Abstract
Cotton (Gossypium hirsutum L.) is crucial for the textile industry worldwide. Among the diseases attacking cotton, Verticillium wilt caused by Verticillium dahliae Kleb. is the most significant. Isolates of V. dahliae can be classified into defoliating and non-defoliating pathotypes. Thirty-two isolates of the non-defoliating pathotype and one isolate of a virulent, defoliating pathotype were analysed by the amplified fragment length polymorphism (AFLP) method. Three hundred and forty AFLP fragments were obtained with nine primer combinations. The number of total bands per primer pair ranged from 16 to 81, with an average of 37.7. The average polymorphism information content (PIC) value for the AFLP products was 0.50. Using the genotypic data, genetic distance analysis was performed. The maximum variation was found between isolates (Vd11) Nazilli and (Vd16) Soke, at a value of 0.79 and the minimum variation was found between isolates (Vd20) Aydin and (Vd14) Soke, at 0.24. The unweighted paired group method with arithmetic averages cluster analysis (UPGMA) was used to discriminate the V. dahliae isolates into five subgroups. Defoliating pathotypes (Vd33) from Soke province formed a single subgroup. As a result, it was found that there was significant variation among Verticillium isolates. AFLP analysis is an efficient and effective marker technology for determining genetic relationships among Verticillium isolates.
Résumé
Dans presque tous les pays, le coton (Gossypium hirsutum L.) est essentiel à l'industrie du textile. Parmi les maladies qui l'affectent, la flétrissure verticillienne, causée par Verticillium dahliae Kleb., est la plus dommageable. Les isolats de V. dahliae peuvent être classés en pathotypes défoliants et non défoliants. Trente-deux isolats du pathotype non défoliant et un isolat d'un pathotype défoliant virulent ont été analysés par la technique du polymorphisme de longueur de fragments amplifiés (AFLP). Trois cent quarante fragments ont été obtenus en fonction de neuf combinaisons d'amorces. Le nombre total de bandes par paire d'amorces variait de 16 à 81, pour une moyenne de 37,7. L'indice PIC (polymorphism information content) de ces produits de l'AFLP était de 0,50. Une analyse de la distance génétique a été effectuée à partir des données génotypiques. La variation maximale (0,79) a été observée entre les isolats (Vd11) Nazilli et (VD16) Soke et la variation minimale (0,24), entre les isolats (Vd20) Aydin et (Vd14) Soke. La méthode des moyennes par paire non pondérée avec moyenne arithmétique (UPGMA) a été utilisée pour répartir les isolats de V. dahliae en cinq sous-groupes. Les pathotypes défoliants (Vd33) de la province de Soke ont formé un seul sous-groupe. En conséquence, on a constaté qu'il existait des variations significatives chez les isolats de la flétrissure. L'analyse AFLP avec marqueurs est une méthode efficiente et efficace pour établir la parenté chez les isolats de la flétrissure.
Introduction
Cotton is an important textile crop used by many industries. In Turkey, a total of 450 000 ha of upland cotton is grown yearly and 631 000 tons of lint are produced in the four main regions – South-eastern Anatolia, Aegean, Cukurova and Antalya. A total of 83 000 ha is planted annually in the Aegean region. This area covers 17% of the cotton-producing region of Turkey, yielding 150 000 tons of lint annually, or approximately 18% of Turkey's cotton production (Anonymous, Citation2010).
There are about 20 diseases that attack cotton. One of them is vascular wilt caused by the fungus Verticillium dahliae Kleb., which represents one of the most important diseases (Pegg, Citation1984). This disease was first identified by C.W. Carpenter in 1914, and later found in Manisa (Turkey) in 1940 (Iyriboz, Citation1941). Verticillium dahliae can survive for a long time in soil. It has been determined that wilt disease of cotton can cause economically significant crop losses, which have been estimated as high as 31% in Turkey when both decreases in yield and quality are considered (Karaca et al., Citation1971).
Verticillium wilt thrives in soil with pH levels between 6 and 9, and a growing temperature of 21–27 °C. The symptoms first appear on the leaves of the cotton plant. The symptoms, in the form of wilting and discolouration, spread from the bottom leaves to the upper leaves. The leaves turn yellow and dry, and over time the plant is defoliated as the leaves die. The cells of the xylem tissue turn brown, but the pathogen does not move to the phloem.
