Abstract
Switchgrass mosaic virus (SwMV) was identified in switchgrass (Panicum virgatum) and was proposed as a new marafivirus based on its genome sequence and comparison with its closest relative, Maize rayado fino virus (MRFV), a type member of the genus Marafivirus. MRFV only infects maize (Zea mays) and its wild relatives, and is naturally transmitted by a corn leafhopper, Dalbulus maidis. Proving that SwMV is transmitted by a different species of leafhopper than D. maidis would provide additional evidence that SwMV is a new species of the genus Marafivirus. In 2010, three leafhopper species were identified in switchgrass fields near Champaign, Illinois – Graminella aureovittata, Graminella mohri and Flexamia atlantica. Switchgrass mosaic virus was detected by two independent RT–PCR assays in 100% of G. mohri and F. atlantica, and 95% of G. aureovittata. Twenty specimens of each species were evaluated. Of the three leafhoppers, only G. aureovittata transmitted the virus to ‘Cave in Rock’ switchgrass plants in a growth chamber. Transmission efficiency was 80% and only one of the eight SwMV-infected plants displayed mosaic/yellow streak symptoms. Switchgrass mosaic virus was detected in 78% and 83% of the switchgrass plants in the two fields from which the leafhoppers were collected. The detection of a leafhopper vector of SwMV will facilitate the transmission of the virus and the study of its impact on switchgrass biomass yield.
Résumé
Le virus de la mosaïque du panic raide (VMPr) a été détecté chez le panic raide (Panicum virgatum) et, en se basant sur sa séquence génomique ainsi qu'en le comparant à son plus proche parent, le virus Rayado Fino du maïs (VRFM), un représentant typique du genre Marafivirus, le VMPr a été reconnu comme nouveau marafivirus. Le VRFM ne s'attaque qu'au maïs (Zea mays) et aux plantes sauvages qui lui sont apparentées. Il est naturellement transmis par une cicadelle du maïs, Dalbulus maidis. La preuve que le VMPr est transmis par une autre espèce de cicadelle serait une preuve supplémentaire qu'il s'agit d'une nouvelle espèce du genre Marafivirus. En 2010, trois espèces de cicadelles ont été décelées dans des champs de panic raide près de Champaign en Illinois : Graminella aureovittata, Graminella mohri et Flexamia atlantica. Le virus de la mosaïque du panic raide a été détecté, à l'aide de deux analyses de sources indépendantes par RT-PCR, chez tous les spécimens de G. mohri et F. atlantica (100 %) et chez 95 % de ceux de G. aureovittata. Vingt spécimens de chaque espèce ont été évalués. Des trois cicadelles, seulement G. aureovittata a transmis le virus aux plants de panic raide ‘Cave Rock’ cultivés en chambre de croissance. L'efficacité de la transmission était de 80 % et seulement un des huit plants infectés par le VMPr a affiché les stries jaunes caractéristiques du symptôme de la mosaïque. Le virus de la mosaïque du panic raide a été détecté sur 78 % et 83 % des plants de panic raide dans les deux champs où les cicadelles avaient été collectées. La détection d'une cicadelle en tant que vecteur de dispersion du VMPr facilitera sa transmission et par conséquent l’étude de ses effets sur le rendement du panic raide en tant que biomasse.
Introduction
Switchgrass (Panicum virgatum L.) is a perennial, warm season, C-4 grass native to North America. It has potential as a biofuel feedstock because of high yield and its ability to sequester atmospheric carbon dioxide (Lewandowski et al., Citation2003). Production of cellulosic ethanol is affected by the yield of biomass feed stocks. Pests and pathogens have the potential to reduce biomass, which in turn will reduce ethanol yield. Recently, a virus was detected in switchgrass plants displaying mosaic/yellow streak symptoms on leaves. The detected virus was most related to but distinct from Maize rayado fino virus (MRFV), a type member of the genus Marafivirus, and was proposed as a new virus species named Switchgrass mosaic virus (SwMV) (Agindotan et al., Citation2010a , Citation2012a ). The complete genome of the virus has been sequenced (Agindotan et al., Citation2012a ). The nucleotide and the amino acid sequences of its polyprotein were 76% and 81% identical, respectively, to those of MRFV in GenBank.
