Abstract
Five laboratory-isolated Bacillus subtilis strains: CB1, CGB, JHB1, SSB1 and WW4; and five commercial biocontrol agents: Actinovate® (Stretomyces lydicus strain WYEC108), Serenade® MAX (Bacillus subtilis strain QST713), Mycostop® (Streptomyces griseoviridis strain K61), RootShield® (Trichoderma harzianum strain T-22), and Prestop® WP (Gliocladium catenulatum strain J1446) were evaluated for their potential to inhibit Fusarium foetens both in vitro in Petri dishes and in the greenhouse using potted begonia plants. Bacillus subtilis strain JHB1 inhibited F. foetens mycelial growth by 76% compared with the untreated dishes after seven days of dual culturing; and also provided significant protection to potted begonia plants against F. foetens under greenhouse conditions (two trials) when applied as two soil drenches prior to pathogen inoculation. Compared with the pathogen-inoculated control, disease severity (DS) was reduced by 33% and 50% by JHB1 in trial one and two, respectively. All of the commercial biocontrol agents significantly reduced DS compared with the control when applied as soil drenches prior to pathogen inoculation. Most of the commercial products delayed plant disease symptom development by seven or more days. Moreover, Mycostop, Prestop, Actinovate, RootShield and Serenade all significantly reduced the DS, and increased the shoot dry weights and chlorophyll content index for over 28 days after inoculation compared with the control.
Résumé
Cinq souches de Bacillus subtilis isolées en laboratoire (CB1, CGB, JHB1, SSB1 et WW4) et quatre agents de lutte biologique produits commercialement (ActinovateMD [Streptomyces lydicus, souche WYEC108], SerenadeMD MAX [Bacillus subtilis, souche QST713], MycostopMD [Streptomyces griseoviridis, souche K61], RootShieldMD [Trichoderma harzianum, souche T-22] et PrestopMD WP [Gliocladium catenulatum, souche J1446]) ont été évalués pour leur capacité potentielle à inhiber Fusarium foetens in vitro, dans des boîtes de Petri et en serre sur des bégonias en pot. La souche JHB1 de B. subtilis avait inhibé la croissance mycélienne de F. foetens de 76 % au bout de sept jours de double culture, comparativement aux boîtes de Petri non traitées. Elle a également protégé adéquatement deux bégonias cultivés en pot en serre (deux essais) contre F. foetens lorsqu'elle était appliquée par trempage du sol préalablement à l'inoculation. Comparativement au témoin inoculé, la gravité de la maladie (GM) était réduite de 33 % et de 50 % par la souche JHB1 au cours des essais un et deux, respectivement. Tous les agents commerciaux de lutte biologique ont réduit significativement la GM comparativement aux témoins lorsqu'ils étaient appliqués par trempage du sol préalablement à l'inoculation. La plupart des produits commerciaux ont retardé le développement des symptômes de la maladie de sept jours ou plus. En outre, comparativement aux témoins, Mycostop, Prestop, Actinovate RootShield et Serenade ont tous significativement réduit la GM et accru le poids sec des pousses ainsi que l'indice normalisé de chlorophylle durant plus de 28 jours après l'inoculation.
Introduction
Soil-borne pathogens can cause serious economic losses for farmers in crop production. Fusarium foetens, a causal agent of wilt in Hiemalis begonia (Begonia × hiemalis Fotsch), has been linked to serious losses in greenhouse and nursery begonia production in the USA, the Netherlands, Germany, Japan and Canada (Elmer & Vossbrinck, Citation2004; Schroers et al., Citation2004; Sekine et al., Citation2008; Tian et al., Citation2010). The symptoms of this disease normally appear on leaves two to three weeks after infection, then progress to the stems and flowers of Hiemalis begonia, often resulting in plant death after six to eight weeks (Tian et al., Citation2012).
Biocontrol measures have found a niche in the greenhouse industry due to the paucity of registered fungicides. Concerns about potential negative effects of chemical pesticides on the environment, increases in pathogen resistance to pesticides, greater regulation and human safety issues have attracted more attention on using biocontrol agents to manage greenhouse ornamental plant diseases (Bélanger, Citation2006; Elliott et al., Citation2009).
