Abstract
Zebra chip disease (ZCD) of potato (Solanum tuberosum L.) has caused significant economic losses to the potato industry in the USA. ‘Candidatus Liberibacter solanacearum’ Liefting et al. has been associated with ZCD. This non-culturable bacterium dwells within the phloem of plants where other endophytic bacteria may also live. Knowledge concerning phloem colonization by ‘Ca. L. solanacearum’ and other endophytic bacteria may provide a better understanding about disease biology and could facilitate the development of novel disease management strategies. In this study, endophytic bacteria in potato tubers afflicted with ZCD were evaluated by scanning electron microscopy (SEM), in vitro cultivation, and PCR analyses. Bacillus, coccus, and filamentous bacterial cells were observed by SEM in phloem tissue of ZCD-diseased tubers, but were absent in healthy potato tubers. Fifty-one bacterial isolates were obtained in vitro from ZCD-affected tubers and 34 isolates were from non-ZCD tubers. Comparison of 16S rDNA sequences identified bacteria belonging to 16 genera. Seven (Brachybacterium, Enterobacter, Microbacterium, Paenibacillus, Staphylococcus, Stenotrophomonas and Variovorax) were only isolated from ZCD-affected potato tubers, whereas four (Bosea, Nocardia, Sphingomonas and Sphingopyxis) were only isolated from non-ZCD potato tubers. However, a more sensitive PCR analysis diminished the specific association of the bacteria to either ZCD or non-ZCD tubers, suggesting that infection by ‘Ca. L. solanacearum’ influenced the titres of other endophytic bacteria in potato tubers.
Résumé
La maladie de la chips zébrée (CZ) de la pomme de terre a causé d'importantes pertes économiques à l'industrie de la pomme de terre aux États-Unis. ‘Candidatus Liberibacter solanacearum’ Liefting et al. a été associé à la CZ. Cette bactérie, impossible à produire par culture, vit dans le phloème des plantes où d'autres bactéries endophytes sont également susceptibles de vivre. Une connaissance plus approfondie de la colonisation du phloème par ‘Ca. L. solanacearum’ et d'autres bactéries endophytes nous fournirait une meilleure compréhension de l'affection biologique et pourrait faciliter l’élaboration de nouvelles stratégies de gestion de la maladie. Dans cette étude, nous avons évalué par microscopie électronique à balayage (MEB), culture in vitro et analyses par PCR les bactéries endophytes qui avaient colonisé des tubercules infectés par la CZ. Des bacilles, des coques et des cellules bactériennes filamenteuses ont été observés par MEB dans le phloème des tubercules atteints de la CZ, mais pas dans celui de tubercules sains. Nous avons obtenu, in vitro, 51 isolats bactériens à partir de tubercules atteints et 34 de tubercules sains. La comparaison de séquences d'ADNr 16S a permis de répertorier des bactéries appartenant à 16 genres. Sept seulement (Brachybacterium, Enterobacter, Microbacterium, Paenibacillus, Staphylococcus, Stenotrophomonas et Variovorax) ont été isolées à partir de tubercules atteints de CZ, tandis que quatre seulement (Bosea, Nocardia, Sphingomonas et Sphingopyxis) ont été isolées à partir de tubercules sains. Toutefois, une analyse par PCR, plus sensible, a permis de minimiser l'association spécifique des bactéries relativement aux tubercules atteints ou pas de CZ, ce qui suggère que l'infection causée par ‘Ca. L. solanacearum’ a influencé les titres d'autres bactéries endophytes dans les tubercules.
Introduction
Potato zebra chip disease (ZCD) drastically reduces quality and value of potato tubers. ‘Candidatus Liberibacter solanacearum’ Liefting et al., a currently unculturable alpha-proteobacterium, has been associated with ZCD (Liefting et al., Citation2009a , Citation2009b ). This proteobacterium resides in the phloem tissues of potato and therefore shares the same ecological niche as other bacterial endophytes. In this study, we refer to endophytes as all bacteria living in the internal plant tissues, although various definitions of endophytic bacteria exist (Hallmann et al., Citation1997; Berg et al., Citation2005; Ryan et al., Citation2008). Knowledge about endophytic bacteria, particularly those capable of inhabiting phloem tissue, could facilitate ZCD research and the development of endophyte-based disease control strategies (Sturz et al., Citation2000).
