Abstract
Irrigation water can harbour propagules of pathogenic oomycetes that may be a primary source of disease outbreak in crop plants. Irrigation ponds associated with vegetable production in southern Georgia, USA, were assessed in this study. Camellia and rhododendron leaves were used as baits for recovery of oomycetes that were further identified by morphological characteristics and analysis of the internal transcribed spacer rDNA regions. Pythium litorale was frequently isolated from all irrigation ponds sampled. Assessment of growth rates of P. litorale isolates at 5–45 °C indicated that the optimum and maximum temperatures were 30 and 40 °C, respectively. It appeared that these isolates were more tolerant to higher temperatures than previously described P. litorale isolates from Europe that had a maximum growth temperature of 35 °C. Pathogenicity assay with the P. litorale isolates showed that they caused fruit rot and seedling damping-off of yellow squash. Pythium litorale was first described as a new species in 2006 and the present study is the first report indicating it is pathogenic on plants. This study provides useful information for a more comprehensive understanding of the nature of P. litorale and its potential impact on vegetable production.
Résumé
L'eau d'irrigation peut héberger des propagules d'oomycètes pathogènes qui peuvent être une source principale d’éclosion de maladies dans les cultures. Au cours de cette étude, les étangs d'irrigation utilisés pour la production de légumes dans le sud de la Géorgie, aux États-Unis, ont été évalués. Des feuilles de camélia et de rhododendron ont été utilisées comme appâts pour recueillir des oomycètes qui, par la suite, ont été identifiés en fonction de leurs caractères morphologiques et par analyse de l'espaceur transcrit interne de l'ADNr. Pythium litorale a été fréquemment isolé dans l'eau de tous les étangs échantillonnés. L’évaluation des taux de croissance des isolats de P. litorale à des températures variant de 5 à 45 °C a indiqué que les températures optimales et maximales étaient de 30 et 40 °C, respectivement. Trois isolats se sont par contre révélés plus tolérants aux températures élevées que ce qui avait été préalablement établi pour des isolats européens de P. litorale dont la température maximale de croissance était de 35 °C. Des tests de pathogénicité effectués sur des isolats de P. litorale ont montré qu'ils ont causé la pourriture du fruit et la fonte des semis chez la courge jaune. Pythium litorale a été décrit en tant que nouvelle espèce pour la première fois en 2006, et cette étude est la première mention du champignon en tant qu'agent pathogène. L’étude fournit des renseignements utiles contribuant à mieux comprendre la nature de P. litorale et ses conséquences possibles sur la production de légumes.
Introduction
Contamination of irrigation water sources with oomycete plant pathogens has become an increasing concern due to the potential threat to crop production. Phytophthora and Pythium are common oomycetes found in irrigation water sources associated with crop production. Hong & Moorman (Citation2005) reported that plant pathogens detected from water sources included 17 species of Phytophthora and 26 species of Pythium. In more recent studies, other species of oomycetes were isolated from irrigation water, including Pythium litorale Nechwatal & Mendgen and a number of Phytophthora species, such as P. hydropathica Hong, P. inundata Brasier, P. insolita Ann & Ko, P. irrigata Hong & Gallegly, P. pini Leonian, P. polonica Belbahri, and P. ramorum Werres (Brasier et al., Citation2003; Nechwatal & Mendgen, Citation2006; Hong et al., Citation2008; Tjosvold et al., Citation2008; Ghimire et al., Citation2009, Citation2011).
