Abstract
Clubroot (Plasmodiophora brassicae) was initially discovered on canola (Brassica napus) in western Canada near Edmonton, Alberta in 2003. Since then, the disease has spread rapidly but is still most common and most destructive on the heavy, slightly acidic soils in the region where the first infested fields were identified. However, there was (and is) concern that the pathogen might continue to spread and become a constraint to canola production across the Canadian prairies. To assess the risk of continued spread, the effect of factors such as temperature and soil type, pH and micronutrients on clubroot development were examined. Temperatures below 17 °C were shown to slow or inhibit the development of P. brassicae at all stages of its life cycle. Alkaline pH also reduced infection and symptom development in both controlled environment and field situations, but alkaline pH did not eliminate clubroot when other conditions were conducive for infection. Differences in the concentration of boron and other micronutrients in soil were shown to affect clubroot development on canola, but the effect of these differences was unlikely to limit the development of clubroot in situations where inoculum concentration was high. Soil moisture, especially in the rhizosphere during primary and secondary infection, had an important impact on clubroot development, but is a difficult factor for most growers to manipulate. Soil type had a small effect on clubroot severity in controlled environment studies, but a strong interaction of soil type with soil moisture is extremely likely under field conditions. Assessment of the interaction of environmental factors affecting infection success with the mechanisms of pathogen dispersal indicated that clubroot on canola has the potential to spread across large portions of the Canadian prairies.
Résumé
La hernie (Plasmodiophora brassicae) a été initialement détectée sur le canola (Brassica napus) dans l’Ouest canadien en 2003, près d’Edmonton en Alberta. Depuis, la maladie s’est répandue rapidement, mais elle est toujours plus courante et destructrice sur les sols lourds et légèrement acides de la région où les premiers champs infestés ont été découverts. Toutefois, il y avait (et il y a toujours) une crainte que l’agent pathogène continue de se propager et qu’il pose des contraintes à la production du canola dans les Prairies canadiennes. Afin d’évaluer le risque de propagation continue, les effets de facteurs tels que la température, le type de sol, le pH et les oligo-éléments sur le développement de la hernie ont été examinés. Il a été démontré que les températures de moins de 17 °C ralentissaient ou inhibaient le développement de P. brassicae, et ce, à tous les stades de son cycle de vie. Le pH alcalin réduit également le développement de l’infection et des symptômes tant dans des conditions contrôlées qu’en champ, mais il n’élimine pas la hernie quand les autres conditions sont propices à l’infection. Il a été également démontré que les différences de concentrations de bore et d’autres oligo-éléments contenus dans le sol influençaient le développement de la hernie chez le canola, mais qu’il est peu probable que les effets de ces différences limitent le développement de la hernie lorsque les concentrations d’inoculum sont élevées. L’humidité du sol, particulièrement au niveau de la rhizosphère lors des infections primaires et secondaires, influence fortement le développement de la hernie, mais, pour la majorité des producteurs, c’est un facteur difficile à gérer. Le type de sol a eu peu d’effets sur la gravité de la hernie dans des études menées dans des conditions contrôlées, mais il est possible qu’en champ il y ait une forte interaction entre le type de sol et son humidité. L’évaluation des interactions qui existent entre les facteurs environnementaux influant sur le succès de l’infection et les mécanismes de propagation de l’agent pathogène indiquent que la hernie du canola peut envahir de vastes portions des Prairies canadiennes.
Introduction
Many factors influence the disease risk associated with a crop pathogen. The key elements include availability and distribution of a susceptible host, suitability of the environment for infection, pathogen reproduction and persistence, and the potential for movement of the pathogen. An assessment of the potential risks associated with clubroot (Plasmodiophora brassicae Woronin) on canola (primarily Brassica napus L.) across the Canadian prairies would be of interest to the agricultural industry in the region. The mechanisms for pathogen dispersal are described in detail elsewhere in this volume (Strelkov & Hwang Citation2013); therefore, this review focuses on the suitability of the prairie environment for pathogen infection and reproduction, and how that might affect the disease risk for clubroot on canola across the region.
Plasmodiophora brassicae was first identified on canola on the Canadian prairies in 2003 near Edmonton, Alberta (Tewari et al. Citation2005). It caused devastating losses and all of the commercial cultivars were highly susceptible (Strelkov et al. Citation2006). Canola is grown on more than 8 million ha on the Canadian prairies each year (Canola Council of Canada Citation2013). The fields used for canola production are typically large (~ 60 ha), often contiguous, and the crop is routinely grown on a short rotation (0 to 2 years between crops) (Rempel et al. Citation2013). Clearly, there is a substantial hectarage of susceptible crop plantings that could potentially be affected by clubroot.
At present, management of clubroot on canola in infested fields on the Canadian prairies relies almost exclusively on deployment of resistant cultivars. Several clubroot-resistant cultivars are available, and intensive research is underway to identify additional sources of resistance and incorporate them into elite canola lines for the Prairie region (reviewed in Rahman et al. Citation2013). However, clubroot resistance has often broken down rapidly when exposed to high disease pressure (reviewed in Diederichsen et al. Citation2009). One line of evidence indicates that a single mechanism of resistance may be operating in several of the resistant cultivars available in Canada (Deora et al. Citation2012, Citation2013b). The implication of this finding is that the resistance gene(s) employed in these cultivars potentially originated from a single source of resistance (Deora et al. Citation2013b). Erosion of clubroot resistance in these cultivars is possible if the resistant cultivars are not used effectively (Strelkov et al. Citation2011).
An initial assessment of the risk of clubroot to canola on the Canadian prairies was based on modelling software called CLIMEX (Turkington et al. Citation2004). CLIMEX matches patterns of climate and other factors associated with epidemic development of the disease in other parts of the world with those same factors in the area of interest. Based on the impact of climate on clubroot development on B. napus and Brassica vegetables in other parts of the world, the program estimated and mapped the risk potential of severe clubroot impact on canola across the Canadian prairies. The indication from this initial analysis was that rainfall during the growing season was too low and soil pH was too high over most of the prairie region to favour severe epidemics of clubroot. As a result, the only regions identified as being at high risk included a small area in north-central Alberta around Edmonton, and the Red River Valley region of southern Manitoba (Turkington et al. Citation2004). However, it quickly became clear that the disease was spreading and causing substantial damage to canola crops in Alberta far beyond the area at risk identified by CLIMEX. Since the initial identification in 2003, clubroot has spread to sites across large areas of Alberta (Strelkov et al. Citation2012). Even more significantly, the pathogen was identified in infested field soil and in symptomatic plants in Saskatchewan (Dokken-Bouchard et al. Citation2010, Citation2012) and from infested field soil in Manitoba in 2012 (Strelkov & Hwang Citation2013).
When clubroot was first identified on canola in western Canada, temperature, pH, soil moisture (Macfarlane Citation1952; Colhoun Citation1953, Citation1958) and micronutrient concentration (Hamilton & Crete Citation1978; Webster & Dixon Citation1991b; Donald & Porter Citation2004) were known to have substantial effects on infection and development of P. brassicae, but specific information on these factors was often surprisingly weak and incomplete. A research programme was undertaken to examine the factors that influence infection and development of P. brassicae in canola, and to determine how these factors might influence the frequency and severity of potential outbreaks of the disease across the Canadian prairies.
Effect of temperature on clubroot development
The first study of the influence of temperature on the development of P. brassicae was conducted nearly 100 years ago. It showed that a minimum temperature of 14 °C was required for germination of the resting spores of P. brassicae (Chupp Citation1917). Symptom development also appeared to be affected by temperature, based on observational studies. The minimum temperature required to produce severe clubroot symptoms on brassica vegetable crops was estimated to be 18–19.5 °C (Colhoun Citation1953; Buczacki et al. Citation1978), and clubroot levels increased as soil temperature increased, with very low levels at or below 14 °C (Thuma et al. Citation1983). Similarly, clubroot severity was highest in short-season brassica vegetable crops harvested in July and August, when soil temperatures ranged between 20–22 °C during the growing period, and lowest for crops harvested in October, when mean air temperatures during the final 10 days before harvest were below 12 °C (McDonald & Westerveld Citation2008).
