant checks used for comparison (Malihipour et al., Citation2008; Cao et al., Citation2009). However, TC 67 is hexaploid and resistant tetraploid germplasm remains scarce.
In the pre-2009 literature, studies that identified and characterized QTLs in spring wheat predominated over those in winter wheat, reflecting in large part the greater availability of spring wheat germplasm with high levels of FHB resistance. Accordingly, the majority of the 52 studies in the seminal review by Buerstmayr et al. (Citation2009) were those that had been conducted on spring wheat populations. In the more recent literature, a large number of winter wheat populations have been analysed, as European and North American breeders alike recognize the advantage of using ‘native’ resistance over the introgression of resistance found in Asian sources (Abate et al., 2008; Wilde et al., Citation2008; Häberle et al., Citation2009a ; Zhang et al., Citation2012a ). Häberle et al. (Citation2009a ) verified a major FHB QTL on 1BL (formerly incorrectly assigned to chromosome 5B) from the European winter wheat ‘Cansas’. Abate et al. (Citation2008) identified QTLs on 3BSc, 4BL and 5AS which conferred DON and FDK resistance in the US soft red winter wheat ‘Ernie’ known for its type II resistance. Zhang et al. (Citation2012a ) found QTLs for type II resistance on chromosomes 3AS, 4DL and 4AL in the US hard winter wheat ‘Heyne’. Wilde et al. (Citation2008) examined the effect of three QTLs singly and in combinations of 0, 2 or 3 in populations derived from two resistant German cultivars. They discovered that at least two QTLs were required to significantly reduce disease severity. Using a similar strategy and examining combinations of QTLs (0, 1, 2 or 3) of Asian origin in NILs, Kang et al. (Citation2011) concluded that a combination of the QTLs on 3BS and 2DL showed value for introgressing FHB resistance into soft red winter wheat. The latter also noted the disadvantage, arising from linkage drag, in using Asian sources of FHB resistance, but they and several others from Europe and North America alike, reported the possibility of selecting winter wheat lines which combined high yield with good FHB resistance derived from Asian sources (Häberle et al., Citation2009b ; von der Ohe et al., Citation2010a ; Kang et al., Citation2011; Salameh et al., Citation2011).
Effectiveness as a tool for breeding FHB-resistant cultivars
The rapidly expanding literature describing QTLs for FHB resistance, of which the articles cited above form just a part, contrasts with the paucity of elite germplasm or registered cultivars that can attribute any improved FHB resistance to the deliberate introgression of QTLs using the tools of MAS. Indeed, much has been written but, so far, little has been sown. This is not to deny the useful progress that has been made in developing cultivars with improved resistance.
The recently registered hard red spring wheat ‘Cardale’ exemplifies the success of a strategy using MAS to combine two QTLs, Fhb1and 5A, for FHB resistance (Fox et al., Citation2013). These two QTLs together also counteracted the negative effects of the dwarfing gene Rht-D1b in the DH population generated from the cross between the FHB-resistant winter wheat Line 265 and the susceptible spring wheat ‘Avle’ (Lu et al., Citation2011). However, the improved resistance in new cultivars often cannot be ascribed to the presence of a specific QTL. The cultivar ‘Waskada’, for example, a Canadian hard red spring wheat registered in 2009, has a moderately resistant reaction to F. graminearum. Examining its pedigree, it would be reassuring to attribute the resistance to the presence of Fhb2 transferred from ‘Sumai 3’ through BW278, but the evidence of the DNA markers does not support this. An examination of the Fhb2 region indicated that recombination had occurred between the flanking markers Xwmc397 and Xwmc398 (Fox et al., Citation2009). The scepticism that these observations might engender concerning the usefulness of tracking QTLs for the improvement of FHB resistance is not new, as more than 12 years ago questions were raised about the value of MAS in plant breeding (Young, Citation1999) and the same questions have been raised, implicitly, more recently (Xu & Crouch, Citation2008; Miedaner & Korzun, Citation2012).
The recently registered winter wheat ‘Emerson’ offers another example where the improvement in FHB resistance is clear but the link to QTLs is not, since the dramatic improvement in resistance over its pedigree parents, and indeed all other members of its cultivar class, cannot be attributed to the presence of any known QTLs (Graf et al., Citation2012). It is the result of the expression of a fortuitous combination of alleles, as was the case of ‘Sumai 3’ derived from the moderately susceptible parents ‘Funo’ and ‘Taiwan wheat’. The moderate resistance to FHB of the US winter wheats ‘Ernie’ and ‘Truman’ likewise cannot be linked to introgression of known FHB resistance QTLs (McKendry et al., 1995, 2005). Tracking QTLs also played no role in the evolution of the resistant Canadian germplasm FL62R1. Instead, repeated rounds of selection following exposure to multiple stresses identified those lines that expressed the most effective resistance (Comeau et al., Citation2008).
An epigenetics-driven perspective on improving FHB resistance
Almost all the research effort to improve the genetic resistance of wheat to FHB starts from the assumption that the target, susceptible germplasm lacks the necessary genes, or more specifically, effective combinations of the correct alleles of these genes. It follows that as resistant lines do possess these gene alleles, enhancing genetic resistance to FHB will depend on introgressing the right genes and selecting the progeny of crosses with the optimal gene combinations. Reviews of progress toward improved FHB resistance have therefore described available sources of resistance genes, research to identify new candidate resistance genes for introgression, methods to facilitate their introgression, and the extent of improvement achieved.
An expanded perspective of how wheat hosts might evolve more effective defences against FHB informs recent work that seeks to move beyond the gene-introgression model. Comeau et al. (Citation2010) have emphasized the opportunities for improving disease resistance that can follow from looking beyond: ‘ … genes as simply repositories of – save for mutations – invariant information whose expression conforms to a small number of simple combinatorial rules’. In the simplest and most practical terms, Comeau's ‘systemic, heuristic approach’ sets forth the theory and practice of selecting resistant progeny from susceptible parents. By seeking to apply McClintock's (1984) insight that as plants respond to other players in their environment, they capture profound effects of stresses on their genomes, this approach distinguishes itself from the introgression model. It treats the complexity of the myriad interacting levels of the control of gene expression as a resource rather than an impediment. Continuing cycles of exposure to complex, interacting biotic and abiotic stresses reveal increasingly optimized expressions of desired traits, such as FHB resistance. A signature example is the development of bread-quality wheat germplasm with high FHB resistance from the AB143 four-way cross, in which a major source of the resistance is derived from the susceptible cultivar parent ‘SS Blomidon’ (Comeau et al., Citation2010, Citation2011).
In presenting a perspective to complement gene introgression, the authors of the systemic, heuristic approach have drawn attention to genetic resources for FHB resistance that may remain unexpressed in elite, susceptible germplasm. A substantial portion of the current literature on improving genetic resistance to FHB in wheat describes research that aims to achieve the resistance of ‘Sumai 3’ in adapted cultivars; yet ‘Sumai 3’ itself is descended from parents which are moderately susceptible (Liu & Wang, Citation1991). While this may be explained by transgressive segregation, invoking this concept does not guide researchers toward the practical objective of choosing the FHB-susceptible elite parents that will have a better likelihood of yielding FHB-resistant, high-quality germplasm.
An unexpected light was shone on this question by the comparison of gene induction in ‘Sumai 3’ and its susceptible near-isogenic lines (Golkari et al., Citation2009), which indicated that the same general plant defence genes were induced in both the resistant and susceptible lines. Since no genes had been introgressed from a resistant parent, and initiating resistance did not depend on recognition phenomena, resistance could be seen as arising from the effective coordination of the diverse plant defence pathways whose underlying genes existed in both the resistant and susceptible lines (Haber et al., Citation2011b ). It follows that altering in a heritable manner the expression of genetic information already present would generate pools of variation in descendant generations, from which lines with superior resistance might be selected. In a recent example, just such epigenetic change, brought about by applying a DNA demethylating agent, revealed in a susceptible rice cultivar a specific resistance to bacterial blight that breeders had previously needed to introgress from wild relatives (Akimoto et al., Citation2007).
In their simplest and most explicit form, these observations indicated that applying a suitable agent of epigenetic modification should enable resistant sublines to be evolved among the direct descendants of susceptible progenitors. However, to be of practical use, the modifying agent(s) should, in contrast to demethylating chemicals, be safe and simple to use. As plant hosts respond to infection with plant viruses by producing short, interfering RNA (siRNA), which is implicated in modifying gene expression (Li, Citation2008), developers of the systemic, heuristic approach have employed wheat streak mosaic virus (WSMV) for this role, as it is capable of systemic infection but does not integrate its genome information in host DNA (Comeau et al., 2010; Haber et al., Citation2011a ).