Isolates of V. dahliae are classified into two main groups based on virulence. The first group consists of defoliating (D) pathotypes, which cause defoliation of the plants and are highly virulent. The other group is non-defoliating (ND) and causes moderately severe symptoms (Schnathorst & Mathre, Citation1966a ; Schnathorst et al., Citation1975). When comparing the different disease intensities, the effects of the D type appear earlier and are more rapid. Also, this pathotype has a severely negative impact on plant growth parameters, causing greater loss of yield. ND pathotypes tend to cause only tenuous wilt and the plants can recover from the symptoms. Management of the disease is best achieved by planting tolerant cotton cultivars, in order to overcome the highly virulent D pathotype (Schnathorst & Mathre, Citation1966b ; Bell, Citation1994). Determination of the V. dahliae pathotypes infecting cotton is of importance for resistance breeding against this disease. Cotton cultivars tolerant to Verticillium wilt develop only minor levels of disease (Klosterman et al., 2009). When this type of resistance is compromised, Verticillium wilt is likely to re-emerge as a significant production problem for cotton crops (Klosterman et al., 2009).
Morphological and physiological methods have traditionally been used to determine genetic variation in V. dahliae (Harris et al., Citation1993; Goud & Termorshuizen, Citation2003). However, these methods can be inconsistent, time-consuming and generally uninformative about diversity of populations of the pathogen. To overcome the problem, different polymerase chain reaction (PCR)-based molecular marker techniques have been used to estimate genetic diversity and relationships among V. dahliae (Koike et al., Citation1996; Messner et al., Citation1996; Pérez-Artés et al., Citation2000b ; Zeise & von Tiedemann, Citation2002; Fahleson et al., Citation2003; Collado Romero et al., Citation2006). Among these, AFLP techniques generate more markers using single primer combinations to characterize populations. Another advantageous feature of the AFLP technique is that no sequence information is required (Rafalski et al., Citation1996). The ability of AFLP analysis to reveal genomic polymorphisms is very high. Likewise, AFLP has been successfully used in the genetic analysis of V. dahliae (Fahleson et al., Citation2003; Wang et al., Citation2004). It has been shown that there was limited genetic variation among Swedish and German isolates of V. longisporum attacking B. napus by using AFLP (Fahleson et al., Citation2003). These results also showed that AFLP is a suitable method for studying population structure in Verticillium.
The aim of this study was to determine the extent of genetic variation among V. dahliae populations existing in the extensively cotton growing area of Turkey, using AFLP marker techniques.
Materials and methods
Isolation of V. dahliae Kleb. from affected plants
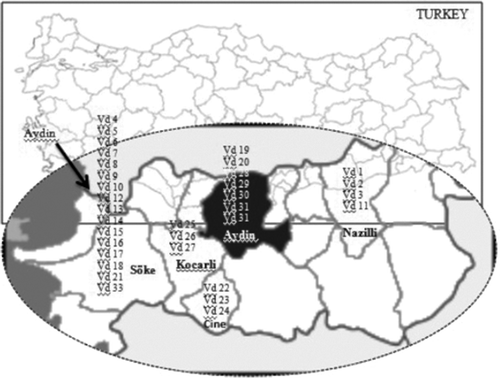
Diseased plant samples were collected from 33 different cotton fields in the Aydin region of Turkey (). The tissues were sampled when Verticillium symptoms appeared on cotton plants from July to September in 2006 (Bora & Karaca, Citation1970). The samples were placed in an icebox and brought to the laboratory, and cultured within 24 h. Infected tissues (stems) were aseptically cut into pieces of approximately 1–2 cm in length and surface-disinfested in 0.5% NaOCl solution for 2 min, rinsed twice with sterile distilled water and dried between sterile filter papers.
Disinfested tissues were plated on ethanol streptomycin agar (ESA), consisting of 8 g of agar, 135 mg of streptomycin, 6 mL of 95% ethanol, in 1 L of distilled water. The Petri dishes (9 cm diameter) were incubated at 23–24 °C in the dark for 5–7 days. After incubation, hyphal plugs of each growing colony were transferred to potato dextrose agar (PDA) and incubated in the dark at 23–24 °C for 10 days. Colonies forming microsclerotia were identified as V. dahliae according to the taxonomic features of the fungus via microscopic examination (Melouk et al., 1992). Single-spore isolates of V. dahliae were obtained on water agar (WA) and maintained in vials containing PDA at 4 °C. Thirty-three V. dahliae isolates were collected from different locations of the Aydin region and their origins are listed in .