To examine the impact of temperature on infection and pathogen development in more detail, a series of studies were conducted using Shanghai pak choy (B. rapa L. subsp. chinensis (Rupr.) var. communis Tsen and Lee) as a model crop. It is a small, short-season vegetable that is highly susceptible to clubroot. Each life stage of P. brassicae (primary infection, secondary infection, colonization and symptom development) exhibited a similar response; development was very slow at temperatures below 17 °C, increased quickly to a maximum at about 23–26 °C, and then declined as temperature increased to 30 °C (Sharma et al. Citation2011a, Citation2011b). This same pattern of response to temperature was also demonstrated for primary infection and symptom severity on canola under controlled conditions (Gossen et al. Citation2012a) and for Shanghai pak choy in field trials (Gossen et al. Citation2012b). Finally, a study of the impact of fluctuating temperatures on development of P. brassicae in canola seedlings demonstrated that daily fluctuations of up to 10 °C had little or no effect on infection success or subsequent pathogen development (Gludovacz Citation2013). This last result supports the use of fixed temperature in studies to estimate the impact of temperature on clubroot under field conditions.
In previous studies, there was no correlation between root hair infection and clubroot incidence (Macfarlane Citation1952; Naiki & Dixon Citation1987). However, when conditions were maintained near the optimum for infection, root hair infection was highly correlated with symptom development in susceptible brassica crops (Hwang et al. Citation2011a, Citation2011b). This indicates that the incidence of root hair infection is a useful indicator of inoculum potential (levels of resting spores in soil) when assessed under controlled conditions (Hwang et al. Citation2011a), but is likely to be much less useful under the much more variable conditions found in the field.
The effect of temperature on this pathogen has implications for disease management. A recent study demonstrated that clubroot severity declines as the initial infection of seedlings is delayed by as little as one week (Hwang et al. Citation2011b). Therefore, seeding into cool soils to delay infection may be a useful approach to clubroot management. Early (or late) seeding to avoid periods of high soil temperature has been shown to provide effective management of clubroot in short-season brassica vegetable crops (Gossen et al. Citation2012b). In canola, early seeding reduced clubroot severity and increased seed yield slightly (Hwang et al. Citation2012a). However, early seeding is already used routinely to maximize yield potential of the crop, so little or no change in seeding date is possible.
Effect of soil pH on clubroot development
The impact of soil pH on clubroot incidence has been known for centuries. An early report by Ellis in 1750 notes that English farmers applied calcium-rich soil (marl) to suppress clubroot before planting turnip (cited in Wellman Citation1930). Germination of resting spores occurred readily in acidic soil, but was slower in alkaline soil (Bremer 1924 cited in Karling Citation1968). As a result, the standard recommendation for clubroot management has been to raise the soil pH to 7.2 or above by applying lime (Colhoun Citation1953; Hamilton & Crete Citation1978; Fletcher et al. Citation1982).
Reduction in root hair infection and subsequent reduction in clubroot development in brassicas at alkaline pH has been reported in many crops (Samuel & Garrett Citation1945; Hamilton & Crete Citation1978; Crute et al. Citation1981; Myers & Campbell Citation1985; Webster & Dixon Citation1991b; Donald & Porter Citation2004). For example, development of clubbing symptoms on turnip was inhibited under alkaline conditions (Palm Citation1963) and severe clubbing in broccoli was observed at pH values up to 7.2, but no clubbing occurred at pH 8.0 (Myers & Campbell Citation1985). Similarly, clubroot levels in fields surveyed in Sweden (Wallenhammar Citation1996) and Finland (Rastas et al. Citation2012) were highest in soils with acidic pH. In a study where clubroot incidence was suppressed by application of manure or compost, the authors concluded that application of organic matter played an important role in clubroot suppression by increasing soil alkalinity (Niwa et al. Citation2007).
Application of calcium, magnesium or boron to raise soil pH above 7.2 under controlled conditions reduced root hair infection and subsequent symptom development (Webster & Dixon Citation1991a, Citation1991b; Donald & Porter Citation2004; Rashid et al. Citation2013). However, infection of root hairs was not affected by high pH in the absence of calcium (Myers & Campbell Citation1985). Reduction in primary infection and clubroot incidence at pH ≥ 7.2 has been attributed to inhibition of primary plasmodia prior to the release of secondary zoospores (Myers & Campbell Citation1985).
It is interesting to note that the pattern of low clubroot at neutral or slightly alkaline pH is not consistently observed in field situations (Karling Citation1968; McDonald et al. Citation2004). Even heavy liming did not prevent clubroot if moisture and spore load were sufficiently high (Macfarlane Citation1952; Colhoun Citation1953). Therefore, a study was conducted under controlled conditions to assess the interaction of pH and temperature on root hair infection by P. brassicae and subsequent clubroot incidence and severity (Gossen et al. Citation2013a). This study demonstrated that slightly alkaline soil conditions reduced but did not completely stop root hair infection, secondary colonization by the pathogen, or development of clubroot symptoms in susceptible canola seedlings when conditions were highly conducive for the pathogen (moderate concentration of resting spores, and warm (20–27 °C) wet soil). Similarly, assessment of soil pH in 267 commercial canola fields in Alberta infested with P. brassicae showed that soil pH was weakly correlated with clubroot severity (Gossen et al. Citation2013a). This indicates that other factors may have an important impact on clubroot levels in many fields.
Effect of soil type, rainfall and drainage on clubroot development
Studies indicate that clubroot incidence and severity may be influenced by the organic matter content, moisture, pH, and physical and chemical properties of soil, but different studies often provide conflicting results. In one study, high levels of clubroot were reported in heavy, acidic loam soils relative to lighter soils (Colhoun Citation1953), while in another study, light soil, sandy soil, humus-rich soil and clay soil favoured clubroot development (Karling Citation1968).
The effect of organic matter in soil on clubroot levels is also not well understood. High levels of clubroot have been associated with low organic matter content (Wallenhammar Citation1996; Murakami et al. Citation2000), and adding organic amendments to field soils has been shown to reduce clubroot (Niwa et al. Citation2007). Organic matter improved soil texture and increased soil microbial activity and nutrient availability, which may be associated with pathogen suppression (Dixon & Tilston Citation2010). In contrast, high levels of clubroot developed each year in soils with very high (70%) organic matter content (Thuma et al. Citation1983; McDonald & Westerveld Citation2008; Gossen et al. Citation2012b).
Soil moisture has an important and consistent influence on clubroot levels. However, the use of different methods of measuring and expressing soil moisture (gravimetric vs. volumetric; World Meteorological Organization Citation2008) have made comparisons difficult. An early study reported that high soil moisture maintained for a short period was sufficient for clubroot infection to occur (Wellman Citation1930). A subsequent report indicated that 60–70% soil moisture was most favourable for clubroot development at optimum temperature, but that low levels of infection occurred even when soil moisture was low (Colhoun Citation1953). Also, an increase in soil moisture increased clubroot severity on turnip (Ayers Citation1944). When the impact of soil type on clubroot was examined under low soil matric potential, almost 100% infection occurred in silty loam soil, sandy loam soil and muck soil (Dobson et al. Citation1982). This indicated that clubroot was most severe in soil with a high capacity for moisture retention. In another study, clubroot developed on cabbage at soil moisture content as low as 60% (w/w) in muck soil and 9% in mineral soil, which was equivalent to about 25% moisture-holding capacity in both soils (Hamilton & Crete Citation1978). Clubroot incidence in these studies increased as soil moisture increased. Similarly, rainfall was positively correlated with clubroot severity on radish, brassica vegetables and canola on muck soil (Thuma et al. Citation1983, Adhikari Citation2010; Gludovacz Citation2013).
