Abstract
In central Alberta, stripe rust (Puccinia striiformis) of wheat and barley has become more prevalent and this disease is generally more severe on winter wheat than spring wheat. This study was carried out to determine the potential role of winter wheat in overwintering and transmission of inoculum to subsequent spring crops. Field plants of winter wheat, infected in the autumn, were sampled during January to May from 2008 to 2011 in central Alberta. The pathogen's viability was established using in vivo growth parameters and pathogen detection was determined using PCR. Approximately 4–20% of urediniospores sampled from pustules on winter wheat during January to March–April of each year were viable. Latent mycelia were intermittently observed on winter wheat sampled from January to May in each of 2008 and 2010. The fungus was detected using PCR intermittently from winter to early spring. Persistent snow cover was found to be critical for the survival of overwintering inoculum, as viable pustules and urediniospores rarely survived after snow melt. There was higher stripe rust severity in spring wheat or barley seeded near winter wheat compared with the same crops seeded near spring wheat, for all years of field testing. The epidemiological significance of overwintering inoculum is discussed in relation to stripe rust management in central Alberta.
Résumé
Dans le centre de l'Alberta, la rouille jaune du blé et de l'orge (Puccinia striiformis) est devenue plus courante et elle frappe habituellement plus durement le blé d'hiver que le blé de printemps. Cette étude a été menée pour déterminer le rôle possible que joue le blé d'hiver dans la survie hiémale de l'inoculum et dans sa transmission aux cultures printanières subséquentes. De 2008 à 2011, dans le centre de l'Alberta, des plants de blé d'hiver cultivés en champ, infectés à l'automne, ont été échantillonnés de janvier à mai. La viabilité de l'agent pathogène a été établie à l'aide de paramètres de croissance in vivo et il a été détecté par PCR. De 4 à 20 % environ des urédiniospores échantillonnés sur des pustules formées sur du blé d'hiver de janvier à mars-avril de chaque année étaient viables. Du mycélium latent a été observé par intermittence sur du blé d'hiver échantillonné de janvier à mai en 2008 et 2010. Le champignon a été détecté par PCR utilisée sporadiquement de l'hiver au début du printemps. La persistance du couvert nival a semblé déterminante pour la survie hiémale de l'inoculum étant donné que les pustules et les urédiniospores semblaient rarement survivre après la fonte des neiges. La gravité de la rouille jaune était plus intense chez le blé ou l'orge semés à proximité du blé d'hiver, contrairement aux mêmes cultures semées près du blé de printemps, et ce, durant toutes les années d'expérimentation en plein champ. La signification épidémiologique de la survie hiémale de l'inoculum est discutée en fonction de la gestion de la rouille jaune dans le centre de l'Alberta.
Introduction
Stripe rust of wheat caused by Puccinia striiformis Westend. f. sp. tritici Eriks. & Henn. (Pst) is an important disease of wheat worldwide. The pathogen is a biotrophic, polycyclic heteroecious rust fungus. Until recently, Berberis chinensis Poir. was identified to be an alternative host for Pst to complete sexual reproduction (Jin et al., Citation2010). Although this alternative host may play a role in the development of pathogen variability, the pathogen mainly relies on the infection of susceptible hosts by its airborne urediniospores to survive and spread. The disease is most prevalent in cool and humid temperate conditions. Optimal temperatures for germination and growth in the host are significantly lower than that of other cereal rusts (Roelfs et al., Citation1992).
Since 2000, the Pst population in North America has become dominated by an invasive aggressive strain and its descendents (Markell & Milus, Citation2008; Chen et al., Citation2010). These isolates are capable of overcoming novel combinations of resistance genes, are more aggressive, and are better adapted to growth and survival in warmer conditions (Milus et al., Citation2009; Chen et al., Citation2010). As such, the disease has become more severe outside of its traditional areas in North America (Chen et al., Citation2010). Barley and triticale can also suffer stripe rust infections, primarily caused by P. striiformis f. sp. hordei Eriks. & Henn., Psh and Pst, respectively. The two diseases are less frequently observed than wheat stripe rust in Alberta and information on overwintering of stripe rust in barley and triticale is lacking.
Due to its airborne ureidiniospores, Pst is well adapted for long-distance dispersal either through long-distance movement or a series of shorter movements within the same season (Brown & Hovmøller, Citation2002). This allows the pathogen to overwinter in warmer southern regions and then move north, thereby providing exogenous inoculum to central Alberta. Within North America, parts of Mexico, the southern USA and particularly the Pacific north-west (PNW) experience mild winter conditions that are conducive to overwintering of the stripe rust pathogen which then can migrate into Alberta (Chen et al., Citation2002; Chen, Citation2005). Similar situations are found in other regions such as China where the disease can overwinter in southern regions and then spread northwards to cause epidemics (Zeng & Lou, Citation2006; Wang et al., Citation2010).
