Abstract
Select biofungicides and fungicides, used alone or with cultivar resistance or crop rotation, were assessed for their potential in integrated management of clubroot disease. The synthetic fungicides pentachloronitrobenzene, fluazinam and cyazofamid showed activities against Plasmodiophora brassicae. The biofungicides Serenade® and Prestop® also suppressed the disease on canola via antibiosis and induced host resistance under controlled-environment conditions. Granular and seed-treatment formulations were developed to facilitate the delivery of biofungicide in field trials. Where P. brassicae resting spore populations were large in the soil, neither biofungicides nor synthetic fungicides were sufficiently effective when applied in the seed furrow. They occasionally reduced clubroot severity on Chinese cabbage. More than 5000 soil microbial isolates indigenous to the Canadian prairies were screened for potential clubroot control, but none showed consistent efficacy. Resistant cultivars reduced clubroot severity and canola yield losses significantly. A 2-year break from canola reduced P. brassicae resting spore concentrations by 90% relative to growing continuous canola or only a 1-year break in heavily infested field plots. This 2-year break alleviated disease impact on plant growth and development in a susceptible canola cultivar. Despite the substantial inoculum reduction after 2 years, the levels were still too high to obtain commercially acceptable yields in a susceptible cultivar. In a resistant cultivar, >2-year breaks increased yields by up to 25% relative to continuous growing of canola. A 2-year interval with non-hosts between canola crops, together with use of resistant cultivars, is recommended to reduce the inoculum load of P. brassicae in soil and achieve maximum yields of canola.
Résumé
Des biofungicides et des fongicides de synthèse sélectionnés, utilisés seuls ou conjointement avec des cultivars résistants ou la rotation des cultures, ont été évalués en fonction de leur efficacité dans le cadre de la gestion intégrée de la hernie. Les fongicides de synthèse pentachloronitrobenzène, fluazinam et cyazofamide se sont avérés efficaces contre Plasmodiophora brassicae. Les biofongicides SerenadeMD et PrestopMD ont également enrayé la maladie chez le canola, en milieu contrôlé, par antibiose et résistance induite de l’hôte. Des formulations granulaires et d’autres applicables directement aux semences ont été développées pour en faciliter l’application lors des essais en champs. Dans les sols où il y avait de fortes populations de spores dormantes de P. brassicae, ni les biofongicides ni les fongicides de synthèse se sont avérés efficaces lorsqu’appliqués directement dans le sillon des semences. Ils ont à l’occasion réduit la gravité de la hernie chez le pé-tsai. Plus de 5000 isolats microbiens provenant d’échantillons de sol prélevés sur les Prairies canadiennes ont été criblés en vue d’évaluer leurs effets possibles sur la gestion de la hernie, mais aucun n’a affiché de réelle efficacité. Les cultivars résistants ont permis de réduire significativement la gravité de la hernie et les pertes de rendement. Dans les parcelles de terrain fortement infestées, une rotation de deux ans sans canola a permis de réduire de 90% les concentrations de spores dormantes de P. brassicae, comparativement à une culture continuelle ou à une rotation d’une année seulement sans canola. Cette pause de deux ans a réduit les effets de la maladie sur la croissance et le développement d’un cultivar réceptif à la hernie. Malgré la réduction substantielle de la quantité d’inoculum au bout de deux années, les taux étaient encore trop élevés pour obtenir des rendements commercialement acceptables avec un cultivar de canola réceptif. Chez les cultivars résistants, des pauses de plus de deux années ont accru les rendements de 25%, comparativement à une culture ininterrompue. Une rotation de deux années, au cours desquelles des plantes résistantes sont cultivées entre les semis de canola, utilisée conjointement à des cultivars résistants, est recommandée pour réduire la charge de l’inoculum de P. brassicae dans le sol et obtenir des rendements maximaux.
Introduction
Integration of strategies including use of host resistance, crop rotation, nutrient supplements and fungicides are often proposed for management of clubroot disease (Dixon Citation2003; Donald & Porter Citation2009). Host resistance is a cornerstone of clubroot control (Hirai Citation2006; Diederichsen et al. Citation2009), especially on large-hectarage crops such as canola or oilseed rape (Brassica napus L.). Calcium, boron and pH levels in the soil (Webster & Dixon Citation1991a, Citation1991b; Dixon & Page Citation1998; Deora et al. Citation2011; Gossen et al. Citation2013a) or application of fungicides (Suzuki et al. Citation1995; Donald et al. Citation2001; Takeshi et al. Citation2004) may also reduce the severity of clubroot disease. Several previous studies have shown that naturally occurring microorganisms (Arie et al. Citation1998; Narisawa et al. Citation1998; Joo et al. Citation2004) or biofungicides (Peng et al. Citation2011b) may also have potential in clubroot management. This review examines the effect of crop rotation, cultivar resistance and biofungicide or fungicide treatments against this disease, with a focus on recent research activities on canola in Canada. Results from three field studies will be presented to illustrate the attempt to integrate cultivar resistance with biofungicides or crop rotation for management of clubroot disease.
A wide range of fungicides have been evaluated for efficacy against this pathogen, primarily on Brassica vegetable crops (Doyle & Clancy Citation1987). A soil-drench treatment with Terraclor (pentachloronitrobenzene) controlled P. brassicae on cole crops (Finlayson & Campbell Citation1971), while fluazinam and cyazofamid, so-called reduced-risk products, have shown efficacy on several cruciferous vegetable crops (Mitani et al. Citation2003; Gossen et al. Citation2013b). Investigations of the use of microbes for management of clubroot disease have been conducted (Arie et al. Citation1998; Narisawa et al. Citation1998; Cheah et al. Citation2000; Joo et al. Citation2004). This approach is attractive because if the biocontrol agent (BCA) can colonize the root and/or rhizosphere, durable root protection may be achieved. There are few reports on the efficacy of BCAs for control of clubroot disease at the field level, especially on large-hectarage crops like canola. No microbial biofungicides have been registered for clubroot disease, although several have been available in Canada for the control of other soil-borne diseases. These BCAs include Serenade® (Bacillus subtilis), Prestop® [Clonostachys rosea f. catenulate (Gilman & Abbott) Schroers], Mycostop® (Streptomyces griseoviridis) and Root Shield® (Trichoderma harzianum Rifai). It is undetermined, however, if any of the biofungicides are effective against clubroot disease.
Genetic resistance to clubroot has been found in all of the major Brassica species except B. juncea and B. carinata (Diederichsen et al. Citation2009), providing an important management tool through resistance breeding. Most of the resistant genotypes identified so far appear to be race or pathotype-specific, although some have shown a broader resistance spectrum (Crute et al. Citation1980; Toxopeus et al. Citation1986). Clubroot-resistant cultivars have been developed for cruciferous vegetables (Hirai Citation2006; Saito et al. Citation2006; Sakamoto et al. Citation2008; Kamei et al. Citation2010) and oilseed rape (Gowers Citation1982; Diederichsen et al. Citation2006). Since 2009, several clubroot-resistant canola cultivars have been introduced in Canada, which has allowed for the production of canola crops even in fields where severe clubroot damage occurred just recently. Multiple races (Williams Citation1966), populations (Buczacki et al. Citation1975) or pathotypes (Crute et al. Citation1980) of P. brassicae may exist in a given geographical region (Kuginuki et al. Citation1999; Hatakeyama et al. Citation2006), which serve as sources of variation that can lead to the erosion of host resistance (Seaman et al. Citation1963). Almost all clubroot resistant cultivars have single gene-based resistance (Diederichsen et al. Citation2009) and so are vulnerable to shifts in the virulence structure of the pathogen populations. Depending on the resistance gene involved, clubroot disease resistance can be eroded rapidly, as observed on oilseed rape (Diederichsen et al. Citation2003; Oxley Citation2007) and Chinese cabbage (Piao et al. Citation2004; Hatakeyama et al. Citation2006). In western Canada, P. brassicae pathotypes 2, 3, 5 and 8, based on the differentials of Williams (Citation1966) have been reported, with pathotype 3 being the most prevalent (Xue et al. Citation2008; Cao et al. Citation2009) and virulent (Strelkov et al. Citation2006, Citation2007) on canola. Substantial erosion in clubroot disease resistance in one of the Canadian canola cultivars occurred after only two cycles of inoculation with P. brassicae pathotype 3 (LeBoldus et al. Citation2012). This indicates that resistance stewardship should be an important element in the sustainable production of canola.