Pathogenicity tests
The pathogenicity of the 33 isolates was tested by injecting a conidial suspension (3 × 107 conidia per mL of sterile water) of each isolate of V. dahliae directly into the xylem tissues of 4–6 week old cotton plants of the cultivar ‘Cukurova 1518’ using a hypodermic needle, as described in Bejarano-Alcazar et al. (Citation1996). Control plants were treated with sterile distilled water. Plants were grown in a growth chamber at 25–27 °C by day and 18–22 °C by night with 75% relative humidity, under a 14 h photoperiod with white fluorescent lights. The number of diseased plants was observed two weeks after injection of the conidia. Disease severity was assessed for each plant on a 0 to 4 rating scale according to the percentage of foliage affected by acropetal chlorosis, necrosis, wilt and/or defoliation (where: 0 = healthy plant, 1 = 1–33% of foliage affected, 2 = 34–66% of foliage affected, 3 = 67–97% of foliage affected, 4 = dead plant) (Bejarano-Alcazar et al., Citation1995). Each isolate was replicated three times in a randomized complete design, which included three plants per pot for each isolate. Disease severity (%) was calculated and the data obtained were subjected to Arcsin for transformation (Booth, Citation1970). Data analysis was conducted using JMP software (version 3.1, SAS Institute Citation1995, Cary, USA). When P values showed a significant difference, means were ranked by least significant different (LSD) test at P ≤ 0.05 level.
Pathotype tests
Besides differing in the symptoms caused in different cotton cultivars, pathotypes of V. dahliae that attack cotton can be distinguished by optimum temperature for growth (Schnathorst et al., Citation1975) and by the formation of elongated or round microsclerotia in water agar (Blanco Lopez et al., Citation1989). Growth rates were determined in three separate trials by plating each isolate on PDA and incubating duplicate plates at temperatures between 24 and 27 °C. Colony diameters were measured at four day intervals for 16 days, and the growth rate at each temperature was converted to mm day−1. To determine the shape of microsclerotia, each isolate was grown on water agar for 14 days at 24 °C and examined under a microscope. The differential pathotypes of V. dahliae were determined according to Schnathorst et al. (Citation1975) and Blanco Lopez et al. (Citation1989) using the cultivars ‘Acala- SJ-1’ (susceptible to defoliating strain) and ‘Deltapine 15-21’ (mildly susceptible to defoliating type). For this purpose, the seeds of cultivars ‘Acala-SJ-1’ and ‘Deltapine 15-21’ were sown and germinated in a styrofoam tray filled with a sterile mixture of peat and perlite (1 : 1; vol/vol) in a growth chamber. Then the seedlings were transferred to 20 cm diameter plastic pots (five plants per pot) filled with a non-sterilized mixture of sand/clay-loam/peat (2 : 1 : 2, vol/vol/vol). The seedlings were grown in the growth chamber with 75% relative humidity, at 22–26 °C, with a 14 h photoperiod of fluorescent light of 216–270 μEm−2s−1 intensity and watered as needed. Six-week-old plants were inoculated with 5 μL of a 3 × 106 conidia mL−1 suspension in sterile distilled water (SDW) as described previously. Control plants were treated similarly with SDW. The plants were observed daily for the development of foliar symptoms and defoliation. Since the cultivar ‘Acala-SJ-1’ is susceptible to defoliating strain, the plants of cultivar ‘Acala-SJ-1’ were dead and their leaves were defoliated. In contrast, since the cultivar ‘Deltapine 15-21’ is mildly susceptible to defoliating strain, the plants were also dead but the leaves remained attached on the plants.
DNA isolation
Verticillium dahliae isolates were grown on PDA plates at 25 °C for a week and total genomic DNA was extracted from ground mycelia, as per Doyle & Doyle (Citation1987). Isolated DNA was run on 1% (w/v) agarose gels. DNA bands were visualized by ethidium bromide staining. The concentration of the isolated DNA was determined spectrophotometrically by measuring absorbance at 260 nm and 280 nm using a Nanodrop ND-1000 (Thermo Sci. Co, Lafayette, CO, USA).
AFLP analysis
The AFLP analysis of nine primer combinations () was performed with a LI-COR AFLP Kit (catalog number: 830-06195 AFLP 2-DYE Selective Amplification Kit, LI-COR Biosciences, Lincoln, NE, USA), as described in the manufacturer's protocol. DNA (200 ng) was digested with EcoRI and MseI restriction enzymes (LI-COR Biosciences) at 37 °C for 2 h. Digested DNA fragments were then ligated with enzyme adaptors in the presence of T4 DNA ligase. The ligation reaction was incubated at 20 °C for 2 h. After ligation, the reaction mixture was diluted with ultrapure water in a 1 : 10 ratio. This was followed by a pre-amplification step using non-selective primers, including one additional selective nucleotide at the 3′ end of the MseI primer (MseI + C) and the EcoRI primer (EcoRI + A). Primer combinations are named according to the restriction enzyme initials; for instance, in the case of M-CA/E-GG, ‘M’ stands for MseI and ‘CA’ stands for the nucleotid extentions, and ‘E’ stands for the EcoRI enzyme. For selective amplification, EcoRI primers were labelled with two different fluorescent dyes (IRD 700 or 800) at the 5′ ends, and used in a 1 : 1 ratio with the unlabelled primer (MseI). The selective PCR products were loaded on an 8% denaturing polyacrylamide gel in 1X TBE (Tris-borate-EDTA) buffer and electrophoresed at 1500 V and 40 mA. For further determination of polymorphisms, the PCR products were run on an Li-Cor 4300s DNA Analyzer (LI-COR Biosciences). Imaging process of the AFLP fragments were performed using SAGA software (LI-COR Biosciences).