Different methodologies have been used to study bacterial endophytes. Use of cultivation-independent techniques has significantly increased our knowledge of the composition, distribution and population dynamics of these endophytes (Garbeva et al., Citation2001; Reiter et al., Citation2002; Berg et al., Citation2005; Ryan et al., Citation2008). For example, based on a cultivation-independent terminal restriction fragment length polymorphism analysis of 16S rDNA, Reiter et al. (Citation2002) described a range of organisms belonging to several distinct phylogenetic groups. These endophytic bacterial populations were shown to significantly increase in potato plants infected by Pectobacterium atrosepticum Gardan et al. (Erwinia caraotovora subsp. atroseptica Hellmers), the causal agent of blackleg disease (Reiter et al., Citation2002).
A disadvantage of DNA-based techniques is that they do not reveal bacterial morphology and the locations within the host where bacteria reside. Scanning electron microscopy (SEM) resolves these problems (Gantar et al., Citation1991; van Doorn et al., Citation1991; Sardi et al., Citation1992). A limitation of SEM is the difficulty in detection at low titres and uneven distribution of endophytic bacteria. The average population densities of bacterial endophytes were estimated to be 1 × 104 – 1 × 105 colony forming units(CFU) g−1 tissue, whereas SEM detection limits were estimated to be about 1 × 106 CFU g−1 tissue (Hallmann et al., Citation1997). However, bacterial titres after pathogen infection could increase (Reiter et al., Citation2002). Therefore, despite the threshold of SEM detection nearing the limits in healthy plants and the uneven distribution, it may still be possible to observe bacterial endophytes in planta using SEM.
Pure cultures of endophytic bacteria are fundamental for research on bacterial–host interactions, genetic analyses, taxonomy and biological manipulation. Cultivable bacteria also could be developed as biological control agents for diseases. Several endophytic bacteria were isolated from potato and evaluated for ability to inhibit pathogen growth, such as Pseudomonas sp. to limit growth of P. carotovorum (E. carotovora) (Kloepper, Citation1983), and Curtobacterium luteum Komagata to limit Phytophthora infestans de Bary (Sturz et al., Citation1999). However, to the best of our knowledge, using endophytic bacteria to mitigate ZCD has yet to be explored.
The objective of this research was to characterize and compare bacterial endophytes in ZCD-afflicted and healthy (non-ZCD) potato tubers. To accomplish this objective, three approaches were used: (i) endophytic bacteria in phloem tissue of potato tubers were observed by SEM; (ii) endophytic bacteria were isolated from ZCD and non-ZCD tubers and cultured in vitro to compare bacterial populations; and (iii) PCR-based techniques were utilized to confirm whether endophytes which could be cultivated from either healthy or ZCD-afflicted tubers were truly associated with ZCD disease status.
Materials and methods
Source of potato tubers
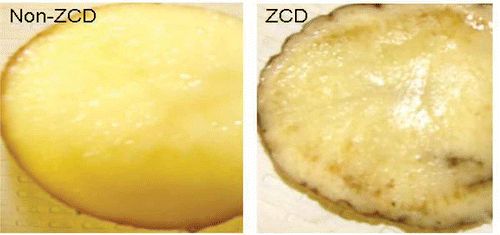
Fresh potato tubers ‘Atlantic’ were collected in April 2009 from research fields in Rio Grande Valley, Texas, and were sent to San Joaquin Valley Agricultural Sciences Center (SJVASC), Parlier, CA. Upon arrival, they were stored at 4–10 °C and experiments were performed within 3 months. ZCD tubers exhibited necrotic symptoms whereas healthy tubers did not (). Presence of ‘Ca. L. solanacearum’ was determined by PCR using primer set OA2 (5′-GCGCTTATTT TTAATAGGAG CGGCA-3′)/OI2c (5′-GCCTCGCGAC TTCGCAACCC AT-3′) (Liefting et al., Citation2009a ) with the PCR procedure described below.