Several modes of action have been described to explain the activity of biocontrol agents. These include competition for nutrients and niches, antibiosis, mycoparasitism and rhizosphere colonization (Hassanein et al., Citation2006; Siddiqui & Akhtar, Citation2007; Bailey et al., Citation2008). Recently, species belong to the genera Pseudomonas, Bacillus, Penicillium, Streptomyces and Trichoderma have been registered as commercial biocontrol products whose mode of action involves the induction of plant host defences or plant-growth promotion (Kucey, Citation1987; Kwok et al., Citation1987; Handelsman et al., Citation1990; Kim et al., Citation1997; Mathre et al., Citation1999; Kloepper et al., Citation2004). The proposed mechanisms involved in the induction of resistance in plants include increased production of endogenous levels of oxidative enzymes (e.g. polyphenol oxidase and peroxidase), enhanced lignification of host tissues, increased salicylic acid (SA) levels in plants and induction of pathogeneses-related proteins (PR-1), chitinase and β-1,3 glucanase (Jetiyanon et al., Citation1997; De Meyer et al., Citation1998).
Many different biocontrol agents have been employed for suppression of damping-off and root and stem rot diseases in greenhouse production (Punja, Citation1997; Gracia-Garza et al., Citation2003; Rose et al., Citation2003). In Canada, Mycostop® was the first biological control agent to be registered (in 2000) for use against root pathogens on greenhouse crops. This was followed by Actinovate® (2007) and Senerade® (2007), Prestop® (2008) and RootShield® (2011). Mycostop, Prestop and RootShield were reported to reduce the severity of fusarium root and stem rot on cucumbers grown in rockwool under greenhouse conditions (Rose et al., Citation2003). Actinovate reduced the severity of powdery mildew and other diseases and increased the yield and quality of greenhouse tomatoes when applied as a seed drench followed by repeated application through drip irrigation (Obispo, Citation2011).
Currently, there is no information available on the efficacy of biocontrol agents for F. foetens in begonia. The objectives of this study were to evaluate the antagonistic activities of five laboratory-isolated Bacillus subtilis strains against F. foetens, and to examine the efficacy of these B. subtilis strains and five commercially available biological control products: Actinovate® (Streptomyces lydicus), Mycostop® (Streptomyces griseoviridis), Prestop® (Gliocladium catenulatum), RootShield® (Trichoderma harzianum), Serenade® MAX (Bacillus subtilis) in suppressing Fusarium wilt in Hiemalis begonia under greenhouse conditions.
Materials and methods
In vitro inhibition activity
Bacillus subtilis strains (CB1, CGB, JHB1, SSB1 and WW4; ) were obtained from Dr Allen Xue (Agriculture and Agri-Food Canada, Oilseeds and Cereal Research Centre, Ottawa). Bacteria and F. foetens were evaluated in dual culture on potato dextrose agar (PDA) at 25 °C as follows: a 0.5 cm diameter agar plug of Bacillus was placed on one side of a Petri dish and a 0.5 cm diameter plug of F. foetens was placed 4 cm away from the Bacillus plug. Plates were sealed with Parafilm and incubated in the dark at 25 °C. Plates were examined daily and the radial growth of the F. foetens colony was measured after seven days in two directions: proximal radius (i.e. facing bacteria) and distal radius (tangential to bacteria). Inhibition was represented by the comparison of these radii to the control treatment where a water agar plug was used in place of the bacteria. The experiment had four replicates in each treatment and was repeated once.
Table 1. Description of the biocontrol products employed in this study
Isolation of F. foetens and plant inoculation
The isolate of F. foetens was originally obtained from diseased Begonia × hiemalis in 2010. Diseased stems were surface-sterilized in 70% ethanol for one min, rinsed twice in sterile water, transferred to PDA amended with antibiotics (100 mg streptomycin and 50 mg tetracycline per L), and incubated at 25 °C in the dark for seven days. Pure cultures were stored at 4 °C. Inoculum was prepared by culturing the fungus on potato carrot agar (PCA) for five days in the dark. Conidia were then collected by flooding the plates with sterile water and gently scraping the surface with an inoculation loop. The suspension was adjusted to a final concentration of 1× 103 conidia mL−1. Begonia × hiemalis ‘Batik’ plants were inoculated by drenching 30 mL of the spore suspension onto each pot three weeks after transplanting. Uninoculated control plants were drenched with the same volume of sterile water.
