Abstract
The introduction of the sugarcane white leaf (SCWL) phytoplasma into Yunnan Province, China was confirmed for the first time by nested PCR. Sequencing and analysis of 17 nested PCR products of the 16S rDNA of the phytoplasma from random samples showed that these sequences were identical. A BLAST search indicated that the sequences were highly homologous (99.05–100% similarity) to the corresponding genome region in GenBank. Field surveys showed that disease incidence among affected cultivars was 13–52% and up to 100% in seriously infected fields. The sugarcane cultivar ‘Yuetang86-368’ was highly susceptible. SCWL is a destructive disease on sugarcane that requires preventive quarantine management measures to prevent its further spread.
Résumé
L'introduction du phytoplasme de la maladie de la feuille blanche de la canne à sucre (FBCS) dans la province du Yunnan, en Chine, a été confirmée pour la première fois par PCR par amorces incluses. Le séquençage et l'analyse de 17 produits découlant de la PCR par amorces incluses des séquences d'ADNr 16s de phytoplasmes provenant d’échantillons aléatoires ont montré que ces séquences étaient identiques. Une recherche BLAST a indiqué que les séquences étaient très homologues (de 99,05 à 100 % de similarité) à la région correspondante du génome de la GenBank. Des études menées sur le terrain ont montré que l'incidence de la maladie chez les cultivars touchés était de 13 à 52 % et pouvait atteindre jusqu’à 100 % dans les champs gravement infectés. Le cultivar de la canne à sucre ‘Yuetang86-368’ était hautement réceptif. En ce qui a trait à la canne à sucre, la FBCS est une maladie destructrice qui nécessite l'application de mesures préventives de quarantaine pour éviter qu'elle se propage davantage.
Introduction
Sugarcane white leaf (SCWL) disease is one of the most important diseases caused by phytoplasmas in sugarcane (Nakashima et al., Citation1994; Wongkaew et al., Citation1997; Hanboonsong et al., Citation2002, Citation2006; Wongkaew & Fletcher, Citation2004). This disease was first reported in 1954 in Lumpang Province in the northern part of Thailand (Marcone, Citation2002). Currently, SCWL is known to occur in many sugarcane-growing areas such as India, Pakistan, Sri Lanka, Japan, Myanmar and the Philippines (Ediso, Citation1976; Tran-Nguyena et al., Citation2000; Kumarasinghe & Jones, Citation2001; Rao et al., Citation2005; Thein et al., Citation2012). In Taiwan, China, SCWL was first reported in 1958 (Ling, Citation1962). SCWL can cause destructive damage to sugarcane. In Thailand, the disease accounted for annual losses of over 20 million US dollars, with disease incidence rates of 5–35% (Hanboonsong et al., 2006). In New Guinea, SCWL brought about considerable economic damage to the sugar industry, with losses of 100% in the cultivar ‘Ragna’ (Cronje & Bailey, Citation1999; Rao et al., 2005).
SCWL phytoplasma generally occurs in the sieve tubes of phloem tissues. Since sugarcane is a vegetatively propagated crop, the pathogen can spread across great distances by infected planting material. Quarantine measures are considered as the most effective prevention measures for SCWL (Chen, Citation1980; Hanboonsong et al., 2002). In the field, SCWL phytoplasma can be transmitted by the leafhoppers Matsumuratettix hiroglyphicus (Matsumura) and Yamatotettix flavovittatus (Hanboonsong et al., Citation2002, 2006). In China, SCWL was detected in 1987 by electron microscopy in some cultivars that were grown in Fujian Province, Guangxi Province and Yunnan Province in China (Zhou et al. Citation1987). Since the 1990s, a quarantine system has been established successively in these three provinces to screen imported sugarcane varieties/materials for SCWL. In Guangxi and Yunnan, once SCWL was found in the imported quarantine materials, the diseased material was destroyed immediately (Deng, Citation1997; Lu et al., Citation2012). In 2012, sugarcane plants suspected of harbouring SCWL occurred in Baoshan of Yunnan Province. In the present study, we detected phytoplasma in 36 suspected SCWL samples by nested PCR and surveyed the incidence rate, damage and loss in the cane fields caused by SCWL.