Scanning electron microscopy
Pieces (5 mm3) of potato tuber tissue showing necrotic symptoms were fixed in 2.5% glutaraldehyde and 2.5% formaldehyde prepared from paraformaldehyde in 0.1 m phosphate buffer (pH 7.2) (Karnovsky, Citation1965) for 24 h at 4 °C. A mild vacuum was applied to ensure the rapid infiltration of fixative into the specimens. The fixed specimens were washed three times with 0.1 m phosphate buffer (pH 7.2), incubating at 23 °C for 30 min each time. The specimens were infiltrated overnight with a cryoprotectant, 30% glycerol in water. Specimens were plunge-frozen in liquid N2, then cross-sectioned with a scalpel blade. Specimens were post-fixed in 1% OsO4 in 0.1 m phosphate buffer (pH 7.2) for 1 h, and then washed three times in deionized water, incubating 30 min for each wash. The specimens were dehydrated in an ethanol series to 100% ethanol, then critical-point dried. The dried specimens were mounted on aluminium stubs and sputter-coated with gold. Observations and digital photos were made with a Hitachi S-3500N SEM at 15 kV.
In vitro bacterial isolation
For in vitro assays, periwinkle wilt (PW) medium (Davis et al., Citation1983) was used, since it supports growth of many bacterial species, even the nutritionally fastidious Xylella fastidiosa Wells et al. Twelve ZCD-affected and non-affected potato tubers were selected, washed with tap water, and air-dried. Tubers were dipped in 100% ethanol and flame-sterilized twice. Tubers then were cut by a sterilized scalpel, with a small piece (5 mm3) placed in a sterile Petri dish and crushed into tiny pieces. Into the Petri dish, 0.2 mL of PW broth was immediately added and mixed. One loopful of the mixture was streaked on the surface of PW medium solidified with GelRite (Sigma-Aldrich Co., St. Louis, MO) (the plating-only method) or inoculated into 10 mL of PW broth for later plating (the broth-plating method). All cultures were placed in an incubator set at 28 °C. For the plating-only method, single bacterial colonies that appeared from overnight to 30 days were selected and transferred to fresh PW plates to generate pure cultures. For the broth-plating method, one loopful of 7-day-old culture was streaked onto PW plates to generate single colonies in order to obtain pure cultures. Colonies from pure cultures were placed into 25% glycerol PW broth and maintained as an archived voucher collection in a −80 °C freezer at San Joaquin Valley Agricultural Sciences Center, Parlier, CA.
DNA extraction and PCR amplification
Template bacterial DNA for PCR was prepared either from pure cultures or from potato tissue. For bacterial cultures, DNA templates were prepared from suspensions made by adding a loop of cultured bacteria in 100 μL of sterile water. For potato tuber tissues, after flame-sterilization, small vascular blocks (5 mm3) were cut out by a scalpel, and these were placed in a labelled paper envelope and freeze-dried overnight in the Freezone 2.5 Freeze Dry System (Labconco Corp., Kansas City, MO). The freeze-dried samples were pulverized in a Fast-Prep machine (FP120, Qbiogene, Inc. Carlsbad, CA) for 20 s. The bacterial suspensions or powdered tuber tissues were used for DNA isolation with the cetyltrimethylammonium bromide (CTAB) method (Murray & Thompson, Citation1980).
PCR primers and their sources are listed in . Primers were designed based on 16S rDNA sequences. PCR reactions were carried out with a TaKaRa Hot Start Taq system (Clontech Laboratories, Inc., Madison, WI) in 25 μL mixtures (Chen et al., 2005) containing 2.5 μL of 10× DNA polymerase buffer, 2.5 μL of 2.5 mm deoxynucleoside triphosphates (dNTPs), 0.5 μL of each of forward and reverse PCR primer (10 μm), 2 μL of template DNA, 0.2 μL of Taq DNA polymerase at 5 U μL−1, and 16.8 μL of H2O. An MJ Research DNA Engine Tetrad 2 was programmed with an initial denaturing step of 96 °C for 1 min and 35 cycles of the following conditions: 96 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s. A final extension of 72 °C for 4 min was set after the last cycle. PCR products were electrophoresed in a 1.5% agarose gel and visualized by ethidium bromide staining under UV light.