The importance or possibility of stripe rust overwintering by surviving cold winter temperatures is not new. Early reports of stripe rust overwintering in central and northern Europe started with Eriksson & Henning in 1896 and became more frequent in the early 1900s [reviewed in Zadoks (Citation1961) and Humphrey et al. (Citation1924)]. Up to the late 1960s, there have been over 60 reports of stripe rust overwintering (Burleigh & Hendrix, Citation1970). Although it is possible that stripe rust could overwinter as either uredia and urediniospores or mycelia in infected hosts, most research suggests that mycelia is hardier than the urediniospores. There have been reports of viable uredia occuring throughout the winter in mild climates such as that of the PNW region of the USA (Hungerford, Citation1923), but this has been viewed as unlikely and not observed in harsher climates (Zadoks, Citation1961). Despite this, there is evidence that urediniospores are well adapted to cold conditions, capable of germinating and infecting winter wheat down to −4 °C (Burleigh & Hendrix, Citation1970). There have been reports of latent infections surviving the winter period within host tissues for durations of 120–160 days (Zadoks, Citation1961; Sharp & Hehn, Citation1963; Burleigh & Hendrix, Citation1970). As an obligate pathogen, survival of the fungus is contingent on survival of the host. As a result, conditions that result in winter kill will also result in the mortality of the fungus. Winter wheat leaves with uredia have been reported to be more vulnerable to frosts and cold conditions than leaves with only latent mycelia (Gassner & Pieschel, Citation1934; Zadoks, Citation1961). Potential overwintering of latent P. striiformis infections are difficult to detect based on visual observations. Molecular diagnostic techniques have been developed to provide early detection and, thereby, identify latent infections of overwintering stripe rust in winter wheat (Wang et al., Citation2008).
There have been previous reports of overwintering (Sanford & Broadfoot, Citation1929) and disease development in winter wheat from potential overwintering inoculum in southern Alberta (Conner et al., Citation1988). Elevated stripe rust severity in winter wheat has become a common occurrence in central Alberta (Xi et al., Citation2011). While the cause of the epidemics in southern Saskatchewan in 2012 was unknown, high inoculum loads observed in winter wheat in the autumn of 2011 may have contributed to the disease outbreak in 2012 (Gehl, personal communication). Furthermore, epidemics in 2006 and 2011 have been attributed to overwintering of the pathogen in southern Alberta (D. Gaudet, unpublished results, cited from Randhawa et al., Citation2012). Stripe rust that overwinters in winter wheat will begin producing urediniospores in the cool, wet conditions the following spring, which will spread to the emerging spring wheat crop. Despite these observations, no studies have been performed to examine the overwintering of the aggressive stripe rust strain which now dominates the pathogen populations in North America or the potential for elevated disease severity on spring cereal crops seeded near the source of inoculum such as winter wheat.
In the present study, we determined if P. striiformis inoculum overwinters in central Alberta winter wheat by examining pustule development, determining the viability of overwintered urediniospores, testing for viable latent mycelium and monitoring the pathogen using a molecular-based technique. Additionally, we determined the relative contribution of early infection of winter and spring cereals to disease progression in adjacent seeded spring cereal crops during the following spring and summer. This was done by comparing the onset and development of stripe rust in spring wheat, triticale and barley seeded near spring wheat with those seeded near winter wheat.
Materials and methods
Winter survival of Puccinia striiformis
Multiple field experiments were conducted to determine if P. striiformis could survive winter conditions in Lacombe (52°28′ N, 113°44′ W), central Alberta. The first experiment (Experiment 1) was conducted during the winters of 2007/2008, 2008/2009, 2009/2010 and 2010/2011. Plots were seeded with the winter wheat cultivars ‘AC Bellatrix’, ‘AC Tempest’, ‘CDC Falcon’, ‘CDC Osprey’ and ‘Radiant’ in late August or early September from 2007 to 2009, and only ‘AC Bellatrix’ was seeded in the middle of September in 2010. Plots were 4.6 m in length and 3 m in width consisting of eight rows with 0.14 m row spacing. The experiment was conducted using a three-replicate randomized complete block design. ‘AC Bellatrix’ was also seeded as border rows around the field plots. The inoculum for plot infection originated from natural sources. Additionally, in 2008/2009 and 2009/2010, the cultivars ‘Radiant’, ‘AC Bellatrix’ and ‘CDC Osprey’ were inoculated with a composite of four P. striiformis f. sp. tritici isolates, collected in central Alberta since 2007 (Kumar et al., Citation2012). The cultivars were seeded in plastic pots (12 cm diameter) containing Pro-Mix BX®, a soilless potting mix nutrient medium (Premier Horticulture Inc., Quakertown, PA) with 10 seeds per pot and 15 pots per cultivar. Two weeks after inoculation, when the pustules on the leaves began to develop, five of these inoculated pots were transplanted to one side of each established field plot of the same cultivar, in early November. During the 2010/2011 season, ‘AC Bellatrix’ growing in the field plots was inoculated at the two-leaf stage with a composite of three Pst isolates. The inoculation procedure was the same as described by Kumar et al. (Citation2012).
Beginning from the seedling stage of winter wheat in Experiment 1 and before snowfall, plants with or without pustules were tagged for sampling. During January to May from 2008 to 2011, plants with or without pustules of each cultivar were removed from the ground at monthly intervals for the determination of winter survival of the fungal pathogen using microscopic examinations. Winter survival of P. striiformis was determined as follows: (i) the number of pustules by natural infection or artificial inoculation was recorded; (ii) viability of urediniospores was tested using the leaves with pustules from the sampled plants when sufficient and uncontaminated urediniospores were available. In this test, urediniospores scraped from the infected leaves were spread on 0.5% water agar (Bacto, Difco, Detroit, MI) using a glass rod and incubated under ambient laboratory conditions for 24 h. Mean percentage of spore germination was obtained by examining spore counts in three microscopic fields at 10× magnification per sample. Spores with germtubes longer than the diameter of spore were considered to have germinated; and (iii) the plants sampled at monthly intervals were transplanted to pots containing potting mix and kept at 4 °C for 12 h in the dark and then moved to a misting chamber under high humidity at 10 °C for 24 h in the dark. The plants were subsequently incubated in a growth chamber programmed at 16 h light at 16 °C and 8 h dark at 12 °C for 4 weeks. The plants were then examined for the development of new pustules for the determination of the presence or absence of viable latent mycelium.