Most studies have characterized oomycetes in recirculated irrigation water used for ornamental crops (Hong & Moorman, Citation2005), while studies to recover oomycete plant pathogens from irrigation water associated with vegetable production are limited. A study conducted three decades ago indicated that more than 20 Pythium spp. were recovered from surface irrigation ponds in Georgia, USA, among which some common plant pathogenic species, including P. aphanidermatum (Edson) Fitzp., P. dissotocum Drechsler, P. irregulare Buis. and P. spinosum Sawada, were shown to be pathogenic on cabbage, pea and tomato (Shokes & McCarter, Citation1979). Studies of oomycetes found in irrigation water that provide inoculum for vegetable diseases have focused on Phytophthora capsici Leonian in recent years. This pathogen attacks a range of vegetable crops and causes plant wilt, root rot, crown rot, seedling damping-off, leaf and stem blight and fruit rot (Erwin & Ribeiro, Citation1996; Sholberg et al., Citation2007). Studies indicate that P. capsici survives in tailwater (surface run-off water), river system, ponds and ditches in a few states in the United States and irrigation water may serve as an important inoculum source (Roberts et al., Citation2005; Gevens et al., Citation2007). In a study to detect and characterize P. capsici in irrigation ponds in southern Georgia, the majority of irrigation ponds investigated were contaminated with this pathogen (Wang et al., Citation2009).
In 2012, studies were conducted to characterize oomycetes other than P. capsici in irrigation ponds associated with vegetable production in southern Georgia. Pythium litorale was frequently isolated from all ponds investigated. Pythium litorale is a newly classified species and belongs to the clade K species (Nechwatal & Mendgen, Citation2006). Isolates in the clade K species of Pythium have more characteristics similar to Phytophthora than other Pythium species (Lévesque & de Cook, Citation2004; Nechwatal & Mendgen, Citation2006).
The objective of this study was to characterize P. litorale recovered from irrigation ponds in southern Georgia and determine pathogenicity on yellow squash (Cucurbita pepo L.), an important vegetable crop highly susceptible to P. capsici. Studies on the biology and pathogenicity of P. litorale in irrigation water would contribute to a more comprehensive understanding of this organism and its potential role in vegetable production.
Materials and methods
Isolation and identification of Pythium litorale
Studies were initiated in January 2012 to isolate oomycetes from five vegetable irrigation ponds located in two counties, Tift and Worth, in southern Georgia. Water samples were taken monthly and results from January to April 2012 were included in this study. Water samples were taken from four locations in each pond, mixed in equal volumes, and a 1 L sample was placed in a sterile plastic bottle. To isolate Phytophthora and Pythium species, camellia and rhododendron leaf discs (1 cm2) were surface-sterilized by dipping in 70% ethanol for 60 s, and 5 discs of each plant species were floated on water in each bottle and incubated under laboratory conditions (22–24 °C) for 5–7 days. Leaf discs were then transferred onto PARPH-V8 agar plates (Jeffers & Martin, Citation1986) and incubated at 22–24 °C under continuous light. Colonies growing from leaf discs were transferred to new PARPH-V8 agar plates, and pure isolates were obtained by subculturing hyphal tips on V8 agar plates. The isolates were identified based on morphological characteristics (Waterhouse, Citation1968; Van der Plaats-Niterink, Citation1981) and sequencing of the internal transcribed spacer (ITS) regions of rDNA with the primers ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) using the colony polymerase chain reaction (PCR) technique (Kong et al., Citation2005).
Pathogenicity assay
Seeds of squash ‘Gentry’ were placed inside plastic containers, 3.8 cm in diameter and 21 cm in height (Stuewe & Sons, Corvallis, OR), that were filled with a commercial potting mix (Miracle-Gro LLC, Marysville, OH) and placed in a greenhouse. Inoculum of P. litorale was prepared using the methods described previously (Ji et al., Citation2012). Briefly, 1 L flasks containing 250 mL of vermiculite were autoclaved twice on two consecutive days and 125 mL of autoclaved V8 juice (V8 juice, 50 mL; CaCO3, 2 g; distilled water, 950 mL) was added to each flask. Isolates of P. litorale were grown on V8 agar plates at 25 °C in the dark for seven days. Eight 7-mm-diameter agar plugs were taken from the margin of the colony and placed in each flask containing the vermiculite. The flasks were incubated at 25 °C in the dark for one week, with daily shaking by hand. Squash seedlings were inoculated one week after seedling emergence by placing 1.5 mL of vermiculite inoculum around the stem (0.5 cm away) below the soil surface. Four isolates of P. litorale (PL-1-1-2, PL-2-2-24, PL-3-3-5a and PL-4-4-3) were evaluated and four seedlings were inoculated with each isolate. Seedlings receiving blank V8-amended vermiculite were used as a control. The seedlings were kept in the greenhouse (23±2 oC) and disease incidence and severity were recorded 3–7 days after inoculation. Disease severity was quantified using a 0–5 scale: 0 = no disease symptoms; 1 = 0–20% plants wilted; 2 = 21–40% plants wilted; 3 = 41–60% plants wilted; 4 = 61–80% plants wilted; and 5 = 81–100% plants wilted or completely dead. The experiment was repeated once under the same conditions.