Plant growth
Begonia × hiemalis ‘Batik Orange’ was obtained from a commercial greenhouse in southern Ontario. Rooted plug cuttings at the 3-leaf stage were transplanted into 12 cm diameter plastic pots filled with a standard commercial growing medium: BM6 (Berger Peat Moss, Quebec) and maintained on greenhouse benches with a drip irrigation system. Drip irrigation was applied every other day as required with a nutrient solution (N:P2O5:K2O 7-11-27) with a pH of 5.5 ± 0.5 and an electrical conductivity (EC) of 1.0–1.5 ms cm−1. Excess nutrient solution was leached from the bottom of pots. Both trials were conducted for eight weeks with 22–24 /20 °C day/night. The experiments used a randomized complete block design with four replicates in each treatment. Two trials were conducted, one each during the spring and summer of 2012 in the University of Guelph research greenhouse complex.
Treatment with biocontrol agents
To prepare B. sublitis inoculum, strains from stock cultures in 15% (v/v) glycerol at −80 °C were cultured in flasks containing Luria Bertani (LB) broth (10 g Bacto-tryptone, 5 g yeast extract, 10 g sodium chloride in 1 L distilled, pH adjusted to 7.5) on a shaker/incubator at 125 rpm for 72 h. The final concentration was adjusted to108 CFU mL−1 based on optical density reading (0.5) at 600 nm with a spectrophotometer (UV-1201, Shimadzu Scientific Instruments Inc., USA). The begonias were inoculated with 100 mL bacterial spore suspension twice at 14-day intervals applied as a drench, with the initial inoculation at five weeks after transplanting.
Five commercial biocontrol agents () were used according to the manufacturer's instructions as follows: PreStop® WP applied at 5.0 g of product L−1 (1.25 g product per m2), 10 mL per plant; Mycostop® applied 0.5 g of product L−1, 10 mL per plant; RootShield® WP applied 0.9 g L−1, 10 mL per plant; Actinovate® applied 0.5 g L−1, 10 mL per plant; Serenade® ASO™ applied 1% solution, 10 mL per plant. All biocontrol agents were applied as a soil drench five weeks after transplanting, and again after 14 days.
Efficacy of biocontrol agents on Fusarium wilt in begonia
Plants were inoculated with F. foetens seven days after the second application of the biocontrol agents. Disease severity (DS) was assessed three weeks after inoculation and compared with the non-inoculated control. The percentage of plants exhibiting symptoms (disease incidence) was recorded and the disease severity was assessed using a disease severity rating scale of 1–5 according to Elmer (Citation2008): 1 = no symptoms, 2 = slight dull-green colouration of the foliage, 3 = dull-green wilted foliage, 4 = stunted and wilting plants with distinct darkened lesions on basal stem sections and 5 = dead or near death. Ratings were recorded weekly for up to eight weeks post-inoculation. DS was calculated according to the formula given by Tarig et al. (Citation1998):
Chlorophyll content index (CCI) was measured on the youngest fully expanded leaf weekly according to Zheng et al. (Citation2005) with a portable chlorophyll content meter (CCM-200, Opti-Sciences, Tyngsboro, MA) three weeks after inoculation and continued weekly up to final harvest. Plants were harvested 12 weeks after transplanting in both trials. Plant materials were dried to a constant mass at 65 °C in an oven for three days and shoot dry weights were measured.
Data analysis
Analysis of variance was performed using the GLM procedure of SAS version 9.1 (SAS Institute Inc., Cary, NC, USA). When a significant treatment effect was observed, mean separation was done using Fisher's least significant difference (LSD, P = 0.05). The data were analysed as a single experiment for each trial.