Infection by MRFV has been reported to occur naturally on the host [maize (Zea mays L.)] and related wild relatives by the corn leafhopper vector, Dalbulus maidis (Family: Cicadellidae) (DeLong & Wolcott) (Nault & Delong, Citation1980; Nault et al., Citation1980; Gamez & Leon, Citation1985). In the absence of the vector, the virus is not naturally transmitted (Madriz-Ordenaña et al., Citation2000). One of the criteria established by the International Committee on Taxonomy of Viruses (ICTV) for speciation of marafiviruses is vector specificity. Therefore, establishing that SwMV is a different species from its closest relative, MRFV, includes proving transmission of SwMV by a vector other than D. maidis.
All marafiviruses are transmitted by leafhoppers, including Oat blue dwarf mosaic virus (OBDV), Citrus sudden death-associated virus (CSDaV), Bermuda grass etched-line virus (BELV) and Grapevine Syrah virus 1 (GSyV-1) (Adams & Antoniw, Citation2005). The three main objectives of this research were to: (i) identify the leafhopper vector(s) of SwMV; (ii) determine the symptoms induced by the virus in switchgrass; and (iii) estimate the incidence of the virus in switchgrass plants in the field.
Materials and methods
Switchgrass and leafhopper samples
The switchgrass variety ‘Cave-in-Rock’ was used throughout this experiment. Switchgrass plants for transmission studies were grown from seeds in an insect-free growth chamber. SwMV-positive control switchgrass plants were obtained from growth-chamber propagated rhizomes of SwMV-positive switchgrass taken from a switchgrass field in July 2010 at the University of Illinois SoyFACE field at Savoy, near Champaign, IL. Both parent and daughter plants tested negative to Sugarcane mosaic virus (SCMV) and Panicum mosaic virus (PMV).
In July 2010, leafhoppers were also collected from switchgrass fields at SoyFACE field plots, established in 2003 and 2004. The three most abundant leafhoppers in the switchgrass fields were: Graminella aureovittata (Sanders & DeLong), Graminella mohri (DeLong) and Flexamia atlantica (DeLong), which all belong to the family Cicadellidae. A planthopper, Myndus ovatus Ball (Family, Cixiidae), was also identified. About 50–100 individuals of each of the leafhopper and planthopper species were flash frozen and stored at −80 °C.
Switchgrass leaf samples were randomly collected from two of the research fields at SoyFACE in July 2010, from which leafhoppers and planthoppers were also collected. Each field was 30.5 m × 61 m. An open quadrant (∼16 cm2) was thrown from eight points about a field (four edges and four mid-sides), and there were five throws per point. The youngest leaves from five plants were collected per throw. Two of the plants were collected at point of throw and at least 10 cm apart, while the rest were at least 10 cm apart from where the quadrant landed. Two hundred leaf samples from different plants were collected from each field and labelled 1–200. Fifty random numbers were picked from these numbers and the corresponding leaf samples screened for SwMV, to determine the incidence of the virus in switchgrass plants in each field.
Transmission studies
In July 2010, 30–40 live leafhoppers of each of the three species: G. aureovittata, G. mohri and F. atlantica taken directly from SwMV-infected switchgrass fields described above, were separately maintained for two weeks on four week old switchgrass plants in a growth chamber (25 °C; 16 h light : 8 h dark). Two weeks later, composite leaf tissues from the plants infested with each species were tested for SwMV. Graminella aureovittata, G. mohri and F. atlantica were allowed to acquire SwMV by restricting feeding on SwMV-infected switchgrass for seven days. The leafhoppers were then transferred and maintained on three week old healthy switchgrass seedlings for three weeks in a growth chamber as described above. For each leafhopper species, one individual leafhopper was allowed to feed on one potted plant and 10 potted plants per each leafhopper species were used for the experiment.