Table 1. Primer sets and their properties used in this study
Bacterial sequence analyses and identification
The PCR products of bacterial 16S rDNA were sequenced directly in both orientations using BigDye Terminator v3.1 Cycle Sequencing Kit in a 3130xl Genetic Analyzer (Applied Biosystems, Inc.) with the same PCR primers (). Sequences were used for similarity searches through the BLASTn application of GenBank maintained by the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov). Putative bacterial identities were assigned according to high similarity (> 97%) at the genus level.
Results
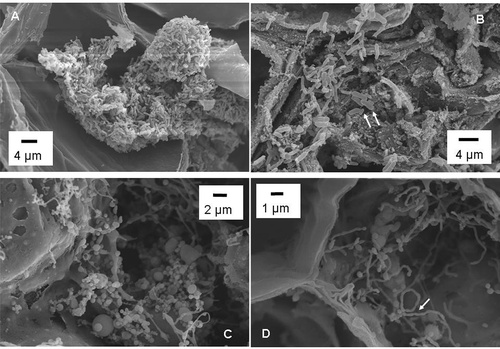
Phloem necrosis, a characteristic symptom of ZCD, is shown in No bacteria were observed in any of the non-necrotic phloem tissue examined by SEM. Only about 5% of the examined necrotic phloem tissues showed bacteria (). The bacteria differed in morphology, and included bacilli (, ), cocci (), filamentous and pleomorphic shapes (). Bacilli bacteria formed clumps (). Two distinctive bacillus cells were present side by side, with the larger cells having an average dimension of 2.31 ± 0.23 × 0.7 ± 0.06 μM (n = 5) and the smaller bacterial cells having an average dimension of 0.97 ± 0.06 × 0.40 ± 0.39 μM (n = 5) (). Diameters of the cocci ranged from 0.2 μM to 5 μM or even larger (). Some of the long filamentous cells were observed undergoing transitions to chain-like morphology (). It is likely that at least one of the observed bacterial morphology was ‘Ca. L. solanacearum’, since only potato tubers that tested positive for the bacterium were used for SEM.
Fig. 2. Scanning electron micrographs of phloem tissue of potato tubers with zebra chip disease. (A) Clumps of bacilli bacteria; (B) Bacilli bacteria of different sizes (arrows); (C) Cocci or cocci-like bacteria; and (D) Chain-like structure of bacterial cells (arrow).
Of the two in vitro culture methods (plating-only or broth-plating), the broth-plating method was far more successful, with 94% (15 of 16) of tuber tissue segments yielding bacteria, compared with 25% (4 out of 16) using the plating-only method in initial tests. Therefore, in vitro endophyte isolation was primarily conducted using the broth-plating method. A total of 28 tissue samples from 12 non-ZCD potato tubers and 29 samples from 12 ZCD-affected potato tubers were used for in vitro isolation. In total, 85 bacterial isolates were recovered, with 34 isolates from non-ZCD tubers and 51 isolates from ZCD-affected tubers (). The 85 bacterial isolates were representative of the major phylogenetic groups of bacteria, including α-proteobacteria, β-proteobacteria, γ-proteobacteria, Firmicutes and Actinobacteria ().
Table 2. Comparison of taxonomic distribution of endophytic bacteria from potato tubers
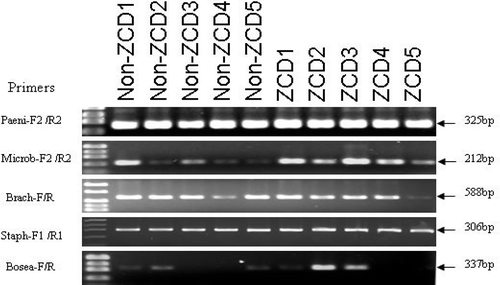
Based on comparative analyses of 16S rDNA sequences, the bacterial isolates fell into 16 genera. Six isolates could not be identified. One of these six isolates matched with an uncultured bacterium and the other five had no match in the current GenBank database according to default specifications (). Seven bacterial genera (Brachybacterium, Enterobacter, Microbacterium, Paenibacillus, Staphylococcus, Stenotrophomonas and Variovorax) were only recovered from ZCD-affected potato tubers, and four (Bosea, Nocardia. Sphingomonas and Sphingopyxis) were only isolated from non-ZCD potato tubers. The related bacterial sequences were submitted to GenBank with accession numbers listed in . GenBank currently only accepts sequences over 200 bp. The 188 bp sequence from Variovorax sp. was not submitted.