Applying WSMV inoculation as an ‘epigenetic modifying agent’ to evolve heritable de novo resistance to WSMV (Haber et al., Citation2011a ) and leaf rust (Haber et al., Citation2011b ) from susceptible progenitors showed this approach was feasible. An arguably larger prize, the expression of strong, heritable FHB resistance in hitherto refractory wheat backgrounds, became the next objective as success here might validate the more general applicability of this alternative approach to crop improvement. The cultivar ‘McKenzie’ was chosen as it was particularly amenable to the de novo evolution of altered, heritable traits and because, as a DH cultivar, none of its descendants should show any effects of gene re-assortment if care were taken to avoid accidental out-crossing (Haber et al., Citation2011a ). Starting from a single seed, successive generations of selfed lines (that had been inoculated with WSMV in the first, second, third and fifth cycles) evolved resistance to virus infection. With repeated rounds of selection, the virus resistance which had originally evolved de novo became genetically fixed as evidenced by its ability to perform like a Mendelian source of a single, dominant gene in crosses to the virus-susceptible ‘McKenzie’ progenitor. Some of the sublines which expressed resistance to WSMV also evolved improved FHB resistance, which proved stable in 3 years of testing in field nurseries and in controlled, indoor tests (Haber et al., Citation2011a ).
A more ambitious demonstration of how altering expression might serve as an alternative or complement to gene introgression is to evolve sublines that express strong FHB resistance from a cultivar such as ‘Roblin’, which is so susceptible that it serves as a check in evaluating the FHB resistance of its cultivar class (, ). After nine cycles of a protocol of applied stress similar to that used to evolve de novo WSMV resistance and improved FHB resistance in sublines descended from ‘McKenzie’, sublines descended from ‘Roblin’ were selected whose FHB resistance (, ) in closely controlled trials both in field and greenhouse approached those of resistant checks (Haber & Gilbert, Citation2012; Haber et al., Citation2013).
Where suitable sources of genetic resistance are altogether lacking, as for example in cultivated durum wheat germplasm, resistance cannot be improved by gene introgression. A feasible alternative may be to select, in an iterative manner, for improved resistance in descendant populations that express the existing gene pool in an altered manner. Early work has shown that sublines expressing stronger FHB resistance can be evolved from highly susceptible Canadian durum wheat cultivars and advanced breeding lines (Haber et al., Citation2012), but the stability of such resistance needs more extensive confirmation.
Changing the expression of genes already present, which is essential to the success of this alternative approach, appears to be accompanied by shifts in simple-sequence repeat (SSR) alleles of DNA markers for genomic regions of FHB-resistance QTLs (Haber et al., Citation2013). While the mechanism driving these shifts remains unexplained, the very occurrence of such changes is not obviously compatible with conventional models of inheritance and gene introgression. An interpretation that allows for both the genome localization of QTLs for FHB resistance and the seemingly discordant findings described above would be that mapping studies which identified QTLs had perhaps identified genomic regions which are responsive to epigenetic effects. Thus from the pools of heritable variation generated by such effects, the founders of resistant sublines might be selected (Haber et al., Citation2013).
Summary and conclusions
In the continuing struggle against the ravages wrought by FHB on wheat, there are essentially two available strategies: fight the pathogen and strengthen the host's defence.
Fighting the pathogen
The experience of those who have confronted FHB over extended periods is captured in what they have recommended as cultural practices that can mitigate the losses FHB causes. If control of FHB were the sole consideration, practices such as complete tillage would be recommended. However, there are many larger factors, such as control of erosion and retention of soil moisture that override such an approach. Given that contemporary cultural practices encourage producers to retain residues of harvested crops, control efforts must identify those rotations least conducive to the build-up of inoculum. No dramatic advances should be expected on this front as current best practices already reflect the findings of extensive research.
The judicious use of fungicides has achieved great successes in fighting the fungal pathogens that can cause substantial losses. In high-input cropping systems, for example, fungicides such as tebuconazole have proven cost-effective in combating losses from cereal rusts. Unfortunately, F. graminearum is a wily opponent that does not yield easily to single applications of a single fungicide. Research has identified the fungicide application regimes with respect to mixing of agents with different modes of action, and timing of sprays that do achieve effective control of FHB disease and DON accumulation. There will probably only be incremental improvements in control that arise from continuing empirical studies until fungicides with entirely different modes of action are discovered and validated.
On the face of it, biocontrol agents should offer safe and effective disease control which respects the environment. The essential aspect of applying this approach to fighting the pathogen is recognizing that evolution has already selected for effective antagonism among the players in the ecosystem. However, the challenge lies in devising practical control methods that deliver potent formulations which can be stored in a warehouse, poured in a tank, applied easily, and which will then safely degrade in the environment.
Strengthening the host's defence
Over evolutionary time, host populations that defended themselves more successfully against attacks by pathogens such as F. graminearum increasingly prevailed. In identifying sources of genetic resistance, modern wheat workers seek to capture and transfer this benefit to cultivated varieties. This strategy has succeeded very clearly in the control of epidemics caused by rust pathogens. By identifying discrete resistance genes in the germplasm pool and introgressing them into adapted, high-quality wheat lines, breeders have developed the modern cultivars that successfully resist these important pathogens. In seeking to repeat the success achieved in obtaining effective host genetic resistance to rusts, there was initially considerable optimism that, having identified promising sources of resistance, FHB in commercial cultivars might soon be controlled by introgressing the resistance. Within a decade, however, the scope of the challenge became clearer. The distribution of resistant phenotypes in the progeny of crosses soon showed that the resistance trait was not simply determined by a small number of discrete genes. Among the progeny generated by such crosses, it was proving more difficult to select good candidate lines. Such lines would need to retain superior agronomic and quality traits while expressing consistent resistance, sufficient to protect against losses to yield and quality over the range of spatial and temporal environmental variation the crops would encounter.
It is clear that large populations need to be examined in order to identify the small number of near-optimal combinations of numerous minor genes for resistance as well as all the determinants necessary for acceptable agronomic and quality traits. It was a happy coincidence that the technology for identifying and tracking DNA markers became available to wheat workers at just about the time when the full scope of this challenge became clear. Instead of needing to determine detailed phenotypes of large numbers of individual plants or lines over several years, the purportedly closely linked DNA markers of QTLs for FHB resistance could be tracked with all the advantages digital information offers. Having identified a relatively small number of likely promising candidates, phenotypic testing would then confirm that MAS-QTLs had indeed pointed to the lines with optimal gene combinations.
After nearly two decades, it is emerging that even the powerful expedient of MAS-QTLs has so far failed to generate FHB-resistant cultivars. It is not clear that identifying yet more QTLs and better means of tracking their introgression will soon achieve this goal. Recall that the goal requires the expression of resistance, and not simply the carrying of DNA regions associated with that resistance in other lines. The question of whence exactly ‘Sumai 3’ derives its resistance has not been properly put, let alone addressed. If an answer remains elusive it may be time to put the question differently.
‘Sumai 3’ and its susceptible NILs contain, by definition, nearly identical DNA. Moreover, they all respond to attack by F. graminearum by inducing the same sets of pathogenesis-related proteins, yet their expressions of resistance are dramatically different. Was this difference really entirely explained by a genetic difference? This question could be addressed if one examined the lines descended by selfing from a DH cultivar that expressed altered phenotypic traits in a stable, inheritable pattern. Here altered phenotypes are expressed by genotypes that should be unchanged. Thus, the direct descendants of an individual DH wheat plant should be genetically identical, yet lines which express such traits of disease resistance as near-immunity to WSMV, have been derived from susceptible progenitors. Such seemingly discordant observations might begin to be reconciled if one ascribes epigenetic factors to the control of expression, and the subsequent genetic fixation by mechanisms that are as yet unexplained.
In proposing a counterpoint to the currently dominant perspective on how to improve the effectiveness of host defences against FHB, we are suggesting that much needs to be learned about how the host and pathogen evolve in their environment. By wedding the best contemporary analytical tools to observations interpreted with disciplined imagination, we might find ways to grasp a larger portion of the full range of adaptation and inheritance mechanisms accumulated by evolution.
Notes
Contribution No. XXXX
References
- Abate , Z.A. , Liu , S. and Mckendry , A.L. 2008 . Quantitative trait loci associated with deoxynivalenol content and kernel quality in the soft red winter wheat ‘Ernie’ . Crop Sci. , 48 : 1408 – 1418 .
- Akimoto , K. , Katakami , H. , Kim , H.J. , Ogawa , E. , Sano , C.M. wada , Y . 2007 . Epigenetic inheritance in rice plants . Ann. Bot. , 100 : 205 – 217 .
- Anderson , J.A. 2007 . Marker-assisted selection for Fusarium head blight resistance in wheat . Int. J. Food Microbiol. , 119 : 51 – 53 .