Band scoring and data analysis
Each polymorphic AFLP band was scored manually as present (1) or absent (0) across all 33 genotypes for each primer-pair combination and the values were used to compile a binary data matrix. Only bright, clearly distinguishable bands were used in the genetic analysis. Genetic distance estimates were calculated using Jaccard's coefficient of distance (Jaccard, Citation1908). JMP software (version 3.1, SAS Institute Citation1995) was used to calculate distances and a dendrogram was generated. The isolates were grouped by cluster analysis using the unweighted pair-group method (UPGMA). PIC (polymorphism information content) was calculated from the 1/0 data matrix. The PIC value refers to the relative value of each marker with respect to the amount of polymorphism it exhibits. PIC was calculated by the following formula (De Riek, Citation2001) : 1−Σpi2 : where i = individual p and pi = the allele frequencies of the loci.
Table 1. Results of pathogenicity tests of V. dahliae isolates (the isolate number is ranked according to decreasing disease severity)
Results and discussion
Pathogenicity and pathotype assay
Values for disease severity after inoculation with the 33 V. dahliae isolates were analysed and the differences among isolates were determined to be statistically significant (LSD at P ≤0.05, ). While pathogenicity values varied between 21.6–84.6%, the most virulent isolate was Vd33 (84.6%), followed by Vd11 (75.0%) and Vd12 (73.3%). It was determined that Vd22, Vd27, Vd28, Vd31 and Vd32 isolates caused approximately 60% disease severity, while the other 25 V. dahliae isolates caused between 21.6–49.1% disease severity on the cotton plants (). Among the 33 V. dahliae isolates, only one isolate obtained from Soke was identified as belonging to the defoliating pathotype, while the remaining 32 isolates were classified as belonging to non-defoliating pathotypes. In this experiment, the isolate of the defoliating pathotype (Vd33) was inoculated onto test plants of the cultivar ‘Cukurova 1518’, and the first symptoms occurred 5–6 days after inoculation. The leaves on plants inoculated with the defoliating pathotype dropped off at 12 days and only the stem remained.
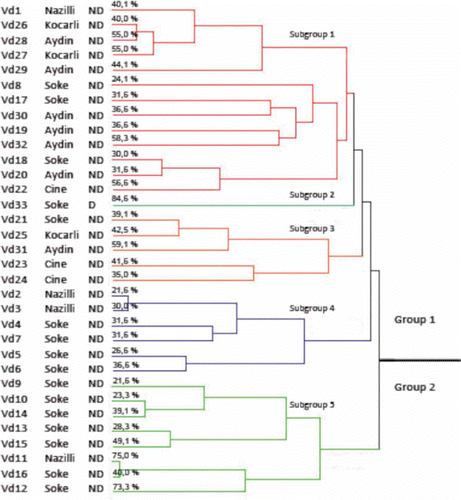
When the distribution of the isolates in the dendrogram () was evaluated, in general, the isolates formed clusters according to the level of disease severity that they caused. For instance, Vd1, Vd26, Vd27, Vd28, Vd29 formed a cluster within group 1 and they caused disease severities between 40 and 55%. In contrast, Vd2, Vd3, Vd4,Vd5, Vd6, Vd7 formed a cluster within group 1, and they caused disease severities ranging between 21 and 36%. We also found that Vd33, belonging to a defoliating pathotype, formed a single cluster in group 1 and that this isolate caused the highest disease severity. However, some exceptions in the dendrogram were detected with respect to the clustering of the isolates by disease severity. For example, although the isolates Vd11 and Vd16 were the closest two pathotypes among the isolates collected, they caused disease severities of 75 and 40%, respectively. Possibly, this could be explained by those isolates being derived from each other (Subbarao et al., Citation1995; Bhat & Subbarao, Citation1999).