On a large-hectarage crop like canola, options for managing clubroot disease are limited and many measures used successfully in production of high-value vegetable crops, such as soil liming or drenching with a fungicide, are impractical (Gossen et al. Citation2013b). Crop rotation is one of the valuable tools used for disease management in western Canada (Kutcher et al. Citation2013), but there is limited information on its effectiveness against clubroot disease. Based on soil samples from fields with a 4- to 6-year break from a Brassica crop, Wallenhammar (Citation1996) estimated that this lengthy break would substantially reduce the clubroot disease potential. This 4- to 6-year break, however, will be difficult to implement in western Canada, where the break between successive canola crops has been shortened in recent years, and some to even just 1 year in favour of canola production (Kutcher et al. Citation2013). Based on bioassay results, the ‘half-life’ of P. brassicae resting spores in field soils has been estimated at about 3.5–5 years (Wallenhammar Citation1996; Hwang et al. Citation2013), and this poses a dilemma regarding the value and practicality of crop rotation for managing clubroot disease on canola, especially in severely infested fields. A study using micro-plots seems to indicate a faster declining rate of P. brassicae resting spores when infested plots were planted with non-host crops or left fallow for 1–3 years (Robak Citation1994). There has been no information on the effect of crop rotation in alleviating clubroot impact on canola development and yield. This review will examine the potential of biofungicides/fungicides, host resistance and crop rotation for management of clubroot disease.
Synthetic fungicides
Most growers of brassica vegetables are unable to follow recommendations for management of clubroot that require a long crop rotation because of limited available land, while liming the soil to raise its pH is not always effective (McDonald et al. Citation2004; Gossen et al. Citation2013a). As a result, fungicides represent an attractive option for management of P. brassicae. A range of fungicides with efficacy against P. brassica have been identified. The old fungicide Calomel (mercurous chloride) reduced clubroot severity and increased the yield of Chinese cabbage [Brassica rapa L. subsp. pekinensis (Lour.) Hanelt] (Doyle & Clancy Citation1987), but mercury-based fungicides have been banned in North America for years due to their environmental toxicity. Hymexazol and procymidone also reduced clubroot severity but did not affect the yield, while thiophanate-methyl increased the yield even though it had little effect on the severity of clubbing symptoms. Cyazofamid, fluazinam, and fenamidone + mancozeb reduced the severity of clubbing on Chinese cabbage when applied as a soil-drench treatment after transplanting (Townley & Fox Citation2003). Benomyl, calcium cyanamide (mainly a fertilizer), pentachloronitrobenzene (PCNB), and trichlamide reduced root-hair infection and clubroot development. While the infection by secondary zoospores was inhibited most effectively by benomyl, trichlamide provided the longest residual activity (Naiki & Dixon Citation1987). Trichlamide, flusulfamide and fluazinam were effective when used against a weakly virulent P. brassicae population, but less effective against a highly virulent pathogen population (Tanaka et al. Citation1999). Clubroot was reduced with irrigation water amended with 200 mg L−1 chlorine, but this treatment was also phytotoxic (Datnoff et al. Citation1987). A post-planting soil-drench treatment with 0.14% (a.i.) of phosphonate fungicide delivered in 55 000 L ha−1 water reduced clubroot severity in pak choy [B. rapa L. subsp. chinensis (Rupr.) var. communis Tsen and Lee] and cabbage (B. oleracea L. var. capitata) in Ontario, Canada (Abbasi & Lazarovits Citation2006).
In recent years, the fungicides that received the most attention in Canada have been fluazinam (Allegro®, Omega®) and cyazofamid (RanmanTM); fluazinam is registered against P. brassicae on vegetable crops, while cyazofamid is registered for the control of other diseases of vegetables excluding clubroot in Canada. Cyazofamid when applied as a soil drench to Shanghai pak choy (B. rapa L. subsp. chinensis) and Chinese flowering cabbage (B. rapa subsp. chinensis) grown on a high organic-matter (om) soil [~70% om, pH 6.7] reduced clubroot incidence and disease severity substantially (7 of 10 trials) where inoculum pressure was high (Gossen et al. Citation2012). The reduction of disease severity ranged from 44 to 89% based on the area under a disease progress curve. In a trial with directly seeded Chinese cabbage grown in a similar high-organic matter soil, fluazinam reduced the disease severity index (DSI) from 44 to 20% while cyazofamid reduced DSI to 8% (Peng et al. Citation2011b). In controlled-environment trials, these two fungicides provided 100% clubroot control on Shanghai pak choy (Adhikari Citation2010).
These fungicides, however, are not always effective in controlling P. brassicae under field conditions. No reduction of clubroot incidence or an increase in yield was observed when fluazinam (2.9 L Allegro® 500F ha−1) or cyazofamid (1.5 L Ranman® SC400 ha−1) was applied as a soil drench to cabbage transplants on muck or mineral soils (Adhikari Citation2010; Saude et al. Citation2012). Although these fungicide rates were suggested by the manufacturers, the effective water volume for soil drench varies. It is possible that the water volume used in these trials (100 mL per plant) did not provide sufficient coverage around the transplant. Also, the fungicides may degrade or be leached from the root zone. The effectiveness of fungicide application to vegetable crops may depend on the crop type and the method of application. Fluazinam and cyazofamid appear to be more effective on short-season crops such as pak choy and Chinese flowering cabbage. If used on longer-season crops that are grown from transplants, a high water volume may be required to improve efficacy. Indeed, research in Australia has shown that fluazinam is most effective when incorporated into the soil in a band prior to transplanting (Donald et al. Citation2001).
The modes of action for most of the fungicides against infection and symptom development caused by P. brassicae largely remain unknown. Flusulfamide (NebijinTM SSC) has been reported to inhibit germination of resting spores of P. brassicae (Tanaka et al. Citation1999). Incubating resting spores with NebijinTM SSC for 2 days, however, did not reduce resting spore viability or their ability to cause clubroot disease on Chinese cabbage ‘Granaat’ (S.F. Hwang, unpublished data). It was also believed that cyazofamid would be able to inhibit resting spore germination, root-hair infection and development of clubroot symptoms (Mitani et al. Citation2003). Considering the vulnerability of zoospores in the pathogen life cycle (Dixon Citation2009), it is likely that most fungicides would act against primary and/or secondary zoospores.
On canola, a soil-drench application of fungicide is not feasible because of the large volumes of water required. The fungicides fluazinam and cyazofamid were not effective when applied in-furrow at 500 L water ha−1 (Peng et al. Citation2011b). Cyazofamid was most effective on canola when incorporated into the soil prior to seeding, but was only effective at low to moderate inoculum pressures (Hwang et al. Citation2008). Similarly, fluazinam was effective under low inoculum pressure (Ahmed et al. Citation2011). PCNB (Terraclor 75% WP), when incorporated into the soil before seeding, reduced clubroot severity more consistently than other fungicides in heavily infested fields (Hwang et al. Citation2008). None of these fungicides are likely to be used in commercial canola production; fluazinam and cyazofamid are too expensive and the use of PCNB is restricted due to health concerns (Health Canada Citation2010).
Fungicide seed treatments have also been examined. Dynasty 100 FS (azoxystrobin), NebijinTM SSC, Vitavax RS (carbathiin + thiram), Prosper FX (carbathiin + trifloxystrobin + metalaxyl), and Helix Xtra (difencazole + metalaxyl + fludioxonil) reduced infection under controlled greenhouse conditions, but none of the treatments was effective in field trials (Hwang et al. Citation2011a). Seed treatments may have a role only in reducing the risk of seed-borne dissemination of P. brassicae carried on seed produced in infested fields (Rennie et al. Citation2011). These results indicate that fungicide seed treatments are ineffective in heavily infested fields. In general, limitations in product delivery and the lack of an option for soil-drench applications could contribute to the low efficacy of fungicides observed on canola relative to vegetable crops.
Microbial biofungicides
The life cycle of P. brassicae consists of primary infection in root hairs and secondary infection in the root cortex. The primary and secondary zoospores that initiate each phase of the infection are fragile, short-lived, and appear to be a weak link in the pathogen life cycle (Dixon Citation2006). Biofungicide treatment should target the peak releases of these zoospores, which can occur shortly after seeding in infested and moist soils (Hwang et al. Citation2011b; Sharma et al. Citation2011).
Extensive studies have been conducted in Canada to investigate the potential of biocontrol for management of clubroot using indigenous microorganisms or commercial biofungicides. An initial project evaluated more than 5000 soil microorganisms indigenous to the Canadian prairies using a two-tiered process. First, in vitro evaluation was conducted against an indicator pathogen (Pythium ultimum Trow) for potential properties of antibiosis or competition, and then in vivo evaluation for suppression of clubroot disease was performed on canola. Most of these indigenous isolates showed no biocontrol potential, but three endophytic fungal isolates and four rhizobacteria reduced clubroot disease severity by >75%, relative to the inoculated control, when applied as a soil-drench treatment under controlled-environment conditions (G. Peng, unpublished data).