Pathogenicity of the four isolates was also evaluated on squash fruit. A mycelial plug (7 mm diameter) prepared as described above was placed on an unwounded squash fruit. Squash fruits inoculated with V8 agar plugs were used as a control. The fruits were placed in plastic crisper boxes lined with moistened paper towels and incubated at 25 °C in the dark for five days until lesions developed. A fruit skin plug (7 mm diameter) taken from the advancing margin of the lesions was transferred to a healthy squash fruit. Three fruits were inoculated with each isolate while control fruits received plugs from healthy fruit. All fruits were incubated as mentioned above and development of lesions on the fruit was recorded 3 to 7 days after inoculation. Lesion sizes were calculated by multiplying the semi-length (length/2), semi-width (width/2), and π (3.14) (the lesions were oval or round). Samples of diseased seedlings and fruits were plated on V8 agar to isolate and confirm infection by P. litorale through observation of morphological characteristics and ITS sequencing.The experiments were conducted twice under the same conditions.
Growth at different temperatures
Growth of the four P. litorale isolates was determined at different temperatures. The isolates were grown on V8 agar at 25 °C in the dark for seven days. A mycelial plug (5 mm in diameter) taken from the edge of the colony was placed in the centre of a V8 agar plate. Three plates were used for each isolate and the plates were placed in incubators at 5, 10, 15, 20, 25, 30, 35, 40 and 45 °C in the dark. Colony diameter was measured in two perpendicular directions three days after incubation and averaged for analysis. The diameter of the agar plug was subtracted from the total colony diameter for calculating actual diameter of colony growth. Daily growth rates (mm day−1) of the isolates were calculated. The experiment was repeated once under same conditions.
Production and characteristics of sporangia
A method was developed for sporangial production after unsuccessful attempts to produce sporangia by placing mycelial plugs in sterile water, filtered pond water, and soil extract under light. A mycelial plug (5 mm in diameter) taken from the edge of an actively growing colony of P. litorale was placed in the centre of a V8 agar plate and incubated at 25 °C in the dark for seven days. Two plates of each isolate were placed approximately 30 cm away from a tap spout of running tap water so that droplets splashed onto the plates overnight (approximately 15–20 h). Production and characteristics of sporangia and zoospores were observed under a compound microscope.
Sequence analysis
Genomic DNA was extracted from the four P. litorale isolates. The internal transcribed spacer regions of rDNA were amplified and sequenced with the universal primers ITS1 and ITS4. The PCR conditions used were as follows: initial denaturation at 95 °C for 3 min followed by 40 cycles of 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 1 min, and the final extension at 72 °C for 5 min. Sequences of ITS1, 5.8S and ITS2 of related clade K Pythium species (Lévesque & de Cook, Citation2004) were obtained from the National Center for Biotechnology Information (NCBI) GenBank database. They were compared with sequences of the four isolates of P. litorale recovered in this study. Multiple sequence alignment of these isolates were done using CLUSTAL-W program (http://www.genome.jp/tools/clustalw). Subsequently, the neighbour-joining method (Saitou & Nei, Citation1987; Tamura et al., Citation2004) using the software MEGA5 (Tamura et al., Citation2011) was applied. The evolutionary distances were computed using the Maximum Composite Likelihood method and expressed as number of base substitutions per site. The analysis involved 20 nucleotide sequences. Codon positions included were 1st+2nd+3rd+Noncoding. The optimal tree with the sum of branch length = 0.899 is shown.