Materials and methods
Collection of suspected SCWL samples
In October 2012, 36 suspected SCWL samples were collected from Shidian County and Longyang District, Baoshan City, Yunnan Province. The suspected samples exhibited symptoms including softened leaf texture, whole-leaf, striated or mottled chlorosis due to a decrease in chlorophyll content, significantly increased numbers of tillers, stunted and attenuated stalks, shortened internodes and top-clumped leaves (). Detection and identification were performed in a laboratory after sample collection. SCWL phytoplasma DNA (provided by Yunnan Key Laboratory of Sugarcane Genetic Improvement, Kaiyuan, China) was used as a positive control, and healthy sugarcane plants that were free of symptoms were used as a negative control; double distilled water was used as a blank control.
Investigation of incidence rates and damage in fields
A preliminary investigation on the occurrence and damage by SCWL was investigated in October 2012. For each diseased cultivar, an average of five rows was measured. For each row, the number of diseased plants and the total number of plants in a 10-m section were counted. The diseased plant rate was calculated as following:
Diseased plant rate (%) = (Diseased plants/Total plants) × 100%.
Nested PCR detection
Two primer sets were designed to amplify the 210-bp 16S rDNA sequence of SCWL phytoplasma (Namba et al., Citation1993; Wongkaew et al., Citation1997). The first set of primers were as follows: MLOX: 5′-GTTAGGTTAAGTCCTAAAACGAGC-3′, MLOY: 5′-GTGCCAAGGCATCCACTGTATGCC-3′; the second set of primers were as follows: P1: 5′-GTCGTAACAAGGTATCCCTACCGG-3′, P2: 5′-GGTGGGCCTAAATGGACTTGAACC-3′. The primers were synthesized by Sangon Biotech Co., Ltd. (Shanghai, China).
An improved CTAB procedure described by Harrison et al. (Citation1994) was used to extract total DNA from 0.2 g of leaf tissue for each tested sample. A nested PCR method described by Lu et al. (Citation2012) for detection of SCWL phytoplasma was adopted to amplify the DNA from each sample. The first PCR reaction was performed in a total volume of 20 μL containing 1 μL total DNA template, 2.5 μL 10× PCR buffer, 1 μL MLOX (20 μmol L−1), 1 μL MLOY (20 μmol L−1), 0.5 μL MgCl2 (25 mmol L−1), 1 μL dNTPs (10 mmol L−1), 0.2 μL Taq enzyme (5 U μL−1) and 12.8 μL ddH2O. The thermal cycling conditions were as follows: 5 min at 94 °C followed by 25 cycles of denaturation for 1 min at 94 °C, annealing for 1 min at 55 °C and extension for 1 min at 72 °C, followed by a final extension for 10 min at 72 °C. The nested PCR assays were carried out using 1 μL of the first PCR product (diluted 1 : 100 in sterile deionized water) as template in the same reaction mixture as for the first PCR, 1 μL P1 (20 μmol L−1) and 1 μL P2 (20 μmol L−1). The thermal cycling conditions were as follows: 5 min at 94 °C followed by 35 cycles each consisting of denaturation at 94 °C for 1 min, annealing at 62 °C for 1 min and extension at 72 °C for 1 min, followed by extension for 10 min at 72 °C to complete the amplification. The PCR products were separated on 1.5% agarose gels.
All nested PCR amplicons were purified from agarose gels using an Axy Prep DNA Gel Purification Kit (Axygen Biotechnology, Hangzhou, China). DNA sequencing of the PCR amplicons was performed at BGI Sequencing Co. (Beijing, China). After performing a BLAST search in GenBank, the sequence information was analysed using DNAMAN 6.0 and Clustal 2.0 software.