Results
Efficacy of B. subtilis for control of Fusarium wilt
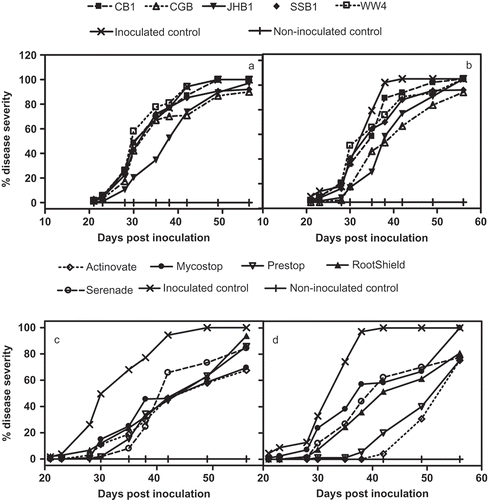
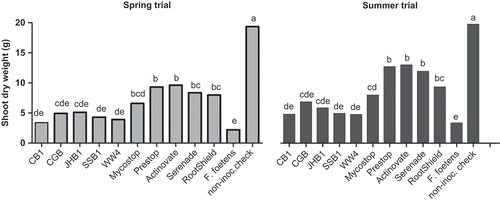
Four out of five B. subtilis strains significantly inhibited F. foetens mycelia growth after seven days of dual culturing. Inhibition rates were between 31% and 76% compared with the control (). In greenhouse trials, only strains JHB1 and CGB of B. subtilis reduced wilt disease severity (DS) in Hiemalis begonia ( a, 1b). JHB1 reduced DS by 10–33% from 28 days post-inoculation (dpi) until 49 dpi in the spring trial ( a); by 21–50% when rated on and before 35 dpi in the summer trial ( b). Strain CGB reduced the DS by 20–49% when rated on and before 35 dpi in the summer trial ( b). CGB delayed the disease symptom development for 7 days compared with the control (). In both trials, Fusarium-inoculated, control plants exhibited an 88% reduction of above-ground dry weight compared with the non-inoculated checks (17.1 ± 2.88 g). There were no differences in above-ground dry weights when comparing the CB1, CGB, JHB1, SSB1 or WW4 treated plants to the pathogen-inoculated controls (2.4 ± 0.92 g) ().
Table 2. Inhibitory effect of B. subtilis strains on F. foetens mycelial growth after 7-day dual culturing on PDA
Table 3. Days post-inoculation for the first observed Fusarium wilt symptom on Begonia × hiemalis treated by B. subtilis strains and commercial products under greenhouse conditions
Efficacy of commercial biocontrol products for control of Fusarium wilt
All the biocontrol products showed significant reduction of Fusarium wilt in greenhouse begonia plants in both trials. In the spring trial, differences in DS between the biocontrol-treated plants and the pathogen-inoculated control became apparent (P = 0.05) on or before 28 dpi; and most of the biocontrol treatments delayed the onset of disease symptoms for seven or more days (). Mycostop, Prestop and Actinovate provided significant protection from F. foetens for over 35 days after inoculation; while RootShield and Serenade were less effective and provided protection for over 28 days after inoculation ( c).
Similarly, in trial two, the differences (P = 0.05) in DS between the biocontrol-treated plants and the pathogen-inoculated controls became apparent 28 dpi. Additionally, the biocontrol treatments except Mycostop® delayed the onset of disease symptoms for 7–21 days (). Prestop, RootShield, Actinovate, Mycostop and Serenade provided significant (P > 0.05) reduction in disease severity caused by F. foetens for the plants past 28 days ( d). Plants treated with the commercial biological control products showed 58–77% increases in above-ground dry weight over that of the untreated, pathogen-inoculated controls (2.4 ± 0.92 g) (). Actinovate reduced DS up to 49% and 97% and delayed the disease symptom development for 7 and 21 days in the spring and summer trials, respectively.