Fig. 2. Dendrogram derived from cluster analysis (UPGMA) of AFLP data obtained from amplification products using nine selective primers of combination in 33 Verticillium dahliae isolates. The values of % indicate the disease severity (in ).
Our results showed the existence of genetically distinct subpopulations of V. dahliae. Jiménez-Díaz et al. (Citation2006) found that subgrouping occurs among isolates based on genetic and molecular differences, and that this subgrouping also correlated with the virulence of the isolates on the cotton hosts. Korolev et al. (Citation2001) also found disease severities that ranged between 7.4% (ND isolates) and 45.9% (D isolates) among V. dahliae isolates.
Marker analysis
The total number of assays conducted was nine EcoRI+NN/MseI+NN primer combinations for AFLP, as listed in . In total, 340 amplification products were found to be polymorphic from nine primer combinations. Similarly, Fahleson et al. (Citation2003) found 349 polymorphic bands from two primer combinations. On the other hand, Radišek et al. (Citation2003) obtained 1268 scorable bands from 39 primer combinations. Radišek et al. (Citation2003) and Fahleson et al. (Citation2003) obtained more bands because they used a two-base extension of selective primers. Selective primers with two base extentions can produce more bands than those with three base extensions. Xingfen et al. (Citation2007) found the number of polymorphic bands to be 214 in their study. The number of polymorphic bands per primer ranged from 16 (M-CA/E-GG) to 81 (M-CA/E-GA). An average number of 37.7 polymorphic bands per assay unit were identified by AFLP in this study. Radišek et al. (Citation2003) identified an average number of 32 polymorphic bands per primer combination while Fahleson et al. (Citation2003) obtained an average of 174 bands per primer. A high number of polymorphic bands per primer combination occurrs among individuals in populations that exhibit high genetic variation (Collado-Romero et al., Citation2006), suggesting that the isolates studied by Fahleson et al. (Citation2003) were genetically more distinct from each other.
Table 2. Number of bands and PIC values of the primers used for AFLP analysis of V. dahlia e isolates
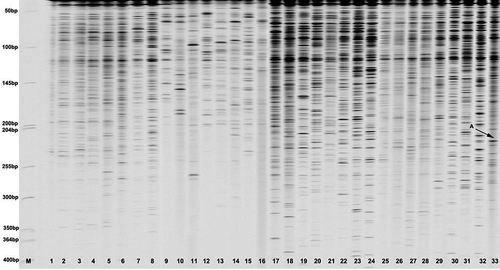
A representative gel obtained from the primer combinations is presented in . The size of the bands scored in all the 33 isolates varied from approximately 50 bp to 400 bp. Collado-Romero et al. (Citation2006) found band sizes of between 50 to 490 bp, which are close to those in this study. AFLP profiles showed the genetic polymorphism detected among V. dahliae isolates using the selective primer combination (M-CA/E-AT) were the highest.
Fig. 3. AFLP pattern of 33 Verticillium dahliae DNA samples. The M line shows molecular weight standard (50–700 bp ladder, Li-Cor Biosciences). The ‘A with arrow’ stands for the unique bands from Vd33 sample coded as 1.
The polymorphism information content values, which measure the usefulness of markers to discriminate one from another, varied from 0.33 to 0.64, and the nine primer combinations used had a PIC exceeding 0.5, demonstrating a good discriminatory power for these primer combinations (). Therefore, the data suggest that AFLP with two selected primers is a powerful procedure to survey V. dahliae isolates.
Verticillium isolates were clustered using the UPGMA method to determine relationships in the dendrogram (). There was a clear relationship between groups. Within each cluster, individuals from the same geographical origins tended to group together as shown in the AFLP dendrogram. The dendrogram revealed two distinct groups. The first group was divided into four subgroups. The first subgroup consisted of isolates from Nazilli, Soke, Aydin, Cine and Kocarli. The second subgroup included only one isolate (Vd33) from Soke province, which belongs to the defoliating pathotype. The third subgroup contained five isolates from Soke, Cine, Kocarli and Aydin. Four isolates from Soke and two isolates from Nazilli were found in the last subgroup. Group 2 contained a range of isolates isolates from Soke and one from Nazilli (Vd11).