Reverse transcription–polymerase chain reaction (RT–PCR)
Both sets of RT-PCR reactions described below targeted the capsid protein of SwMV genome. For total RNA extraction, a single leafhopper was placed in a 2 mL tube containing 0.5 g ceramic beads (1.4 mm, MP Biomedicals, Salon, OH) and extracted in 1 mL RNApro™ solution (MP Biomedicals) containing 0.05% mercapto-ethanol, following the manufacturer's protocol. The samples were kept cold with dry-ice during homogenization with FastPrep-24 at program 5 (60 m s−1 for 5 min). Similarly, total RNA was extracted from 0.1 g of leaf tissue per plant except that Plant RNA mini kit (Qiagen, Valencia, CA) was used. The total RNA was suspended or eluted in 50 μL of nuclease-free water for both methods and RT-PCR done as described in Agindotan et al. (Citation2010a ). Briefly, 20 μL RT reaction included 3 μL of total RNA, 0.5 μm B88 (GCC CAC AGG TCT TGT GGC CGA CCT GTT ACC; JF727261.2:6408-6379), and incubation was at 42 °C. The subsequent 25 μL PCR reaction mixture consisted of 2 μL of RT product, 1× Hot Start Green PCR mix (ThermoScientific, Walthan, MA), 0.2 μm each of B88 and B89 (GCTATTCCTGCTCCTCCTCGTGTGGTTGAAACC; JF727261.2: 5755-5787nt). Amplification cycling conditions were: 94 °C for 4 min, 40 cycles of denaturation at 94 °C for 30 s, annealing at 60 °C for 30 s, and elongation at 72 °C for 45 s; and final extension at 72 °C for 20 min. The expected amplicon size was 653 bp.
A second set of RT–PCRs was done using a primer B283 (an Oligo dT anchor primer: GACCACGCGTATCGATGTCGACTTTTTTTTTTTTTTTTV) for reverse transcription (RT) and a pair of B88 and B308 (anchor primer: GACCACGCGTATCGATCGATGTCGAC) for PCR. Reverse transcription and amplification conditions were the same as above, except that RT was done at 37 °C. Selected amplicons were gel-purified and sequenced directly. The amplicon size with B89/B308 was expected to be slightly longer than 653 bp because of the polyA tail at the 3′-end of the genome (Agindotan et al., Citation2012a ).
Results and discussion
SwMV was detected in three leafhopper species (Family: Cicadellidae) and one planthopper (Family: Cixiidae) in two independent sets of RT–PCRs (one set: RT with B88 and PCR with B88 and B89 primer pair; and second set: RT with B283 and PCR with B89 and B308 primers). The results were identical and were as follow: 100% of G. mohri and F. atlantica, and 95% of G. aureovittata, were SwM-positive. The virus was also detected in 100% of the planthopper species, Myndus ovatus. Twenty specimens of each species were evaluated in four batches. shows the result from one of the batches. One amplicon (second set of RT–PCR) from each species was directly sequenced. The following are the GenBank accession numbers: KF061098 (SwMV from F. atlantica), KF061099 (SwMV from G. aureovittata), KF061100 (SwMV from G. mohri) and KF061101 (SwMV from M. ovatus). The nucleotide sequences were 98.9% to 99.7% identical to the most related SwMV (JQ768542.1) in GenBank, and 98.8% to 99.9% to each other. Thus, the virus sequences from switchgrass and the insects were identical.
Fig. 1. Detection of Switchgrass mosaic virus in individual leafhoppers. Panel A: RT–PCR using B287 primer for RT and B88 and B89 primer pairs for PCR. Panel B: RT–PCR using B287 primer for RT and B305 and B89 for PCR. M = GeneRuler 1Kb plus (Fermentas); Lanes 1–5: Fleximiana atlantica (FA); Lanes 6–10: Graminella morhi (GM); Lanes 11–15: G. aureovitatta (GA); Lanes 16–20: Myndus ovatus (MO); Lane 16: Switchgrass leaf from plant on which FA obtained directly from the field were maintained; Lane 17: Switchgrass leaf from plant on which FA obtained directly from the field were maintained; Lane 18: Switchgrass leaf from plant on which GA obtained directly from the field were maintained; Lane 19: Switchgrass leaf from plant on which GM obtained directly from the field were maintained; Lane 20: leaf from uninfested, SwMV-negative switchgrass plant (negative control). FA, GA, GM were leafhoppers, while GO was a planthopper.