The efficacy of selected BCAs was compared with that of several commercial biofungicides, including Serenade (Bayer CropScience, Germany), Prestop (Verdera Oy, Finland), Mycostop (Verdera Oy), Actinovate® (Streptomyces lydicus, Natural Industries, USA) and Root Shield (BioWorks Inc. USA) that are registered in Canada, as well as against the synthetic fungicides fluazinam and cyazofamid. Serenade and Prestop were generally more effective than the indigenous BCAs and as effective as the synthetic fungicides, especially under low P. brassicae inoculum pressure (Peng et al. Citation2011b). Serenade and Prestop, with 85–100% reduction in clubroot disease severity when applied as a soil drench after seeding, were also more effective than the other biofungicides in controlled environments (Peng et al. Citation2011b). In contrast, Mycostop and Actinovate were more effective on Shanghai pak choy in a greenhouse trial (Adhikari Citation2010). Frequently, Serenade or Prestop were slightly less effective than the fungicides fluazinam and cyazofamid when disease pressure was high (Peng et al. Citation2011b). Both biofungicides contain secondary metabolites that have a role in their activity against clubroot. Additionally, the microbes in these products also colonize the rhizosphere of canola, and induce plant resistance through up-regulation of the genes that are involved in phenylpropanoid (BnOPCL, BnCCR), jasmonic acid (BnOPR2) and ethylene (BnACO) pathways (Lahlali et al. Citation2011, Citation2013; Lahlali & Peng Citation2013). Development of P. brassica and consequently clubroot symptoms may be partially suppressed by these biofungicides via induced systemic resistance, which is reflected to some extent by the reduced amount of P. brassicae DNA in the root detected using quantitative PCR (qPCR) at 2 weeks after inoculation. This level of DNA also is positively correlated with reduced clubroot severity. These biofungicides do not reduce the germination or viability of P. brassicae resting spores (Lahlali et al. Citation2011; Lahlali & Peng Citation2013), so suppression of clubroot disease probably results from the reduction of root-hair and/or cortical infection by primary and secondary zoospores (Lahlali et al. Citation2013), as well as the colonization of cortex by plasmodia.
Vegetable crops have a higher value per hectare than field crops such as canola, so growers may be able to afford more expensive inputs. Some brassica vegetables are grown typically from transplants, and therefore a biofungicide can be applied to the seedlings in plug trays, allowing time for BCA to colonize the roots before the plants are transplanted into the infested soil. Serenade and Prestop reduced clubroot severity by about 50% on Chinese cabbage grown on a high organic matter soil, when applied in the seed furrow. This efficacy was equivalent to that of fluazinam (Peng et al. Citation2011b). The biofungicides Root Shield and Serenade were ineffective when applied as a soil drench treatment around cabbage transplants, as were the fungicides tested (Adhikari Citation2010; Kasinathan Citation2012).
Serenade and Prestop were tested initially as an aqueous formulation applied in seed furrow at 500 L ha−1 in two field trials on canola. The test sites did not receive any rain for 4 weeks after seeding, and this extended dry period was detrimental to the efficacy of the biofungicides (Peng et al. Citation2011b). When rain finally came, the conditions became conducive for P. brassicae infection and clubroot severity was close to 80%. None of the treatments, including fluazinam and cyazofamid, substantially reduced clubroot severity. In a controlled environment, Serenade and fluazinam appeared to be more sensitive than Prestop and cyazofamid to dry soil conditions (<30% water content and 23/18 °C with a 16-h photoperiod), especially when the situation persisted for more than 2 weeks (Peng et al. Citation2011b).
The efficacy of the biofungicides varied among trials, especially across crops and test sites. For instance, in a controlled environment, Serenade and Prestop reduced clubroot severity on canola grown in a soil-less mix (Peng et al. Citation2011b) but were ineffective on Shanghai pak choy grown in a similar growing medium. On Shanghai pak choy, however, Mycostop was more consistently effective while Actinovate was effective only at low inoculum levels (Adhikari Citation2010).
Soil types were examined for potential influence on the efficacy of Serenade and Prestop against clubroot severity on canola and Shanghai pak choy. The biofungicides were applied to four types of growth media: muck soil (pH 6.2, 68% organic matter), mineral soil, (pH 6.8, 3.5% organic matter), non-calcareous coarse sand (pH 6.5, no organic matter), and soil-less mix (pH 6.0 and an unknown amount of organic matter) (Sunshine LB-2 Mix Basic, Sun Gro Horticulture Canada Ltd, Seba Beach, AB). These media were selected to represent the soil types on which field trials had been conducted previously (mineral and muck soils), or the growing media used for controlled-environment studies (sand and soil-less mix). The efficacy of both biofungicides was influenced by the growing medium. Prestop reduced clubroot on canola in muck and mineral soils by 40%, while Serenade was only effective on sand (Kasinathan Citation2012). In a further trial where the growing media were watered with only light sprays that resulted in noticeably less compaction, the biofungicides reduced clubroot severity, with Serenade being slightly more effective than Prestop. This indicates that the excessive amount of water used in earlier trials to stimulate infection by P. brassicae might have limited the activity of the biofungicides, perhaps by leaching some of the product components away from the root zone. It is possible that the efficacy of biofungicides would not vary substantially when used in mineral and muck soils, but adding the sand and soil-less mix in controlled-environment tests may produce additional variations in levels of organic matter, moisture, and possibly compaction, which appear to increase the complexity of microbial interactions and variation in the outcome of biocontrol.
A soil-drench application with aqueous formulations of biofungicides or synthetic fungicides will not be practical for managing clubroot disease in canola. A formulation that balances the need of BCA for survival and antagonism against the target pathogen would enhance the performance of the biofungicide. Formulation ingredients are important for preserving the viability and efficacy of biopesticides, and also need to be economical and compatible with standard application equipment (Burges Citation1998; Hynes & Boyetchko Citation2011).
For managing clubroot disease, formulations that deliver the BCA to the rhizosphere as a seed dressing or granular inoculant would provide the most efficient root protection. Bacillus subtilis, which produces spores resistant to adverse conditions, has a distinct advantage with regard to the selection of formulation ingredient and processes (Schisler et al. Citation2004). A seed-treatment formulation was developed using the complex coacervate reaction to microencapsulate B. subtilis (Serenade) (Mayya et al. Citation2003; Hynes et al. Citation2010), and was applied to the seed coat at a rate of 1 × 105 cfu seed−1. Also, a soil-applied granular formulation (0.7 mm dia.) made of corn starch and peat mixed with a B. subtilis suspension (1× 1011 cfu mL−1) via extrusion and spheronization (Hynes & Boyetchko Citation2011) was developed. There was no change in the B. subtilis titre with these formulations after 8–14 wk of storage at room temperature, and their efficacy was equivalent to that of Serenade in aqueous form under controlled conditions (R.K. Hynes, unpublished data).
Cultivar resistance
In general, sources of clubroot resistance are limited (Hirai Citation2006). Before 2009, most Canadian canola cultivars were highly susceptible to pathotype 3, which dominated in Alberta, but were resistant to pathotype 6, common in eastern Canada (Strelkov et al. Citation2006; Deora et al. Citation2012, Citation2013). Pathotype 2 is also common in eastern Canada (Cao et al. Citation2009) on vegetable (Ayers & Lelacheur Citation1972; Chiang & Crête Citation1985, 1989) and canola crops (Vigier et al. Citation1989; Pageau et al. Citation2006). In Europe, the resistant oilseed rape cultivars ‘Mendel’ and ‘Tosca’ were developed in the early 2000s, with each carrying a single pathotype-specific resistance gene. The resistance in these cultivars broke down quickly in the UK under high clubroot disease pressure (Diederichsen et al. Citation2003; Oxley Citation2007). In Canadian studies, the resistance in ‘Mendel’ appeared to be effective against the five P. brassica pathotypes (2, 3, 5, 6 and 8) found in Canada (Rahman et al. Citation2013), while ‘Tosca’ was susceptible to pathotype 3 but resistant to pathotype 2 (Peng et al. Citation2013). These results indicate that the resistance spectrum may vary and some of the R genes may be ineffective in certain regions depending on the dominant pathotypes present.