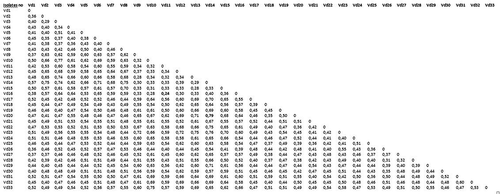
The UPGMA matrix was used to calculate genetic distance (Jaccard, Citation1908). The minimum variation (GD = 0.24) was between (Vd11) Nazilli and (Vd16) Soke, and the maximum variation (GD = 0.79) was detected between isolate (Vd14) Soke and isolate (Vd20) Aydin (). The results showed that large genetic variation exists among the isolates studied. This rate is similar to that reported in the study of Collado-Romero et al. (Citation2006) on V. dahliae diversity. They found the percentage of total variance to be between 2.69% and 88.05% within V. dahliae. In previous studies, RAPD analyses indicated high molecular similarity among D isolates from cotton in the southern part of the Guadalquivir Valley in Spain (Bejarano-Alcázar et al., 1996; Pérez-Artés et al., Citation2000a ; Korolev et al., Citation2001). The RAPD analyses revealed minor genetic variation among isolates from different V. dahliae hosts or VCGs (Vegetative Compatibility Groups) (Bhat & Subbarao, Citation1999; Bhat et al., Citation2003).
Fig. 4. Genetic distance matrix computed according to Jaccard's (Citation1908) coefficient based on AFLP data.
The current study also indicated that V. dahliae isolates (D and ND) clustered according to their disease severity, and demonstrated that the results from the genetic analyses and pathogenity testing were correlated. Compared with other V. dahliae studies conducted using AFLP with isolates collected from very close geographical regions where cotton is extensively grown, a very high degree of variation was shown in this study. Collado-Romero et al. (Citation2006) studied V. dahliae isolates from east-central Spain, including 53 isolates from artichoke, 96 isolates from cotton, seven from soil where cotton was grown, and 45 from olive trees. They found a maximum genetic variation of 88% for all isolates and a genetic variation of 9.26% within the isolates from cotton. This extent of variation in the cotton isolates is very narrow, relative to the results from the current study. The maximum genetic variation of 0.79 in our study is closer to the value that was obtained by Collado-Romero et al. (Citation2006), who also collected isolates from very diverse crops. Dervis et al. (Citation2005) studied molecular variation in 21 V. dahliae isolates collected from watermelon, cotton and eggplant in the southeastern part of Turkey using five polymorphic RAPD primers. They found that genetic distance ranged between 18 and 40% although they collected the V. dahliae isolates from very distant locations. Thus, we found greater genetic variation results than Dervis et al. (Citation2005).
In conclusion, we observed a large amount of genetic variation among V. dahliae isolates collected from cotton in a narrow geographical region of Turkey using AFLP analysis. Cotton is extensively grown in the study area, and cotton growers use the same agro-chemicals against Verticillium for a number of growing seasons. These management practices might lead to selection pressure in the pathogen population, and could contribute to the development of fungicide resistance in V. dahliae. Similarly, intensive cropping of cotton could lead to the emergence of new pathotypes from this region. This bears phytopathological significance and is worth investigating further using molecular analyses that will provide a higher resolution for characterization of pathogen strains. It also will be very important to study further mechanisms leading to high levels of genetic variation among the isolates of V. dahliae from cotton. The present study supports the earlier suggestions that AFLPs provide good insight into the genetic diversity of V. dahliae. The large amount of genetic variation that exists in Turkey may result in a breakdown of resistance in the cotton cultivars, namely ‘Carmen’, ‘Cloudia’ and ‘Gloria’ (Bayer Crop Science, Germany), which are resistant to the pathogen, and which have been cultivated for approximately 10 years. Thus, continuous cotton breeding programmes aimed at developing cultivars resistant to newly emerging pathotypes are needed.
Acknowledgements
We acknowledge the Cotton Research Institute of Nazilli (Turkey) for their contribution to collecting Verticillium samples. Special thanks to Dean Cuming for his critical review and editing of the manuscript.
References
- Statistics for Plant Production , Anonymous (2010). Turkish Statistics Agency. www.tuik.gov.tr [cited on:13.09.2011].
- Bejarano-Alcazar , J. , Melero-Vara , J.M. , Blanco-Lopez , M.A. and Jimenez-Diaz , R.M. 1995 . Influence of inoculum density of defoliating and nondefoliating pathotypes of Verticillium dahliae on epidemics of Verticillium wilt of cotton in southern Spain . Phytopathology , 85 : 1474 – 1481 .
- Bejarano-Alcazar , J. , Blanco , L.M.A. , Melero , V. and Jimenez , D.R.M. 1996 . Etiology, importance and distribution of Verticillium wilt of cotton in southern Spain . Plant Dis , 80 : 1233 – 1238 .
- Bell , A.A. 1994 . “ Mechanisms of disease resistance in Gossypium species and variation in Verticillium dahliae ” . In Challenging the Future: Proceedings of the World Cotton Research Conference , Edited by: Constable , G.A. and Forrester , NW. Vol. 1 , 225 – 235 . Melbourne : CSIRO .
