Abstract
The aim of this study was to characterize 93 isolates of Monilinia from stone fruit orchards of Paraná State Brazil, and make inferences concerning their physiological aspects. A synoptic key was utilized at 10 days after incubation for morphological characterization, which allowed us to identify three Monilinia species (M. laxa, M. fructigena and M. fructicola). Molecular analysis was later performed for isolates representative of the morphological species. The polymerase chain reaction (PCR) confirmed only M. fructicola to be present for all isolates collected in Paraná State and contradicted the synoptic key identification. Additionally, isolates were tested physiologically in order to support Monilinia segregation. However, no significant differences were found among the isolates for optimum growth temperature and lesion size of inoculated pear fruit. We concluded that the synoptic key cannot segregate isolates of Monilinia species from southern Brazil and that PCR with specific primers was the best approach.
Résumé
Le but de cette étude était de caractériser 93 isolats de Monilinia obtenus de vergers de fruits à noyau de l’État du Paraná, au Brésil, et d’en déduire les aspects physiologiques. Dix jours après incubation, une clé synoptique a été utilisée en vue d’une caractérisation morphologique, ce qui nous a permis d’identifier trois espèces de Monilinia (M. laxa, M. fructigena et M. fructicola). Une analyse moléculaire a par la suite été effectuée sur les isolats représentatifs de l’espèce morphologique. La réaction en chaîne de la polymérase (RCP) a confirmé que seul M. fructicola avait été trouvé dans tous les isolats collectés dans l’État du Paraná et a contredit l’identification découlant de la clé synoptique. De plus, les isolats ont été testés physiologiquement afin d’appuyer la ségrégation de Monilinia. Toutefois, aucune différence notable n’a été observée parmi les isolats en ce qui a trait à la température optimale de croissance et à la taille des lésions chez les poires inoculées. Nous en avons conclu que la clé synoptique ne permet pas de ségréguer les isolats des espèces de Monilinia du sud du Brésil que la RCP avec amorces appropriées était la méthode la plus efficace.
Introduction
Stone fruits, such as peaches (Prunus persica (L.) Batsch), nectarines (P. persica var. nucipersica (L.) Batsch), and Japanese plums (Prunus salicina Lindley) are cultivated in temperate and subtropical regions where well-defined seasons occur. Most of the stone fruits from Brazil are produced in the southern States of Rio Grande do Sul, Santa Catarina, Paraná and São Paulo. In the 2011 season, the peach production in Brazil was 222.18 tons year−1 and Rio Grande do Sul accounted for 58.2% of peaches destined for the processing industry and fresh consumption (Andrade Citation2011). São Paulo, Santa Catarina and Paraná produced 15.3%, 10% and 7.3% respectively, of the national production (Andrade Citation2011). Despite the similar yields obtained by Paraná (11.13 tons ha−1) with the national average (11.02 tons ha−1), in the last 10 years Paraná has increased production by 25.8%, while in Brazil only an increase of 12.7% in production was achieved (Andrade Citation2011). The peach production in Paraná is 16,260 tons produced on 1460 ha (Andrade Citation2011). Furthermore, Paraná has the benefit of proximity to the largest fruit market in Brazil. Recently, peach and other stone fruit growers in Paraná have been encouraged to increase production by using newer technologies and better agricultural practices to increase yields and boost income. However, there remain unfavourable conditions that need to be overcome for a market that demands high quality of fresh fruit and efficient disease management (Fachinello et al. Citation2000).
Brown rot disease is threatening stone fruit production and causing economic losses worldwide (Adaskaveg et al. Citation2008). Most severe losses occur from pre-harvest until post-harvest, when fruits are stored or displayed in the market (Moreira & May De Mio Citation2007). Brown rot disease is caused by three Monilinia species: M. fructicola (Wint) Honey, M. laxa (Aderh and Ruhl.) Honey, and M. fructigena (Aderh and Ruhl.) Honey. The first species is commonly found in all production areas of Brazilian stone fruit orchards, while M. laxa was first reported in São Paulo peach orchards in 2008 (Souza et al. Citation2008). Despite colony differences observed under laboratory conditions, the brown rot symptoms on fruits are difficult to differentiate to species. The similarity of morphological characteristics makes the distinction of M. laxa from M. fructicola difficult. Conidial size, hyphae diameter, germ tube formation, colony colouration and shape are characteristics that facilitate the species distinction (van Leeuwen & van Kesteren Citation1998). However, these characteristics are easily affected by culture conditions that can compromise the comparative species diagnosis (Byrde & Willetts Citation1977). Considering the difficulties to obtain an accurate diagnosis for Monilinia species, identification protocols were developed to elucidate phenotypical differences based on quantitative colony characteristics (van Leeuwen & van Kesteren Citation1998) and a synoptic key (Lane Citation2002).