Avoiding compacted soils is a standard recommendation for clubroot management, but data on the effect of soil compaction on disease are very limited (McDonald et al. Citation2004). Soil compaction has been associated with root disease in many host–pathogen systems (Raghavan et al. Citation1982; Moots et al. Citation1988; Carter & Johnston Citation1989). In general, clubroot development is reduced in well-drained soil, but heavily water-logged and compacted soils favour the disease (Dixon & Tilston Citation2010). This may be related to the sizes, frequency and continuity of soil pores, which are crucial for zoospore movement (Cook & Papendick Citation1972).
A study was conducted to examine the impact of soil texture on infection and development of clubroot on canola (Kasinathan Citation2012). Canola was grown in tall pots filled with muck soil (pH 6.2, organic matter 68%), mineral soil (pH 6.8, organic matter 3.5%), autoclaved non-calcareous coarse sand (pH 6.5, organic matter 0%), or soil-less mix (pH 6.0). Mineral and muck soil were selected to represent field soils, and sand and soil-less mix to represent growth media used in research studies. The concentration of inoculum used in this experiment was 5 × 106, which reflects the concentration of resting spores in infested commercial canola fields in Alberta (Ahmed et al. Citation2011).
In one experiment, the soil/media treatments were allowed to drain normally. When the study was repeated, the soil/media treatments were maintained at saturation for 3 weeks after inoculation. This change in moisture level had little effect on clubroot severity. Clubroot severity was high in both muck soil and mineral soil, intermediate on sand, and low on soil-less mix (Kasinathan Citation2012). The low severity on soil-less mix may be associated with its larger pore space, which made it difficult to maintain saturation in the root zone. This result supported the observation that severe clubroot can develop on both muck and mineral soil when moisture is adequate.
Also, an experiment was conducted to determine if pore size affected the development of clubroot severity (Kasinathan Citation2012). Mineral soil had a higher bulk density than muck soil or soil-less mix, and pore volume was larger in soil-less mix than in the other growth media, so soil-less mix presumably had the greatest moisture-holding capacity. Clubroot incidence and severity were highest on mineral soil, intermediate on muck soil and lowest in soil-less mix (Kasinathan Citation2012). There was a negative correlation between bulk density and micro-pore volume, and a positive correlation between bulk density and clubroot severity. This may indicate that soil pore volume or pore size affect the success of primary infection, possibly via an effect on soil moisture content (Dodson et al. Citation1982).
Effect of boron and other soil micronutrients on clubroot development
Boron
Application of mineral nutrients can be an effective tool for management of many crop pathogens (Datnoff et al. Citation2007). One of the most successful applications of this approach has been the use of boron, calcium and nitrate nitrogen to suppress clubroot in brassica vegetable production (Stangoulis & Graham Citation2007; Dixon Citation2009).
Canola and other brassica crops require large amounts of boron to achieve optimum growth (Asad et al. Citation2000, Citation2002). A deficiency of boron can reduce foliar and root growth, flower retention and pollen germination (Asad et al. Citation2000; Stangoulis et al. Citation2001). Although crop tolerance to excess boron is higher than in cereals and pulse crops (Gupta Citation1993), the range of concentration between deficiency and phytotoxicity is narrow (Tanaka & Fujiwara Citation2008). Excess boron can cause phytotoxic effects such as necrosis on leaf edges and upward cupping of older leaves (Brown & Shelp Citation1997; Brown & Hu Citation1998).
Boron application in brassica vegetables generally reduced clubroot incidence by up to 50% and increased yield by 30% (Dixon Citation1996; Nott et al. Citation1999; Ruaro et al. Citation2009). Application rate and timing can be important; prolonged exposure to a low concentration of boron had an equivalent effect on symptom development as short exposure to a high concentration (Webster & Dixon Citation1991b).
Application of boron inhibited the development of primary plasmodia in root hairs into sporangia, and maturation of secondary plasmodia in the root cortex. This inhibition reduced clubroot severity in Chinese cabbage (B. rapa ssp. pekinensis) under field conditions (Webster & Dixon Citation1991b). Boron also had a role in strengthening plant cell walls. Up to 90% of cellular boron was localized in the cell walls, where it formed cross-links with pectin polymers that enhanced cell wall stability and rigidity (Loomis & Durst Citation1992) and stabilized the structure of plasma membranes (Cakmak et al. Citation1995). Stabilization of these structural components may interfere with penetration of zoospores or development of P. brassicae (Dixon Citation1996), or may retard the movement of the pathogen through the root cortex (Graham & Webb Citation1991). Also, boron concentration may influence the cellular environment. This may occur through induction of secondary metabolites that are toxic to a wide range of pathogens, such as phenolic and peroxidase compounds (Ruiz et al. Citation1998; Camacho-Cristobal et al. Citation2008), or via an increase in the pH of cytoplasm to create conditions that are less favourable for P. brassicae.
The concentration of boron in soil is adequate for canola production across most of the prairie regions of Canada, but the potential to use additional boron applications to reduce clubroot on canola had not been assessed. Therefore, studies were conducted to assess (i) phytotoxicity on canola and pathogen inhibition in response to rates of boron, (ii) the effect of application timing on efficacy against clubroot under controlled environment conditions, and (iii) efficacy in field trials.
In controlled environment studies on canola seedlings grown in sand, primary infection in root hairs and secondary development in the root cortex was inhibited with increasing rates of boron, irrespective of boron formulation (Deora et al. Citation2011). The timing of boron application (pre- or post-emergence) had no effect on clubroot development. This indicates that the rate of boron applied is more important than the timing of application. Rates > 2 kg ha−1 of boron resulted in phytotoxicity, but the seedlings grew out of the symptoms within about 10 days (Deora et al. Citation2011).
Broadcast applications of boron (range 2–32 kg ha−1) before seeding for suppression of clubroot in canola were examined on naturally infested mineral soils in Alberta and Quebec, and on a muck soil in Ontario (Deora et al. Citation2013a). High rates of boron (16 and 32 kg ha−1) reduced clubroot severity by 25–35% on muck soil, but even high rates of boron application did not reduce severity on mineral soil. The difference in response may reflect the difference in organic matter content (~3% vs. 70%). Organic matter is a storehouse of boron because of the binding ability of dihydroxy compounds of humus, the major component of organic matter in soil (Parks & White Citation1952; Goldberg Citation1997). More boron is available to the plant for a longer period during the growing season in soil with high organic matter than in mineral soil, where boron leaches out more quickly (Parks & White Citation1952; Yermiyahu et al. Citation1988). The longer period of availability of boron on muck soil was likely an important factor in the higher efficacy against clubroot compared with mineral soil (Gupta & Cutcliffe Citation1978; Deora et al. Citation2013a).
Canola is grown almost exclusively on mineral soils in Canada, so application of boron will not provide effective clubroot management for standard cultivars. Instead, boron-tolerant cultivars have been suggested as a component of clubroot management programmes (Nott et al. Citation1999; Porth et al. Citation2003). Boron-tolerant genotypes can efflux excess boron from their roots, reducing boron transport to the shoot and subsequent phytotoxicity, so high rates of application are possible (Hayes & Reid Citation2004). Also, the taproot of tolerant genotypes retains a high proportion of total plant boron (40% vs. 1%) compared with sensitive genotypes (Kaur et al. Citation2006). These high levels likely can inhibit the growth of P. brassicae at the site of colonization but additional work in this area is required.
Calcium and nitrogen
Application of lime has been used since Roman times for management of clubroot. Liming makes soils more alkaline, and also increases levels of available calcium. Alkaline pH reduced root-hair infection and the rate of maturation of the pathogen, and calcium also inhibits the pathogen (Webster & Dixon Citation1991a). The efficacy of lime is affected by the type of lime applied, the interval before planting, and frequency of application (Wellman Citation1930; Campbell et al. Citation1985; Campbell & Greathead Citation1996). It is also affected by environmental factors and inoculum density (Colhoun Citation1958).