Results
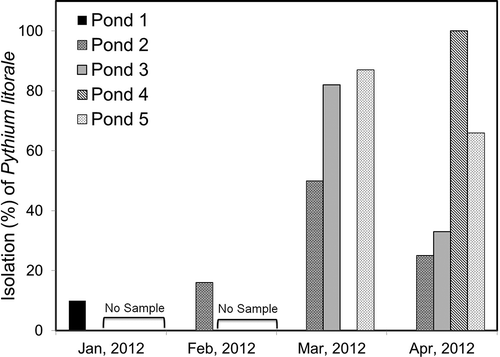
Pythium litorale was isolated monthly during January–April 2012 from at least one of the ponds tested and isolation frequency is presented in Approximately 24 % of oomycetes (Phytophthora and Pythium) isolated during this period of time were P. litorale based on morphological characteristics and ITS sequence analysis. The remaining isolates belonged to Phytophthora (16%) and other Pythium species (60%) that are being further identified.
Fig. 1. Isolation frequency of Pythium litorale (percent of total Pythium and Phytophthora spp.) from five vegetable irrigation ponds in southern Georgia.
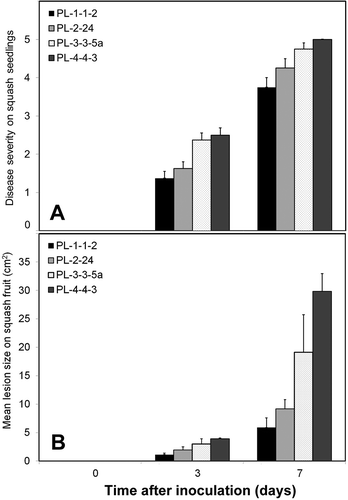
The four P. litorale isolates evaluated in pathogenicity assays caused damping-off, root rot, dark brown sunken lesions on the crown area of the stem, or wilting of squash seedlings. Disease incidence was 100% for all isolates seven days after inoculation, and mean disease severity ranged from 1.4 to 2.5 after three days and 3.8 to 5.0 after seven days (). The isolates also caused light brown, water-soaked lesions on all inoculated squash fruit. Lesion sizes ranged from 1.1 to 3.9 cm2 after three days and 5.9 to 29.8 cm2 after seven days ().
Fig. 2. Severity of disease caused by four Pythium litorale isolates on (A) squash seedlings and (B) on fruit. Disease severity on squash seedlings was quantified using a 0–5 scale (see methods). Values are means of two repeated experiments. Error bars represent standard errors of the means.
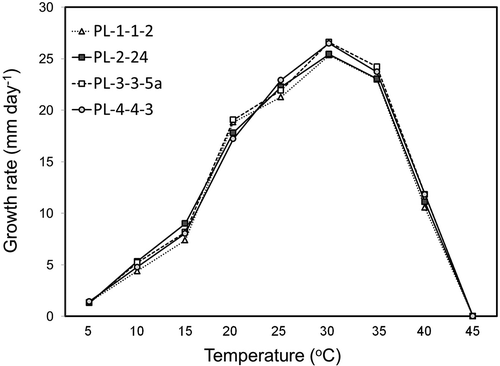
The four isolates grew similarly at different temperatures (). Growth occurred between 5–40 °C, with an optimum temperature of 30 °C. At 30 °C, the average daily growth rate was 26.0 mm. At 10 and 40 °C, the average growth rates were 4.9 and 11.3 mm a day, respectively. None of the isolates grew at 45 °C.
Fig. 3. Colony growth rates (mm day−1) of four isolates of Pythium litorale (PL-1-1-2, PL-2–24, PL-3-3-5a, and PL-4-4-3) from vegetable irrigation ponds on V8 agar medium at different temperatures. Values are means of two repeated experiments.