The second experiment (Experiment 2) for determining pathogen winter survival was conducted during January to May in 2010 in Lacombe, at a different site than Experiment 1. Naturally infected ‘AC Bellatrix’ plants were marked prior to snowfall then plants were collected monthly from the field test. Spore germination was determined using the same procedure described above except that no assessment of viable latent mycelia was conducted.
Viability of the urediniospores collected from the sampled plants from the two experiments was also tested by occasionally inoculating wheat seedlings of a highly susceptible cultivar in the growth chamber. This was done by inoculating ‘Morocco’ with urediniospores from field samples of ‘AC Tempest’ in 2008 from Experiment 1 and ‘AC Bellatrix’ in 2010 from Experiment 2 and examining plants 2 weeks following inoculation according to Kumar et al. (Citation2012). Stripe rust symptoms were subsequently examined after incubation. From the two field experiments, leaves from all collected plants that were asymptomatic were sampled for PCR analysis (see below) to detect latent infections. Because a destructive sampling techinque was employed, different sampled plants were used for each test. Snow depth was recorded at 10 locations in the field at monthly intervals and the average was reported.
Table 1. Comparison of spring cereal genotypes for stripe rust severity when seeded adjacent to either winter or spring wheat from 2007 to 2012, Lacombe, AB
PCR detection of Puccinia striiformis
From both winter-survival experiments during January to May of 2009, 2010 and 2011, asymptomatic leaves were sampled and DNA was subsequently isolated and subjected to PCR analysis. To reduce sampling error resulting from a single leaf, 0.2–0.4 g was taken from 8–10 asymptomatic leaves, washed with distilled water, and ground to a powder in liquid nitrogen. DNA was extracted using the CTAB method with a chloroform : isoamyl alcohol extraction as reported by Alaei et al. (Citation2009). Two previously described primer pairs specific to P. striiformis, PS F&R that amplifies the internal transcribed spacer region (Zhao et al., Citation2007) and YRNT1&2 that amplifies the β-tubulin gene (Fraaije et al., Citation2001), were used. PCRs were performed with a 25 μL reaction volume according to the specifications of Zhao et al. (Citation2007) and Fraaije et al. (Citation2001). DNA extracted from non-infected greenhouse-grown plants of each winter wheat cultivar and sterile water was included as negative controls with each PCR reaction. One ng of pure P. striiformis DNA was included as a positive control with each PCR reaction. Following PCR, the reaction products (8 μL) were electrophoresed in 1.5% agarose gels containing 0.1 μg μL−1 ethidium bromide, in 0.5× Tris–borate–EDTA buffer (TBE). DNA was visualized under UV light. Successful amplification of the target PCR products was interpreted as confirmation of the presence of the pathogen in the corresponding leaf samples.
Stripe rust severity on spring cereal crops seeded near winter and spring wheat
In the breeding nursery at Lacombe, AB, from 2007 to 2012, a range of spring wheat, barley and triticale genotypes () were seeded adjacent to a spring wheat field and a winter wheat field. There was 100–200 m distance between the two fields where each of the two sets of crops was seeded. Each seeded plot was 4.6 m in length, but trimmed to 2.5 m in length and 1.5 m in width and consisted of eight rows with 0.14 m row spacing. The two sets of experimental plots were seeded in early May on the same day or one day apart in 2007, 2008, 2009 and 2011; and nine days apart in 2010; and four days apart in 2012. The seeding rate was approximately 11 g seed m−2 for two-row and six-row hulled barley; 12.7 g m−2 for hulless barley; 12.2 g m−2 for spring triticale and 11.6 g m−2 for spring wheat. The experiments were conducted using a three-replicate randomized complete block design. The susceptible wheat ‘AC Crystal’ was seeded to border rows and between blocks to facilitate natural infection by P. striiformis. Stripe rust severity caused by natural infection in each plot was compared between the spring wheat and winter wheat fields for the growing seasons.
Table 2. Sampling date, disease observation, spore germination, viable latent mycelium, PCR detection, and snow depth for Puccinia striiformis winter survival in Experiment 1 from 2008 to 2010, Lacombe, AB
One assessment was made for final disease levels at GS 90 (ripening, Zadoks et al., Citation1974) towards the end of the 2007 season. For the 2008, 2009, 2010, 2011 and 2012 seasons, the first disease assessment was made at GS 60 (anthesis) in late July, while subsequent assessments were done at 1-week intervals until the final assessment at GS 90. All assessments for disease severity were made using the Cobb scale (Peterson et al., Citation1948). An arcsine transformation was applied to percentage severity data to equalize the variance for analysis of variance. Data for the 2007 season were analysed based on final disease severity, while those for the 2008, 2009 and 2012 seasons were analysed based on the area under the disease progress curve (AUDPC) obtained by integrating disease severity curves over time using the formula described by Shaner & Finney (Citation1977). Data from each trial were analysed using the PROC GLM procedure of SAS 9.1 (SAS Institute Inc., Cary, NC, 2002–2003) with field and crop as fixed effects.