Genetic resistance has become a cornerstone for managing clubroot disease in canola. The first resistant canola cultivar adapted for Canada, ‘45H29’ (Pioneer Hi-Bred Canada), was released in 2009. Since then, several more resistant canola cultivars have also been introduced to the marketplace. There has been a rapid uptake of these cultivars in regions where clubroot is most common, and they now occupy a substantial proportion of the hectarage in heavily infested areas. The genetic basis for clubroot resistance in these cultivars may be quite narrow (Deora et al. Citation2013); several cultivars are believed to use a resistance gene derived from ‘Mendel’, and a small proportion of plants of these cultivars developed small galls under high pathogen inoculum pressure (LeBoldus et al. Citation2012). These lightly diseased plants may reflect segregation or incomplete gene penetrance, but also may represent the first stage of pathogen adaptation to the resistance gene(s) in these cultivars. It is anticipated that the deployment of these cultivars across a large hectarage will exert selection pressure on the pathogen population. The identification and development of new sources of clubroot resistance for deployment in canola is the primary focus for several research groups in Canada, and a high priority for several seed companies.
Little is known about the mechanisms of clubroot resistance. In the root cortex, P. brassicae plasmodia develop quickly in susceptible plants but are extremely limited in resistant cultivars (Kroll et al. Citation1983; Deora et al. Citation2012, Citation2013). A hypersensitive response was linked to restricting the development of P. brassicae in the root cortex of turnip and Arabidopsis (Dekuijzen Citation1979; Kobelt et al. Citation2000), but was not observed in radish, Chinese cabbage or cauliflower (Kroll et al. Citation1983; Tanaka et al. Citation2006; Donald et al. Citation2008). No hypersensitive response was detected in canola, but the accumulation of reactive oxygen species near the endodermis was suppressed by infection and colonization by P. brassicae (Deora et al. Citation2013). When inoculated with an avirulent P. brassicae pathotype, resistant canola plants exhibited reduced growth and delayed development, relative to un-inoculated plants, despite being free of clubbing symptoms (Hwang et al. Citation2012; Deora et al. Citation2013). Similarly, clubroot resistant cabbage cultivars grown in a low inoculum field site produced higher marketable yields than the same cultivars grown in a high inoculum area of the same field. The plants showed no symptoms of clubroot disease at either site (Gludovacz Citation2013). This indicates that there may be a ‘metabolic cost’ to the expression of resistance under high inoculum pressure. Based on these observations, our question is: Would management practices that potentially reduce the inoculum load in soil, such as crop rotation, benefit a resistant canola cultivar in a heavily infested field? This will be addressed in the third field study to be presented in the last part of this review.
The durability of resistance is a major concern for canola producers. Prior experience indicates that host resistance based on a single gene is unlikely to be durable. The P. brassicae population in many Alberta fields appears to be genetically heterogeneous (Cao et al. Citation2009), and none of the cultivars are immune to infection (LeBoldus et al. Citation2012). A recent study showed that there were no substantial differences in responses among clubroot-resistant canola cultivars to the P. brassicae pathotypes prevalent in Canada (Deora et al. Citation2012, Citation2013). Since most sources of resistance are pathotype-specific, this represents one line of evidence that these cultivars may possess similar types of resistance. At the same time, pathogen populations repeatedly cycled on one canola cultivar, which showed increased virulence on that cultivar, did not show similar increases in virulence when other clubroot-resistant canola cultivars were inoculated (LeBoldus et al. Citation2012). This indicates that more than one mechanism may be present in these resistant cultivars. These apparently contradicting results highlight the need for direct genetic studies to elucidate clearly the relationships of the resistance genes incorporated into the various canola cultivars.
Cropping systems and crop rotation
Crop rotation has been recognized for many years for its benefits to crop production (Cook Citation2006). In western Canada, greater crop diversity has been promoted since the 1980s to replace systems consisting primarily of cereals and fallow. Increased cropping diversity has helped reduce the need for tillage (Gan et al. Citation2010) and alleviated the risk to canola production posed by blackleg disease [Leptosphaeria maculans (Desmaz.) Ces. & De Not] (Guo et al. Citation2005). Over the last 10 years, the hectarage devoted to canola crops in western Canada has expanded substantially, and crop diversity has been reduced in many areas. A 3-year rotation of canola with cereals and pulses is considered sustainable (Cathcart et al. Citation2006), but a 2-year rotation of canola with a cereal crop or even continuous canola is no longer uncommon (Hartman Citation2012). This increasingly intensive canola production has been driven primarily by increased market demand, and facilitated, at least in part, by the availability of disease-resistant and herbicide-tolerant canola cultivars.
Although the use of the short cereal–canola rotation or even continuous canola is a relatively recent development, their sustainability from the perspective of management of important diseases such as blackleg (Kutcher et al. Citation2013) and clubroot (Peng et al. Citation2013) is being evaluated. The increasing use of short rotations between successive canola crops challenges conventional husbandry that calls for a 3- or 4-year rotation in canola production and raises several important questions. First, do 3-year crop rotations with resistant cultivars produce real benefits to canola growers? Second, can such cultivars be used with crop rotation and/or biofungicides to maximize the yield in heavily infested fields? The following three field studies were used to address these questions.
Field Study 1: potential synergy between cultivar resistance and biofungicides
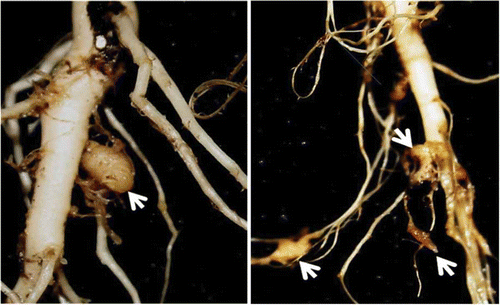
The introduction of resistant cultivars allowed canola to be grown in many Alberta fields that were heavily infested with resting spores of P. brassicae. None of these cultivars is immune and small clubs can develop on some of the plants (), especially under high inoculum pressure (Peng et al. Citation2011a). In field trials, a resistant canola cultivar showed an average DSI of 11% compared with 100% in a susceptible one (Peng et al. Citation2011b), but biofungicide and fungicide treatments did not reduce clubroot severity substantially. The small clubs that develop on resistant cultivars are a cause for concern. In western Canada, where mixed populations of P. brassicae pathotypes are known to occur (Xue et al. Citation2008; Cao et al. Citation2009), the potential risk for selection of pathogen genotypes capable of overcoming clubroot resistance appears to be high (Jones et al. Citation1982; Fahling et al. Citation2003). A study was initiated to explore the potential synergy between a biofungicide treatment and host resistance. The resistant cultivar can effectively reduce inoculum load in the soil (Hwang et al. Citation2013) and the biofungicide may potentially decrease the incidental infection on these cultivars (Peng et al. Citation2011a), thus slowing the development of virulent pathotypes and contributing to resistance stewardship.
Fig. 1. (Colour online) Small clubs (arrow) developing on roots of a resistant canola cultivar 4 wk after inoculation with a resting spore suspension of Plasmodiophora brassicae at 1 × 107 spores cc−1 soil under controlled-environment conditions.
In a controlled environment, the biofungicide Serenade® was applied to susceptible (S), moderately resistant (MS) and resistant (R) canola cultivars exposed to an extremely high dose of pathogen inoculum (approximately 1 × 108 resting spores cc−1 soil). There was a slight interaction between Serenade and cultivar resistance. The biofungicide substantially reduced clubroot severity on S and MR cultivars relative to non-treated controls (Peng et al. Citation2011a). On the R cultivar, the symptom on non-treated plants was negligible under controlled conditions and application of the biofungicide did not reduce the disease level any further. On the MR cultivar, which showed a moderate level of clubbing, Serenade reduced the DSI to the level observed on the resistant cultivar (Peng et al. Citation2011a).
To validate this promising but still preliminary result observed under controlled conditions, field trials were carried out at three locations in 2010. The canola cultivars ‘45H26’ (S) and ‘45H29’ (R) were seeded in heavily infested fields near Leduc and Edmonton, Alberta and Normandin, Quebec. The predominant strain of P. brassicae is pathotype 3 in Alberta but pathotype 2 in Quebec. A granular formulation of B. subtilis (Serenade® ASO 13 L ha−1) in a starch and peat mixture (as described earlier) was applied in-furrow to the susceptible and resistant cultivars. This granular formulation disintegrated rapidly when hydrated, releasing the active ingredients into the soil (Hynes & Boyetchko Citation2011). Commercial corn-cob grits coated with Serenade® ASO (13 L ha−1) were also assessed as a low-cost formulation for B. subtilis as well as for fluazinam and cyazofamid. Each formulation was pre-mixed with canola seed and applied at 50 kg ha−1 at seeding. All of the formulations suppressed clubroot disease in controlled growth cabinet trials. Clubroot severity was assessed on 25 plants per plot at late flowering using a standard 0–3 scale (Strelkov et al. Citation2006) and seed yield per plot was assessed at crop maturity.