To evaluate the apparent specific associations from in vitro cultivation experiments, four genera (Microbacterium, Paenibacillus, Staphylococcus and Brachybacterium) classified as ZCD-only and one bacterium (Bosea) classified as non-ZCD-only, were selected for further PCR amplification. The three ZCD-associated bacteria determined by in vitro cultivation were detected in non-ZCD tubers by PCR (). Similarly, the non-ZCD Bosea was detected in ZCD tubers (). Therefore, PCR analyses diminished the specificity of these bacteria to ZCD status based on the in vitro culture method.
Discussion
A few bacterial endophytes present within potato tuber phloem of ZCD plants were observed using SEM. The presence of morphological distinct types of bacteria () suggests the coexistence of multiple endophytes and raises an interesting question whether these bacteria interact with each other. Using coinoculation, Quadt-Hallmann et al. (Citation1997) reported that Enterobacter asburiae JM22 and Arthrobacter agilis did not affect the internal population density of JM22 in cotton plants. However, coinoculation using JM22 and another endophyte (Paenibacillus macerans) resulted in reduced population densities of both endophytes.
The morphology of ‘Ca. L. solanacearum’ has only been described by transmission electron microscopy (TEM) (Liefting et al., Citation2009a ; Secor et al., Citation2009). In general, the bacterium was considered as rod shaped (Liefting et al., Citation2009b ) and detailed morphological features are currently lacking. Liefting et al. (Citation2009a ) observed bacteria as long as 4 μM and the presence of continuous intermittent short rod-shaped or spherical cells. The latter could be from the cross-section of chain-like bacteria similar to those seen in in this study. Since the SEM samples were PCR-positive for ‘Ca. L. solanacearum’, it is possible that the chain-like cells in are ‘Ca. L. solanacearum’. Our current research with ‘Ca. L. solanacearum’ maintained in tomato plants also suggests the filamentous/pleomorphic morphology is common (data not shown). If so, this study revealed a previously undescribed morphological feature of ‘Ca. L. solanacearum’. Morphological characterization is important in fastidious prokaryote research. It was the recognition of spiroform morphology that facilitated the in vitro cultivation of corn stunt spiroplasma (Chen & Liao, Citation1975; Williamson & Whitcomb, Citation1975).
As shown in , it is interesting that a significant number of actinobacteria were identified and most of them (74% or 20/27) were associated with ZCD potato tubers. In an effort to cultivate ‘Ca. L. asiaticus’, the presumptive pathogen of citrus Huanglongbing (HLB) and close relative to ‘Ca. L. solanacearum’, Davis et al. (Citation2008) reported that the co-inhabitance of an actinobacterium could play a role in maintaining ‘Ca. L. asiaticus’ over 10 weekly passages. The relationship of actinobacteria with ‘Ca. L. solanacearum’ deserves further research in the future, particularly in light of in vitro cultivation of ‘Ca. L. solanacearum’.
The isolated/identified bacteria observed in this study represented many major groups of eubacteria, and the species coverage was found to be comparable to that reported previously (Reiter et al., Citation2002; Sessitsch et al., Citation2002, 2004; Berg et al., Citation2005). However, no Pseudomonas was identified even though it is a common endophyte of potato that has been identified by both in vitro cultivation and molecular techniques (De Boer & Copeman, Citation1974; Hallmann et al., Citation1997; Sturz et al., Citation1999; Reiter et al., Citation2002; Sessitsch et al., Citation2004; Berg et al., Citation2005). The lack of Pseudomonas species may reflect the heterogeneity of endophytic bacterial populations from different sources and the influence of uncontrolled biotic/abiotic factors on endophytes.