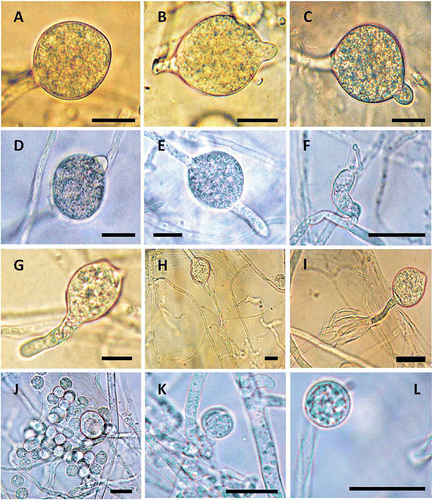
Numerous sporangia were produced within 24 h after placing 1-week-old cultures of P. litorale on V8 agar under splashing water overnight. Some sporangia had no papillae (), but the majority of them were lemon-shaped with papillae (–D). Some sporangia had longer discharge tubes (–I), presumably emerged from the papilla as cytoplasmic movement occurred to discharge the contents of sporangia. The average width and length of 50 sporangia was 29.6 × 31.8 μm. After zoospores were released through the discharge tube from papilla, new sporangia developed through internal proliferation (, I). It appeared that zoospores were released during the overnight incubation near splashing tap water and groups of zoospores were observed around the sporangia. Twenty zoospores were measured and the diameter of the spores averaged 10.8 μm (–L). The P. litorale isolates did not produce oospores or chlamydospores on V8 agar plates after several weeks of incubation in the dark when distilled water or pond water was used to make the medium (Parkunan & Ji, unpublished).
Fig. 4. Sporangia and zoospores of Pythium litorale. (A) sporangium without papilla; (B–D) sporangia with papillae; (E and G) sporangia with papillae emerging to discharge tubes; (F) discharge tube with undifferentiated protoplasm inside; (H and I) development of new sporangia by internal proliferation; (J) group of newly encysted zoospores; (K–L) a single encysted zoospore. Bar = 20 μm.
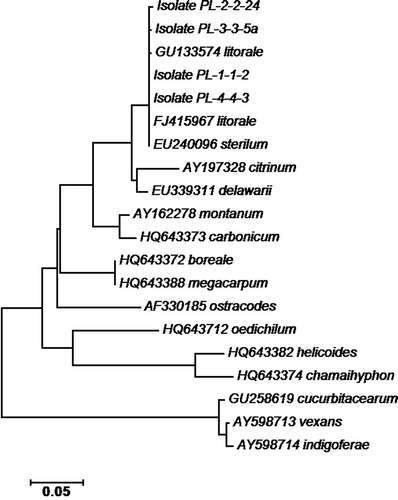
ITS sequences of several Pythium species in clade K were used for the phylogenetic analysis. The species and GenBank accession numbers included Pythium litorale (FJ415967 and GU133574), P. citrinum (AY197328), P. sterilum (EU240096), P. montanum (AY162278), P. carbonicum (HQ643373), P. oedichilum (HQ643712), P. boreale (HQ643372), P. megacarpum (HQ643388), P. ostracodes (AF330185), P. helicoides (HQ643382), P. chamaihyphon (HQ643374), P. vexans (AY598713), P. indigoferae (AY598714), P. cucurbitacearum (GU258619) and P. delawarii (EU339311). Sequences of the ITS1, 5.8S and ITS2 region of the rDNA from the four P. litorale isolates recovered from ponds in this study matched with that of five P. litorale isolates in GenBank (accession numbers JQ898465, JQ013487, FJ415967, GU133574 and FJ415966; 99 to 100% similarity). The Megablast result indicated that the 5 P. litorale isolates are the top five matches to the isolates from the irrigation ponds with total scores of 1439–1445 and query coverage of 100%. Hence the four isolates from the irrigation ponds were identified as P. litorale. The four isolates are very close to P. sterilum and have 99–100% similarity to an isolate of P. sterilum (accession number EU240096). However, the Megablast result indicated that total scores of five closest P. sterilum isolates are lower (1406–1421) with query coverage of only 97–99%. Sequences of the four isolates from irrigation ponds were submitted to GenBank (accession numbers: JX985743, JX985744, JX985746 and JX985747). Phylogenetic positions of the four isolates with other clade K taxa are shown in The closest relatives to P. litorale in clade K are taxa P. sterilum, P. delawarii and P. citrinum.