Weather conditions were generally conducive to clubroot development at each of the trial locations. The disease pressure was high, with DSI ranging from 69% to 98% on the non-treated S cultivar (). The trends in clubroot severity and canola yield were similar across the locations; DSI for cultivar ‘45H29’ was consistently low (<15%) and the seed yield of the resistant cultivar was 73% to 81% higher than the S cultivar. None of the biofungicide or synthetic fungicide formulations reduced DSI on S or R cultivars. The synergy between Serenade and moderate resistance observed previously under controlled conditions was not apparent in these field trials, possibly due to the lack of efficacy of the biofungicide formulations.
Fig. 2. Clubroot disease severity index and seed yield of a susceptible (grey bars) and resistant (black bars) canola cultivar treated with a fungicide or biofungicide formulation in a field trial near Leduc, Alberta. The field had been heavily infested by Plasmodiophora brassicae for several years.
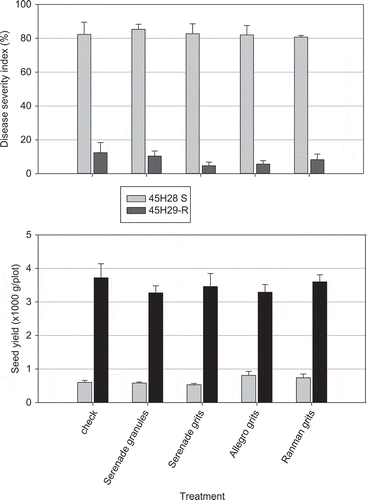
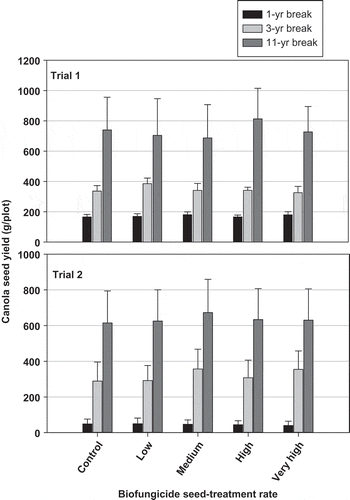
Fig. 3. Effect of biofungicide (Bacillus subtilis) seed treatment and crop-rotation (1-, 3-, or 11-year break from a canola crop) on canola yield in a field near Normandin, Quebec that was heavily infested by Plasmodiophora brassicae since 1990s. The biofungicide rates from low to very high were in four equal increments from 1 × 105 to 5 × 106 cfu seed−1.
The peat and starch granular formulation of B. subtilis biofungicide has a relatively long shelf-life (R.K. Hynes, unpublished data) and was easy to apply. This formulation did not enhance the efficacy of the biofungicide substantially over an aqueous form used in previous trials, where poor survival of BCA in the dry soil was suspected (Peng et al. Citation2011b). The longevity of BCA in a granular formulation in field soil has not been determined, but an effective dose of Serenade throughout the period of primary and secondary infection is required to provide maximum protection against clubroot disease (Lahlali et al. Citation2013). Based on the effect of temperature on the timing of primary and secondary infection (Hwang et al. Citation2011b; Sharma et al. Citation2011), the formulation may need to maintain the biocontrol effect for up to 3 weeks after seeding. Efficient distribution of the BCA can also be a challenge – even at a high application rate of 50 kg ha−1, there was hardly any direct contact of granules with canola seed after seeding (G. Peng, personal observations). A seed treatment may be more efficient in delivering the BCA to where it is needed most, i.e. on the radicle of the germinating seedling, compared with a granular formulation.
In conclusion, this study demonstrated that resistant cultivars should be used as the first line of defence for management of clubroot disease on canola. Also, the development of more effective formulations for biofungicides and synthetic fungicide is required to increase their efficacy before they can be considered for use in commercial canola production. Finally, seed treatments should be considered for more efficient and effective field delivery of BCAs against clubroot disease.
Field Study 2: biofungicide seed dressing and crop rotation
To improve the efficiency of delivery and potential efficacy of biocontrol, two seed treatment formulations of B. subtilis were evaluated in controlled environment studies. The complex coacervate microencapsulation (Mayya et al. Citation2003; Hynes et al. Citation2010) was used to apply Serenade (B. subtilis QST 713) to seed of a susceptible (S) canola cultivar at 1 × 105 cfu seed−1. Similarly, the biofungicide Kodiak® (B. subtilis GB03, Bayer CropScience) with a commercial seed-treatment formulation L1782 (Bayer CropScience) was applied to seed of an S cultivar at rates from 1 × 105 to 5 × 106 cfu seed−1. The treated seed was planted in soil-less potting mix infested with resting spores of P. brassicae at 1 × 104 and 1 × 105 resting spores cc−1. The seed treatments were moderately effective at the lower inoculum rate, but ineffective at the higher rate.
Seed treatments with BCAs have several potential advantages over granular formulations, including ease of application, lower cost and proximity to the root during seed germination. Despite these advantages, the relatively low efficacy and sensitivity to inoculum pressure indicate that seed treatment cannot be used as a single option for management of clubroot disease. Seed treatment with BCAs might have potential when used in combination with cropping rotations that reduce the inoculum level in soil. To assess this possibility, trials were conducted in a pathogen-infested field where a crop-rotation study had been continuing for the previous 12 years. The objective was to assess the potential for use of a biofungicide seed treatment in situations where inoculum concentration is low, e.g. reduced by crop rotation.
The study site was located on the AAFC Normandin Experimental Farm, Quebec. Severe clubroot had been observed at the site since the early 1990s. In 2011, a susceptible canola cultivar was treated with Kodiak biofungicide plus L1782 at rates from 1 × 105 to 5 × 106 cfu seed−1. Three crop rotations were used: a 12-year break (continuous barley), 3-year break (canola–barley–field pea–barley), and 1-year break (canola–barley) from the previous susceptible canola crop. Treatments with 1- and 3-year breaks would represent 2-year and 4-year crop rotations in canola production. Plots that had been sown to barley for 12 years were used to represent an extremely long break from canola, for comparison purposes only.
Nitrogen (N), phosphorus (P2O5) and potassium (K2O) fertilizers were applied according to soil analysis (Mechlich-3 extraction) to obtain 90 kg N, 50 kg P2O5, and 50 kg K2O ha−1 (Guide de référence en fertilisation Citation2010). Fertilizers were broadcast onto the soil surface and incorporated by cultivation before seeding. Soil samples were taken from the top 15 cm at five random sites of each rotation block, and the inoculum potential of P. brassicae was estimated using a bioassay. In addition, five plants were pulled randomly from the control plots within each crop-rotation block at 3 weeks after seeding, and DNA content of P. brassicae in the roots was estimated using qPCR (Sundelin et al. Citation2010; Lahlali et al. Citation2011). Clubroot severity was assessed at flowering as described previously. In addition, the impact on above-ground crop development was also evaluated on a 0–4 scale, where: 0 = plants develop normally, and 4 = >75% plants showing stunting and wilting symptoms, and the plot is thinned. Canola seed yield was taken at crop maturity. The trial was repeated by planting a second crop of canola in a different area of the same field one week after the initial trial began.
Inoculum potential declined as the break from canola increased; the 1-year break resulted in the highest DSI in the bioassay and the greatest amount of P. brassicae DNA in the roots of young plants in field trials. The DSI in field trials exceeded 90% in all of the treatments and none of the biofungicide seed treatments reduced clubroot severity effectively (Fig. 3). Plants in the 3- and 12-year break treatments produced slightly smaller clubs, developed more normally (less stunting and wilting), and had higher yield than those in the 1-year break treatment (Peng et al. Citation2013). For example, the 3-year break increased seed yield over that of 1-year break by >100%, although the yield per unit was still low in all rotation treatments, even for the 12-year break.
In conclusion, this study indicated that a 3-year break (growing canola in a 4-year rotation with non-host crops) reduced pathogen inoculum as well as the clubroot disease impact on canola, even in a heavily infested field. It is important to note that the yield of the susceptible cultivar was much lower than required for acceptable commercial production, even after an 11-year break from canola. The impact of crop rotation on reducing inoculum potential and increasing canola yield merits further investigation.
Field Study 3: interaction of resistant cultivars and crop rotation
A study was carried out at the Normandin site in 2012 to assess the impact of length of cropping rotation (0-, 1-, 2-, 3- or 4-year break from canola) on S, MS and R canola cultivars. The materials and methods used were similar to that used in the 2011 trial. A second trial was seeded at a different location of the same field 1 week later.