Calcium applied as calcium sulphate (CaSO4) or calcium carbonate (CaCO3), or in combination with nitrogen as calcium cyanamide (CaCN2), calcium nitrate (CaNO3), or calcium ammonium nitrate (Ca(NO3)2 NH4NO3 10H2O), reduced clubroot severity on brassica vegetables (Fletcher et al. Citation1982; Dixon & Wilson Citation1983; Klasse, Citation1999; Donald et al. Citation2002, Citation2004; Porth et al. Citation2003) and rapeseed mustard under field conditions (Bhattacharya & Mandal Citation2006). High levels of calcium salts increased spore dormancy (Macfarlane Citation1970). Also, salts that increased calcium and raised pH were associated with a larger reduction in clubroot symptoms than those that affected pH alone. The efficacy of several calcium and nitrogen fertilizers to reduce clubroot on brassica vegetables has been assessed and substantiated in Canada (Bélec & Tremblay Citation2004; McDonald et al. Citation2004; Tremblay et al. Citation2005; Abbasi & Lazarovits Citation2006) and on canola in Australia (Donald et al. Citation2002, Citation2004).
Both calcium and nitrate nitrogen inhibited the development of plasmodia into sporangia in root hairs (Webster & Dixon Citation1991a; Dixon & Page Citation1998), similar to the effect of boron. In addition to an effect on pathogen development, calcium fertilizers, alone or in combination with nitrogen as calcium nitrate, reduced the viability of P. brassicae resting spores and the ability of primary zoospores to invade the host (Naiki & Dixon Citation1987; Webster & Dixon Citation1991a; Page Citation2001). Application of calcium cyanamide, which degrades into calcium and nitrate nitrogen in the soil, had a similar effect.
Several mechanisms for suppression of P. brassicae by calcium and nitrogen have been suggested. A high calcium concentration decreased membrane permeability of root cells, which could affect the growth and reproduction of P. brassicae as it proliferates within the host. Alternatively, the alkaline environment created by the addition of large amounts of calcium could affect primary and secondary infection, cortical migration and cell hypertrophy. Calcium ions may also have a role in induced cell death in response to infection by P. brassicae (Takahashi et al. Citation2002, Citation2006). Nitrate nitrogen is known to stimulate arginine- or lysine-rich histones in plant cells. These histones may inhibit RNA polymerases in the pathogen, and ultimately block the pathogen’s access to gene products needed for pathogenesis (Webster Citation1986). Another possible mechanism is that a high supply of nitrate nitrogen influenced the activity of P. brassicae via a shortage of cofactors such as NADPH (nicotinamide adenine dinucleotide phosphate) that are needed for conversion of nitrate to ammonium (Webster Citation1986).
Levels of calcium in soils across most of the Canadian prairies are generally high (Soils of Saskatchewan Citation2012), and a wide range of sources of nitrogen are used routinely on canola with no clear pattern of efficacy against P. brassicae. Therefore, the very limited efficacy against clubroot resulting from application of calcium and nitrogen on canola (Hwang et al. Citation2011c) indicates that this will not be a useful strategy for clubroot management in the region. Also, specialized fertilizers such as calcium cyanamide were generally too costly to be used routinely for clubroot management on the Canadian prairies (Strelkov et al. Citation2011). However, the high levels of calcium common in the native soils across this region likely have a strong negative effect on the initial progress of clubroot infestation.
Factors affecting the movement of P. brassicae
The factors that affect the dissemination of inoculum of P. brassicae have been reviewed elsewhere in this volume (Strelkov & Hwang Citation2013). Although the initial source of the clubroot introduction in 2003 has not been identified, it appears that the main mechanism of movement from that initial infestation was on contaminated field equipment (Cao et al. Citation2009). This proved to be an effective means of dispersing the pathogen not only to adjacent fields, but also across the province (Gossen et al. Citation2013b).
In areas where P. brassicae is not yet widespread, producers can reduce the risk of introducing the pathogen to clubroot-free fields by cleaning their field equipment before it is moved from an infested field. Unfortunately, existing methods of removing or killing all of the resting spores from a large piece of equipment each time that it is moved between fields are too time-consuming and exacting for routine use by producers (Hwang et al. Citation2013). Therefore, it appears to be inevitable that P. brassicae will continue to spread from field to field on machinery. However, cleaning of equipment prior to use in a clubroot-free area represents a realistic measure that has the potential to delay the long-distance transmission of the pathogen.
Another potential avenue of long-distance movement of P. brassicae is on seed of crops grown in infested fields. A low potential for seed-to-seedling transmission from contaminated canola seed has been demonstrated under controlled conditions. This low risk can be reduced even further by commercial seed cleaning and seed treatment with fungicides (Rennie et al. Citation2011). Therefore, contaminated seed likely represents a very small risk for long-distance dispersal of the pathogen.
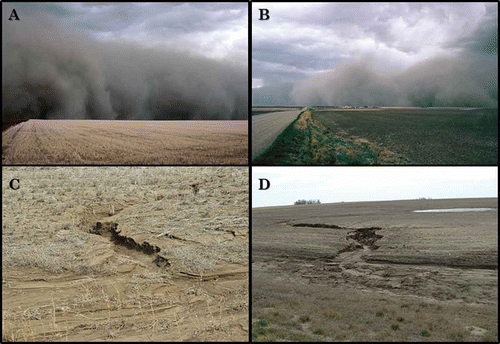
In contrast, movement of resting spores with soil from infested fields by wind and water may be an important means of dispersal (). When dust traps were positioned adjacent to infested fields, spores were routinely collected at more than 30 cm above the soil surface (Strelkov & Hwang Citation2014). Air-borne spores and soil are readily moved upward into the air column and have the potential to be moved tens or even hundreds of kilometres before being re-deposited. Little is known about the ability of these resting spores to survive such movement, but we can infer from the response of resting spores to sanitation treatments (Hwang et al. Citation2013) that they are likely to be very resilient. Also, there is evidence that transmission of wind-borne spores deposited in water used to irrigate seedlings for transplant resulted in rapid spread of clubroot in parts of Australia (Donald et al. Citation2006). Given the large hectarage of fields infested and the high concentration of resting spores (> 106 spore g−1) in some fields in Alberta (Cao et al. Citation2009), the number of spores that could potentially be moved by a single windstorm is staggering (). It appears likely that at least some of the infestations of P. brassica at widely dispersed sites across the region are the result of movement of spores by wind.
The risk of continued spread of clubroot on the Canadian prairies
Since its discovery near Edmonton in 2003, P. brassicae has been identified and confirmed at sites hundreds or even thousands of kilometres away from the initial focus of infection (Dokken-Bouchard et al. Citation2010, Citation2012; Strelkov & Hwang Citation2013). This indicates that the pathogen is spreading quickly (Strelkov & Hwang Citation2013). This rapid spread is driven by a combination of factors: (i) a highly susceptible crop (canola) grown in large, often contiguous fields, (ii) short intervals between successive susceptible canola crops, (iii) movement of resting spores within and among fields by farm machinery, and (iv) development of high concentrations of resting spores in many fields before the disease was discovered (Howard et al. Citation2010). This rapid spread is of particular concern because clubroot causes severe losses in susceptible canola crops, and many of the management methods used to reduce clubroot in high-value brassica vegetable crops are not economical for canola (Gossen et al. Citation2013b; Hwang et al. Citation2013).
Long-distance dispersal of the pathogen may occur in infested soil on farm machinery and miscellaneous vehicles/equipment, via wind or water erosion, with infested seed and inputs such as manure, and other mechanisms (Strelkov & Hwang Citation2013). The relative importance of wind- and waterborne dispersal has yet to be established definitively, but the wide geographic distribution of new sites of infestation indicated that at least some long-distance dispersal was occurring. If the pathogen has been dispersed by wind, low levels of resting spores are almost certainly already present at the soil surface across much of the region.