Results
Winter survival of Puccinia striiformis
The presence of pustules on winter wheat plants before snow cover was indicative of the successful autumn infection by natural inoculum or artificial inoculation in both experiments during 2008–2011. Urediniospores collected from the sampled field plants from 2008–2011were tested for germination. The number of spores observed ranged from 4 to 50 per microscopic field at 10× magnification. Infrequent sporulation occurred on old leaves (leaves 1 to 4) of the sampled plants after incubation for 4 weeks in the growth chamber. In all four years at both experimental sites, as soon as the snow melted, the frequency of visible pustules, and the viability of spores and latent mycelia infections were drastically reduced ( and ), suggesting that snow cover was associated with pathogen survival in winter wheat. However, the quantitative relationship between snow cover and pathogen survival, if any, remains to be determined. The experimental results based on each season are described in detail as follows.
Stripe rust pustules were observed on the winter wheat plants during January to April 2008 in Experiment 1 (). Urediniospores from pustules collected from the sampled plants had approximately 10–25% germination from January to March. Although pustules were found on ‘AC Bellatrix’, ‘AC Tempest’, and ‘Radiant’ in the January and April sampling, spore germination was not determined due to insufficient urediniospores and/or contamination caused by icy and muddy conditions on the plants. Cultivar ‘AC Tempest’ but not the other four cultivars sampled in March showed sporulation four weeks after incubation under controlled conditions, indicating the presence of viable latent mycelia (). Pustules developed on ‘Morocco’ about 20 days after inoculation with urediniospores collected from diseased plants of ‘AC Tempest’ in the field plot during March 2008 (data not shown). No pustules were observed on any plants sampled in May and, therefore, no spore germination was determined this month. However, after incubation, pustules developed on all sampled cultivars except for ‘CDC Osprey’ in May 2008 ().
Table 3. Sampling date, disease observation, spore germination, viable latent mycelium, PCR detection and snow depth for Puccinia striiformis winter survival using ‘AC Bellatrix’ in Experiment 1 in 2011 and in Experiment 2 in 2010 at Lacombe, AB
For the 2009 season, pustules were observed on the inoculated and transplanted wheat cultivars sampled until March, but not on those without inoculation during the same period. Urediniospores sampled from inoculated plants during January to March showed 8–58% germination. The plants transplanted to the field in the autumn did not survive after being transplanted back to the growth chamber for incubation, as a result of experiencing adverse growth conditions in the field. The lack of symptom development from the dead plants was viewed as the absence of viable latent mycelia in the plants sampled from January to May (). Puccinia striiformis was detected by PCR in the leaves of ‘AC Bellatrix’, ‘CDC Osprey’, ‘CDC Falcon’, ‘AC Tempest’ and ‘Radiant’ only during January and February ().
During the 2010 season at the Experiment 1 site, pustules were observed on the field plants in January and February but no pustules were observed afterwards (). Less than 10% of urediniospores were determined to be viable in each sample taken during January and February. Spore germination was not assessed for ‘Radiant’ as a result of insufficient spores that were collected from this cultivar in January. Viable latent mycelia were detected by incubation of winter wheat cultivars sampled during January, February and May. Some plants were found to be positive for latent infections by PCR testing from January up to the end of sampling in May ().
In Experiment 2, pustules were found on ‘AC Bellatrix’ from January to March 2010 and urediniospores were determined to be viable (). The samples were positive for latent infections from March to May, based on the PCR analysis. Stripe rust symptoms developed on ‘Morocco’ after inoculating using urediniospores from ‘AC Bellatrix’ collected only during February 2010 from the field plots (data not shown). During the 2011 season in Experiment 1, pustules on ‘AC Bellatrix’ were observed from January to April (). Although viable spores were found, no viable latent mycelia sporulated from the samples collected throughout the testing period. The samples were positive for latent infection in January and March based on PCR analysis.
Table 4. Analysis of variance of arcsine transformed final percentage stripe rust severity for 2007 and for the area under the disease progress curves for 2008, 2009 and 2012, Lacombe, AB
Stripe rust severity on spring cereal crops seeded near either winter or spring wheat
Differences in disease severity observed between the two sites in 2010 may have resulted from the nine day difference between the seeding dates, which may have influenced disease development. Consequently, disease severity data for 2010 were excluded from the analysis. The test for 2011 was also abandoned due to plot damage from flooding in the winter wheat field. Significantly higher severities of stripe rust were found in the spring crops seeded adjacent to winter wheat compared with spring wheat in all four seasons including 2007, 2008, 2009 and 2012 (, and ). This appeared to be primarily caused by higher final disease severity in ‘AC Crystal’ and ‘AC Barrie’ in 2007 (); the greater AUDPC in barley lines H98077001 and H99027002, in 2008; the greater AUDPC in ‘AC Crystal’ in 2009 and in ‘AC Crystal’, ‘AC Barrie’ and ‘AC Foremost’ in 2012 (). Stripe rust levels were lowest in 2012 among the four years of testing, resulting in similar final levels of disease in the cultivars and lines evaluated by the end of season (). However, rapid development of stripe rust on susceptible wheat and barley contributed to the significantly higher AUDPC near the winter wheat field in comparison with plots near the spring wheat field (). Using the multiple comparison procedure described by Littell et al. (Citation2002), the three wheat cultivars each had significantly higher severity (P < 0.05) near the winter wheat field compared with the spring wheat field in 2012. There were no significant differences for the barley and triticale between the two fields of the same trial in 2012 (data not shown).