- Anon. (2006). Summaries of EU legislation, Food Safety, Contamination and Environmental Factors http://europa.eu/legislation_summaries/food_safety/contamination_environmental_factors/l21290_en.htm (http://europa.eu/legislation_summaries/food_safety/contamination_environmental_factors/l21290_en.htm)
- Aoki , T. and O'donnell , K. 1999 . Morphological and molecular characterization of Fusarium pseudograminearum sp. nov., formerly recognized as the group 1 population of F. graminearum . Mycologia , 91 : 597 – 609 .
- Audenaert , K. 2010 . Hydrogen peroxide induced by the fungicide prothioconazole triggers deoxynivalenol (DON) production by Fusarium graminearum . BMC Microbiol. , 10 : 112
- Basnet , B.R. , Glover , K.D. , Ibrahim , A.M.H. , Yen , Y. and Chao , S. 2012 . A QTL on chromosome 2DS of ‘Sumai 3’ increases susceptibility to Fusarium head blight in wheat . Euphytica , 186 : 91 – 101 .
- Bergamini , E. , Catellani , D. , Dall'asta , C. , Galaverna , G. , Dossena , A. Marchelli , R. 2010 . Fate of Fusarium mycotoxins in the cereal product supply chain: the deoxynivalenol (DON) case within industrial bread-making technology . Food Addit. Contam. Part A. , 27 : 677 – 687 .
- Beyer , M. and Verreet , J.A. 2005 . Germination of Gibberella zeae ascospores as affected by age of spores after discharge and environmental factors . Eur. J. Plant Pathol. , 111 : 381 – 389 .
- Beyer , M. , Klix , M.B. , Klink , H. and Verreet , J.A. 2006 . Quantifying the effects of previous crop, tillage, cultivar and triazole fungicides on the deoxynivalenol content of wheat grain – a review . J. Plant Dis. Protect. , 113 : 241 – 246 .
- Binder , E.M. 2007 . Managing the risk of mycotoxins in modern feed production . Anim. Feed Sci. Technol. , 133 : 149 – 166 .
- Blandino , M. , Pilati , A. and Reyneri , A. 2009 . Effect of foliar treatments to durum wheat on flag leaf senescence, grain yield, quality and deoxynivalenol contamination in North Italy . Field Crops Res. , 114 : 214 – 222 .
- Bonin , C.M. and Kolb , F.L. 2009 . Resistance to Fusarium head blight and kernel damage in a winter wheat recombinant inbred line population . Crop Sci. , 49 : 1304 – 1312 .
- Bowden , R.L. , Leslie , J.F. , Lee , J. and Lee , Y.-H. Dec. 11–15, 2004 2004 . “ Cross fertility of Gibberella zeae ” . In Proceedings of the Second International Symposium on Fusarium Head Blight, Incorporating the 8th European Fusarium Seminar Edited by: Canty , S.M. , Boring , T. , Wardell , J. and Ward , R.W. Dec. 11–15, 2004 , Orlando , Florida (p. 554)
- Buerstmayr , H. , Ban , T. and Anderson , J.A. 2009 . QTL mapping and marker-assisted selection for Fusarium head blight resistance in wheat: a review . Plant Breeding , 128 : 1 – 26 .
- Buerstmayr , M. , Lemmens , M. , Steiner , B. and Buerstmayr , H. 2011 . Advanced backcross QTL mapping of resistance to Fusarium head blight and plant morphological traits in a Triticum macha × T. aestivum population . Theor. Appl. Genet. , 123 : 293 – 306 .
- Cai , X. , Elias , E. , Xu , S., S., K., S., Z. and Chao , S. Dec. 4–6, 2011 2011 . “ Fusarium head blight resistance in durum wheat – progress and challenge ” . In Proceedings of the 2011 National Fusarium Head Blight Forum Edited by: Canty , S. , Clark , A. , Anderson-Scully , A. and Van Sanford , D. Dec. 4–6, 2011 , St. Louis , MO (p. 12)
- Cao , W. , Fedak , G. , Armstrong , K. , Xue , A. and Savard , M.E. 2009 . Registration of spring wheat germplasm TC 67 resistant to Fusarium head blight . J. Plant Regist. , 3 : 104 – 106 .
- Cativelli , M. , Appendino , M.L. and Lewis , S. 2011 . “ A novel QTL for type II Fusarium head blight resistance mapped on chromosome 7D of wheat ” . In 21st International Triticeae Mapping Initiative Workshop Edited by: Dreisigacker , S. and Singh , S. Mexico City , Mexico (p. 76) CIMMYT
- Cavinder , B. and Trail , F. 2012 . Role of Fig1, a component of the low-affinity calcium uptake system, in growth and sexual development of filamentous fungi . Eukaryot. Cell , 11 : 978 – 988 .
- Cavinder , B. , Hamam , A. , Lew , R.R. and Trail , F. 2011 . Mid1, a mechanosensitive calcium ion channel affects growth, development, and ascospore discharge in the filamentous fungus Gibberella zeae . Eukaryot. Cell , 10 : 832 – 841 .
- CCCF . 2011 . Levels for deoxynivalenol (DON) and its acetylated derivatives in cereals and cereal-based products , 26 Codex Alimentarius Commission .
- CFIA . 2010 . Food Safety Action Plan : Report 2009–2010 Targeted Surveys Chemistry: Ochratoxin A and deoxynivalenol in selected foods , 16 Canadian Food Inspection Agency . TS-CHEM-09/10-04
- Chakraborty , S. and Newton , A.C. 2011 . Climate change, plant diseases and food security: an overview . Plant Pathol. , 60 : 2 – 14 .
- Chan , Y. , Savard , M.E. , Reid , L.M. , Cyr , T. , Mccormick , W.A. and Seguin , C. 2009 . Identification of lipopeptide antibiotics of a Bacillus subtilis isolate and their control of Fusarium graminearum diseases in maize and wheat . BioControl , 54 : 567 – 574 .
- Chu , C. , Niu , Z. , Zhong , S. , Chao , S. , Friesen , T. Halley , S. 2011 . Identification and molecular mapping of two QTLs with major effects for resistance to Fusarium head blight in wheat . Theor. Appl. Genet. , 123 : 1107 – 1119 .
- Comeau , A. , Langevin , F. , Caetano , V.R. , Haber , S. , Savard , M.E. Voldeng , H. 2008 . A systemic approach for the development of FHB resistant germplasm accelerates genetic progress . Cer. Res. Commun. , 36 : 5 – 9 .
- Comeau , A. , Caetano , V.R. , Haber , S. , Langevin , F. , Levesque , M. and Gilbert , J. 2010 . “ Systemic heuristic approaches generate better wheat germplasm faster and at lower cost ” . In Genome Instability and Trans-generational Effects , Edited by: Kovalchuk , I. and Kovalchuk , O. 401 – 446 . Hauppauge , NY : Nova Science Publishers .
- Comeau , A. , Langevin , F. , Dion , Y. , Rioux , S. and Voldeng , H. 2011 . Systemic germplasm development delivers more fusarium head blight resistance together with maximum test weight in bread wheat . Can. J. Plant Pathol. , 33 : 328
- Couture , L. 1982 . Receptivity of spring cereals to contamination of grains in the inflorescence by Fusarium spp . Can. J. Plant Sci. , 62 : 29 – 34 .
- Del Ponte , E.M. , Fernandes , J.M.C. , Pavan , W. and Baethgen , W.E. 2009 . A model-based assessment of the impacts of climate variability on Fusarium head blight seasonal risk in southern Brazil . J. Phytopathol. , 157 : 675 – 681 .
- Dill-Macky , R. 2008 . Cultural control practices for Fusarium head blight: problems and solutions . Cer. Res. Commun. , 36 : 653 – 657 .
- Dunlap , C.A. , Schisler , D.A. , Price , N.P. and Vaughn , S.F. 2011 . Cyclic lipopeptide profile of three Bacillus subtilis strains; antagonists of Fusarium head blight . J. Microbiol. , 49 : 603 – 609 .
- Edwards , S.G. 2009 . Fusarium mycotoxin content of UK organic and conventional wheat . Food Addit. Contam. Part A. , 26 : 496 – 506 .
- Edwards , S.G. and Godley , N.P. 2010 . Reduction of Fusarium head blight and deoxynivalenol in wheat with early fungicide applications of prothioconazole . Food Addit. Contam. Part A. , 27 : 629 – 635 .
- Engle , J.S. , Lipps , P.E. , Graham , T.L. and Boehm , M.J. 2004 . Effects of choline, betaine, and wheat floral extracts on growth of Fusarium graminearum . Plant Dis. , 88 : 175 – 180 .
- Fernando , W.G.D. , Paulitz , T.C. , Seaman , W.L. , Dutilleul , P. and Miller , J.D. 1997 . Head blight gradients caused by Gibberella zeae from area sources of inoculum in wheat field plots . Phytopathology , 87 : 414 – 421 .