As in 2011, a longer break from canola generally reduced inoculum levels in the soil; a 2-year break reduced the concentration of resting spores by >90% relative to that in the continuous canola or 1-year break plots, based on qPCR results (Peng et al. Citation2013). Although the longer break did not reduce DSI on S or MS cultivars relative to that in continuous canola, the disease impact on crop development was reduced and the yield of canola was increased substantially compared with continuous canola. The 3-year or a longer break increased the yield of S and MR/MS cultivars relative to shorter rotations, but the yield was still very low. On the R cultivar, however, a 2-year break increased the yield by 25% over continuous canola, despite the similar DSI and crop impact ratings (Peng et al. Citation2013). This result appears consistent with previous observations of an apparent metabolic cost to the expression of resistance under high inoculum pressure on canola in controlled environments (Hwang et al. Citation2011a; Deora et al. Citation2012) and on cabbage under field conditions (Gludovacz Citation2013).
Host resistance is often an active response that involves the up-regulation of genes and activation of metabolic pathways to produce phytoalexins, phenols, lignin and other compounds involved in resisting pathogen invasion (Schneider et al. Citation1996; Siemens et al. Citation2006; Kaur et al. Citation2011). Data from several studies support the hypothesis that resistance to clubroot disease is an active process in brassica crops and that there is a physiological cost to the resistance reaction under heavy pathogen inoculum conditions. For example, seedlings of four resistant canola cultivars inoculated with high doses of P. brassicae resting spores were smaller and developed more slowly than un-inoculated controls under controlled conditions, although no clubbing symptoms were observed in any of these plants (Deora et al. Citation2013). The pathogen colonized the root hairs extensively and some secondary development in the root cortex was also observed (Deora et al. Citation2013). It appears likely that the infected plants expended energy reserves and other metabolic resources to resist the infection that might otherwise have contributed to the growth and yield. The current study demonstrates the value of using a resistant cultivar in conjunction with a 3-year crop rotation (2-year break) to maximize the yield potential of canola in heavily infested fields.
Conclusions
Host resistance is clearly a cornerstone for the effective management of clubroot disease on canola, where it represents the only viable management approach to allow re-cropping of canola in the heavily infested fields in central Alberta. Due to the diversity of P. brassicae populations and a possible narrow genetic background of resistant canola cultivars, resistance stewardship is needed to ensure the long-term effectiveness of this important management approach. Integrated strategies may be considered for clubroot disease management in the future, but practical measures are limited. At this time, a resistant canola cultivar used in conjunction with a 3-year crop rotation is likely to be the most effective and practical strategy for clubroot disease management; this practice reduces the inoculum loads in the field and allows the resistant cultivar to reach maximum yield potential. This recommendation also affirms the merit of a 3-year crop rotation already utilized by some producers in western Canada. Until better formulations are developed and greater efficacy achieved, use of synthetic fungicides or biofungicides is not yet commercially feasible for management of clubroot disease on canola based on the results from our research.
Acknowledgements
We thank Linda McGregor, Dan Hupka, Jon Geissler (AAFC, Saskatoon, SK), George Turnbull (AARD, Edmonton, AB), Isabelle Morasse (AAFC, Normandin, QC), Laura Riches (Muck Crop Research Station, Univ. Guelph, Kettleby, ON), and Derek Rennie (Univ. Alberta, Edmonton, AB) for technical support in conducting the studies reported in this review. Financial support from SaskCanola (2008–2010), the Saskatchewan Agriculture Development Fund (2010–2013, Project #20090359) and AAFC Clubroot Risk Mitigation Initiatives under the Growing Forward program of the Canadian Federal Government (2009–2013) to the senior author is acknowledged.
References
- Abbasi PA, Lazarovits G. 2006. Effect of soil application of AG3 phosphonate on the severity of clubroot of Bok Choy and cabbage caused by Plasmodiophora brassicae. Plant Dis. 90:1517–1522.
- Adhikari KKC. 2010. Effect of temperature, biofungicides and fungicides on clubroot of selected Brassica crops. [M.Sc. Thesis]. Guelph (ON): University of Guelph.
- Ahmed HU, Hwang SF, Strelkov SE, Gossen BD, Peng G, Howard RJ, Turnbull G. 2011. Assessment of bait crops to reduce inoculum of clubroot (Plasmodiophora brassicae) of canola. Can. J. Plant Sci., 91:545–551.
- Arie T, Koayashi Y, Okada G, Kono Y, Yamaguchi I. 1998. Control of soilborne clubroot disease of cruciferous plants by epoydon from Phoma glomerata. Plant Pathol. 47:743–748.
- Ayers GW, Lelacheur KE. 1972. Genetics of resistance in rutabaga to two races of Plasmodiophora brassicae. Can J Plant Sci. 52:897–900.
- Buczacki ST, Toxopeus H, Mattusch P, Johnston TD, Dixon GR, Hobolth LA. 1975. Study of physiologic specialization in Plasmodiophora brassicae: Proposals for attempted rationalization through an international approach. Trans. Br. Mycol. Soc., 65:295–303.
- Burges HD. 1998. Formulation of microbial biopesticides: beneficial microorganisms, nematodes and seed treatments. Dordrecht: Kluwer Academic Publishers. 412 pages.
- Cao T, Manolii VP, Hwang SF, Howard RJ, Strelkov SE. 2009. Virulence and spread of Plasmodiophora brassicae (clubroot) in Alberta, Canada. Can J Plant Pathol. 31:321–329.
- Cathcart RJ, Topinka AK, Kharbanda P, Lange R, Yang RC, Hall LM. 2006. Rotation length, canola variety and herbicide resistance system affect weed populations and yield. Weed Sci. 54:726–734.
- Cheah LH, Verakone S, Kent G. 2000. Biological control of clubroot on cauliflower with Trichoderma and Streptomyces spp. NZ Plant Prot. 53:18–21.
- Chiang MS, Crête R. 1985. Acadie: a clubroot resistant cabbage cultivar. Can. J. Plant Sci., 65:233–235.
- Chiang MS, Crête R. 1989. Richelain: a clubroot-resistant cabbage cultivar. Can. J. Plant Sci., 69:337–340.
- Cook RJ. 2006. Toward cropping systems that enhance productivity and sustainability. Proc. Nat. Acad Sci. USA. 103:18389–18394.
- Crute IR, Gray AR, Crisp P, Buczacki ST. 1980. Variation in Plasmodiophora brassicae and resistance to clubroot disease in Brassicas and allied crops – A critical review. Plant Breed Abstr. 50:91–104.
- Datnoff LE, Kroll TK, Lacy GH. 1987. Efficacy of chlorine for decontaminating water infested with resting spores of Plasmodiophora brassicae. Plant Dis. 71:734–736.
- Dekuijzen HM. 1979. Electron microscopic studies on the root hairs and cortex of a susceptible and a resistant variety of Brassica campestris infected with Plasmodiophora brassicae. Neth J Plant Pathol. 85:1–17.
- Deora A, Gossen BD, Walley FL, McDonald MR. 2011. Boron reduces development of clubroot in canola. Can J Plant Pathol. 33:475–484.
- Deora A, Gossen BD, McDonald MR. 2012. Infection and development of Plasmodiophora brassicae in resistant and susceptible canola cultivars. Can J Plant Pathol. 34:239–247.
- Deora A, Gossen BD, McDonald MR. 2013. Cytology of infection, development, and expression of resistance to Plasmodiophora brassicae in canola. Ann Appl Biol. 163:56–71.
- Diederichsen E, Beckmann J, Schondelmeier J, Dreyer F. 2006. Genetics of clubroot resistance in Brassica napus ‘Mendel’. Acta Hort. 706:307–311.
- Diederichsen E, Deppe U, Sacrista`n MD. 2003. Characterization of clubroot resistance in recent winter oilseed rape material. In: Proceedings of the 11th International Rapeseed Congress, 2003 Jul 6; Copenhagen 68–70.
- Diederichsen E, Frauen M, Linders EGA, Hatakeyama K, Hirai M. 2009. Status and perspectives of clubroot resistance breeding in crucifer crops. J Plant Growth Regul. 28:265–281.
- Dixon GR. 2003. Clubroot, a case for integrated control. Plantsman. 2:79–183.
- Dixon GR. 2006. The biology of Plasmodiophora brassicae Wor. – a review of recent advances in United Kingdom. Acta Hort. 706:271–282.
- Dixon GR. 2009. The occurrence and economic impact of Plasmodiophora brassicae and clubroot disease. J Plant Growth Regul. 28:194–202.
- Dixon GR, Page LV. 1998. Calcium and nitrogen eliciting alterations to growth and reproduction of Plasmodiophora brassicae (clubroot). Acta Hort. 459:343–349.
- Donald EC, Porter IJ. 2009. Integrated control of clubroot. J. Plant Growth Regul. 28:289–303.
- Donald EC, Porter IJ, Lancaster RA. 2001. Band incorporation of fluazinam (Shirlan) into soil to control clubroot of vegetable brassica crops. Austral J Exp Agric. 41:1223–1226.