A recent study that examined the impact of pH on development of clubroot on canola demonstrated that slightly alkaline soil pH alone did not eliminate the risk of clubroot when other conditions were conducive for pathogen infection and development (Gossen et al. Citation2013a). Similarly, assessments of soil type and organic matter under controlled conditions (Kasinathan Citation2012) indicated that these factors had a limited impact on development of clubroot when other conditions were conducive for the pathogen.
Soil moisture has been shown to consistently affect clubroot development (Thuma et al. Citation1983; Adhikari Citation2010; Kasinathan Citation2012; Gludovacz Citation2013). The body of observational reports on this topic (few controlled studies are available) indicate that the interaction of soil moisture with soil pH, soil type and micronutrient content may have an important impact on clubroot risk. Little is known about the nature of these interactions, but areas of low rainfall with well-drained soils are at much lower overall risk of clubroot than regions with high rainfall and heavy, compacted soils that drain slowly. However, even sites with low mean rainfall occasionally experience periods or even seasons of above-normal rainfall, and such fluctuations may have an important impact on the risk of clubroot establishment at a particular site. Also, high levels of clubroot can develop even after prolonged drought in a growing season. For example, the heavy rains in mid-June of 2009 that ended a severe spring drought in central Alberta were associated with a severe epidemic of clubroot in field trials (Peng et al. Citation2011) and commercial fields (Strelkov et al. Citation2010).
Inoculum concentration is also an important factor in clubroot risk (Macfarlane Citation1952; Hildebrand & McRae Citation1998; Hwang et al. Citation2012b). Although susceptible plants can be infected by a single resting spore (Xue et al. Citation2008), a concentration of ≥ 1000 spores g−1 soil is needed to produce consistent clubroot symptoms under conditions that are highly conducive for infection (Hwang et al. Citation2011b). As conditions in the soil become increasingly unfavourable for the pathogen, based on combinations of low or fluctuating soil moisture, alkaline soil pH, high boron or calcium concentration, and other factors, the resting spore concentration required to produce consistent symptoms will also increase.
The need for a larger number of resting spores to initiate infection as conditions become less conducive for the pathogen is expected have a substantial influence on the success of pathogen transmission associated with the various methods of dispersal. On the one hand, the minute quantities of resting spores that are introduced to a field in the dust on wheat seed harvested from an infested field and used to seed a subsequent wheat crop would almost certainly require optimum conditions to have any chance of infecting a susceptible canola crop grown in that field one or two years later. If conditions are even slightly less than ideal, there is little or no risk of successful establishment of the pathogen from this mode of transmission. In contrast, trillions of spores can be introduced to a field in each of the large clumps of infested soil that fall off contaminated field equipment. Over the potential lifetime of these long-lived resting spores, conditions would have to be highly unfavourable to reduce the risk of pathogen establishment if susceptible hosts (crops or weeds) are present over several years.
The concentration of spores deposited by wind is likely to be much lower than the numbers of spores deposited in clumps of soil fallen from farm machinery. Weather systems occasionally pick up and deposit large volumes of soil () at a considerable distance from the initial infested field. In general, however, deposition of wind-borne spores would be higher at sites close to a heavily infested field than at sites further out along the dispersal gradient. Therefore, conditions for infection would generally need to be highly favourable for the pathogen to establish at a site far from a large source of resting spores. Therefore, the risk of pathogen establishment from wind dispersal of spores would be higher at sites close to large sources of inoculum or in regions where conditions are highly conducive for the pathogen (e.g., high rainfall and heavy, acidic soils) and lower in areas where conditions are unfavourable (low rainfall, light or alkaline soils, high calcium or boron).
Long-distance dispersal of spores in water could occur in situations where run-off from infested fields is used to irrigate a susceptible crop. This was shown to be an important mechanism of dispersal in Australia (Donald et al. Citation2006) and in paddy irrigation systems in China (Yu et al. Citation2013). Rapid spread of the pathogen in an area by flooding has also been reported in Canada (Canadian Department of Agriculture Citation1956), but dispersal of resting spores by water on the Canadian prairies is more likely to occur over relatively short distances (), associated with localized flooding.
Based on this analysis, we conclude that the risk of a clubroot epidemic is closely tied to weather, climate, soil characteristics, and the mechanism that brings resting spores to a particular site. Large areas of Saskatchewan appear to be at a relatively low risk that P. brassicae will become established and develop into an important constraint to canola production in the near future. Low mean rainfall, the presence of unfavourable soil characteristics (especially neutral to alkaline pH and high calcium), and the physical distance from existing infested fields, all contribute to this low risk. These analyses indicate that it would be very difficult for the pathogen to become established in these areas from wind- or seed-borne resting spores. However, it appears likely that introduction of large amounts of heavily infested soil from contaminated machinery into a field could allow the pathogen to establish. Once introduced, the pathogen has the potential to build up on susceptible crops and weeds whenever conditions are conducive for infection. Dissemination of the pathogen from an undetected site of infestation to other sites within the field and to adjacent fields would likely occur quickly on susceptible canola cultivars, based on the rate of expansion of infested sites observed in Alberta.
In large areas of Manitoba, conditions of precipitation, soil pH and calcium concentration are much more conducive for the pathogen than many parts of Saskatchewan and southern Alberta. The risk of transmission of P. brassicae via wind and water is currently low in Manitoba because of the physical distance from existing infested fields, but P. brassicae appears to have the potential to be a serious constraint to canola production in Manitoba if it becomes established in that region.
The development of resistant cultivars provides producers with options for clubroot management. Where populations of resting spores in a field are low, even a short break between canola crops has a positive impact on yield of resistant cultivars (Peng et al. Citation2013). Maintaining resting spore populations in the soil at low levels will also reduce the risk of a rapid breakdown of genetic resistance in these cultivars (Diederichsen et al. Citation2003; Peng et al. Citation2011). However, low populations of susceptible off-types, volunteer plants from susceptible cultivars, or even susceptible weeds, will serve to maintain high levels of resting spores (Hwang et al. Citation2012b) in many infested fields. The best option in areas that are currently free of clubroot is to keep the pathogen out by ensuring that all equipment (including that of oil companies, utilities, custom contractors, and even hunters and other occasional visitors) is free of contaminated soil from infested areas.
Acknowledgements
We thank the Clubroot Risk Mitigation Initiative of Agriculture and Agri-Food Canada and the Canola Council of Canada, the Agriculture Development Fund of Saskatchewan, and the Farm Innovation Program through the support of the Fresh Vegetable Growers of Ontario, for partial funding of the project.
References
- Abbasi PA, Lazarovits G. 2006. Effect of soil application of AG3 phosphonate on the severity of clubroot of bok choy and cabbage caused by Plasmodiophora brassicae. Plant Dis. 90:1517–1522.
- Adhikari KKC. 2010. Effect of Temperature, Biofungicides and Fungicides on Clubroot of Selected Brassica Crops [dissertation]. Guelph (ON): University of Guelph.
- Ahmed HU, Hwang SF, Strelkov SE, Gossen BD, Peng G, Howard R, Turnbull GD. 2011. Assessment of bait crops to reduce inoculum of clubroot (Plasmodiophora brassicae) of canola. Can J Plant Sci. 91:545–551.
- Asad A, Bell RW, Dell B. 2000. Uptake and distribution of boron in canola (Brassica napus L.) at vegetative and early flowering stages using boron buffered solution culture. Comm Soil Sci Plant Anal. 31:2233–2249.
- Asad A, Blamey FPC, Edwards DG. 2002. Dry matter production and boron concentrations of vegetative and reproductive tissues of canola and sunflower plants grown in nutrient solution. Plant Soil. 243:243–252.
- Ayers GW. 1944. Studies on the life history of the club root organism, Plasmodiophora brassicae. Can J Res C. 22:143–149.