- Bhat , R.G. and Subbarao , K.V. 1999 . Host range specificity in Verticillium dahliae . Phytopathology , 89 : 1218 – 1225 .
- Bhat , R.G. , Smith , R.F. , Koike , S.T. , Wu , B.M. and Subbarao , K.V. 2003 . Characterization of Verticillium dahliae isolates and wilt epidemics of pepper . Plant Dis , 87 : 789 – 797 .
- Blanco Lopez, M.N., Alcazer, J.B., Malero-Vera, J.M., & Jimenez-Diaz, R.M. (1989). Current status of Verticillium wilt of cotton in southern Spain: pathogen variation and population in soil. In B.C. 475 Tjamos & C. Beckman (Eds.). Vascular Wilt Diseases of Plants (pp. 123–132). NATO ASI Series H: Cell Biology, Vol. H-28, Springer- Verlag, New York.
- Booth , J.A. 1970 . “ Verticillium albo-atrum ” . In Crop Loss Assessment Methods: FAO Manual on the Evaluation and Prevention of Losses by Pests, Diseases and Weeds , Edited by: Chiarappa , L. 50 – 51 . Farnham Royal : Commonwealth Agriculture Bureau .
- Bora , T. and Karaca , I. 1970 . Identification of disease and disease severity in cultured plants . Ege University Agriculture Faculty Textbook , 167 : 43
- Carpenter , C.W. 1914 . The Verticillium wilt problem . Phytopathology , 4 : 393 Abstr
- Collado-Romero , M. , Mercado-Blanco , J. , Olivares-Garcia , C. , Valverde Corredor , A. and Jiménez-Diaz , R.M. 2006 . Molecular variability within and among Verticillium dahliae vegetative compatibility groups determined by fluorescent amplified fragment length polymorphism and polymerase chain reaction markers . Phytopathology , 96 : 485 – 495 .
- De Riek , J. , Calsyn , E. , Evraert , I. , Van Bockstaele , E. and De Loose , M. 2001 . AFLP based alternatives for the assessment of distinctness, uniformity and stability of sugar beet cultivars . Theor. Appl. Genet , 103 : 1254 – 1265 .
- Dervis , S. , Kurt , S. and Bicici , M. 2005 . Molecular characterization of Verticillium dahlia isolates in southern Turkey . Pak. J. Biol. Sci , 8 : 1233 – 1236 .
- Doyle , J.J. and Doyle , J.L. 1987 . A rapid DNA isolation procedure for small quantities of fresh leaf tissue . Phytochem. Bull , 19 : 11 – 15 .
- Fahleson , J. , Lagercrantz , U. , Hu , Q. , Steventon , L.A. and Dixelius , C. 2003 . Estimation of genetic variation among Verticillium isolates using AFLP analysis . Eur. J. Plant Pathology , 109 : 361 – 371 .
- Goud , J.C. and Termorshuizen , A.J. 2003 . Quality of methods to quantify microsclerotia of Verticillium dahliae in soil . Eur. J. Plant Pathology , 109 : 523 – 534 .
- Harris , D.C. , Yang , Y.R. and Ridout , M.S. 1993 . The detection and estimation of Verticillium dahliae in naturally infested soil . Plant Pathol , 42 : 238 – 250 .
- Iyriboz , N. 1941 . Mahsul Hastalıkları . Ziraat Vekaleti Neşriyatı , Umum No. 237
- Jaccard , P. 1908 . Nouvelles recherchers sur la distribution florale . Soc. Vaudoise Sci. Nat , 44 : 233 – 270 .
- Jiménez-Díaz , R.M. , Mercado-Blanco , J. , Olivares-Garcia , C. , Collado-Romero , M. , Bejarano-Alcázar , J. Rodriguez-Jurado , D. 2006 . Genetic and virulence diversity in Verticillium dahliae populations infecting artichoke in eastern-central Spain . Phytopathology , 96 : 288 – 298 .
- Karaca , I. , Karcilioglu , A. and Ceylan , S. 1971 . Wilt disease of cotton in the Ege region of Turkey . J. Turkish Phytopathol , 1 : 4 – 11 .
- Ann. Rev. Phytopathol , Klosterman, S.J., Atallah, Z.K., Vallad, G.E., & Subbarao, K.V. (2009). Diversity, pathogenicity, and management of Verticillium species. 47, 39–62.
- Koike , M. , Fujita , M. , Nagao , N. and Ohshima , S. 1996 . Random amplified polymorphic DNA analysis of Japanese isolates of Verticillium dahliae and V. albo-atrum . Plant Dis , 80 : 1224 – 1227 .