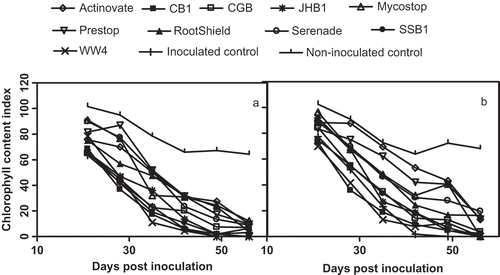
Fusarium disease symptoms appeared as a dull green colour on leaves followed by leaf and stem wilt. Leaf chlorophyll content index (CCI) measurements showed that F. foetens-inoculated plant leaves had less chlorophyll compared with that of the non-inoculated healthy controls (), but plants treated with commercial biocontrol products and subsequently inoculated with F. foetens all maintained higher CCI when compared, at 21 dpi, to the pathogen-inoculated controls.
Discussion
In the present study, considerable variation was observed in the efficacy of the laboratory-isolated B. subtilis strains in controlling F. foetens. In vitro plate studies demonstrated that the strains of bacteria inhibited the growth of F. foetens to varying degrees, with the most effective strains inhibiting growth by up to 76%. However, only two strains (JHB1 and CGB) showed a significant reduction in disease severity under greenhouse conditions. Bacillus spp. have been shown previously to inhibit growth of various plant pathogens (Zheng & Sinclair, Citation2000; Siddiqui & Akhtar, Citation2007). The mechanisms of the antagonism are likely due to competition, antibiosis and induced resistance. A number of Bacillus-based biocontrol products are commercially available for management of a wide range of diseases (Cawoy et al., Citation2011).
Among the biocontrol agents evaluated in this study that suppressed F. foetens, Prestop WP was consistently the most effective. Gliocladium catenulatum, the active ingredient in Prestop WP, has been previously reported as a mycoparasite of Fusarium species (Huang, Citation1978; McQuilken et al., Citation2001) and shown to reduce development of fusarium root and stem rot on greenhouse cucumber plants (Rose et al., Citation2003).
Serenade also significantly suppressed F. foetens on begonia. Serenade is registered for the suppression of botrytis blight, powdery mildew and other diseases on fruit and vegetable crops (Elmhirst et al., Citation2011). It was shown that Serenade can effectively suppress powdery mildew development on summer squash and cantaloupe under greenhouse and field conditions (Zhang et al., Citation2011). The primary modes of action of B. subtilis, the active ingredient in Serenade, are by antibiosis and induction of system acquired resistance (Stein, Citation2005).
Streptomyces lydicus WYEC108, the active ingredient in Actinovate, produces antifungal antibiotics and extracellular chitinases. This actinomycete can colonize plant roots and remain viable in the soil (Beyer & Diekmann, Citation1985; Yuan & Crawford, Citation1995). Actinovate is compatible with the formulations of other biocontrol agents which permits increased flexibility when mixing formulations. This provides broader control of diverse fungal pathogens (Elliott et al., Citation2009). When applied alone, Actinovate has been shown to provide adequate powdery mildew suppression for many crops such as melon and cantaloupe (Matheron & Porchas, Citation2008).
Both Mycostop and RootShield significantly suppressed F. foetens in this study. The biocontrol agents S. griseoviridis and T. harzianum in Mycostop and RootShield, respectively, are reported to be effective against damping-off and root rot diseases caused by Pythium spp. (Paulitz & Bélanger, Citation2001). Mycostop® is a biofungicide based on the K61 strain of S. griseoviridis isolated from sphagnum peat (White et al., Citation1990). Streptomyces spp. are active in the rhizosphere and the modes of action include antibiosis, lysis of fungal cell walls, competition and hyperparasitism (Tapio & Pohto-Lahdenperä, Citation1991). The commercial product based on strain K61 is effective in suppressing root rot and wilt diseases caused by Pythium spp., Fusarium spp., Rhizoctonia spp. and Phytophthora spp. in vegetables and ornamentals, if colonization of the rhizosphere occurs prior to colonization by pathogens. Streptomyces griseoviridis is currently part of an integrated control programme to control soil-borne diseases in greenhouse-grown tomatoes (Minuto et al., Citation2006). RootShield® was reported to effectively reduce Fusarium wilt incidence on tomato plants in greenhouses, with the greatest reduction occurring when the biocontrol agent was applied seven days before the pathogen was applied (Srinivasan et al., Citation2009).