Fig. 2. Disease progress curves for stripe rust severity in ‘AC Barrie’, ‘AC Crystal’ and ‘AC Foremost’ (spring wheat) and H98077001 and H99027002 (barley) seeded near winter wheat or spring wheat during 2008, 2009 and 2012, Lacombe, AB. The disease progress curves for ‘AC Ultima’ and 94L04400 (spring triticale) are not presented due to lack of disease development. Standard deviations based on individual treatments are shown for each curve at the date of observation.
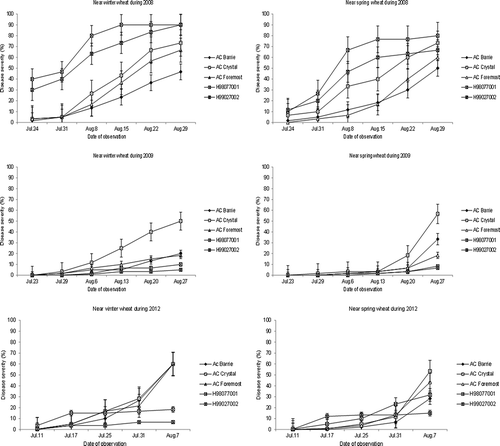
Fig. 1. Final stripe rust severity on ‘AC Barrie’, ‘AC Crystal’ and ‘Glenlea’(spring wheat), ‘AC Ultima’ (spring triticale) and HB522 and ‘Samson’ (barley) planted near either winter or spring wheat on 2 August 2007, Lacombe, AB. Standard deviations are shown for each bar diagram.
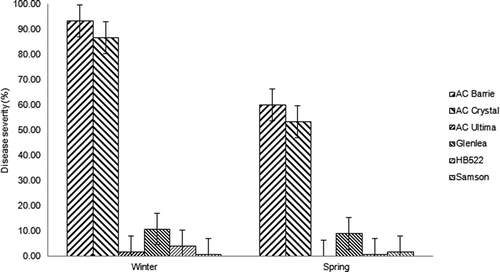
There were no significant interactions between crop and field for 2008 and 2009, but there were for 2007 and 2012 (). Visual examination showed that this interaction for 2007 was likely caused by several genotypes including H99027002, H98077001, ‘AC Ultima’ and 94L04009 displaying different but very low levels of final disease severity between the two experiments (). This interaction disappeared after all four genotypes with low disease severity were removed from the ANOVA (data not shown). This indicates that this interaction was attributable to these genotypes and those susceptible cultivars such as ‘AC Crystal’ and ‘AC Barrie’ exhibited similar differential severity levels between winter and spring seeded fields. The crop × field interaction was also highly significant for 2012 (). Among the three susceptible spring wheat cultivars seeded adjacent to the winter wheat, stripe rust severity was similar, especially for the last rating date (). In contrast, when planted adjacent to spring wheat ‘AC Barrie’ had lower disease severity, especially for the last two rating dates when compared with ‘AC Crystal’ and ‘AC Foremost’. The significant interaction may have also been caused by the barley line H99027002, which had a larger AUDPC near the spring field than near the winter field. Overall, average final stripe rust severity in the barley lines was considerably lower (approximately 30%) than in the three spring wheat cultivars (60%) ().
Discussion
The results from this study suggest that there is potential for the current Pst population present in North America to survive winters in central Alberta, albeit at a low frequency. In all four years from 2008 to 2011, uredia were present with viable spores in the last month with snow cover, typically until March or April. The importance of snow cover for protecting stripe rust infections throughout winter has long been recognized (Humphrey et al., Citation1924; Sanford & Broadfoot, Citation1932; Sharp & Hehn, Citation1963). In the current study, viable urediniospores were rarely observed after snow melt, suggesting that snow cover in the spring is critical to their survival. The survival of urediniospores after the melting of snow cover is considered unlikely. The sensitivity of P. striiformis spores to high humidity and wetting and their ease of being washed off leaves (Becker, Citation1928, cited in Zadoks, Citation1961) likely reduce survival of the spores in the spring. The relevance of the survival of urediniospores throughout the winter in relation to the continuation of disease in the spring is debatable. It is possible that during the brief period after the snow has melted, the urediniospores could infect other winter wheat leaves on the same plant or neighbouring plants and thus perpetuate the disease from one growing season to the next. Urediniospores of stripe rust have been shown to be capable of infecting plants at temperatures just above and potentially slightly below freezing (Burleigh & Hendrix, Citation1970) and could theoretically infect plants at temperatures occurring immediately after the loss of the snow cover. However, the epidemiological significance of the winter survival of urediniospores is currently unknown.
In all years, at least some plants tested positive for P. striiformis by PCR detection until the end of May when sampling ended, suggesting that the pathogen survived as latent infections throughout the winter and into spring. Previous reports have indicated that the latent period of stripe rust can last for up to approximately 160 days (Sharp & Hehn, Citation1963) which is similar to the time frames in the current study. The PCR procedure was more sensitive than spore germination assessments and the incubation technique used in the present study. However, the positive amplification of stripe rust DNA within the host is not irrefutable proof of the survival as a latent infection. It is possible that the pathogen died in its host, and that some of its DNA remained amplifiable. This was probably most likely during the earlier part of the winter where temperatures remained below freezing. If the low temperature conditions did result in the mortality of the pathogen, they would have also likely preserved the DNA for sampling. It is also possible that in April and May, the positive detections were not only due to infections present before winter, but also early spring infections caused by exogenous inoculum. The development of pustules on plants recovered up to April during 2008–2011 after being transferred into controlled environments suggests that the pathogen survived the winter at least until spring. The infrequent sporulation observed on the symptomless plants after incubation also suggests that the latent infection was most likely due to dormant mycelia that survived the winter, given that sporulation occurred on old leaves that had been present prior to snowfall. However, it is possible that extremely early spring infections caused by exogenous inoculum could have been responsible for the symptoms observed on plants sampled in the early spring when there was no longer snow cover. Sharp & Hehn (Citation1963) found that the percentage of plants containing live mycelium was significantly reduced by spring. Zadoks & Bouwman (Citation1965) considered the overwintering of a single lesion per hectare sufficient to initiate an epidemic if environmental conditions in the spring were conducive to the disease. Such low, but potentially epidemiologically significant, levels of winter survival could easily escape experimental detection.