Results and discussion
SCWL is an important systemic disease which can be transmitted rapidly and cause serious losses in cane yield and sucrose content of infected sugarcane. In 2011, the sporadic occurrence of SCWL within 5 hectares was first observed in cane-growing fields of Shidian County, Baoshan City, Yunnan Province. Until the investigations in October 2012, the SCWL occurrence area had extended to 80 hectares, and sporadic occurrence was also observed in fields of Longyang District, Baoshan City. Field surveys showed that cultivars ‘Yuetang86-368’, ‘Yuetang93-159’, ‘Yunzhe86-161’, ‘Yunzhe03-194’ and ‘PY3120’ were infected with SCWL, and ‘Yuetang86–368’ suffered the most serious damage. Field investigations showed that the diseased plant rates were 13–52%, and up to 100% in seriously infected fields. In fields heavily infected with SCWL, sugarcane might completely lose its yield in the year following infection ().
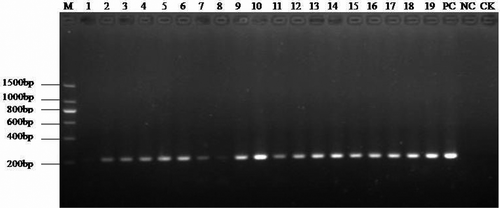
Thirty-six suspected sugarcane samples were subjected to detection of SCWL phytoplasma by nested PCR using the primer pairs MLOX/MLOY and P1/P2, which were designed for 16S rDNA. The results indicated that the 210-bp target band was present in all 36 of the suspected samples and in the positive control, but not in the negative control (healthy plants) and the blank control (double distilled water; ). These results suggest that SCWL phytoplasma were present in all 36 of the suspected sugarcane samples.
Fig. 2. Nested PCR detection results of suspected SCWL samples. M: Marker; Lanes 1–19: samples from No. BS1–BS19; PC: Positive control; NC: Negative control; CK: Blank control.
Seventeen nested PCR products were randomly selected from positive samples and sequenced. The results showed that the sequences were all 210 bp in length and were all identical to each other (GenBank accession number: KC662509). A BLAST search indicated that the sequences obtained in this study were consistent with the sequence of the 16S rDNA of phytoplasma that causes SCWL in sugarcane. Complete similarity (100%) was identified between the sequences identified in the present study and corresponding gene regions published in GenBank from Thailand and Myanmar (accession numbers: HQ917068 and AB646271, respectively), while 99.46% and 99.5% homology were identified between the sequences identified in the present study and those of isolates from India (accession number: JX862179) and Sri Lanka (accession number: JF754446), respectively, which differed in the insertion of one base. In addition, 99.05% similarity was found between the sequences identified in the present study and that of isolates from Hawaii (accession number: JN223448), which differed in the mispairing of one base and the deletion of one base.
As a disease which can be easily transmitted by vegetative propagation, SCWL is a potential threat to the development of the sugar industry. Therefore, it is necessary to prevent the spread of SCWL from the onset by enhanced quarantine measures and monitoring of sugarcane plants in propagation bases, as well as imported/exported sugarcane varieties/materials, to ensure pathogen-free materials are used.
SCWL has a relatively long incubation period, which ranges from 30 days (in a few cases) to 2–3 months (in most cases) and even as long as one year. Thus, the early stage of SWCL infection is difficult to detect by simply observing symptoms. In the present study, only one cane-growing area in Baoshan City, Yunnan Province was monitored for SCWL. To provide a basis for technical support for the effective control of SCWL, further studies should focus on the occurrence and distribution of this disease in all cane-growing regions of China, as well as epidemiological dynamics of SCWL in the field and early monitoring and disease-control methods
Acknowledgements
This work was supported by grants from the Earmarked Fund for China Agricultural Research System (CARS-20-2-2) and the Earmarked Fund for Yunnan province Agriculture Research System.
References
- Chen , C.T. 1980 . Sugarcane white leaf disease in Taiwan . Taiwan Sugar , 27 : 56 – 61 .
- Cronje , C.P.R. and Bailey , R.A. 1999 . The phytoplasma associated with Ramu stunt disease of sugarcane is closely related to the white leaf phytoplasma group . Plant Dis. , 83 : 588 – 588 .
- Deng , ZY. 1997 . Present situation and prospect of sugarcane quarantine for introducing varieties in Guangxi . Guangxi Sugarcane & Canesugar , 4 : 20 – 22 .