Fig. 5. Neighbour-Joining method from the CLUSTAL-W multiple sequence alignment inferred from 1000 replicates to represent the distance among species in Pythium clade K as compared to four representative isolates of Pythium litorale (PL-1-1-2, PL-2-2-24, PL-3-3-5a, and PL-4-4-3) based on the rDNA ITS regions using the software MEGA5. GenBank accession numbers are followed by names of Pythium species. The optimal tree with the sum of branch length = 0.899 is shown. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree.
Discussion
Pythium litorale was first isolated from littoral soils and reeds in Lake Constance, Germany and described by Nechwatal & Mendgen (Citation2006). It proved to be non-pathogenic to reed (Phragmites australis) when tested on detached reed stems, a predominating plant growing in Lake Constance (Nechwatal & Mendgen, Citation2006). More recently, P. litorale was found in rivers and streams in the United States (Preuett et al., Citation2012); however, its pathogenicity on plants was not reported. In the present study, P. litorale was isolated from irrigation ponds in southern Georgia, and the isolates caused seedling damping-off, crown and root rot, and fruit rot of squash under greenhouse and laboratory conditions. This is the first report demonstrating pathogenicity of P. litorale on plants. Isolates of P. litorale recovered in this study had similar morphological and physiological characteristics with those from Germany but were different in certain aspects. Our isolates could grow at 40 °C while the maximum growth temperature of the isolates from Germany was 35 °C. Daily growth rate of our isolates was 26.0 mm at the optimum temperature of 30 °C, which was almost twice that of the German isolates (13.3 mm). Additionally, our isolates did not produce sporangia when mycelial plugs were placed in sterile water and incubated at 20 °C as described by Nechwatal & Mendgen (Citation2006). Hence a new method was used in this study by placing the cultures 30 cm away from the tap under dripping tap water overnight, which resulted in production of large numbers of sporangia. It appeared that zoospores were not released at the same time for all sporangia, as some sporangia were surrounded by zoospores and some had no zoospores but internal proliferation occurred after releasing zoospores. Further study is needed to identify factors favourable for zoospore release of P. litorale.
The phylogeny of 116 species and varieties of Pythium has been described, with 11 clades recognized including clade K (Lévesque & de Cook, Citation2004). Most species in clade K share some common characters such as the ovoid shape of sporangia that often have a papilla and proliferate internally, and all species in this group have high optimum (approximately 30 °C) and maximum (35–40 °C) growth temperatures (Lévesque & de Cook, Citation2004). Nechwatal & Mendgen (Citation2006) placed P. litorale in clade K since it shares several morphological characteristics with other species in the clade and phylogenetically it groups with several clade K species. The four isolates from irrigation ponds in Georgia had 99–100% similarity to P. litorale and some species in clade K based on ITS sequences. The four isolates also share some common biological and morphological characteristics with clade K species such as optimum and maximum growth temperatures and nature of sporangium production. Species in clade K have some common characteristics with Phytophthora species (Lévesque & de Cook, Citation2004). For instance, most of the clade K species appear to be hymexazol insensitive, a common feature of Phytophthora species (Nechwatal & Mendgen, Citation2006). This includes Pythium litorale which is hymexazol insensitive as well (Nechwatal & Mendgen, Citation2006). Since clade K Pythium species share some characteristics of Phytophthora and Pythium, a newly erected genus, Phytopythium, was proposed for the Pythium species in clade K (Bala et al., Citation2010). These species stand morphologically and phylogenetically between Pythium and Phytophthora (Bala et al., Citation2010). Sporangia of these Pythium species commonly proliferate internally with globose to ovoid shape, which is similar to Phytophthora spp. However, zoospore discharge of Phytopythium is similar to Pythium spp.