- Fox , S. , Thomas , J. , Wise , I.L. , Smith , M.A.H. , Humphreys , D.G. Brown , P.D. 2009 . Waskada hard red spring wheat . Can. J. Plant Sci. , 89 : 929 – 936 .
- Fox , S. , Humphreys , D.G. , Brown , P.D. , Mccallum , B.D. , Fetch , T.G. Menzies , J.G. 2013 . Cardale hard red spring wheat . Can. J. Plant Sci. , In press.
- Francl , L. , Shaner , G. , Bergstrom , G. , Gilbert , J. , Pedersen , W. Dill-Macky , R. 1999 . Daily inoculum levels of Gibberella zeae on wheat spikes . Plant Dis. , 83 : 662 – 666 .
- Gaffoor , I. , Brown , D.W. , Plattner , R. , Proctor , R.H. , QI , W. and Trail , F. 2005 . Functional analysis of the polyketide synthase genes in the filamentous fungus Gibberella zeae (anamorph Fusarium graminearum) . Eukaryot. Cell , 4, 1926 1933
- Gale , L.R. , Ward , T.J. , Balmas , V. and Kistler , H.C. 2007 . Population subdivision of Fusarium graminearum sensu stricto in the upper midwestern United States . Phytopathology , 97 : 1434 – 1439 .
- Gervais , L. , Dedryver , F. , Morlais , J.Y. , Bodusseau , V. , Negre , S. Bilous , M. 2003 . Mapping of quantitative trait loci for field resistance to Fusarium head blight in a European winter wheat . Theor. Appl. Genet. , 106 : 961 – 970 .
- Gilbert , J. , Tekauz , A. , Kaethler , R. , Mueller , E. and Kromer , U. 1996 . 1995 survery of fusarium head blight in Manitoba . Can. Plant. Dis. Surv , 76 : 89
- Gilbert , J. , Tekauz , A. , Kaethler , R. , Mccallum , B. , Mueller , E. and Kromer , U. 1999 . 1998 survery of fusarium head blight in Manitoba . Can. Plant. Dis. Surv , 76 : 90
- Gilbert , J. and Tekauz , A. 2000 . Recent developments in research on fusarium head blight of wheat in Canada . Can. J. Plant Pathol. , 22 : 1 – 8 .
- Gilbert, J., & Tekauz, A. (2011). Strategies for management of fusarium head blight (FHB) in cereals. , 4, 97–104 http://www.prairiesoilsandcrops.ca/index.html (http://www.prairiesoilsandcrops.ca/index.html)
- Gilbert , J. , Woods , S.M. and Kromer , U. 2008 . Germination of ascospores of Gibberella zeae after exposure to various levels of relative humidity and temperature . Phytopathology , 98 : 504 – 508 .
- Gilbert , J. , Clear , R.M. , Ward , T.J. , Gaba , D. , Tekauz , A. Turkington , T.K. 2010 . Relative aggressiveness and production of 3- or 15-acetyl deoxynivalenol and deoxynivalenol by Fusarium graminearum in spring wheat . Can. J. Plant Pathol. , 32 : 146 – 152 .
- Gilbert , J. , Clear , R. , Patrick , S. , Slusarenko , K. and Wolfe , C. 2013 . Ratio of 3-ADON and 15-ADON isolates of Fusarium graminearum recovered from wheat kernels in fusarium head blight field nurseries at Glenlea, Manitoba from 2008 to 2011 . Can. J. Plant Pathol. , 35 : 110
- Giraud , F. , Pasquali , M. , El-Jarroudi , M. , COCCO , M. , Delfosse , P. Hoffmann , L. 2011 . Timely fungicide application: a strategy to minimize Fusarium head blight and associated mycotoxin production in winter wheat . J. Plant Pathol. , 93 : S1
- Glenn , A.E. 2007 . Mycotoxigenic Fusarium species in animal feed . Anim. Feed Sci. Technol. , 137 : 213
- Golkari , S. , Gilbert , J. , Ban , T. and Procunier , J.D. 2009 . QTL-specific microarray gene expression analysis of wheat resistance to Fusarium head blight in Sumai-3 and two susceptible NILs . Genome , 52 : 409 – 418 .
- Graf , R.J. , Beres , B.L. , Laroche , A. , Gaudet , D.A. , Eudes , F. Pandeya , R.S. 2013 . Emerson hard red winter wheat . Can. J. Plant Sci , 93 (in press) Submitted
- Guenther , J.C. and Trail , F. 2005 . The development and differentiation of Gibberella zeae (anamorph: Fusarium graminearum) during colonization of wheat . Mycologia , 97 : 229 – 237 .
- Guo , X.W. , Fernando , W.G.D. , Bullock , P. and Sapirstein , H. 2010 . Quantifying cropping practices in relation to inoculum levels of Fusarium graminearum on crop stubble . Plant Pathol. , 59 : 1107 – 1113 .
- Haber , S. and Gilbert , J. 2012 . How to make fusarium head blight-susceptible spring wheat resistant – the example of ‘Roblin’ . Can. J. Plant Pathol. , 34 : 149
- Haber , S. , Gilbert , J , Seifers , D.L. and Comeau , A. 2011a . Epigenetics serves genetics: Fusarium head blight (FHB) resistance in elite wheat germplasm . Am. J. Plant Sci. Biotechnol. , 5 : 95 – 100 .
- Haber , S. , Seifers , D.L. , Seto-Goh , P. and Mccallum , B. 2011b . Evolution de novo of stable, heritable resistance to leaf rust (Puccinia triticina) in susceptible wheat following cycles of infection with wheat streak mosaic virus . Can. J. Plant Pathol. , 33 : 278
- Haber , S. , Gilbert , J. and Singh , A. 2012 . On the express route to fusarium head blight resistance in durum wheat . Can. J. Plant Pathol. , 34 : 149
- Haber , S. , Malihipour , A. , Gilbert , J. and Robinson , S. 2013 . What ‘FHB-resistance QTLs’ might really be telling us: the curious tale of shifts in SSR-marker alleles in resistant wheat lines descended from susceptible progenitors . Can. J. Plant Pathol. , 35 : 113
- Häberle , J. , Holzapfel , J. , Schweizer , G. and Hartl , L. 2009a . A major QTL for resistance against Fusarium head blight in European winter wheat . Theor. Appl. Genet. , 119 : 325 – 332 .
- Häberle , J. , Schweizer , G. , Schondelmaier , J. , Zimmermann , G. and Hartl , L. 2009b . Mapping of QTL for resistance against Fusarium head blight in the winter wheat population Pelikan//Bussard/Ning8026 . Plant Breeding , 128 : 27 – 35 .
- Hager , H. 2011 . New DON toxin standards awaited . Top Crop Manager East , October 2011 : 6 – 8 .
- Haidukowski , M. 2012 . Effect of prothioconazole-based fungicides on Fusarium head blight, grain yield and deoxynivalenol accumulation in wheat under field conditions . Phytopathol. Mediterr. , 51 : 236
- Haidukowski , M. , Pascale , M. , Perrone , G. , Pancaldi , D. , Campagna , C. and Visconti , A. 2005 . Effect of fungicides on the development of Fusarium head blight, yield and deoxynivalenol accumulation in wheat inoculated under field conditions with Fusarium graminearum and Fusarium culmorum . J. Sci. Food Agric. , 85 : 191 – 198 .
- Hallen , H.E. and Trail , F. 2008 . The L-type calcium ion channel cch1 affects ascospore discharge and mycelial growth in the filamentous fungus Gibberella zeae (anamorph Fusarium graminearum) . Eukaryot. Cell , 7 : 415 – 424 .
- Hallen , H.E. , Huebner , M. , Shiu , S.H. , Güldener , U. and Trail , F. 2007 . Gene expression shifts during perithecium development in Gibberella zeae (anamorph Fusarium graminearum), with particular emphasis on ion transport proteins . Fungal Genet. Biol. , 44 : 1146 – 1156 .
- Hallen-Adams , H.E. , Wenner , N. , Kuldau , G.A. and Trail , F. 2011 . Deoxynivalenol biosynthesis-related gene expression during wheat kernel colonization by Fusarium graminearum . Phytopathology , 101 : 1091 – 1096 .
- He , J. , Boland , G.J. and Zhou , T. 2009 . Concurrent selection for microbial suppression of Fusarium graminearum, Fusarium head blight and deoxynivalenol in wheat . J. Appl. Microbiol. , 106 : 1805 – 1817 .
- Heier , T. , Jain , S.K. , Kogel , K.H. and Pons-Kuhnemann , J. 2005 . Influence of N-fertilization and fungicide strategies on Fusarium head blight severity and mycotoxin content in winter wheat . J. Phytopathol. , 153 : 551 – 557 .