- Bélec C, Tremblay N. 2004. Liming and calcium cyanamid for clubroot control in cauliflower. Acta Hort. 635:41–46.
- Bhattacharya TK, Mandal NC. 2006. Management of clubroot (Plasmodiophora brassicae) of rapeseed and mustard by nitrogenous fertilisers. Ann Plant Prot Sci. 14:260–261.
- Brown PH, Hu H. 1998. Boron mobility and consequent management in different crops. Better Crops. 82:28–31.
- Brown PH, Shelp BJ. 1997. Boron mobility in plants. Plant Soil. 193:85–101.
- Buczacki ST, Ockendon JG, Freeman GH. 1978. An analysis of some effects of light and soil temperature on clubroot disease. Ann Appl Biol. 88:229–238.
- Cakmak I, Kurz H, Marschner H. 1995. Short-term effects of boron, germanium and high light intensity on membrane permeability in boron deficient leaves of sunflower. Physiol Plant. 95:11–18.
- Camacho-Cristobal JJ, Rexach J, Gonzalez-Fontes A. 2008. Boron in plants: deficiency and toxicity. J Integr Plant Biol. 50:1247–1255.
- Campbell RN, Greathead AS. 1996. Control of clubroot of crucifers by liming. In: Engelhar AW, editor. Soil Borne Pathogens: Management of Diseases with Macro- and Microelements. St. Paul, MN: APS Press; p. 90–101.
- Campbell RN, Greathead AS, Myers DF, DeBoer GJ. 1985. Factors related to control of clubroot of crucifers in the Salinas Valley of California. Phytopathology. 90:769–774.
- Canada Department of Agriculture. 1956. 36th Annual report of the Canadian plant disease survey. Can Plant Dis Surv. 36:52–53.
- Canola Council of Canada. 2013. Canadian canola harvested acreage. [Internet]. [revised 2013 Oct 4; cited 2013 Oct 17]. Available from http://www.canolacouncil.org/markets-stats/statistics/harvest-acreage/
- Cao T, Manolii VP, Hwang SF, Howard RJ, Strelkov SE. 2009. Virulence and spread of Plasmodiophora brassicae [clubroot] in Alberta, Canada. Can J Plant Pathol. 31:321–329.
- Carter M, Johnston H. 1989. Association of soil macroporosity and relative saturation with root rot severity of spring cereals. Plant Soil. 120:149–152.
- Chupp C. 1917. Studies on clubroot of Cruciferous plants. Bull Cornell Univ Agric Exp Stn. 387:419–452.
- Colhoun J. 1953. A study of the epidemiology of club-root disease of Brassicae. Ann Appl Biol. 40:262–283.
- Colhoun J. 1958. Club Root Disease of Crucifers Caused by Plasmodiophora brassicae Woron A Monograph Phytopathology Paper No 3. Kew (UK): Commonwealth Mycol. Inst.
- Cook RJ, Papendick RI. 1972. Influence of water potential of soils and plants on root disease. Ann Rev Phytopathol. 10:349–374.
- Crute IR, Buczacki ST, Karen S. 1981. A solution culture method for observing the development of clubroot symptoms on young Brassica plants. Ann Appl Biol. 99:241–245.
- Datnoff LE, Elmer WH, Huber DM, editors. 2007. Mineral Nutrition and Plant Disease. St. Paul (MN): APS Press.
- Deora A, Gossen BD, Hwang SF, Pageau D, Howard RJ, Walley F, McDonald MR. 2013a. Effect of boron on clubroot of canola in organic and mineral soils and on residual toxicity to rotational crops. Can J Plant Sci. 93:xxx–xxx (in press).
- Deora A, Gossen BD, McDonald MR. 2012. Infection and development of Plasmodiophora brassicae in resistant and susceptible canola cultivars. Can J Plant Pathol. 34:239–247.
- Deora A, Gossen BD, McDonald MR. 2013b. Cytology of infection, development, and expression of resistance to Plasmodiophora brassicae in canola. Ann Appl Biol. 163:56–71.
- Deora A, Gossen BD, Walley F, McDonald MR. 2011. Boron reduces development of clubroot in canola. Can J Plant Pathol. 33:475–484.
- Diederichsen E, Deppe U, Sacristán MD. 2003. Characterization of clubroot resistance in recent winter oilseed rape material. In Proceedings of the 11th International Rapeseed Congress, Vol. 1, 2003 Jul 6; Copenhagen, Denmark; 68–70.
- Diederichsen E, Frauen M, Linders EGA, Hatakeyama K, Hirai M. 2009. Status and perspectives of clubroot resistance breeding in Crucifer crops. J Plant Growth Regul. 28:265–281.
- Dixon GR. 1996. Repression of the morphogenesis of Plasmodiophora brassicae Wor. by boron – a review. Acta Hort. 407:393–401.
- Dixon GR. 2009. The occurrence and economical impact of Plasmodiophora brassicae and clubroot disease. J Plant Growth Regul. 28:194–202.
- Dixon GR, Page LV. 1998. Calcium and nitrogen eliciting alterations to growth and reproduction of Plasmodiophora brassicae (clubroot). Acta Hort. 459:343–349.
- Dixon GR, Tilston EL. 2010. Soil–borne pathogens and their interactions with the soil environment. In Dixon GR, Tilston EL, editors. Soil Microbiology and Sustainable Crop Production. Dordrecht, Netherlands: Springer; p. 197–271.
- Dixon GR, Wilson F. 1983. Evaluation of calcium cyanamide for control of Plasmodiophora brassicae (clubroot). Tests of agrochemicals and cultivars no. 4. Ann Appl Biol. 102:50–51.
- Dobson R, Gabrielson RL, Baker AS. 1982. Soil water matric potential requirements for root-hair and cortical infection of Chinese cabbage by Plasmodiophora brassicae. Phytopathology. 72:1598–1600.
- Dokken-Bouchard FL, Anderson K, Bassendowski KA, Bouchard A, Brown B, Cranston R, Cowell LE, Cruise D, Gugel RK, Hicks L, Ippolito J, Jurke C, Kirkham CL, Kruger G, Miller SG, Moats E, Morrall RAA, Peng G, Phelps SM, Platford G, Schemenauer I, Senko S, Stonehouse K, Strelkov SE, Urbaniak S, Vakulabharanam V. 2012. Survey of canola diseases in Saskatchewan in 2011. Can Plant Dis Surv. 92:125–129.
- Dokken-Bouchard FL, Bassendowski KA, Boyle T, Cowell LE, Gugel RK, Ippolito J, Kirkham CL, Kutcher HR, Lewchuk Z, Miller SG, Morrall RAA, Phelps SM, Schemenauer I, Sommerfeld S, Vakulabharanam V. 2010. Survey of canola diseases in Saskatchewan, 2009. Can Plant Dis Surv. 90:127–130.
- Donald EC, Lawrence JM, Porter IJ. 2004. Influence of particle size and application method on the efficacy of calcium cyanamide for control of clubroot of vegetable brassicas. Crop Prot. 23:297–303.
- Donald EC, Porter IJ. 2004. A sand–solution culture technique used to observe the effect of calcium and pH on root hair and cortical stages of infection by Plasmodiophora brassicae. Australas Plant Pathol. 33:585–589.
- Donald EC, Porter LJ, Faggian R, Lancaster RA. 2006. An integrated approach to the control of clubroot in vegetable brassica crops. Acta Hort. 706:283–300.
- Donald EC, Porter IJ, Lancaster RA. 2002. Strategic application of lime, fertilisers and fungicides for improved control of clubroot. Cruciferae Newsl. 24:81–82.
- Fletcher JT, Hims MJ, Archer FC, Brown A. 1982. Effects of adding calcium and sodium salts to field soils on the incidence of clubroot. Ann Appl Biol. 100:245–251.