The present study showed very low detection of latent infections after snow melt. Winter kill in central Alberta can be caused by several factors including low temperatures and snow moulds (Gaudet & Gossen, Citation2003). Since P. striiformis establishes an obligate biotrophic association with the host, death of plant cells would lead to death of the fungus (Agrios Citation1978). As a result, winter kill will contribute to fungal mortality, resulting in few detectable latent infections. The low positive detection was due presumably to the drastic reduction in survival of the pathogen that had been exposed to winter conditions, the relatively small sample sizes used, and the small percentages of infected leaves that were likely missed for sampling. Although the multiple detection methods employed were all used on the same plants, they were used on different leaves of the same plants. PCR detection was used for only a few asymptomatic leaves of the plant as it was unnecessary to employ the technique on leaves with visible pustules. In addition, due to the destructive nature of the technique, any leaves sampled could not be incubated to detect latent mycelium. This resulted in the detection of viable latent mycelium being done only on leaves that did not have pustules at the time of removal from the field and these leaves were not used for PCR. However, they were from the same sampled plants. Furthermore, different plants were sampled at each time period due to the necessity to remove them from the field. The PCR method has been used to successfully detect P. striiformis at the dormant stage in the wheat leaves during winter (Wang et al., Citation2008), and specifically to detect the pathogen in asymptomatic wheat leaves sampled around stripe rust foci within wheat fields (Zhao et al., Citation2007).
The present study demonstrated that epidemics can be more severe in spring crops seeded adjacent to a winter wheat field compared with those seeded adjacent to a spring wheat field. The observed elevated disease severity in spring crops planted near winter wheat could have potentially resulted from endogenous inoculum that survived the winter, exogenous inoculum that was transported into the region, or a combination of both. Although the field experiments, as conducted, do not allow the two potential sources to be separated, the elevated severity in spring wheat and barley near winter wheat compared with near spring wheat does suggest that local overwintering inoculum, possibly together with urediniospores arriving in the early spring, leads to early season rust development in winter wheat which then poses a threat to adjacent spring cereal crops. The selection of wheat and barley genotypes for the present study was primarily based on their known variable levels of susceptibility to stripe rust (Anonymous, Citation2012a ). Puccinia striiformis f. sp. tritici was likely the predominant causal agent in 3 of 4 years of the field tests given that wheat stripe rust has been epidemic whereas barley stripe rust primarily caused by Psh was of minor concern during those years. However, the two formae speciales are known to overlap in pathogenicity between wheat and barley (Chen et al., Citation1995). In addition, cross infection between highly susceptible wheat and barley has been demonstrated in greenhouse studies and observed in central Alberta commercial fields (Xi et al., Citation2011).
Due to the short growing season in central Alberta, planting and harvesting of spring wheat and winter wheat may overlap in this region (Anonymous, Citation2012b ). This overlapping may result in year-round cropping that can provide living hosts known as a green bridge (Anonymous, Citation2010a ) for survival of the stripe rust pathogen, with subsequent infection and development of stripe rust (Zadoks, Citation1961). If the stripe rust pathogen can survive the winter in infected winter wheat, the infection cycle can start sooner in the spring on winter wheat and subsequently spread to susceptible spring wheat cultivars. The present study suggested that stripe rust can overwinter on winter wheat and locally overwintering inoculum together with exogenous inoculum can lead to stripe rust developing the following spring in central Alberta. Although winter wheat acreage was small in Alberta in 2010 (Anonymous, Citation2010b ), the potential role of overwintering and transmission of inoculum from winter wheat represented an important source of inoculum for subsequent infection of spring wheat crops. Many commercial winter wheat cultivars are susceptible to stripe rust (Anonymous, Citation2012a ) and one resistant winter wheat, ‘Radiant’, became susceptible as a result of the resistance gene Yr10 being overcome by a change in pathogen virulence (Puchalski et al., Citation2010). Furthermore, most cultivars evaluated in Experiments 1 and 2 in the present study appeared to be susceptible at the seedling stage irrespective of the level of susceptibilty or resistance that may be expressed at late stages. Despite the low frequency of positive detection of latent infections at the seedling stage, such a low infection level may still potentially play a role in epidemics, as demonstrated in the present study. Enhanced levels of resistance at the seedling stage in winter wheat cultivars may help to reduce infection levels, thereby reducing inoculum spread to adjacent spring cereal crops growing in May and June. An integrated approach using resistance and appropriate cultural practices could help to limit the risk of stripe rust infection. For example, avoiding planting spring wheat adjacent to winter wheat fields may help to break the green bridge effect, helping to reduce disease development. Delaying the seeding of winter wheat until spring wheat is mature may break the green bridge. However, late seeding may result in reduced yields due to the short growing season in central Alberta (Anonymous, Citation2012b ). Foliar fungicide application to infected winter wheat crops in the early spring would limit further disease development and thus eliminate a potential source of disease for spring cereal crops. Moreover, recognition of an emerging stripe rust epidemic in winter wheat crops could serve as an early warning sign for potential problems in the spring wheat crop, thus giving producers time to plan for a foliar application of fungicide during the growing season. Ultimately, a combination of stripe rust-resistant winter and spring wheat cultivars would be the most effective disease management strategy.