- Henry, M.H. (2006). Mycotoxins in Feeds: Center for Veterinary Medicine's Perspective. http://www.fda.gov/AnimalVeterinary/Products/AnimalFoodFeeds/Contaminants/ucm050974.htm (http://www.fda.gov/AnimalVeterinary/Products/AnimalFoodFeeds/Contaminants/ucm050974.htm)
- Hollingsworth , C.R. and Motteberg , C.D. Dec. 11–15, 2004 2004 . “ Uniform fungicide trial on FHB of hard red spring wheat in Minnesota ” . In Proceedings of the 2004 National Fusarium Head Blight Forum , Dec. 11–15, 2004 , Orlando : Florida (pp. 324–326) .
- Hollingsworth, C.R., Motteberg, C.D., & Thompson, W.G. (2006). Assessing fungicide efficacies for the management of Fusarium head blight on spring wheat and barley. Plant Health Progress, http://www.plantmanagementnetwork.org/pub/php/research/2006/fusarium/ (http://www.plantmanagementnetwork.org/pub/php/research/2006/fusarium/)
- Inch , S. and Gilbert , J. 2007 . Effect of Trichoderma harzianum on perithecial production of Gibberella zeae on wheat straw . Biocontrol Sci. Technol. , 17 : 635 – 646 .
- Inch , S. and Gilbert , J. 2011 . Scanning electron microscopy observations of the interaction between Trichoderma harzianum and perithecia of Gibberella zeae . Mycologia , 103 : 1 – 9 .
- Inch , S. , Fernando , W.G.D. and Gilbert , J. 2005 . Seasonal and daily variation in the airborne concentration of Gibberella zeae (Schw.) Petch spores in Manitoba . Can. J. Plant Pathol. , 27 : 357 – 363 .
- Jayatilake , D.V. , Bai , G.H. and Dong , Y.H. 2011 . A novel quantitative trait locus for Fusarium head blight resistance in chromosome 7A of wheat . Theor. Appl. Genet. , 122 : 1189 – 1198 .
- JECFA72 . 2011 . Report of the Fifth Session of the Codex Committee on Contaminants in Foods , FAO/WHO Food Standards Programme . 52 pages
- Jennings , P. , Coates , M.E. , Walsh , K. , Turner , J.A. and Nicholson , P. 2004 . Determination of deoxynivalenol- and nivalenol- producing chemotypes of Fusarium graminearum isolated from wheat crops in England and Wales . Plant Pathol. , 53 : 643 – 652 .
- Jiang , J. 2011a . A type 2C protein phosphatase FgPtc3 is involved in cell wall integrity, lipid metabolism, and virulence in Fusarium graminearum . PLoS One , 6, e : 25311
- Jiang , J. 2011b . Involvement of a velvet protein FgVeA in the regulation of asexual development, lipid and secondary metabolisms and virulence in Fusarium graminearum . PLoS One , 6, e : 28291
- Jochum , C.C. , Osborne , L.E. and Yuen , G.Y. 2006 . Fusarium head blight biological control with Lysobacter enzymogenes strain C3 . Biol. Control , 39 : 336 – 344 .
- Jones , R.K. 2000 . Assessments of Fusarium head blight of wheat and barley in response to fungicide treatment . Plant Dis. , 84 : 1021 – 1030 .
- Jurgenson , J.E. , Bowden , R.L. , Zeller , K.A. , Leslie , J.F. , Alexander , N.J. and Plattner , R.D. 2002 . A genetic map of Gibberella zeae (Fusarium graminearum) . Genetics , 160 : 1451 – 1460 .
- Kang , J. , Clark , A. , Van Sanford , D. , Griffey , C. , Brown-Guedira , G. Dong , Y. 2011 . Exotic scab resistance quantitative trait loci effects on soft red winter wheat . Crop Sci. , 51 : 924 – 933 .
- Kazan , K. , Gardiner , D.M. and Manners , J.M. 2012 . On the trail of a cereal killer: recent advances in Fusarium graminearum pathogenomics and host resistance . Mol. Plant Pathol. , 13 : 399 – 413 .
- Khan , M.R. and Doohan , F.M. 2009a . Bacterium-mediated control of Fusarium head blight disease of wheat and barley and associated mycotoxin contamination of grain . Biol. Control , 48 : 42 – 47 .
- Khan , M.R. and Doohan , F.M. 2009b . Comparison of the efficacy of chitosan with that of a fluorescent pseudomonad for the control of Fusarium head blight disease of cereals and associated mycotoxin contamination of grain . Biol. Control , 48 : 48 – 54 .
- Khan , N.I. , Schisler , D.A. , Boehm , M.J. , Lipps , P.E. and Slininger , P.J. 2004 . Field testing of antagonists of Fusarium head blight incited by Gibberella zeae . Biol. Control , 29 : 245 – 255 .
- Lee , J. , Lee , T. , Lee , Y.W. , Yun , S.H. and Turgeon , B.G. 2003 . Shifting fungal reproductive mode by manipulation of mating type genes: obligatory heterothallism of Gibberella zeae . Mol. Microbiol. , 50 : 145 – 152 .
- Lee , J. , Myong , K. , Kim , J.-E. , Kim , H.-K. , Yun , S.-H. and Lee , Y.-W. 2012 . FgVelB globally regulates sexual reproduction, mycotoxin production, and pathogenicity in the cereal pathogen Fusarium graminearum . Microbiology , doi: 10.1099/mic.0.059188–0
- Leslie , J.F. and Bowden , R.L. 2008 . Fusarium graminearum: when species concepts collide . Cer. Res. Commun. , 36 : 609 – 615 .
- Lesnik , M. , Cencic , A. , Vajs , S. and Simoncic , A. 2008 . Milling and bread baking techniques significantly affect the mycotoxin (deoxynivalenol and nivalenol) level in bread . Acta Aliment. , 37 : 471 – 483 .
- Li , L.-C. 2008 . “ Small RNA-mediated-gene activation ” . In RNA and the Regulation of Gene Expression: A Hidden Layer of Complexity , Edited by: Morris , K.V. 189 – 200 . Norwich : Caister Academic Press .
- Li , S. , Jochum , C.C. , Yu , F. , Zaleta-Rivera , K. , DU , L. Harris , S.D. 2008 . An antibiotic complex from Lysobacter enzymogenes strain C3: antimicrobial activity and role in plant disease control . Phytopathology , 98 : 695 – 701 .
- Li , T. , Bai , G. , Wu , S. and Gu , S. 2011 . Quantitative trait loci for resistance to fusarium head blight in a Chinese wheat landrace Haiyanzhong . Theor. Appl. Genet. , 122 : 1497 – 1502 .
- Li , T. , Bai , G. , Wu , S. and Gu , S. 2012 . Quantitative trait loci for resistance to Fusarium head blight in the Chinese wheat landrace Huangfangzhu . Euphytica , 185 : 93 – 102 .
- Liu , J. , Zanardi , S. , Powers , S. and Suman , M. 2012 . Development and practical application in the cereal food industry of a rapid and quantitative lateral flow immunoassay for deoxynivalenol . Food Control , 26 : 88 – 91 .
- Liu, X. (2012). Involvement of FgERG4 in ergosterol biosynthesis, vegetative differentiation and virulence in Fusarium graminearum. Mol. Plant Pathol., http://onlinelibrary.wiley.com/doi/10.1111/j.1364-3703.2012.00829.x/pdf (http://onlinelibrary.wiley.com/doi/10.1111/j.1364-3703.2012.00829.x/pdf)
- Liu , Z.Z. and Wang , Z.Y. 1991 . “ Improved scab resistance in China: sources of resistance and problems ” . In Proceedings of the International Conference: Wheat for the Non-Traditional Warm Areas , Edited by: Saunders , D.A. 178 – 187 . Brazil : Foz do Iguaco .
- Lu , Q. , Szabo-Hever , A. , Bjornstad , A. , Lillemo , M. , Semagn , K. Mesterhazy , A. 2011 . Two major resistance quantitative trait loci are required to counteract the increased susceptibility to fusarium head blight of the Rht-D1B dwarfing gene in wheat . Crop Sci. , 51 : 2430 – 2438 .
- Luongo , L. , Galli , M. , Corazza , L. , Meekes , E. , De Haas , L. Van Der Plas , C.L. 2005 . Potential of fungal antagonists for biocontrol of Fusarium spp. in wheat and maize through competition in crop debris . Biocontrol Sci. Technol. , 15 : 229 – 242 .
- Lutz , M.P. , Feichtinger , G. , Defago , G. and Duffy , B. 2003 . Mycotoxigenic Fusarium and deoxynivalenol production repress chitinase gene expression in the biocontrol agent Trichoderma atroviride P1 . Appl. Environ. Microbiol. , 69 : 3077 – 3084 .