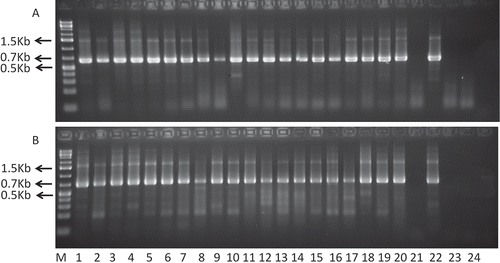
The detection of SwMV in 95–100% of the specimens evaluated was greater than the reported 80% detection of MRFV in D. maidis (Gamez & Leon, Citation1985, Citation1988) and 81% detection of ODBV in aster leafhoppers (Azar & Bantarri, Citation1981). In both cases, the detection method was ELISA, which is generally less sensitive than RT–PCR. The detection of SwMV in field samples of the three leafhopper species (G. mohri, G. aureovittata and F. atlantica) and planthopper species (Myndus ovatus) suggests the virus was either circulating or propagating within them (Nault, Citation1989).
Dalbulus maidis was not found in any of the switchgrass plots. This species is not native to Illinois but has been collected occasionally in central Illinois during the past 15 years (Dietrich, unpublished). Nevertheless, D. maidis is a host plant specialist on corn and related species of Zea and Tripsacum, and is not known to feed on switchgrass (Triplehorn & Nault, Citation1985; Nault, Citation1990).
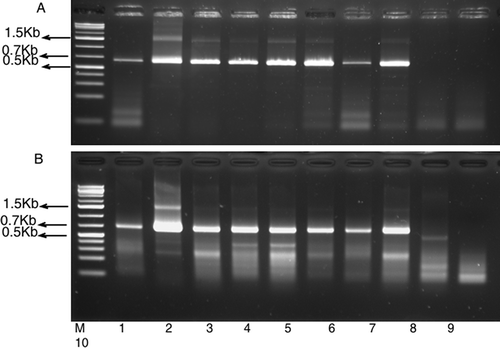
Preliminary results showed that composite leaf samples of switchgrass plants on which G. aureovittata, directly collected from switchgrass plots, was maintained tested positive to SwMV, while those of switchgrass plants on which G. mohri and F. atlantica were maintained, tested negative to SwMV (). The results of a follow-up transmission study showed that eight of 10 (80%) leafhoppers ‘G. aureovittata’ transmitted SwMV to switchgrass (). However, of the eight plants that tested positive to SwMV, only one had mosaic (yellow streaks); the rest were asymptomatic (see ). Two sets of independent RT-PCR confirmed the results above ( and ). The amplicon from the sample with mosaic symptoms was sequenced (GenBank accession number: KF061102) and it was 98.9% identical to SwMV (JQ768542.1).
Fig. 3. Transmission of Switchgrass mosaic virus by Graminella aureovittata. A. Healthy: Asymptomatic leaf from plant not exposed to leafhoppers). Infected: Mosaic (yellow streak) leaf symptom after SwMV transmission by G. aureovittata. Both plants were switchgrass var. Cave-in-Rock. B. The other two leafhopper species did not transmit the virus to switchgrass, as their infested plants were both asymptomatic and SwMV-negative.