It was interesting that ZCD-associated bacteria (Microbacterium, Paenibacillus, Staphylococcus and Brachybacterium), as determined by in vitro cultivation, were detected in non-ZCD tubers by PCR. This is in contrast to ‘Ca. L. solanacearum’ which could be detected by PCR only in ZCD hosts. This suggests that the bacterial endophytes were less likely to be associated with ZCD symptoms. In fact, it is more plausible that ‘Ca. L. solanacearum’ infection resulted in an increase in bacterial titres of these species, allowing culturing to be successful. Likewise, the opposite situation could be assumed for the non-ZCD associated Bosea, i.e. ZCD apparently suppressed the titre of this species. The results from this study demonstrate that ‘Ca. L. solanacearum’ infections can affect population dynamics of certain potato endophytes, either positively or negatively. The mechanism of the possible bacteria–bacteria or bacterial pathogen–host–bacterial endophyte interactions that may have resulted in these observations are worthy of future research.
Acknowledgements
This research was supported by a USDA Specialty Crop Research Initiative grant for ZCD research. We thank R. Huerta, G. Phillips, X. Wang and D. Margosan for technical assistance in the execution of this research.
Notes
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
References
- Baker , G.C. , Smith , J.J. and Cowan , D.A. 2003 . Review and re-analysis of domain-specific 16S primers . J. Microbiol. Meth. , 55 : 541 – 555 .
- Berg , G. , Krechel , A. , Ditz , M. , Sikora , R.A. , Ulrich , A. and Hallmann , J. 2005 . Endophytic and ectophytic potato-associated bacterial communities differ in structure and antagonistic function against plant pathogenic fungi . FEMS Microbiol Ecol. , 51 : 215 – 229 .
- Chen, J., Groves, R., Civerolo, E. L., Viveros, M., Freeman, M., & Zheng, Y. (2005). Two Xylella fastidiosa genotypes associated with almond leaf scorch disease on the same location in California. Phytopathology, 95, 708–714.
- Chen , T.A. and Liao , C.H . 1975 . Corn stunt spiroplasma: isolation, cultivation, and proof of pathogenicity . Science , 188 : 1015 – 1017 .
- Davis , M.J. , Raju , B.C. , Brlansky , R.H. , Lee , R.F. , Timmer , L.W. , Norris , R.C. and Mccoy , R.E. 1983 . Periwinkle wilt bacterium: axenic culture, pathogenicity, and relationships to other Gram-negative, xylem-inhabiting bacteria . Phytopathology , 73 : 1510 – 1515 .
- Davis , M.J. , Mondal , S.N. , Chen , H. , Rogers , M.E. and Brlansky , R.H. 2008 . Co-cultivation of ‘ Candidatus Liberibacter asiaticus’ with actinobacteria from citrus with huanglongbing . Plant Dis. , 92 : 1547 – 1550 .
- De Boer , S.H. and Copeman , R.J. 1974 . Endophytic bacterial flora in Solanum tuberosum and its significance in bacterial ring rot disease . Can. J. Plant Sci. , 54 : 115 – 122 .
- Gantar , M. , Kerby , N.W. and Rowell , P. 1991 . Colonization of wheat (Triticum vulgare L.) by N2-fixing cyanobacteria: II. An ultrastructural study . New Phytol. , 118 : 485 – 492 .
- Garbeva , P. , Van Overbeek , L.S. , Van Vuurde , J.W.L. and Van Elsas , J.D. 2001 . Analysis of endophytic bacterial communities of potato by plating and denaturing gradient gel electrophoresis (DGGE) of 16S rDNA based PCR fragments . Microb. Ecol. , 41 : 369 – 383 .
- Hallmann , J. , Quadt-Hallmann , A. , Mahaffee , W.F. and Kloepper , J.W. 1997 . Bacterial endophytes in agricultural crops . Can. J. Microbiol. , 43 : 895 – 914 .