- Maldonado-Ramirez , S.L. , Schmale III , D.G. , Shields , E.J. and Bergstrom , G.C. 2005 . The relative abundance of viable spores of Gibberella zeae in the planetary boundary layer suggests the role of long-distance transport in regional epidemics of Fusarium head blight . Agric. For. Meterol. , 132 : 20 – 27 .
- Malihipour , A. , Gilbert , J. , Fedak , G. and Brûlé-Babel , A. 2008 . Resistance to fusarium head blight (FHB) in a population derived from the cross of bread wheat and Triticum timopheevii . Cer. Res. Commun. , 36 : 131 – 133 .
- Malihipour , A. , Gilbert , J. , Piercey-Normore , M. and Cloutier , S. 2012 . Molecular phylogenetic analysis, trichothecene chemotype patterns, and variation in aggressiveness of fusarium isolates causing head blight in wheat . Plant Dis. , 96 : 1016 – 1025 .
- Mao , S.-L. , Wei , Y.-M. , Cao , W. , Lan , X.-J. , Yu , M. Chen , Z.-M. 2010 . Confirmation of the relationship between plant height and Fusarium head blight resistance in wheat (Triticum aestivum L.) by QTL meta-analysis . Euphytica , 174 : 343 – 356 .
- Matarese , F. , Sarrocco , S. , Gruber , S. , Seidl-Seiboth , V. and Vannacci , G. 2012 . Biocontrol of Fusarium head blight: interactions between Trichoderma and mycotoxigenic Fusarium . Microbiology , 158 : 98 – 106 .
- Mccartney , C.A. , Somers , D.J. , Fedak , G. , Depauw , R.M. , Thomas , J. Fox , S.L. 2007 . The evaluation of FHB resistance QTLs introgressed into elite Canadian spring wheat germplasm . Mol. Breed. , 20 : 209 – 221 .
- Mcclintock , B. 1984 . The significance of responses of the genome to challenge . Science , 226 : 792 – 801 .
- Mckendry , A.L. , Berg , J.E. , Tague , D.N. and Kephart , K.D. 1995 . Registration of ‘Ernie’ wheat . Crop Sci. , 35 : 1513
- Mckendry , A.L. , Tague , D.N. , Wright , R.L. , Tremain , J.A. and Conley , S.P. 2005 . Registration of ‘Truman’ wheat . Crop Sci. , 45 : 421 – 423 .
- Mcmullen , M. , Halley , S. , Schatz , B. , Meyer , S. , Jordahl , J. and Ransom , J. 2008 . Integrated strategies for Fusarium head blight management in the United States . Cer. Res. Commun. , 36 : 563 – 568 .
- Mcmullen , M. , Bergstrom , G. , De Wolf , E. , Dill-Macky , R. , Hershman , D. Shaner , G. 2012 . A unified effort to fight an enemy of wheat and barley: Fusarium head blight . Plant Dis. , 96 : 1712 – 1728 .
- Merhej , J. 2012 . The velvet gene, FgVe1, affects fungal development and positively regulates trichothecene biosynthesis and pathogenicity in Fusarium graminearum . Mol. Plant Pathol. , 13 : 363 – 374 .
- Mesterhazy , A. 1995 . Types and components of resistance to Fusarium head blight . Plant Breeding , 114 : 377 – 386 .
- Miedaner , T. and Korzun , V. 2012 . Marker-assisted selection for disease resistance in wheat and barley breeding . Phytopathology , 102 : 560 – 566 .
- Miedaner , T. and Voss , H.H. 2008 . Effect of dwarfing Rht genes on Fusarium head blight resistance in two sets of near-isogenic lines of wheat and check cultivars . Crop Sci. , 48 : 2115 – 2122 .
- Miedaner , T. , Cumagun , C.J.R. and Chakraborty , S. 2008 . Population genetics of three important head blight pathogens Fusarium graminearum, F. pseudograminearum and F. culmorum . J. Phytopathol. , 156 : 129 – 139 .
- Miller , J.D. , Greenhalgh , R. , Wang , Y. and Lu , M. 1991 . Trichothecene chemotypes of three Fusarium species . Mycologia , 83 : 121 – 130 .
- Milus , E.A. 1994 . Evaluation of foliar fungicides for controlling fusarium head blight of wheat . Plant Dis. , 78 : 697
- Milus , E.A. and Weight , C.T. Oct. 26–27, 1998 1998 . “ Efficacy of Quadris and benlate applications on wheat scab in Arkansas ” . In Proceedings of the 1998 National Fusarium Head Blight Forum , Oct. 26–27, 1998 , 51 – 52 . East Lansing , MI : Michigan State University . East Lansing, MI
- Müller , M.E.H. , Steier , I. , Köppen , R. , Siegel , D. , Proske , M. Korn , U. 2012 . Co-cultivation of phytopathogenic Fusarium and Alternaria strains affects fungal growth and mycotoxin production . J. Appl. Microbiol. , 113 : 874 – 887 .
- Naef , A. , Senatore , M. and Defago , G. 2006 . A microsatellite-based method for quantification of fungi in decomposing plant material elucidates the role of Fusarium graminearum DON production in the saprophytic competition with Trichoderma atroviride in maize tissue microcosms . FEMS Microbiol. Ecol. , 55 : 211 – 220 .
- Naji , A.M. , Moghaddam , M. , Ghaffari , M.R. , Irandoost , H.P. , Farsad , L.K. Pirseyedi , S.M. 2008 . Validation of EST-derived STS markers localized on Qfhs.ndsu-3BS for Fusarium head blight resistance in wheat using a ‘Wangshuibai’ derived population . J. Genet. Genomics , 35 : 625 – 629 .
- Nguyen , T.V. , Schäfer , W. and Bormann , J. 2012 . The stress-activated protein kinase FgOS-2 is a key regulator in the life cycle of the cereal pathogen Fusarium graminearum . Mol. Plant-Microbe Interact. , 25 : 1142 – 1156 .
- Nkongolo , K.K. , Dostaler , D. and Couture , L. 1993 . Effet de la bétaïne, de la choline et d'extraits d'anthères du blé sur la croissance du Fusarium graminearum . Can. J. Plant Pathol. , 15 : 81 – 84 .
- O'donnell , K. , Kistler , H.C. , Tacke , B.K. and Casper , H.H. 2000 . Gene genealogies reveal global phylogeographic structure and reproductive isolation among lineages of Fusarium graminearum, the fungus causing wheat scab . Proc. Natl. Acad. Sci. USA , 97 : 7905 – 7910 .
- O'donnell , K. , Ward , T.J. , Geiser , D.M. , Kistler , H.C. and Aoki , T. 2004 . Genealogical concordance between the mating type locus and seven other nuclear genes supports formal recognition of nine phylogenetically distinct species within the Fusarium graminearum clade . Fungal Genet. Biol. , 41 : 600 – 623 .
- O'donnell , K. , Ward , T.J. , Aberra , D. , Kistler , H.C. , Aoki , T. Orwig , N. 2008 . Multilocus genotyping and molecular phylogenetics resolve a novel head blight pathogen within the Fusarium graminearum species complex from Ethiopia . Fungal Genet. Biol. , 45 : 1514 – 1522 .
- Oide , S. , Krasnoff , S.B. , Gibson , D.M. and Turgeon , B.G. 2007 . Intracellular siderophores are essential for ascomycete sexual development in heterothallic Cochliobolus heterostrophus and homothallic Gibberella zeae . Eukaryot. Cell , 6 : 1339 – 1353 .
- Oldenburg , E. , Brunotte , J. and Weinert , J. 2007 . Strategies to reduce DON contamination of wheat with different soil tillage and variety systems . Mycotoxin Res. , 23 : 73 – 77 .
- Palazzini , J.M. , Ramirez , M.L. , Torres , A.M. and Chulze , S.N. 2007 . Potential biocontrol agents for Fusarium head blight and deoxynivalenol production in wheat . Crop Protect. , 26 : 1702 – 1710 .
- Palazzini , J.M. , Ramirez , M.L. , Alberione , E.J. , Torres , A.M. and Chulze , S.N. 2009 . Osmotic stress adaptation, compatible solutes accumulation and biocontrol efficacy of two potential biocontrol agents on Fusarium head blight in wheat . Biol. Control , 51 : 370 – 376 .
- Palazzini , J.M. , Groenenboom-De Haas , B.H. , Torres , A.M. , Köhl , J. and Chulze , S.N. 2012 . Biocontrol and population dynamics of Fusarium spp. on wheat stubble in Argentina . Plant Pathol. , doi: 10.1111/j.1365-3059.2012.02686.x
- Paul , P.A. , Lipps , P.E. , Hershman , D.E. , Mcmullen , M.P. , Draper , M.A. and Madden , L.V. 2008 . Efficacy of triazole-based fungicides for Fusarium head blight and deoxynivalenol control in wheat: a multivariate meta-analysis . Phytopathology , 98 : 999 – 1011 .