The suppressive effects against F. foetens observed in this study were likely due to the ability of these agents, both laboratory-isolated and commercially available products, to pre-colonize the rhizosphere of the begonia plants prior to pathogen access to plant roots. Establishment of the antagonistic population in the soil prior to pathogen establishment was essential for the efficacy of these types of biocontrol agents. In the greenhouse trials, two applications were made as previous trials showed one application may not be as effective (authors, unpublished results). Therefore, under a commercial greenhouse production system, a second application would be necessary to ensure adequate root protection, particularly under conditions of high disease pressure (Rose et al., Citation2003). During the trials, the begonias were inoculated with high concentrations of Fusarium spores. However, in normal greenhouse conditions, especially at the beginning of the production cycle, the disease pressure is much lower. If the biocontrol products are applied early in the production cycle, they may be able to provide more effective protection against Fusarium wilt.
Addition of biological control agents to growing media may offer an alternative disease control strategy if efficacy data were available to support their use. This study has demonstrated that two laboratory-isolated B. subtilis strains and five commercially available biological control agents can suppress fusarium wilt in greenhouse grown begonias. All the tested biocontrol products are registered in Canada, increasing the attractiveness of incorporation of these products within existing practices in the greenhouse industry.
Acknowledgements
Ontario Centres of Excellence, Agriculture and Agri-Food Canada's Growing Forward Program, and The Canadian Ornamental Horticulture Alliance provided financial support for this research. We thank Denis Gaudet for his editorial comments. We thank Quarry Ridge Growers for providing begonia plants and Dr Allen Xue for providing the B. subtilis strains. We thank Plant Products, Koppert Biological Systems and UAP distributor for providing the commercially available biocontrol products.
References
- Bailey , B.A. , Bae , H. , Strem , M.D. , Crozier , J. , Thomas , S.E. , Samuels , G.J. , Vinyard , B.T. and Holmes , K.A. 2008 . Antibiosis, mycoparasitism, and colonization success for endophytic Trichoderma isolates with biological control potential in Theobroma cacao . Biol. Control , 46 : 24 – 35 .
- Bélanger , R.R. 2006 . Controlling disease without fungicides: a new chemical warfare . Can. J. Plant Pathol , 28, S233–S238
- Beyer , M. and Diekmann , H. 1985 . The chitinase system of Streptomyces sp. ATCC 11238 and its significance for fungal cell wall degradation . Appl. Microbiol. Biotechnol , 23 : 140 – 146 .
- Cawoy, H., Bettiol, W., Fickers, P. & Ongena, M. (2011). Bacillus-based biological control of plant diseases. In M. Stoytcheva (Ed.). Pesticides in the Modern World – Pesticides Use and Management (Ch. 13). In Tech. Available from http://www.intechopen.com/books/pesticides-in-themodern-world-pesticides-use-and-management/bacillus-based-biological-control-of-plant-diseases (http://www.intechopen.com/books/pesticides-in-themodern-world-pesticides-use-and-management/bacillus-based-biological-control-of-plant-diseases)
- De Meyer , G. , Bigirimana , J. , Elad , Y. and Hofte , M. 1998 . Induced systemic resistance in Trichoderma harzianum T39 biocontrol of Botrytis cinerea . Eur. J. Plant Pathol , 104 : 279 – 286 .
- Elliott , M. , Shamoun , S.F. , Sumampong , G. , James , D. , Masri , S. and Varga , A. 2009 . Evaluation of several commercial biocontrol products on European and North American populations of Phytophthora ramorum . Biocont. Sci. Technol , 99 : 1007 – 1021 .
- Elmer , W.H. 2008 . Preventing spread of Fusarium wilt of Hiemalis begonias in the greenhouse . Crop Prot , 27 : 1078 – 1083 .