- Gludovacz TV. 2013. Clubroot in Canola and Cabbage in Relation to Soil Temperature, Plant Growth and Host Resistance. [dissertation]. Guelph (ON): University of Guelph.
- Goldberg S. 1997. Reactions of boron with soils. Plant Soil. 193:35–48.
- Gossen BD, Adhikari KKC, McDonald MR. 2012a. Effects of temperature on infection and subsequent development of clubroot under controlled conditions. Plant Pathol. 61:593–599.
- Gossen BD, Adhikari KKC, McDonald MR. 2012b. Effect of seeding date on development of clubroot in vegetable brassica crops. Can J Plant Pathol. 34:516–523.
- Gossen BD, Kasinathan H, Cao T, Manolii VP, Strelkov SE, Hwang SF, McDonald MR. 2013a. Influence of pH and temperature on infection and symptom development of clubroot in canola. Can J Plant Pathol. 35:294–303.
- Gossen BD, McDonald MR, Hwang SF, Strelkov SE, Peng, G. 2013b. Comparison of clubroot (Plasmodiophora brassicae) development and management on canola and Brassica vegetables. Can. J. Plant Pathol. 35:175–191.
- Graham RD, Webb MJ. 1991. Micronutrients and disease resistance and tolerance in plants. In: Mortvedt JJ, Cox FR, Schuman LM, Welch RM, editors. Micronutrients in Agriculture. Madison (WI): Soil Science Society of America; p. 329–370.
- Gupta UC. 1993. Factors affecting boron uptake by plants. In: Gupta UC, editor. Boron and Its Role in Crop Production. Boca Raton (FL): CRC Press; p. 87–104.
- Gupta UC, Cutcliffe JA. 1978. Effects of methods of boron application on leaf tissue concentration of boron and control of brown-heart in rutabaga. Can J Plant Sci. 58:63.
- Hamilton HA, Crete R. 1978. Influence of soil moisture, soil pH, and liming sources on the incidence of clubroot, the germination and growth of cabbage produced in mineral and organic soils under controlled conditions. Can J Plant Sci. 58:45–53.
- Hayes JE, Reid RJ. 2004. Boron tolerance in barley is mediated by efflux of B from the roots. Plant Physiol. 136:3376–3382.
- Hildebrand PD, McRae KB. 1998. Control of clubroot caused by Plasmodiophora brassicae with nonionic surfactants. Can J Plant Pathol. 20:1–11.
- Howard RJ, Strelkov SE, Harding MW. 2010. Clubroot of cruciferous crops – new perspectives on an old disease. Can J Plant Pathol. 32:43–57.
- Hwang SF, Ahmed HU, Strelkov SE, Gossen BD, Feng J, Peng G, Turnbull GD. 2011a. Influence of cultivar resistance and inoculum density on root hair infection of canola (Brassica napus) by Plasmodiophora brassicae. Plant Pathol. 60:820–829.
- Hwang SF, Ahmed HU, Strelkov SE, Gossen BD, Turnbull GD, Peng G, Howard RJ. 2011b. Seedling age and inoculum density affect clubroot severity and seed yield in canola. Can J Plant Sci. 91:183–190.
- Hwang SF, Ahmed HU, Zhou Q, Rashid SE, Strelkov SE, Gossen BD, Peng G, Turnbull GD. 2012b. Assessment of the impact of resistant and susceptible canola on Plasmodiophora brassicae inoculum potential. Plant Pathol. 61:945–952.
- Hwang SF, Cao T, Xiao Q, Ahmed H, Manolii VP, Turnbull G, Gossen BD, Peng G, Strelkov SE. 2012a. Efficacy of fungicide, seeding date and seedling age on clubroot severity, seedling emergence and yield of canola. Can J Plant Sci. 92:1175–1186.
- Hwang SF, Howard RJ, Strelkov SE, Gossen BD, Peng G. 2014. Management of clubroot (Plasmodiophora brassicae) on canola (Brassica napus) in western Canada. Can J Plant Pathol. 36(S1):49–65.
- Hwang SF, Strelkov SE, Gossen BD, Turnbull GD, Ahmed HU, Manolii VP. 2011c. Soil treatments and amendments for amelioration of clubroot on canola. Can J Plant Sci. 91:999–1010.
- Karling JS. 1968. The Plasmodiophorales. 2nd ed. New York: Hafner Publishing Company, Inc.
- Kasinathan H. 2012. Influence of pH, Temperature, and Biofungicides on Clubroot of Canola [dissertation]. Guelph, ON: University of Guelph.
- Kaur S, Nicolas ME, Ford R, Norton RM, Taylor PWJ. 2006. Physiological mechanisms of tolerance to high boron concentration on Brassica rapa. Funct Plant Biol. 33:973–980.
- Klasse HJ. 1999. Calcium cyanamide – a unique source of nitrogen promoting healthy growth and improving crop quality in vegetables. In: Dilek A, Pierre M-P, editors. Improved Crop Quality by Nutrient Management. Dordrecht, GDR: Kluwer Academic; p. 233–235.
- Loomis WD, Durst RW. 1992. Chemistry and biology of boron. Biofactors. 3:229–239.
- Macfarlane I. 1952. Factors affecting the survival of Plasmodiophora brassicae Wor. in the soil and its assessment by a host test. Ann Appl Biol. 39:239–256.
- Macfarlane I. 1970. Germination of resting spores of Plasmodiophora brassicae. Trans Br Mycol Soc. 55:97–112.
- McDonald MR, Kornatowska B, McKeown AW. 2004. Management of clubroot of Asian brassica crops grown in organic soils. Acta Hort. 635:25–30.
- McDonald MR, Westerveld SM. 2008. Temperature prior to harvest influences the incidence and severity of clubroot on two Asian brassica vegetables. HortScience. 43:1509–1513.
- Moots CK, Nickell CD, Gray LE. 1988. Effects of soil compaction on the incidence of Phytophthora megasperma f. sp. glycinea in soybean. Plant Dis. 72:896–900.
- Murakami H, Tsushima S, Shishido Y. 2000. Soil suppressiveness to clubroot disease of Chinese cabbage caused by Plasmodiophora brassicae. Soil Biol Biochem. 32:1637–1642.
- Myers DF, Campbell RN. 1985. Lime and the control of clubroot of crucifers: effects of pH, calcium, magnesium, and their interactions. Phytopathology. 75:670–673.
- Naiki T, Dixon GR. 1987. The effects of chemicals on developmental stages of Plasmodiophora brassicae (clubroot). Plant Pathol. 36:316–327.
- Niwa R, Kumei T, Nomura Y, Yoshida S, Osaki M, Ezawa T. 2007. Increase in soil pH due to Ca-rich organic matter application causes suppression of the clubroot disease of crucifers. Soil Biol Biochem. 39:778–785.
- Nott H, Falloon R, Cheah LH. 1999. Clubroot control from safe chemicals. NZ Commer Grower. 54:24–26.
- Page LV. 2001. Studies of Components for a Potential Integrated Control System for Plasmodiophora brassicae. [dissertation]. Glasgow (Scotland): University of Strathclyde.
- Palm ET. 1963. Effect of mineral nutrition on the invasion and response of turnip tissue to Plasmodiophora brassicae Wor. Contrib Boyce Thompson Inst. 22:91–112.
- Parks WL, White JL. 1952. Boron retention by clay and humus systems saturated with various cations. Soil Sci Soc Amer Proc. 16:298–300.
- Peng G, Lahlali R, Hwang SF, Hynes RK, McDonald MR, Pageau D, et al. 2011. Control of clubroot on canola using the biofungicide Serenade plus cultivar resistance. In Proceedings of the 13th International Rapeseed Congress, 2011 Jun 5. Prague, Czech Republic; p. 1134–1137.
- Peng G, Lahlali R, Hwang SF, Pageau D, Hynes RK, McDonald MR, Gossen BD, Strelkov SE. 2014. Managing clubroot on canola with crop rotation, cultivar resistance, and fungicides/biofungicides. Can J Plant Pathol. 36(S1):99–112.