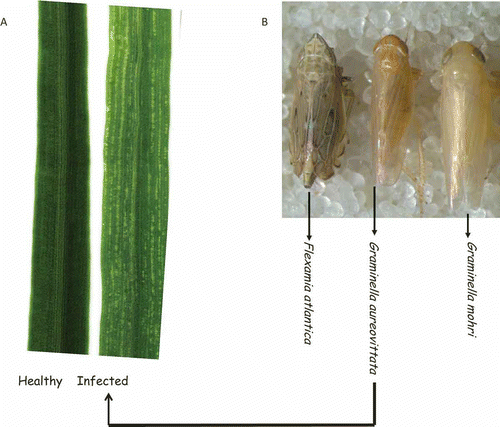
Fig. 2. Detection of Switchgrass mosaic virus in switchgrass transmitted by Graminella aureovittata. Panel A: RT–PCR using B287 primer for RT and B88 and B89 primer pair for PCR. Panel B: RT–PCR using B287 primer for RT and B308 and B89 for PCR. M = GeneRuler 1Kb plus (Fermentas). PCR products 1–8 from eight switchgrass plants exposed to G. aureovittata, while products 9 and 10 were from two switchgrass plants grown from seeds and maintained in insect-free growth chamber. PCR product 1 was from switchgrass plant with foliage mosaic symptom; others were from asymptomatic leaf tissues. Switchgrass variety used was Cave-in-Rock.
Graminella aureovittata transmission of SwMV on switchgrass was greater than the reported 10–34% transmission of MRFV on maize by D. maidis (Gamez & Leon, Citation1985, Citation1988) and closer to 19–61% aster leafhopper transmission of OBDV (Azar & Bantarri, Citation1981). The other leafhopper species, G. mohri and F. atlantica, did not transmit the virus to any of the switchgrass plants as these plants tested negative to SwMV and were asymptomatic. The negative control switchgrass plants were both asymptomatic and negative to SwMV (). The transmission of SwMV by only G. aureovittata, despite the presence of the virus in the other leafhopper species, indicates that detection or propagation of the virus may not be linked with transmission of the virus. Alternatively, the transmission efficiency is so low in the other leafhopper species that a larger sample size of both the leafhoppers and test plants would be needed to detect the virus. For G. aureovittata that transmitted the virus, the elongated acquisition period (three weeks), and the simultaneous inoculation and access periods (three weeks) probably aided in transmission. It has been reported for both MRFV and Oat blue dwarf virus (OBDV) that longer feeding periods by leafhoppers resulted in greater transmission levels (Bantarri & Zeyen, Citation1976; Gamez & Leon, Citation1985, Citation1988).
The incidence of SwMV in plants in two fields in which leafhoppers were collected was 78% (SwMV was detected in 48% of asymptomatic and in 30% of mosaic leaf samples) and 83% (SwMV detected in 54% of asymptomatic and in 29% of mosaic leaf samples). The virus was detected in 69% and 75% of asymptomatic leaf samples in the plots. This was similar to the transmission studies where 87.80% (seven over eight plants) of those plants in which SwMV was successfully transmitted by G. aureovittata showed no symptoms on the foliage. This suggests that a majority of the plants were either tolerant to the virus after infection, or that the virus titre had not reached a threshold to induce symptoms in these plants. The latter is supported by the lower intensity of amplified SwMV bands from asymptomatic switchgrass leaf samples compared with the plant in which mosaic symptom was expressed ( a, b: lanes no. 1, 3–8 compared with lane no. 2).
Further research is needed to fully characterize SwMV transmission by G. aureovittata. Variables that need additional investigation include determining the acquisition, latent and inoculation periods; assessing the effects of sex and age of the leafhopper on SwMV transmission efficiency; and clarifying the effects of switchgrass variety on the transmission rate. Research also needs to be conducted to better understand if G. aureovittata or other leafhopper vector is responsible for transmission of SwMV to Miscanthus species (Agindotan et al., Citation2012b ), and if infection after transmission is systemic or local.
In conclusion, the transmission of SwMV by a different leafhopper species other than the one transmitting MRFV is additional evidence that SwMV is sufficiently distinct from MRFV and qualifies to be considered as a new species in the genus Marafivirus. Identification of the vector of SwMV will aid in the control and management of the disease and in the estimation of the impact of the virus on biomass yield.