- Perez , C. , Dill-Macky , R. and Kinkel , L.L. 2008 . Management of soil microbial communities to enhance populations of Fusarium graminearum-antagonists in soil . Plant Soil , 302 : 53 – 69 .
- Pirgozliev , S.R. , Edwards , S.G. , Hare , M.C. and Jenkinson , P. 2002 . Effect of dose rate of azoxystrobin and metconazole on the development of Fusarium head blight and the accumulation of deoxynivalenol (DON) in wheat grain . Eur. J. Plant Pathol. , 108 : 469 – 478 .
- Placinta , C.M. , D'mello , J.P.F. and Macdonald , A.M.C. 1999 . A review of worldwide contamination of cereal grains and animal feed with Fusarium mycotoxins. Anim . Feed Sci. Technol. , 78 : 21 – 37 .
- Ramarathnam , R. , Bo , S. , Chen , Y. , Fernando , W.G.D. , Xuewen , G. and DE Kievit , T. 2007 . Molecular and biochemical detection of fengycin- and bacillomycin D-producing Bacillus spp., antagonistic to fungal pathogens of canola and wheat . Can. J. Microbiol. , 53 : 901 – 911 .
- Salameh , A. , Buerstmayr , M. , Steiner , B. , Neumayer , A. , Lemmens , M. and Buerstmayr , H. 2011 . Effects of introgression of two QTL for fusarium head blight resistance from Asian spring wheat by marker-assisted backcrossing into European winter wheat on Fusarium head blight resistance, yield and quality traits . Mol. Breed. , 28 : 485 – 494 .
- Sarver , B.A.J. , Ward , T.J. , Gale , L.R. , Broz , K. , Corby Kistler , H. Aoki , T. 2011 . Novel Fusarium head blight pathogens from Nepal and Louisiana revealed by multilocus genealogical concordance . Fungal Genet. Biol. , 48 : 1096 – 1107 .
- Sato , I. , Ito , M. , Ishizaka , M. , Ikunaga , Y. , Sato , Y. Yoshida , S. 2012 . Thirteen novel deoxynivalenol-degrading bacteria are classified within two genera with distinct degradation mechanisms . FEMS Microbiol. Lett. , 327 : 110 – 117 .
- Schisler , D.A. , Slininger , P.J. , Behle , R.W. and Jackson , M.A. 2004 . Formulation of Bacillus spp. for biological control of plant diseases . Phytopathology , 94 : 1267 – 1271 .
- Schisler , D.A. , Khan , N.I. , Boehm , M.J. , Lipps , P.E. , Slininger , P.J. and Zhang , S. 2006 . Selection and evaluation of the potential of choline-metabolizing microbial strains to reduce Fusarium head blight . Biol. Control , 39 : 497 – 506 .
- Schmale , D.G. III , Shields , E.J. and Bergstrom , G.C. 2006 . Night-time spore deposition of the fusarium head blight pathogen, Gibberella zeae, in rotational wheat fields . Can. J. Plant Pathol. , 28 : 100 – 108 .
- Schmale , D.G. , Wood-Jones , A.K. , Cowger , C. , Bergstrom , G.C. and Arellano , C. 2011 . Trichothecene genotypes of Gibberella zeae from winter wheat fields in the eastern USA . Plant Pathol. , 60 : 909 – 917 .
- Schmale , D.G. III , Ross , S.D. , Fetters , T.L. , Tallapragada , P. , Wood-Jones , A.K. and Dingus , B. 2012 . Isolates of Fusarium graminearum collected 40–320 meters above ground level cause Fusarium head blight in wheat and produce trichothecene mycotoxins . Aerobiologia , 28 : 1 – 11 .
- Scudamore , K.A. , Hazel , C.M. , Patel , S. and Scriven , F. 2009 . Deoxynivalenol and other Fusarium mycotoxins in bread, cake, and biscuits produced from UK-grown wheat under commercial and pilot scale conditions . Food Addit. Contam. Part A. , 26 : 1191 – 1198 .
- Semenov , M.A. 2009 . Impacts of climate change on wheat in England and Wales . J. R. Soc. Interface , 6 : 343 – 350 .
- Shali , A. , Ghasemi , S. , Ahmadian , G. , Ranjbar , G. , Dehestani , A. Khalesi , N. 2010 . Bacillus pumilus SG2 chitinases induced and regulated by chitin, show inhibitory activity against Fusarium graminearum and Bipolaris sorokiniana . Phytoparasitica , 38 : 141 – 147 .
- Signorini , M.L. , Gaggiotti , M. , Molineri , A. , Chiericatti , C.A. , Zapata Basilico , M.L.D. Basilico , J.C. 2012 . Exposure assessment of mycotoxins in cow's milk in Argentina . Food Chem. Toxicol. , 50 : 250 – 257 .
- Simpson , D.R. , Weston , G.E. , Turner , J.A. , Jennings , P. and Nicholson , P. 2001 . Differential control of head blight pathogens of wheat by fungicides and consequences for mycotoxin concentration of grain . Eur. J. Plant Pathol. , 107 : 421 – 431 .
- Solfrizzo , M. , Alldrick , A.J. and Egmond , H.P.V. 2009 . The use of mycotoxin methodology in practice: a need for harmonization . Qual. Assur. Safety Crops Foods , 1 : 121 – 132 .
- Somers , D.J. , Fedak , G. and Savard , M. 2003 . Molecular mapping of novel genes controlling Fusarium head blight resistance and deoxynivalenol accumulation in spring wheat . Genome , 46 : 555 – 564 .
- Somers , D.J. , Fedak , G. , Clarke , J. and CAO , W. 2006 . Mapping of FHB resistance QTLs in tetraploid wheat . Genome , 49 : 1586 – 1593 .
- Spolti , P. , Barros , N.C. , Gomes , L.B. , Dos Santos , J. and Del Ponte , E.M. 2012 . Phenotypic and pathogenic traits of two species of the Fusarium graminearum complex possessing either 15-ADON or NIV genotype . Eur. J. Plant Pathol. , 133 : 621 – 629 .
- Srinivasachary, Gosman , N. , Steed , A. , Faure , S. , Bayles , R. Jennings , P. 2008 . Mapping of QTL associated with Fusarium head blight resistancein spring wheat RL4137 . Czech Journal of Genetics and Plant Breeding , 44 : 147 – 159 .
- Srinivasachary, Gosman , N. , Steed , A. , Hollins , T.W. , Bayles , R. Jennings , P. 2009 . Semi-dwarfing Rht-B1 and Rht-D1 loci of wheat differ significantly in their influence on resistance to Fusarium head blight . Theor. Appl. Genet. , 118 : 695 – 702 .
- Starkey , D.E. , Ward , T.J. , Aoki , T. , Gale , L.R. , Kistler , H.C. Geiser , D.M. 2007 . Global molecular surveillance reveals novel Fusarium head blight species and trichothecene toxin diversity . Fungal Genet. Biol. , 44 : 1191 – 1204 .
- Strange , R.N. , Majer , J.R. and Smith , H. 1974 . The isolation and identification of choline and betaine as the two major components in anthers and wheat germ that stimulate Fusarium graminearum in vitro . Physiol. Plant Pathol. , 4 : 277 – 290 .
- Suzuki , T. , Sato , M. and Takeuchi , T. 2012 . Evaluation of the effects of five QTL regions on Fusarium head blight resistance and agronomic traits in spring wheat (Triticum aestivum L.) . Breeding Sci. , 62 : 11 – 17 .
- Tamburic-Ilincic , L. , Somers , D. , Fedak , G. and Schaafsma , A. 2009 . Different quantitative trait loci for Fusarium resistance in wheat seedlings and adult stage in the Wuhan/Nyubai wheat population . Euphytica , 165 : 453 – 458 .
- Trail , F. 2009 . For blighted waves of grain: Fusarium graminearum in the postgenomics era . Plant Physiol. , 149 : 103 – 110 .
- Trail , F. and Common , R. 2000 . Perithecial development by Gibberella zeae: a light microscopy study . Mycologia , 92 : 130 – 138 .
- Trail , F. , Xu , H. , Loranger , R. and Gadoury , D. 2002 . Physiological and environmental aspects of ascospore discharge in Gibberella zeae (anamorph Fusarium graminearum) . Mycologia , 94 : 181 – 189 .
- Trail , F. , Gaffoor , I. , Guenther , J.C. and Hallen , H.E. 2005a . Using genomics to understand the disease cycle of the fusarium head blight fungus, Gibberella zeae (anamorph Fusarium graminearum) . Can. J. Plant Pathol. , 27 : 486 – 498 .
- Trail , F. , Gaffoor , I. and Vogel , S. 2005b . Ejection mechanics and trajectory of the ascospores of Gibberella zeae (anamorph Fusarium graminearum) . Fungal Genet. Biol. , 42 : 528 – 533 .