Acknowledgements
The authors wish to thank Lyla Langford, Susie Albers, Colin Bergen and Linda Vandermaar for technical assistance and the late Dr. Don Salmon and Mazen Alijarrah for their technical advice. The authors express gratitude to the anonymous reviewers for their constructive suggestions and comments to improve the paper. The funding provided by Alberta Crop Industry Development Fund for this project is gratefully acknowledged.
References
- Agrios , G.N. 1978 . Plant Pathology , 2nd , New York : Academic Press. 703 pages .
- Alaei , H. , Baeyen , S. , Maes , M. , Höfte , M. and Heungens , K. 2009 . Molecular detection of Puccinia horiana in Chrysanthemum x morifolium through conventional and real-time PCR . J. Microbiol. Meth , 76 : 136 – 145 .
- Anonymous (2010a). Introduction to plant disease. June 2010. Saskatchewan Ministry of Agriculture. Available fromaccessed 27 March 2013 http://www.agriculture.gov.sk.ca/Default.aspx?DN=c3743d36-98ee-4e12-ba54-6454467cd781 (http://www.agriculture.gov.sk.ca/Default.aspx?DN=c3743d36-98ee-4e12-ba54-6454467cd781)
- Anonymous (2010b). Agri-Food statistical update, preliminary estimates of principal field crops areas Alberta, Issue No. CR10-1. 2010. Available fromupdated 22 December 2011; accessed 27 March 2013 http://www1.agric.gov.ab.ca/$department/deptdocs.nsf/all/sdd13497 (http://www1.agric.gov.ab.ca/$department/deptdocs.nsf/all/sdd13497)
- Anonymous . 2012a . Varieties of cereal and oilseed crops for Alberta . Agdex , 100/32
- Anonymous (2012b). Winter wheat in the Parkland area of Alberta. Agdex 112/11-1. Available fromaccessed 27 March 2013 http://www1.agric.gov.ab.ca/$department/deptdocs.nsf/all/agdex94/$file/112_11-1.pdf (http://www1.agric.gov.ab.ca/$department/deptdocs.nsf/all/agdex94/$file/112_11-1.pdf)
- Becker , J. 1928 . Untersuchungen über die Lebensfähigkeit von Uredosporen von Puccinia glumarum . Kühn-Arch , 19 : 353 – 411 . [cited in Zadoks, 1961]
- Brown , J.K.M. and Hovmøller , M.S. 2002 . Aerial dispersal of pathogens on the global and continental scales and its impact on plant disease . Science , 297 : 537 – 541 .
- Burleigh , J.R. and Hendrix , J.W. 1970 . The winter biology of Puccinia striiformis West. in the Pacific Northwest . Washington State Agricultural Experiment Station Techn. Bull , 65 : 1 – 17 .
- Chen , X.M. 2005 . Epidemiology and control of stripe rust (Puccinia striiformis f. sp. tritici) on wheat . Can. J. Plant Pathol , 27 : 314 – 337 .
- Chen , X.M. , Line , R.F. and Leung , H. 1995 . Virulence and polymorphic DNA relationships of Puccinia striiformis f. sp. hordei to other rusts . Phytopathology , 85 : 1335 – 1342 .
- Chen , X.M. , Moore , M. , Milus , E.A. , Long , D.L. , Line , R.F. , Marshall , D. and Jackson , L. 2002 . Wheat stripe rust epidemics and races of Puccinia striiformis f. sp. tritici in the United States in 2000 . Plant Dis , 86 : 39 – 46 .
- Chen , X. , Penman , L. , Wan , A. and Cheng , P. 2010 . Virulence races of Puccinia striiformis f. sp. tritici in 2006 and 2007 and development of wheat stripe rust and distributions, dynamics, and evolutionary relationships of races from 2000 to 2007 in the United States . Can. J. Plant Pathol , 32 : 315 – 333 .
- Conner , R.L. , Thomas , J.B. and Kuzyk , A.D. 1988 . Overwintering of stripe rust in southern Alberta . Can. Plant Dis. Surv , 2 : 153 – 155 .
- Eriksson , J. and Henning , E. 1896 . Die Getreideroste. Ihre Geschichte und Natursowie Massregein gegen dieselben , 463 Stockholm : P. A. Norstedt and Soner . [cited in Zadoks, 1961]
- Fraaije , B.A. , Lovell , D.J. , Coelho , J.M. , Baldwin , S. and Hollomon , D.W. 2001 . PCR-based assays to assess wheat varietal resistance to blotch (Septoria tritici and Stagonospora nodorum) and rust (Puccinia striiformis and Puccinia recondita) diseases . Eur. J. Plant Pathol , 107 : 905 – 917 .
- Gassner , G. and Pieschel , E. 1934 . Untersuchungen zur Frage der Uredoüberwinterung der Getreideroste in Deutschland . Phytopathol. Z , 7 : 355 – 392 .