- Porth G, Mangan F, Wick R, Autio W. 2003. Evaluation of management strategies for clubroot (Plasmodiophora brassicae Woron) in Brassicaceae. Proc Int Soc Trop Hort. 46:78–80.
- Raghavan GSV, Taylor F, Vigier B, McKyes E, Gauthier L. 1982. Effect of compaction and root rot disease on development and yield of peas. Can Agr Eng. 24:31–34.
- Rahman H, Peng G, Yu F, Falk KC, Kulkarni M, Selvaraj G. 2014. Genetics and breeding for clubroot resistance in Canadian spring canola (Brassica napus L.). Can J Plant Pathol. 36(S1):122–134.
- Rashid A, Ahmed HU, Xiao Q, Hwang SF, Strelkov SE. 2013. Effects of root exudates and pH on Plasmodiophora brassicae resting spore germination and infection of canola (Brassica napus L.) root hairs. Crop Prot. 48:16–23.
- Rastas M, Latvala S, Hannukkala A. 2012. Occurrence of Plasmodiophora brassicae in Finnish turnip rape and oilseed rape fields. Agric Food Sci. 21:141–158.
- Rempel C, Hutton S, Jurke C. 2014. Clubroot and the importance of canola in Canada. Can J Plant Pathol. 36(S1):19–26.
- Rennie DC, Manoli VP, Cao T, Hwang SF, Howard RJ, Strelkov SE. 2011. Direct evidence of surface infestation of seeds and tubers by Plasmodiophora brassicae and quantification of spore loads. Plant Pathol. 60:811–819.
- Ruaro L, Lima Neto VDC, Justiniano P, Júnior R. 2009. Influence of boron, nitrogen sources and soil pH on the control of club root of crucifers caused by Plasmodiophora brassicae. Trop Plant Pathol. 34:231–238.
- Ruiz JM, Bretones G, Baghour M, Ragala L, Belakbir A, Romero L. 1998. Relationship between boron and phenolic metabolism in tobacco leaves. Phytochemistry. 48:269–272.
- Samuel G, Garrett SD. 1945. The infected root-hair count for estimating the activity of Plasmodiophora brassicae (Woron.) in the soil. Ann Appl Biol. 32:96–101.
- Sharma K, Gossen BD, McDonald MR. 2011a. Effect of temperature on primary infection by Plasmodiophora brassicae and initiation of clubroot symptoms. Plant Pathol. 60:830–838.
- Sharma K, Gossen BD, McDonald MR. 2011b. Effect of temperature on cortical infection by Plasmodiophora brassicae and clubroot severity. Phytopathology. 101:1424–1432.
- Soils of Saskatchewan. 2012. Soil management [Internet]; [cited 2013 Oct 28]. Available from http://www.soilsofsask.ca
- Stangoulis JCR, Brown PH, Bellaloui N, Reid RJ, Graham RD. 2001. The efficiency of boron utilization in canola. Aust J Plant Physiol. 28:1109–1114.
- Stangoulis JCR, Graham RD. 2007. Boron and plant disease. In Datnoff LE, Elmer WH, Huber DM, editors. Mineral Nutrition and Plant Disease. St. Paul (MN): APS Press; p. 207–214.
- Strelkov SE, Hwang SF. 2014. Clubroot in the Canadian canola crop: 10 years into the outbreak. Can J Plant Pathol. 36(S1):27–36.
- Strelkov SE, Hwang SF, Howard RJ, Hartman M, Turkington TK. 2011. Progress towards the sustainable management of clubroot (Plasmodiophora brassicae) of canola on the Canadian Prairies. Prairie Soils Crops J. 4:114–121.
- Strelkov SE, Manolii VP, Liu J, Jurke C, Rennie DC, Orchard D, et al. 2012. The occurrence of clubroot on canola in Alberta in 2011. Can Plant Dis Surv. 92:122–124.
- Strelkov SE, Manolii VP, Márquez Zequera I, Manolii E, Hwang SF. 2010. Incidence of clubroot on canola in Alberta in 2009. Can Plant Dis Surv. 90:123–125.
- Strelkov SE, Tewari JP, Smith-Degenhardt E. 2006. Characterization of Plasmodiophora brassicae populations from Alberta, Canada. Can J Plant Pathol. 28:467–474.
- Takahashi H, Ishikawa T, Kaido M, Takita K, Hayakawa T, Okazaki K, Itoh K, Mitsui T, Hori H. 2006. Plasmodiophora brassicae induced cell death and medium alkalisation in clubroot-resistant cultured roots of Brassica rapa. J Phytopathol. 154:156–162.
- Takahashi H, Takita K, Kishimoto T, Mitsui T, Hori H. 2002. Ca2+ is required by clubroot-resistant turnip cells for transient increases in PAL activity that follows inoculation with Plasmodiophora brassicae. J Phytopathol. 150:529–535.
- Tanaka M, Fujiwara T. 2008. Physiological roles and transport mechanisms of boron: perspectives from plants. Eur J Physiol. 456:671–677.
- Tewari JP, Strelkov SE, Orchard D, Hartman M, Lange RM, Turkington TK. 2005. Identification of clubroot of crucifers on canola (Brassica napus) in Alberta. Plant Dis. 67:758–762.
- Thuma BA, Rowe RC, Madden LV. 1983. Relationships of soil temperature and moisture to clubroot (Plasmodiophora brassicae) severity on radish in organic soil. Plant Dis. 67:758–762.
- Tremblay N, Bélec C, Coulombe J, Godin C. 2005. Evaluation of calcium cyanamide and liming for control of clubroot disease in cauliflower. Crop Prot. 24:798–803.
- Turkington TK, Olfert OO, Weiss RM, Clear RM, Xi K, Tewari JP, Strelkov S. 2004. Forecasting the potential distribution and abundance of plant diseases using CLIMEXTM modeling with historical and potential weather scenarios associated with climate change [Internet]. In Proceedings of the Manitoba Agronomy Conference, Winnipeg, MB. [cited 2014 Feb 10]. Available from: http://www.umanitoba.ca/faculties/afs/MAC_proceedings/2004/proceedings.html
- Wallenhammar AC. 1996. Prevalence of Plasmodiophora brassicae in a spring oilseed rape growing area in central Sweden and factors influencing soil infestation levels. Plant Pathol. 45:710–719.
- Webster MA. 1986. pH and Nutritional Effects on Infection by Plasmodiophora brassicae Wor. and on Clubroot Symptoms. Dissertation, University of Aberdeen, Aberdeen, Scotland.
- Webster MA, Dixon GR. 1991a. Calcium, pH and inoculum concentration influencing colonization by Plasmodiophora brassicae. Acta Hort. 95:64–73.
- Webster MA, Dixon GR. 1991b. Boron, pH and inoculum concentration influencing colonization by Plasmodiophora brassicae. Mycol Res. 95:74–79.
- Wellman FL. 1930. Club root of crucifers. USDA Techn Bull No 181. p. 331.
- World Meteorological Organization. 2008. Guide to Meteorological Instruments and Methods of Observation. 7th ed. Chapter 11. Measurement of soil moisture. World Meteorological Organization, Geneva, Switzerland.
- Xue S, Cao T, Howard RJ, Hwang SF, Strelkov SE. 2008. Isolation and variation in virulence of single-spore isolates of Plasmodiophora brassicae from Canada. Plant Dis. 92:456–462.
- Yermiyahu U, Keren R, Chen Y. 1988. Boron sorption on composted organic matter. Soil Sci Soc Am J. 52:1309–1313.
- Yu X-K, Wu Y-X, Mao Z-C, He Y-Q. 2013. Water-mediated dissemination and chemical control of cabbage clubroot disease. J Huazhong Agric Univ. 2013 Issue 1:48–53.