- Vanittanakom , N. 1986 . Fengycin – a novel antifungal lipopeptide antibiotic produced by Bacillus subtilis F–29–3 . J. Antibiot. , 39 : 888
- Vogelgsang , S. , Hecker , A. , Musa , T. , Dorn , B. and Forrer , H.R. 2011 . On-farm experiments over 5 years in a grain maize/winter wheat rotation: effect of maize residue treatments on Fusarium graminearum infection and deoxynivalenol contamination in wheat . Mycotoxin Res. , 27 : 81 – 96 .
- Von Der Ohe , C. , Ebmeyer , E. , Korzun , V. and Miedaner , T. 2010a . Agronomic and quality performance of winter wheat backcross populations carrying non-adapted Fusarium head blight resistance QTL . Crop Sci. , 50 : 2283 – 2290 .
- Von Der Ohe , C. , Gauthier , V. , Tamburic-Ilincic , L. , Brûlé -Babel , A. , Fernando , W.G.D. Clear , R. 2010b . A comparison of aggressiveness and deoxynivalenol production between Canadian Fusarium graminearum isolates with 3-acetyl and 15-acetyldeoxynivalenol chemotypes in field-grown spring wheat . Eur. J. Plant Pathol. , 127 : 407 – 417 .
- Voss , H.H. , Holzapfel , J. , Hartl , L. , Korzun , V. , Rabenstein , F. Ebmeyer , E. 2008 . Effect of the Rht-D1 dwarfing locus on Fusarium head blight rating in three segregating populations of winter wheat . Plant Breeding , 127 : 333 – 339 .
- Waalwijk , C. , Kastelein , P. , De Vries , I. , Kereny , Z. , Van Der Lee , T. Hesselink , T. 2003 . Major changes in Fusarium spp. in wheat in the Netherlands . Eur. J. Plant Pathol. , 109 : 743
- Wang , J. , Liu , J. , Chen , H. and Yao , J. 2007 . Characterization of Fusarium graminearum inhibitory lipopeptide from Bacillus subtilis IB . Appl. Microbiol. Biotechnol. , 76 : 889 – 894 .
- Wang , J.R. , Wang , L. , Gulden , S. , Rocheleau , H. , Balcerzak , M. Hattori , J. 2010 . RNA profiling of fusarium head blight-resistant wheat addition lines containing the Thinopyrum elongatum chromosome 7E . Can. J. Plant Pathol. , 32 : 188 – 214 .
- Ward , T.J. , Bielawski , J.P. , Corby Kistler , H. , Sullivan , E. and O'donnell , K. 2002 . Ancestral polymorphism and adaptive evolution in the trichothecene mycotoxin gene cluster of phytopathogenic Fusarium . Proc. Natl. Acad. Sci. USA , 99 : 9278 – 9283 .
- Ward , T.J. , Clear , R.M. , Rooney , A.P. , O'donnell , K. , Gaba , D. Patrick , S. 2008 . An adaptive evolutionary shift in Fusarium head blight pathogen populations is driving the rapid spread of more toxigenic Fusarium graminearum in North America . Fungal Genet. Biol. , 45 : 473 – 484 .
- Wegulo , S.N. , Bockus , W.W. , Hernandez Nopsa , J. , DE Wolf , E.D. , Eskridge , K.M. Peiris , K.H.S. 2011 . Effects of integrating cultivar resistance and fungicide application on Fusarium head blight and deoxynivalenol in winter wheat . Plant Dis. , 95 : 554 – 560 .
- West , J.S. , Holdgate , S. , Townsend , J.A. , Edwards , S.G. , Jennings , P. and Fitt , B.D.L. 2012 . Impacts of changing climate and agronomic factors on fusarium ear blight of wheat in the UK . Fungal Ecol. , 5 : 53
- Wilde , F. , Schön , C.C. , Korzun , V. , Ebmeyer , E. , Schmolke , M. Hartl , L. 2008 . Marker-based introduction of three quantitative-trait loci conferring resistance to Fusarium head blight into an independent elite winter wheat breeding population . Theor. Appl. Genet. , 117 : 29 – 35 .
- Willyerd , K.T. , Li , C. , Madden , L.V. , Bradley , C.A. , Bergstrom , G.C. Sweets , L.E. 2012 . Efficacy and stability of integrating fungicide and cultivar resistance to manage Fusarium head blight and deoxynivalenol in wheat . Plant Dis. , 96 : 957 – 967 .
- Xiao , J. , Jia , X. , Wang , H. , Zhao , R. , Fang , Y. Gao , R. 2011 . A fast-neutron induced chromosome fragment deletion of 3BS in wheat landrace Wangshuibai increased its susceptibility to Fusarium head blight . Chromosome Res. , 19 : 225 – 234 .
- Xu , X. and Nicholson , P. 2009 . Community ecology of fungal pathogens causing wheat head blight . Annu. Rev. Phytopathol. , 47 : 83 – 103 .
- Xu , X. , Nicholson , P. and Ritieni , A. 2007 . Effects of fungal interactions among Fusarium head blight pathogens on disease development and mycotoxin accumulation . Int. J. Food Microbiol. , 119 : 67 – 71 .
- Xu , Y. and Crouch , J.H. 2008 . Marker-assisted selection in plant breeding: from publications to practice . Crop Sci. , 48 : 391 – 407 .
- Xue , A.G. , Voldeng , H.D. , Savard , M.E. , Fedak , G. , Tian , X. and Hsiang , T. 2009 . Biological control of fusarium head blight of wheat with Clonostachys rosea strain ACM941 . Can. J. Plant Pathol. , 31 : 169 – 179 .
- Yan , W. , Li , H.B. , Cai , S.B. , Ma , H.X. , Rebetzke , G.J. and Liu , C.J. 2011 . Effects of plant height on type I and type II resistance to Fusarium head blight in wheat . Plant Pathol. , 60 : 506 – 512 .
- Yli-Mattila , T. , Gagkaeva , T. , Ward , T.J. , Aoki , T. , Kistler , H.C. and O'Donnell , K. 2009 . A novel Asian clade within the Fusarium graminearum species complex includes a newly discovered cereal head blight pathogen from the Russian Far East . Mycologia , 101 : 841 – 852 .
- Yoshida , M. , Nakajima , T. , Tomimura , K. , Suzuki , F. , Arai , M. and Miyasaka , A. 2012 . Effect of the timing of fungicide application on Fusarium head blight and mycotoxin contamination in wheat . Plant Dis. , 96 : 845 – 851 .
- Young , N.D. 1999 . A cautiously optimistic vision for marker-assisted breeding . Mol. Breed. , 5 : 505 – 510 .
- Yuen , G.Y. and Schoneweis , S.D. 2007 . Strategies for managing Fusarium head blight and deoxynivalenol accumulation in wheat . Int. J. Food Microbiol. , 119 : 126 – 130 .
- Zhang , G. , Mergoum , M. and Stack , R.W. Dec. 10–12, 2006 2006 . “ Grain shattering and FHB-resistance QTLs linkage in wheat ” . In Proceedings of the 2006 National Fusarium Head Blight Forum , Edited by: Canty , S. , Clark , A. and Van Sanford , D. Dec. 10–12, 2006 , NC (pp. 128) : Research Triangle Park .
- Zhang , S.A. , Schisler , D.A. , Boehm , M.J. and Slininger , P.J. 2005 . Carbon-to-nitrogen ratio and carbon loading of production media influence freeze-drying survival and biocontrol efficacy of Cryptococcus nodaensis OH 182.9 . Phytopathology , 95 : 626 – 631 .
- Zhang , S.A. , Schisler , D.A. , Boehm , M.J. and Slininger , P.J. 2007 . Utilization of chemical inducers of resistance and Cryptococcus flavescens OH 182.9 to reduce Fusarium head blight under greenhouse conditions . Biol. Control , 42 : 308 – 315 .
- Zhang , X. , Shen , X. , Hao , Y. , Cai , J. , Ohm , H.W. and Kong , L. 2011 . A genetic map of Lophopyrum ponticum chromosome 7E, harboring resistance genes to Fusarium head blight and leaf rust . Theor. Appl. Genet. , 122 : 263 – 270 .
- Zhang , X. , Bai , G. , Bockus , W. , Ji , X. and Pan , H. 2012a . Quantitative trait loci for Fusarium head blight resistance in U.S. hard winter wheat cultivar Heyne . Crop Sci. , 52 : 1187 – 1194 .
- Zhang , X. , Pan , H. and Bai , G. 2012b . Quantitative trait loci responsible for Fusarium head blight resistance in Chinese landrace Baishanyuehuang . Theor. Appl. Genet. , 125 : 495 – 502 .
- Zinedine , A. and Manes , J. 2009 . Occurrence and legislation of mycotoxins in food and feed from Morocco . Food Control , 20 : 334 – 344 .