- Gaudet , D. and Gossen , B.D. 2003 . “ Overwintering diseases ” . In Diseases of Field Crops of Canada , 3rd , Edited by: Bailey , K.L. , Gossen , B.D. , Gugel , R.K. and Morrall , R.A.A. 214 – 222 . Saskatoon , SK : The Canadian Phytopathological Society, University Extension Press, University of Saskatchewan .
- Humphrey , H.B. , Hungerford , C.W. and Johnson , A.G. 1924 . Stripe rust Puccinia glumarum of cereals and grasses in the United States . J. Agric. Res , 29 : 209 – 227 .
- Hungerford , C.W. 1923 . Studies on the life history of stripe rust, Puccinia glumarum (Schm.) Erikss. & Henn . J. Agric. Res , 24 : 209 – 228 .
- Jin , Y. , Szabo , L.J. and Carson , M. 2010 . Century-old mystery of Puccinia striiformis life history solved with the identification of Berberis as an alternate host . Phytopathology , 100 : 432 – 435 .
- Kumar , K. , Holtz , M.D. , Xi , K. and Turkington , T.K. 2012 . Virulence of Puccinia striiformis on wheat and barley in central Alberta . Can. J. Plant Pathol , 34 : 551 – 561 .
- Littell , R.C. , Stroup , W.W. and Freund , R.J. 2002 . SAS for Linear Models , 4th , Cary , NC : SAS Institute, Inc. 496 pages .
- Markell , S.G. and Milus , E.A. 2008 . Emergence of a novel population of Puccinia striiformis f. sp. tritici in eastern United States . Phytopathology , 98 : 632 – 639 .
- Milus , E.A. , Kristensen , K. and Hovmoller , M.S. 2009 . Evidence for increased aggressiveness in a recent widespread strain of Puccinia striiformis f. sp tritici causing stripe rust of wheat . Phytopathology , 99 : 89 – 94 .
- Peterson , R.F. , Campbell , A.B. and Hannah , A.E. 1948 . A diagrammatic scale for estimating rust intensity of leaves and stem of cereals . Can. J. Res. Sect. C , 26 : 496 – 500 .
- Puchalski , B.J. , Gaudet , D.A. , Randhawa , H. , Graf , R. and Despins , T. The reaction of western Canadian wheat and triticale varieties to stripe rust . Plant Pathology Society of Alberta, 31st Annual Meeting . October 12–14, 2010 .
- Randhawa , H. , Puchalski , B.J. , Frick , M. , Goyal , A. , Despins , T. , Graf , R.J. , Laroche , A. and Gaudet , D.A. 2012 . Stripe rust resistance among western Canadian spring wheat and triticale varieties . Can. J. Plant Sci , 92 : 713 – 722 .
- Roelfs , A.P. , Singh , R.P. and Saari , E.E. 1992 . Rust Disease of Wheat: Concepts and Methods of Disease Management , Mexico , D.F : CIMMYT. 81 pages .
- Sanford , G.B. and Broadfoot , W.C. 1929 . Stripe rust in Alberta . Sci. Agr , 9 : 337 – 345 .
- Sanford , G.B. and Broadfoot , W.C. 1932 . Epidemiology of stripe rust in western Canada . Sci. Agr , 13 : 77 – 96 .
- Shaner , G. and Finney , R. 1977 . The effect of nitrogen fertilization on the expression of slow-mildewing in knox wheat . Phytopathology , 36 : 1307 – 1311 .
- Sharp , E.L. and Hehn , E.R. 1963 . Overwintering of stripe rust in winter wheat in Montana . Phytopathology , 53 : 1239 – 1240 .
- Wang , H. , Yang , X.B. and Ma , Z. 2010 . Long-distance spore transport of wheat stripe rust pathogen from Sichuan, Yunnan, and Guizhou in southwestern China . Plant Dis , 94 : 873 – 880 .
- Wang , X. , Zheng , W. , Buchenauer , H. , Zhao , J. , Han , Q. , Huang , L. and Kang , Z. 2008 . The development of a PCR-based method for detecting Puccinia striiformis latent infections in wheat leaves . Eur. J. Plant Pathol , 120 : 241 – 247 .
- Xi , K. , Holtz , M. , Kumar , K. , Aljarrah , M. , Helm , J. Juskiw , P. 2011 . Control of Cereal Stripe Rust in Alberta using Genetic Resistance, Final Report , Alberta Crop Industry Development Fund, Project #2007F028R .
- Zadoks , J.C. 1961 . Yellow rust on wheat. Studies in epidemiology and physiologic specialization . Tijdschrift over Plantenziekten , 67 : 69 – 256 .
- Zadoks , J.C. and Bouwman , J.J. 1965 . “ Epidemiology in Europe ” . In The Cereal Rusts, Vol. II; Diseases, Distribution, Epidemiology, and Control , Edited by: Roelfs , A.P. and Bushnell , W.R. 329 – 369 . Orlando , FL : Academic Press .
- Zadoks , J.C. , Chang , T.T. and Konzak , C.F. 1974 . A decimal code for the growth stages of cereals . Weed Res , 14 : 415 – 421 .
- Zeng , S.M. and Lou , Y. 2006 . Long-distance spread and interregional epidemics of wheat stripe rust in China . Plant Dis , 90 : 980 – 988 .
- Zhao , J. , Wang , X.J. , Chen , C.Q. , Huang , L.L. and Kang , Z.S. 2007 . A PCR-based assay for detection of Puccinia striiformis f. sp. tritici in wheat . Plant Dis , 91 : 1669 – 1674 .