- Korolev , N. , Perez-Artes , E. , Bejarano-Alcazar , J. , Rodriguez-Jurado , D. , Katan , J. , Katan , T. and Jimenez-Diaz , R.M. 2001 . Comparative study of genetic diversity and pathogenicity among populations of Verticillium dahliae from cotton in Spain and Israel . Eur. J. Plant Pathol , 107 : 443 – 456 .
- Melouk , H.A. 1992 . “ Verticillium ” . In Methods for Research on Soilborne Phytopathogenic Fungi , Edited by: Singleton , L.L. , Mihail , J.D. and Rush , C.M. 175 – 178 . St. Paul , MN : American Phytopathological Society Press .
- Messner , R. , Schweigkofler , W. , IBL , M. , Berg , G. and Prillinger , H. 1996 . Molecular characterization of the plant pathogen Verticillium dahliae Kleb. using RAPD-PCR and sequencing of the 18s rRNA gene . J. Phytopathol , 144 : 347 – 354 .
- Pegg , G.F. 1984 . The impact of Verticillium diseases in agriculture . Phytopathol. Mediter , 23 : 176 – 192 .
- Pérez-Artés , E. , Garcia-Pedrajas , M.D. , Bejarano-Alcázar , J. and Jiménez-Diaz , R.M. 2000a . Differentiation of cotton-defoliating and non-defoliating pathotypes of Verticillium dahliae by RAPD and specific PCR analyses . Eur. J. Plant Pathol , 106 : 507 – 517 .
- Pérez-Artés , E. , Garcia-Pedrajas , M.D. , Bejarano-Alcazar , J. , Korolev , N. , Rodriguez Jurado , D. , Katan , T. , Katan , J. and Jimenez-Diaz , R.M. 2000b . “ Comparative RAPD analysis of cotton isolates of Verticillium dahliae from Israel and Spain ” . In Advances in Verticillium Research and Disease Management , Edited by: Tjamos , E.C. , Rowe , R.C. , Heale , J.B. and Fravel , D.R. 41 – 43 . St. Paul , MN : American Phytopathological Society Press .
- Radišek , S. , Jakše , J. , Simončič , A. and Javornik , B. 2003 . Characterization of Verticillium albo-atrum field isolates using pathogenicity data and AFLP analysis . Plant Dis , 87 : 633 – 638 .
- Rafalski , J.A. , Vogel , J.M. , Morgante , M. , Powell , W. , Andre , C. and Tingey , S.V. 1996 . “ Generating and using DNA markers in plants ” . In Non‐Mammalian Genomic Analysis: A Practical Guide , Edited by: Birren , B. and Lai , E. 75 – 134 . London : Academic Press .
- Sas Institute . 1995 . JMP Statistics and Graphics Guide, Version 3.1 , Cary , NC : SAS Institute .
- Schnathorst , W.C. and Mathre , D.E. 1966a . Cross-protection in cotton with strains of Verticillium albo-atrum . Phytopathology , 56 : 1204 – 1209 .
- Schnathorst , W.C. and Mathre , D.E. 1966b . Host range and differentiation of a severe form of Verticillium albo-atrum in cotton . Phytopathology , 56 : 1155 – 1161 .
- Schnathorst , W.C. , Reeve , T.A. and Fogle , D. 1975 . Verticillium dahliae strains in cotton in the Pahrump valley, Nevada . Plant Dis. Rept , 59 : 863 – 865 .
- Subbarao , K.V. , Chassot , A. , Gordon , T.R. , Hubbard , J.C. Bonello , P. 1995 . Genetic relationships and cross pathogenicities of Verticillium dahliae isolates from cauliflower and other crops . Phytopathology , 85 : 1105 – 1112 .
- Wang , J.-Y. , Cai , Y. , Gou , J.-Y. , Mao , Y.-B. , Xu , Y.-H. , Jiang , W.-H. and Chen , X.-Y. 2004 . VdNEP, an elicitor from Verticillium dahliae, induces cotton plant wilting . Appl. Environ. Microbiol , 70 : 4989 – 4995 .
- Xingfen , W. , Jun , M.A. , Shuo , Y. , Guiyin , Z. and Zhiying , M.A. 2007 . Assessment of genetic diversity among Chinese upland cottons with Fusarium and/or Verticillium wilt resistance by AFLP and SSR markers . Front. Agric , 1 : 129 – 135 .
- Zeıse , K. and Von Tıedemann , A. 2002 . Application of RAPD-PCR for virulence type analysis within Verticillium dahliae and V. longisporum . J. Phytopathol , 150 : 557 – 563 .