- Donald EC, Jaudzems G, Porter IJ. 2008. Pathology of cortical invasion by Plasmodiophora brassicae in clubroot resistant and susceptible Brassica oleracea hosts. Plant Pathol. 57:201–209.
- Doyle OPE, Clancy KJ. 1987. Effects of eight fungicides on clubroot of brassicas, disease severity and plant yield. Ann Appl Biol. 110(Suppl), 50–51.
- Fähling M, Graf H, Siemens J. 2003. Pathotype separation of Plasmodiophora brassicae by the host plant. J Phytopathol. 151:425–430.
- Finlayson DG, Campbell CJ. 1971. Fungicides for preventing clubroot of cauliflower in loam and peat soils. Can Plant Dis Surv. 51:122–126.
- Gan Y, Kutcher HR, Menalled F, Lafond G, Brandt SA. 2010. Crop diversification and intensification with broadleaf crops in cereal-based cropping systems in the Northern Great Plains of North America. In: Malhi SS, Gan Y, Schoenau JJ, Lemke RL, Liebig MA, editors. Recent Trends in Soil Science and Agronomy Research in the Northern Great Plains of North America. Trivandrum, Kerala: Research Signpost; p. 277–299.
- Gludovacz TV. 2013. Clubroot in canola and cabbage in relation to soil temperature, plant growth and host resistance. M.Sc.Thesis, University of Guelph, Guelph (ON). Available from: https://atrium.lib.uoguelph.ca/xmlui/bitstream/handle/10214/6660/Gludovacz_Thomas_201305_Msc.pdf.
- Gludovacz TV, Gossen BD, McDonald MR. 2013. Evidence for tolerance to Plasmodiophora brassicae in a cabbage cultivar. Can J Plant Pathol. 35:111 [Abstr.].
- Gossen BD, Adhikari KK, McDonald MR. 2012. Effect of seeding date on development of clubroot in short-season Brassica crops. Can J Plant Pathol. 34:516–523.
- Gossen BD, Kasinathan H, Cao T, Manolii VP, Strelkov SE, Hwang SF, McDonald MR. 2013a. Influence of pH and temperature on infection and symptom development of clubroot in canola. Can J Plant Pathol. 35:294–303.
- Gossen BD, McDonald MR, Hwang SF, Strelkov SE, Peng G. 2013b. Comparison of clubroot (Plasmodiophora brassicae) development and management on canola and Brassica vegetables. Can J Plant Pathol. 35:175–191.
- Gowers S. 1982. The transfer of characters from Brassica campestris L. to Brassica napus L.: Production of clubroot-resistant oil-seed rape (B. napus ssp oleifera). Euphytica. 31:971–976.
- Guide de référence en fertilisation. 2010. Centre de référence en agriculture et agroalimentaire du Québec (2nd ed.), Sainte-Foy, QC, Canada, 473 pages.
- Guo XW, Fernando WGD, Entz M. 2005. Effects of crop rotation and tillage on blackleg disease of canola. Can J Plant Pathol. 27:53–57.
- Hartman M. 2012. Canola rotation performance. In: Proceedings of the FarmTech; 2012 Jan 24; Red Deer (AB). Available from: http://farmtechconference.com/wp-content/uploads/2013/01/Murray-Hartman-FarmTech2012.pdf.
- Hatakeyama K, Fujimura M, Ishida M, Suzuki T, Sato T. 2006. Classification of pathogenicity of Plasmodiophora brassicae field isolates in Japan based on resistance of F1 cultivars of Chinese cabbage (Brassica rapa L.) to clubroot. Acta Hort. 706:323–328.
- Health Canada. 2010. Re-evaluation Decision RVD2010-06, Quintozene. Available from http://hc-sc.gc.ca/cps-spc/pubs/pest/decisions/rvd2010-06/index-eng.php#re.
- Hirai M. 2006. Genetic analysis of clubroot resistance in Brassica crops. Breed Sci. 56:223–229.
- Hirai M, Harada T, Kubo N, Tsukada M, Suwabe K, Matsumoto S. 2004. A novel locus for clubroot resistance in Brassica rapa and its linkage markers. Theor Appl Genet. 108:639–643.
- Hwang SF, Strelkov SE, Turnbull GD, Manolii V, Howard RJ, Hartman M. 2008. Soil treatments and amendments for management of clubroot on canola in Alberta, Canada. J Plant Pathol. 90:410 [Abstr.].
- Hwang SF, Strelkov SE, Turnbull GD, Manolii V, Howard RJ, Hartman M. 2008. Soil treatments and amendments for management of clubroot on canola in Alberta, Canada. J Plant Pathol. 90:410 [Abstr.].
- Hwang SF, Ahmed HU, Zhou Q, Strelkov SE, Gossen BD, Peng G, Turnbull GD. 2011a. Influence of cultivar resistance and inoculum concentration on root hair infection of canola (Brassica napus) by Plasmodiophora brassicae. Plant Pathol. 60:820–829.
- Hwang SF, Strelkov SE, Gossen BD, Turnbull GD, Ahmed HU, Manolii V, Howard RJ. 2011b. Soil treatments and amendments for amelioration of clubroot of canola. Can J Plant Sci. 91:999–1010.
- Hwang SF, Ahmed HU, Zhou Q, Rashid A, Strelkov SE, Gossen BD, et al. 2012. Assessment of the impact of resistant and susceptible canola on Plasmodiophora brassicae inoculum potential. Plant Pathol. 61:945–952.
- Hwang SF, Howard RJ, Strelkov SR, Gossen BD, Peng G. 2014. Management of clubroot (Plasmodiophora brassicae) on canola (Brassica napus) in western Canada. Can J Plant Pathol. 36(S1):49–65.
- Hynes RK, Boyetchko SM. 2011. Improvements to the pesta formulation to promote survival and dispersal of Pseudomonas fluorescens BRG100, green foxtail bioherbicide. Pest Tech. 5:80–87.
- Hynes RK, Chumala PB, Hupka D, Peng G. 2010. A complex coacervate formulation for delivery of Colletotrichum truncatum 00-003B1. Weed Tech. 24:185–192.
- Jones DR, Ingram DS, Dixon GR. 1982. Characterization of isolates derived from single resting spores of Plasmodiophora brassicae and studies of their interaction. Plant Pathol. 31:239–245.
- Joo GJ, Kim YM, Kim JW, Kim WC, Rhee IK, Choi YH, Kim JH. 2004. Biocontrol of cabbage clubroot by the organic fertilizer using Streptomyces sp. AC-3. Kor J Microbiol Biotechnol. 32:173–178.
- Kamei A, Tsuro M, Kubo N, Hayashi T, Wang N, Fujimura T, Hirai M. 2010. QTL mapping of clubroot resistance in radish (Raphanus sativus L). Theor Appl Genet. 120:1021–1027.
- Kasinathan H. 2012. Influence of pH, temperature, and biofungicides on clubroot of canola. M.Sc. thesis, University of Guelph, Guelph (ON). Available from http://atrium.lib.uoguelph.ca/xmlui/bitstream/handle/10214/3547/Hema Kasinathan Thesis May 1 2012.pdf.
- Kaur P, Jost R, Sivasithamparam K, Barbetti MJ. 2011. Proteome analysis of the Albugo candida–Brassica juncea pathosystem reveals that the timing of the expression of defence-related genes is a crucial determinant of pathogenesis. J Expt Bot. 62:1285–1298.
- Kobelt P, Siemens J, Sacristán MD. 2000. Histological characterisation of the incompatible interaction between Arabidopsis thaliana and the obligate biotrophic pathogen Plasmodiophora brassicae. Mycol Res. 104:220–225.
- Kroll TK, Lacy GH, Moore LD. 1983. A quantitative description of the colonization of susceptible and resistant radish plants by Plasmodiophora brassicae. J Phytopathol. 108:97–105.
- Kuginuki Y, Hiroaki Y, Hirai M. 1999. Variation in virulence of Plasmodiophora brassicae in Japan tested with clubroot-resistant cultivars of Chinese cabbage (Brassica rapa L. sp. pekinensis. Eur J Plant Pathol. 105:327–332.
- Kutcher HR, Brandt SA, Smith EG, Ulrich D, Malhi SS, Johnston AM. 2013. Blackleg disease of canola mitigated by resistant cultivars and four-year crop rotations in western Canada. Can J Plant Pathol 35:209–221.
- Lahlali R, Peng G. 2013. Clonostachys rosea confers suppression of clubroot on canola via antibiosis and induced host resistance. Plant Pathol. 62, DOI: 10.1111/ppa.12112 (in press).