- Ediso , S. 1976 . Comparison of grassy shoot disease (India) with the white leaf disease (Taiwan) of sugarcane . Sugarcane Pathologists’ Newsletter , 17 : 30 – 35 .
- Harrison , N.A. , Richardson , P.A. , Jones , P. , Tymon , A.M. , Eden-Green , S.J. and Mpunami , A.A. 1994 . Comparative investigation of MLOs associated with Caribbean and African coconut lethal decline diseases by DNA hybridization and PCR assays . Plant Dis. , 78 : 507 – 511 .
- Hanboonsong , Y. , Choosai , C. , Panyim , S. and Damak , S. 2002 . Transovarial transmission of sugarcane white leaf phytoplasma in the insect vector Matsumuratettix hiroglyphicus (Matsumura) . Insect Molec. Biol. , 11 : 97 – 103 .
- Hanboonsong , Y. , Ritthison , W. , Choosai , C. and Sirithorn , P. 2006 . Transmission of sugarcane white leaf phytoplasma by Yamatotettix flavovittatus, a new leafhopper vector . J. Econ. Entomol. , 99 : 1531 – 1537 .
- Kumarasinghe , N.C. and Jones , P. 2001 . Identification of white leaf disease of sugarcane in Sri Lanka . Sugar Tech. , 3 : 55 – 58 .
- Ling , K. C . 1962 . White leaf disease of sugarcane . Taiwan Sugar , 9 : 1 – 5 .
- Lu , W. J. , Huang , Y.K. , Li , W. F. , Wang , X.Y. and Luo , Z. M. 2012 . Development and application of nested PCR assay for detection of phytoplasma of sugarcane white leaf . Acta Phytopathol. Sin. , 42 : 311 – 314 .
- Marcone , C. 2002 . Phytoplasma disease of sugarcane . Sugar Tech. , 4 : 79 – 85 .
- Nakashima , K. , Chaleeprom , W. , Womgkaew , P. and Sirithorn , P. 1994 . Detection of mycoplasma-like organisms associated with white leaf disease of sugarcane in Thailand using DNA probes . Japan Inter. Res. Center Agric. Sci. , 1 : 57 – 67 .
- Namba , S. , Oyaizu , H. , Kato , S. , Iwanami , S. and Tsuchizaki , T. 1993 . Phylogenetic diversity of phytopathogenic mycoplasma-like organisms . Intern. J. Syst. Evol. Microbiol. , 43 : 461 – 467 .
- Rao , G.P. , Singh , A. , Singh , H.B. and Sharma , S.R. 2005 . Phytoplasma diseases of sugarcane: characterization, diagnosis and management . Indian J. Plant Pathol. , 23 : 1 – 21 .
- Thein , M.M. , Jamjanya , T. , Kobori , Y. and Hanboonsong , Y. 2012 . Dispersal of leafhoppers Matsumuratettix hiroglyphicus and Yamatotettix flavovittatus (Homoptera: Cicadellidae), vectors of sugarcane white leaf disease . Appl. Entomol. Zoology , 47 : 255 – 262 .
- Tran-Nguyena , L. , Blanchea , K.R. , Eganb , B. and Gibb , K.S. 2000 . Diversity of phytoplasmas in northern Australian sugarcane and other grasses . Plant Pathol. , 49 : 666 – 679 .
- Wongkaew , P. and Fletcher , J. 2004 . Sugarcane white leaf phytoplasma in tissue culture: long-term maintenance, transmission, and oxytetracycline remission . Plant Cell Rep. , 23 : 426 – 434 .
- Wongkaew , P. , Hanboonsong , Y. , Sirithorn , P. , Choosai , C. , Boongkrong , S. , Tinnangwattana , T. , Kitchareonpanya , R. and Damak , S. 1997 . Differentiation of phytoplasmas associated with sugarcane and gramineous weed white leaf disease and sugarcane grassy shoot disease by RFLP and sequencing . Theor. Appl. Gen. , 95 : 660 – 663 .
- Zhou , Z.J. , Lin , Q.Y. and Xie , L.H. 1987 . Sugarcane white leaf disease occurrence and observation of its pathogens by electron microscopic . J. Fujian Agric. Coll. , 16 : 165 – 168 .