- Elmer , W.H. and Vossbrinck , C. 2004 . First report of a wilt disease of Hiemalis begonias caused by Fusarium foetens in the United States . Plant Dis , 88 : 1287
- Elmhirst , J.F. , Haselhan , C. and Punja , Z.K. 2011 . Evaluation of biological control agents for control of botrytis blight of geranium and powdery mildew of rose . Can. J. Plant Pathol , 33 : 499 – 505 .
- Gracia-Garza , J.A. , Little , M. , Brown , W. , Blom , T.J. , Schneider , K. , Allen , W. and Potter , J. 2003 . Efficacy of various biological control agents and biorationals against Pythium root rot in poinsettia . HortTechnology , 13 : 149 – 153 .
- Handelsman , J. , Raffel , S. , Mester , E.H. , Wunderlich , L. and Grau , C.R. 1990 . Biological control of damping-off of alfalfa seedlings with Bacillus cereus UW85 . Appl. Environ. Microbiol , 56 : 713 – 718 .
- Hassanein , A.M. , El-Garhy , A.M. and Mekhemar , G.A.A. 2006 . Symbiotic nitrogen fixation process in faba bean and chickpea as affected by biological and chemical control of root rot . J. Agric. Sci. Mansoura Univ , 31 : 963 – 980 .
- Huang , H.C. 1978 . Gliocladium catenulatum – Hyper-parasite of Sclerotinia sclerotiorum and Fusarium speies . Can. J. Bot , 56 : 2243 – 2246 .
- Jetiyanon , K. , Tuzun , S. and Kloepper , J.W. 1997 . “ Lignification, peroxidase reaction and superoxidase dismutase as early plant defense associated with PGPR-mediated induced systemic resistance ” . In Plant Growth-Promoting Rhizobacteria – Present Status and Future Prospects , Edited by: Ogoshi , A. , Kobayashi , K. , Homma , Y. , Kodama , F. , Kondo , N. and Akino , S. 265 – 268 . Sapporo : Nakanishi Printing .
- Kim , D.S. , Cook , R.J. and Weller , D.M. 1997 . Bacillus sp. L324-92 for biological control of three root disease of wheat grown with reduced tillage . Phytopathology , 87 : 551 – 558 .
- Kloepper , J.W. , Ryu , C.M. and Zhang , S. 2004 . Induced systemic resistance and promotion of plant growth by Bacillus spp . Phytopathology , 94 : 1259 – 1266 .
- Kucey , R.M.N. 1987 . Increased phosphorus uptake by wheat and field beans inoculated with a phosphorus-solubilizing Penicillium bilaii strain and with vesicular-arbuscular mycorrhizal fungi . Appl. Environ. Microbiol , 53 : 2699 – 2703 .
- Kwok , O.C.H. , Gahy , P.C. , Hoitink , H.A.J. and Kuter , G.A. 1987 . Interactions between bacteria and Trichoderma hamatum in suppression of Rhizoctonia damping-off in bark compost media . Phytopathology , 77 : 1206 – 1212 .
- Matheron , M.E. and Porchas , M. 2008 . “ Effectiveness of the biopesticides Actinovate and Kaligreen within a management program for powdery mildew on cantaloupe ” . In Centennial Meeting Proceedings [CD-ROM] , Minneapolis , MN : American Phytopathological Society .
- Mathre , D.E. , Cook , R.J. and Callan , N.W. 1999 . From discovery to use: traversing the world of commercializing biocontrol agents for plant disease control . Plant Dis , 83 : 972 – 983 .
- Mcquilken , M.P. , Gemmell , J. and Lahdenpera , M.L. 2001 . Gliocladium catenulatum as a potential biological control agent of damping-off in bedding plants . J. Phytopathol , 149 : 171 – 178 .
- Minuto , A. , Spadaro , D. , Garibaldi , A. and Gullino , M.L. 2006 . Control of soilborne pathogens of tomato using a commercial formulation of Streptomyces griseoviridis and solarization . Crop Prot , 25 : 468 – 475 .
- Obispo , S.L. 2011 . Evaluation of drip applications and foliar sprays of the biocontrol product Actinovate against powdery mildew and other fungal diseases of tomato , San Luis Obispo , , USA : MS dissertation. California Polytechnic State University .