- Lahlali R, Peng G, McGregor L, Gossen BD, Hwang SF, McDonald MR. 2011. Mechanisms of the biofungicide Serenade® (Bacillus subtilis QST713) in suppressing clubroot. Biocont Sci Technol. 21:1351–1362.
- Lahlali R, Peng G, McGregor L, Yu FQ, Hynes HK, Hwang SF, et al. 2013. Evidence that Bacillus subtilis suppresses clubroot on canola via antibiosis and induced host resistance. Phytopathology. 103:245–254.
- LeBoldus JM, Manolii VP, Turkington TK, Strelkov SE. 2012. Adaptation to Brassica host genotypes by a single-spore isolate and population of Plasmodiophora brassicae [clubroot]. Plant Dis. 96:833–838.
- Mayya KS, Bhattacharyya A, Argillier JF. 2003. Micro-encapsulation by complex coacervation: influence of surfactant. Polymer Intern. 52:644–647.
- McDonald MR, Kornatowski B, McKeown AW. 2004. Management of clubroot of Asian Brassica crops grown on organic soils. Acta Hort. 635:25–30.
- Mitani S, Sugimoto K, Hayashi H, Takii Y, Ohshima T, Matsuo N. 2003. Effects of cyazofamid against Plasmodiophora brassicae Woronin on Chinese cabbage. Pest Manag Sci. 59:287–293.
- Naiki T, Dixon GR. 1987. The effects of chemicals on developmental stages of Plasmodiophora brassicae (clubroot). Plant Pathol. 36:316–327.
- Narisawa K, Tokumasu S, Hashiba T. 1998. Suppression of clubroot formation in Chinese cabbage by the root endophytic fungus, Heteroconium chaetospira. Plant Pathol. 47:206–210.
- Oxley S. 2007. Clubroot disease of oilseed rape and other brassica crops. Technical Note TN 620. Edinburgh (UK). Scottish Agricultural College.
- Pageau D, Lajeunesse J, Lafond J. 2006. Effect of clubroot (Plasmodiophora brassicae) on the productivity and quality of canola. Can J Plant Pathol. 28:137–143.
- Peng G, Lahlali R, Hwang SF, Hynes RK, McDonald MR, Pageau D, et al. 2011a. Control of clubroot on canola using the biofungicide Serenade plus cultivar resistance. In Proceedings of the 13th International Rapeseed Congress; 2011 Jun 5; Prague, Czech Republic (pp. 1134–1137).
- Peng G, McGregor L, Lahlali R, Gossen BD, Hwang SF, Adhikari KKC, et al. 2011b. Potential biological control of clubroot on canola and crucifer vegetable crops. Plant Pathol. 60:566–574.
- Peng G, Pageau D, Strelkov SE, Lahlali R, Hwang SF, Hynes RK, et al. 2013. Assessment of crop rotation, cultivar resistance and Bacillus subtilis biofungicide for control of clubroot on canola. Acta Hort. 1005:591–598.
- Piao ZY, Deng YQ, Choi SR, Park YJ, Lim YP. 2004. SCAR and CAPS mapping of CRb, a gene conferring resistance to Plasmodiophora brassicae in Chinese cabbage (Brassica rapa ssp. pekinensis). Theor Appl Genet. 108:1458–1465.
- Rahman H, Peng G, Yu F, Falk KC, Kulkarni M, Selvaraj G. 2014. Genetics and breeding for clubroot resistance in Canadian spring canola (Brassica napus L). Can J Plant Pathol. 36(S1):122–134.
- Rennie DC, Manolii VP, Cao T, Hwang SF, Howard RJ, Strelkov SE. 2011. Direct evidence of seed and tuber infestation by Plasmodiophora brassicae and quantification of inoculum loads. Plant Pathol. 60:811–819.
- Robak J. 1994. Crop rotation effect on clubroot disease decrease. Acta Hort. 371:223–226.
- Saito M, Kbbo N, Matsumoto S, Suwabe K, Tsukada M, Hirai M. 2006. Fine mapping of the clubroot resistance gene, Crr3, in Brassica rapa. Theor Appl Genet. 114:81–91.
- Sakamoto K, Saito A, Hayashida N, Taguchi G, Matsumoto E. 2008. Mapping of isolate-specific QTLs for clubroot resistance in Chinese cabbage (Brassica rapa L. ssp. Pekinensis). Theor Appl Genet. 117:759–767.
- Saude C, McKeown A, Gossen BD, McDonald MR. 2012. Effect of host resistance and fungicide application on clubroot pathotype 6 in green cabbage and napa cabbage. HortTechnology. 22:311–319.
- Schisler DA, Slininger PJ, Behle RW, Jackson MA. 2004. Formulation of Bacillus spp. for biological control of plant diseases. Phytopathology. 94:1267–1271.
- Schneider M, Schweizer P, Meuwly P, Métraux JP. 1996. Systemic acquired resistance in plants. Inter Rev Cytol. 168:303–340.
- Seaman WL, Walker JC, Larson R. 1963. A new race of Plasmodiophora brassicae affecting Badger Shipper cabbage. Phytopathology. 94:33–43.
- Sharma K, Gossen BD, McDonald MR. 2011. Effect of temperature on cortical infection by Plasmodiophora brassicae and clubroot severity. Phytopathology. 101:1424–1432.
- Siemens J, Keller U, Sarx J, Kunz S, Schuller A, Nagel W. et al. 2006. Transcriptome analysis of Arabidopsis clubroot indicates a key role for cytokinins in disease development. Mol. Plant-Microbe Interact. 19:480–494.
- Strelkov SE, Tewari JP, Smith-Degenhardt E. 2006. Characterization of Plasmodiophora brassicae populations from Alberta, Canada. Can J Plant Pathol. 28:467–474.
- Strelkov SE, Manolii VP, Cao T, Xue S, Hwang SF. 2007. Pathotype classification of Plasmodiophora brassicae and its occurrence in Brassica napus in Alberta, Canada. J Phytopathol. 155:706–712.
- Sundelin T, Christensen CB, Larsen J, Møller K, Lübeck M, Bødker L, Jensen B. 2010. In planta quantification of Plasmodiophora brassicae using signature fatty acids and real time PCR. Plant Dis. 94:432–438.
- Suzuki K, Sugimoto K, Hayashi H, Komyoji T. 1995. Biological mode of action of fluazinam, a new fungicide for Chinese cabbage clubroot. Ann Phytopathol Soc Jpn. 61:395–398.
- Takeshi O, Terumasa K, Shigeru M, Norifusa M, Toshio N. 2004. Development of a novel fungicide, cyazofamid. J Pestic Sci. 29:147–152.
- Tanaka S, Kochi S, Kunita H, Ito S, Kameya-Iwaki M. 1999. Biological mode of action of the fungicide flusulfamide against Plasmodiophora brassicae (clubroot). Eur J Plant Pathol. 105:577–584.
- Tanaka S, Mido H, Ito S. 2006. Colonization by two isolates of Plasmodiophora brassicae with differing pathogenicity on a clubroot-resistant cultivar of Chinese cabbage (Brassica rapa L. subsp. Pekinensis). J Gen Plant Pathol. 72:205–209.
- Townley D, Fox RTV. 2003. Control of clubroot disease using cyazofamid and fluazinam fungicides. In: Proceedings of the 8th International Congress of Plant Pathology; 2003 Feb 2; Christchurch (NZ) (vol. 2, p.72).
- Toxopeus H, Dixon GR, Mattusch P. 1986. Physiological specialization in Plasmodiophora brassicae: an analysis by international experimentation. Trans Brit Mycol Soc. 87:279–287.
- Vigier B, Chiang MS, Hume DJ. 1989. Source of resistance to clubroot (Plasmodiophora brassicae Wor.) in triazine-resistant spring canola (rapeseed). Can Plant Dis Surv. 69:113–115.
- Wallenhammar AC. 1996. Prevalence of Plasmodiophora brassicae in a spring oilseed rape growing area in central Sweden and factors influencing soil infestation levels. Plant Pathol. 45:710–719.
- Webster MA, Dixon GR. 1991a. Boron, pH and inoculum concentration influencing colonization by Plasmodiophora brassicae. Mycol Res. 95:74–79.
- Webster MA, Dixon GR. 1991b. Calcium, pH and inoculum concentration influencing colonization by Plasmodiophora brassicae. Mycol Res. 95:64–73.
- Williams PH. 1966. A system for the determination of races of Plasmodiophora brassicae that infect cabbage and rutabaga. Phytopathology. 56:624–626.
- Xue S, Cao T, Howard RJ, Hwang SF, Strelkov SE. 2008. Isolation and variation in virulence of single-spore isolates of Plasmodiophora brassicae from Canada. Plant Dis. 92:456–462.