- Karnovsky , M.J . 1965 . A formaldehyde-glutaraldehyde fixative of high osmolarity for use in electron microscopy . J. Cell Biol. , 27 : 137A
- Kloepper , J.W. 1983 . Effect of seed piece inoculation with plant-growth promoting rhizobacteria on populations of Erwinia carotovora on potato roots and daughter tubers . Phytopathology , 73 : 217 – 219 .
- Liefting , L.W. , Sutherland , P.W. , Ward , L.I. , Paice , K.L. , Weir , B.S. and Clover , G.R.G. 2009a . A new ‘Candidatus Liberibacter’ species associated with diseases of solanaceous crops . Plant Dis. , 93 : 208 – 214 .
- Liefting , L.W. , Weir , B.S. , Oennycook , S. R. and Clover , G.R.G. (2009b). ‘ . Candidatus Liberibacter solanacearum’ associated with plants in the family Solanaceae . Intern. J. Syst. Evol. Microbiol. , 59 : 2274 – 2276 .
- Murray , M.G. and Thompson , W.F. 1980 . Rapid isolation of high molecular weight plant DNA . Nucleic Acids Res. , 8 : 4321 – 4324 .
- Quadt-Hallmann , A. , Hallmann , J. and Kloepper , J.W. 1997 . Bacterial endophytes in cotton: location and interaction with other plant-associated bacteria . Can. J. Microbiol. , 43 : 254 – 259 .
- Reiter , B. , Pfeifer , U. , Schwab , H. and Sessitsch , A. 2002 . Response of endophytic bacterial communities in potato plants to infection with Erwinia carotovora subsp . atroseptica. Appl. Envir. Microbiol. , 68 : 1168 – 2261 .
- Ryan , R.P. , Germaine , K. , Franks , A. , Ryan , D.J. and Dowling , D.N. 2008 . Bacterial endophytes: recent developments and applications . FEMS Microbiol. Lett. , 278 : 1 – 9 .
- Sardi , R. , Saracchi , M. , Quaroni , S. , Petrolini , B. , Borgonovi , G.E. and Merli , S. 1992 . Isolation of endophytic Streptomyces strains from surface-disinfested roots . Appl. Environ. Microbiol. , 58 : 2691 – 2693 .
- Secor , G.A. , Rivera , V.V. , Abad , J.A. , Lee , I.M. , Clover , G.R.G. , Liefting , L.W. , LI , X. and De Boer , S.H. 2009 . Association of ‘Candidatus Liberibacter solanacearum’ with zebra chip disease of potato established by graft and psyllid transmission, electron microscopy, and PCR . Plant Dis. , 93 : 574 – 583 .
- Sessitsch , A. , Reiter , B. , Pfeifer , U. and Wilhelm , E. 2002 . Cultivation-independent population analysis of bacterial endophytes in three potato varieties based on eubacterial and Actinomycetes specific PCR of 16S rRNA genes . FEMS Microbiol. Ecol. , 39 : 23 – 32 .
- Sessitsch , A. , Reiter , B. and Berg , G. 2004 . Endophytic bacterial communities of field-grown potato plants and their plant-growth-promoting and antagonistic abilities . Can. J. Microbiol. , 50 : 239 – 249 .
- Sturz , V. , Christie , B.R. , Matheson , B.G. , Arsenault , W.J. and Buchanan , N.A. 1999 . Endophytic bacterial communities in the periderm of potato tubers and their potential to improve resistance to soil-borne plant pathogens . Plant Pathol. , 48 : 360 – 369 .
- Sturz , V. , Christie , B.R. and Nowak , J. 2000 . Bacterial endophytes: potential role in developing sustainable system of crop production . Crit. Rev. Plant Sci. , 19 : 1 – 30 .
- Van Doorn , W.G. , Clerkx , A. and Boekestein , A. 1991 . Bacteria as a cause of vascular occlusion in cut fronds of Adiantuym raddianum: a scanning electron microscope study . Sci. Hortic. , 48 : 299 – 309 .
- Williamson , D.L. and Whitcomb , R.F. 1975 . Plant mycoplasmas: a cultivable spiroplasma causes corn stunt disease . Science , 188 : 1018 – 1020 .