- Paulitz , T.C. and Bélanger , R.R. 2001 . Biological control in greenhouse systems . Annu. Rev. Phytopathol , 39 : 103 – 133 .
- Punja , Z.K. 1997 . Comparative efficacy of bacteria, fungi, and yeasts as biological control agents of disease of vegetable crops . Can. J. Plant Pathol , 19 : 315 – 323 .
- Rose , S. , Parker , M. and Punja , Z.K. 2003 . Efficacy of biological and chemical treatments for control of fusarium root rot and stem rot on greenhouse cucumber . Plant Dis , 87 : 1462 – 1470 .
- Schroers , H.J. , Baayen , R.P. , Meffert , J.P. , de , Gruyter, J. , Hooftman , M. and O'Donnell , K. 2004 . Fusarium foetens, a new species pathogenic to begonia elatior hybrids (Begonia × hiemalis) and the sister taxon of the Fusarium oxysporum species complex . Mycologia , 96 : 393 – 406 .
- Sekine , T. , Kanno , H. and Aoki , T. 2008 . Occurrence of leaf and stem rot caused by Fusarium foetens on begonia elatior hybrids (Begonia × hiemalis) . Japn. J. Phytopathol , 74 : 164 – 166 .
- Siddiqui , Z.A. and Akhtar , M.S. 2007 . Biocontrol of a chickpea root rot disease complex with phosphate-solubilizing microorganisms . J. Plant Pathol , 9 : 67 – 77 .
- Srinivasan , K. , Gilardi , G. , Garibaldi , A. and Gullino , M.L. 2009 . Bacterial antagonists from used rockwool soilless substrates suppress Fusarium wilt of tomato . J. Plant Pathol , 91 : 147 – 154 .
- Stein , T. 2005 . Bacillus subtilis antibiotics; structure, synthesis and specific functions . Mol. Microbiol , 56 : 845 – 857 .
- Tapio , E. and Pohto-Lahdenperä , A. 1991 . Scanning electron microscopy of hyphal interaction between Streptomyces griseoviridis and some plant pathogenic fungi . J. Agric. Sci. Fin , 63 : 435 – 441 .
- Tarig , S.A. , Sariah , M. , Sijam , K. and Marziah , M. Enhancement of growth and disease suppression by PGPF, Fusarium oxysporum (FO4) in banana seedlings . First National Banana Seminar . 23–25 November 1998 . Malaysia : Awana Genting .
- Tian , X. , Dixon , M. and Zheng , Y. 2010 . First report of Hiemalis begonias wilt disease caused by Fusarium foetens in Canada . Plant Dis , 94 : 1261
- Tian , X. , Dixon , M. and Zheng , Y. 2012 . Susceptibility of various potted begonias to Fusarium foetens . Can. J. Plant Pathol , 34 : 248 – 254 .
- White , J.G. , Linfield , C.A. , Lahdenpera , M.L. and Uoti , J. Mycostop-A novel biofungicide based on Streptomyces griseoviridis . Proceedings of Brighton Crop Protection Conference – Pests and Diseases . Vol. 1 , 19–22 November 1990 . pp. 221 – 226 . Brighton , , UK
- Yuan , W.M. and Crawford , D.L. 1995 . Characterization of Streptomyces lydicusWYEC108 as a potential biocontrol agent against fungal root and seed rots . Appl. Environ. Microbiol , 61 : 3119 – 3128 .
- Zhang , S. , Vallad , G.E. , White , T.L. and Huang , C.H. 2011 . Evaluation of microbial products for management of powdery mildew on summer squash and cantaloupe in Florida . Plant Dis , 95 : 461 – 468 .
- Zheng , X.Y. and Sinclair , J.B. 2000 . The effects of traits of Bacillus megaterium on seed and root colonization and their correlation with the suppression of Rhizoctonia root rot of soybean . Biol. Control , 45 : 223 – 243 .
- Zheng , Y. , Wang , L. and Dixon , M. 2005 . Greenhouse pepper growth and yield response to copper application . HortScience , 40 : 2132 – 2134 .