Acknowledgement
This project was funded by the Energy Biosciences Institute (EBI).
References
- Adams , M.J. and Antoniw , J.F. 2005 . DPVweb: an open access internet resource on plant viruses and virus diseases . Outlooks Pest Manag , 16 : 268 – 270 .
- Agindotan , B.O. , Ahonsi , M.O. , Domier , L.L. , Gray , M.E. and Bradley , C.A. 2010a . Application of sequence-independent amplification (SIA) for the identification of RNA viruses in bioenergy crops . J. Virol. Meth , 169 : 119 – 128 .
- Agindotan , B.O. , Ahonsi , M.O. , Domier , L.L. , Gray , M.E. and Bradley , C.A. 2010b . Potential viral threats to Miscanthus x giganteus and switchgrass production for bioenergy in the United States . Phytopathology , 100 : S3 Abstr
- Agindotan , B.O. , Gray , M.E. , Hammond , R. and Bradley , C.A. 2012a . Complete genome sequence of switchgrass mosaic virus, proposed as a new species of the genus Marafivirus . Arch. Virol , 157 : 1825 – 1830 .
- Agindotan , B. , Okanu , N. , Oladeinde , A. , Voigt , T. , Long , S. , Gray , M. and Bradley , C. 2013 . Detection of Switchgrass mosaic virus in Miscanthus and other grasses . Can. J. Plant Pathol. , 35 : 81 – 86 .
- Azar , M. and Bantarri , E.E. 1981 . Enzyme-linked immunosorbent assay versus transmission assay for detection of Oat blue dwarf virus in aster leafhoppers . Phytopathology , 71 : 858
- Bantarri , E.E. and Zeyen , R.J. 1976 . Multiplication of the oat blue dwarf virus in the aster leafhopper . Phytopathology , 66 : 896 – 900 .
- Gamez , R. and Leon , P. 1985 . “ Ecology and evolution of a Neotropical leafhopper-virus-maize association ” . In Leafhoppers and Planthoppers , Edited by: Nault , L.R. and Rodriguez , J. 331 – 350 . New York : John Wiley and Sons .
- Gamez , R. and Leon , P. 1988 . “ Maize rayado fino and related viruses ” . In The Plant Viruses , Edited by: Koenig , R. 213 – 223 . New York : Plenum Press .
- Lewandowski , I. , Scurlock , J.M.O. , Lindvall , E. and Christou , M. 2003 . The development and current status of perennial rhizomatous grasses as energy crops in the US and Europe . Biomass Bioenergy , 25 : 335 – 361 .
- Madriz-Ordeñana , K. , Rojas-Montero , R. , Lundsgaard , T. , Ramírez , P. , Thordal-Christensen , H. and Collinge , D. B. 2000 . Mechanical transmission of maize rayado fino marafivirus (MRFV) to maize and barley by means of the vascular puncture technique . Plant Pathol , 49 : 302 – 307 .
- Nault , L.R. 1989 . Evolution of an insect pest: maize and the corn leafhopper, a case study . Maydica , 35 : 165 – 175 .
- Nault , L.R. 1990 . Leafhopper and planthopper transmission of plant viruses . Annu. Rev. Entomol , 34 : 503 – 529 .
- Nault , L.R. and Delong , D.W. 1980 . Evidence for co-evolution of leafhoppers in the genus Dalbulus (Cicadellidae. Hompotera) with maize and its ancestors . Ann. Entomol. Soc. Amer , 73 : 349 – 353 .
- Nault , L.R. , Gringery , R.E. and Gordon , D.T. 1980 . Leafhopper transmission and host range of Maize rayado fino virus . Phytopathology , 70 : 709 – 712 .
- Triplehorn , B.W. and Nault , L.R. 1985 . Phylogenetic classification of the genus Dalbulus (Homoptera: Cicadellidae), and notes on the phylogeny of Macrostelini . Ann. Entomol. Soc. Amer , 78 : 291 – 315 .