Information regarding pathogenicity of the hymexazol-insensitive Pythium species is limited, and it will be interesting to determine if these species cause diseases on crop plants as do Phytophthora spp. In this study, P. litorale was observed to be pathogenic on squash plants and fruit in artificial inoculations. Squash is an important vegetable crop in the United States with an annual farm gate value of more than US$20 million in the state of Georgia alone (Wolfe & Luke-Morgan, Citation2011). It is known that Phytophthora capsici causes severe yield and quality loss on squash and other vegetable crops (Jackson et al., Citation2010; Ji et al., 2012). Disease symptoms caused by P. litorale could be easily confused with symptoms caused by P. capsici on squash. However, whether P. litorale causes diseases on squash and other vegetables in commercial fields is unknown. In addition, research is in progress to study population dynamics of P. litorale in irrigation ponds in different seasons and years, which will contribute to a more comprehensive understanding of the biology of this organism.
Acknowledgement
The authors would like to acknowledge financial support from the Georgia Vegetable Growers Association and Georgia Food Industry Partnership Program.
References
- Bala , K. , Robideau , G.P. , Lévesque , C.A. , de Cock , A.W.A.M. , Abad , Z.G. , Lodhi , A.M. , Shahzad , S. , Ghaffar , A. and Coffey , M.D. 2010 . Phytopythium Abad, de Cock, Bala, Robideau, Lodhi & Lévesque, gen. nov. and Phytopythium sindhum Lodhi, Shahzad and Lévesque, sp. nov . Persoonia , 24 : 136 – 137 .
- Brasier , C.M. , Sanchez-Hernandez , E. and Kirk , S.A. 2003 . Phytophthora inundata sp. nov., a part heterothallic pathogen of trees and shrubs in wet or flooded soils . Mycol. Res. , 107 : 477 – 484 .
- Erwin , D.C. and Ribeiro , O.K. 1996 . Phytophthora Diseases Worldwide , St. Paul , MN : The American Phytopathological Society Press .
- Gevens , A.J. , Donahoo , R.S. , Lamour , K.H. and Hausbeck , M.K. 2007 . Characterization of Phytophthora capsici from Michigan surface irrigation water . Phytopathology , 97 : 421 – 428 .
- Ghimire , S.R. , Richardson , P.A. , Moorman , G.W. , Lea-Cox , J.D. , Ross , D.S. and Hong , C.X. 2009 . An in-situ baiting bioassay for detecting Phytophthora species in irrigation runoff containment basins . Plant Pathol. , 58 : 577 – 583 .
- Ghimire , S.R. , Richardson , P.A. , Kong , P. , Hu , J.H. , Lea-Cox , J.D. , Ross , D.S. , Moorman , G.W. and Hong , C.X. 2011 . Distribution and diversity of Phytophthora species in nursery irrigation reservoir adopting water recycling system during winter months . J. Phytopathol. , 159 : 713 – 719 .
- Hong , C.X. and Moorman , G.W. 2005 . Plant pathogens in irrigation water: challenges and opportunities . Crit. Rev. Plant Sci. , 24 : 189 – 208 .
- Hong , C.X. , Richardson , P.A. and Kong , P. 2008 . Pathogenicity to ornamental plants of some existing species and new taxa of Phytophthora from irrigation water . Plant Dis. , 92 : 1201 – 1207 .
- Jackson , K.L. , Yin , J. , Csinos , A.S. and Ji , P. 2010 . Fungicidal activity of fluopicolide for suppression of Phytophthora capsici on squash . Crop Prot. , 29 : 1421 – 1427 .
- Jeffers , S.N. and Martin , S.B. 1986 . Comparison of two media selective for Phytophthora and Pythium species . Plant Dis. , 70 : 1038 – 1043 .
- Ji , P. , Koné , D. , Yin , J. , Jackson , K.L. and Csinos , A.S. (2012). Soil amendments with Brassica cover crops for management of Phytophthora blight on squash . Pest Manag. Sci. , 68 : 639 – 644 .
- Kong , P. , Richardson , P.A. and Hong , C.X. 2005 . Direct colony PCR-SSCP for detection of multiple pythiaceous oomycetes in environmental samples . J. Microbiol. Meth. , 61 : 25 – 32 .
- Lévesque , C.A. and de Cook , W.A.M. 2004 . Molecular phylogeny and taxonomy of the genus . Pythium. Mycol. Res. , 108 : 1363 – 1383 .
- Nechwatal , J. and Mendgen , K. 2006 . Pythium litorale sp. nov., a new species from the littoral of Lake Constance, Germany . FEMS Microbiol. Lett. , 255 : 96 – 101 .
- Preuett , J. , Collins , D. and Williams , A. 2012 . Stream baiting in South Louisiana for . Phytophthora ramorum. Phytopathology , 102, S4, 94 Abstr.)
- Roberts , P.D. , French-Monar , R.D. , Hoffine , M.S. , Seijo , T.E. and McGovern , R.J. 2005 . Survival and recovery of Phytophthora capsiciand oomycetes in tailwater and soil from vegetable fields in Florida . Ann. Appl. Biol. , 146 : 351 – 359 .
- Saitou , N. and Nei , M. 1987 . The neighbor-joining method: a new method for reconstructing phylogenetic trees. . Mol. Biol. Evol. , 4 : 406 – 425 .
- Shokes , F.M. and McCarter , S.M. 1979 . Occurrence, dissemination, and survival of plant pathogens in surface irrigation ponds in southern Georgia . Phytopathology , 69 : 510 – 516 .
- Sholberg , P.L. , Walker , M.C. , O'gorman , D.T. and JespersEn , G.D. 2007 . First report of Phytophthora capsici on cucurbits and peppers in British Columbia . Can. J. Plant Pathol. , 29 : 153 – 158 .
- Tamura , K. , Nei , M. and Kumar , S. 2004 . Prospects for inferring very large phylogenies by using the neighbor-joining method. . Proc. Natl. Acad. Sci. USA , 101 : 11030 – 11035 .
- Tamura , K. , Peterson , D. , Peterson , N. , Stecher , G. , Nei , M. and Kumar , S. 2011 . MEGA5: Molecular evolutionary genetics analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony methods. . Mol. Biol. Evol. , 28 : 2731 – 2739 .
- Tjosvold , S.A. , Chambers , D.L. , Koike , S.T. and Mori , S.R. 2008 . Disease on nursery stock as affected by environmental factors and seasonal inoculum levels of Phytophthora ramorum in stream water used for irrigation . Plant Dis. , 92 : 1566 – 1573 .
- Van der Plaats-Niterink , A.J. 1981 . Monograph of the genus . Pythium. Stud. Mycol. , 21 : 1 – 242 .
- Wang , Z. , Langston , D.B. , Csinos , A.S. , Gitaitis , R.D. , Walcott , R.R. and Ji , P. 2009 . Development of an improved isolation approach and simple sequence repeat markers to characterize Phytophthora capsici populations in irrigation ponds in southern Georgia . Appl. Environ. Microbiol. , 75 : 5467 – 5473 .
- Waterhouse , G.M. 1968 . The genus Pythium Pringsheim . Mycol. Papers , 110 : 1 – 71 .
- Wolfe, K., & Luke-Morgan, A. (2011). 2010 Georgia Farm Gate Value Report. The Center for Agribusiness and Economic Development, University of Georgia College of Agricultural and Environment Sciences, AR-11-01, November 2011 [online]. Available at http://www.caes.uga.edu/center/caed/pubs/2011/documents/AR-11-01.pdf (http://www.caes.uga.edu/center/caed/pubs/2011/documents/AR-11-01.pdf)