To facilitate accurate species identification, other tools provide fast and reliable results. Banks et al. (Citation1997) developed an immunoassay utilizing monoclonal antibodies. However, this method has shown cross-reactive with another fungi. The polymerase chain reaction (PCR) was used for the development of specific primers for Monilinia fructicola by the amplification of the group-I intron sequence for the small subunit (SSU) rDNA region (Fulton & Brown Citation1997), but this method is no long reliable since some studies showed that the intron-containing PCR product is not amplified in all isolates of M. fructicola (Forster & Adaskaveg Citation2000; Côté et al. Citation2004). In contrast, Malvárez et al. Citation(2001), Côté and Tardif et al. Citation(2004) have successfully identified Monilinia species by using species-specific primers with amplification of corresponding PCR products for each species.
The objectives of this study were to: (i) identify the Monilinia species affecting stone fruit production in Paraná State by using macro-morphological features; (ii) characterize a subset of isolates representing each identified species; and (iii) confirm the synoptic identification throughout the PCR technique.
Materials and methods
A total of 93 Monilinia isolates were collected from symptomatic and mummified fruits from 2000 to 2008 during the harvesting time (November until January) in Paraná State, Brazil. Isolates were obtained from several stone fruits, including peaches, nectarines and Japanese plums. They were purified by single-spore and stored in sterile water vials. Isolates were recovered from the sterile water vial storage by placing mycelial plugs in Petri dishes (60 × 12 mm) containing potato dextrose agar (PDA) (Hi-Media Laboratories, Mumbai) and incubating for 4 days at 25 °C with a 12 h light regime. A 4-mm diameter plug was then transferred from the edge of the colony to a new Petri dish (90 × 15 mm) containing PDA amended with lactic acid (APDA, 2.5 mL of 25% lactic acid [w/v]). Three replicates per isolate were made and incubated under the same conditions described above for 10 days. Seven macro-morphological characters (colony colour, growth rate, sporulation, concentric rings of spore production, lobed colony margin, rosettes and black arcs associated with rosettes) were assessed at the 10th day of incubation according to the synoptic key proposed for Monilinia species identification (Lane Citation2002).
After the subjective analysis with the synoptic keys, a subset of isolates identified as M. laxa, M. fructigena and M. fructicola were characterized with species-specific primers (Côté & Tardif et al. Citation2004). DNA of isolates was extracted with the FastDNA kit (Biogene Inc., Carlsbad, CA) following the manufacturer’s guidelines. PCR reactions were carried out in a Perkin Elmer 480 Thermal Cycler (Applied Biosystems, Foster City, CA) using the following program: initial denaturation at 95 °C for 2 min, followed by 35 cycles of 95 ºC for 15 s, 60 ºC for 15 s, an extension at 72 ºC for 1 min, and at the end of the last cycle, a final extension at 72 °C for 3 min. The primers used were: M0386-5 (common reverse primer) 5′-GCA AGG TGT CAA AAC TTC CA-3′; and three forward primers: MO368-5 (M. fructigena) 5′-AGA TCA ACC ATC GTC CAT CT-3′; MO368-10 (M. fructicola) 5′-AAG ATT GTC ACC ATG GTT GA-3′; and Laxa-R2 (M. laxa) 5′- TGC ACA TCA TAT CCC TCG AC-3′. The reaction mixture consisted of 10 to 50 ng template DNA, 20 mM Tris-HCl, pH 8.4, 50 mM KCl, 200 μM dNTP, 2.5 mM MgCl2, 0.2 μM of each specific primers, and 0.25 units of Taq DNA polymerase in a total volume of 10 μL. PCR products were separated by gel electrophoresis on a 1.5% gel.
From the isolates studied with the synoptic key, 75 isolates were selected from the groups of similarity obtained in this study. The radial growth was evaluated at five temperatures (10 ºC, 15 ºC, 20 ºC, 25 ºC and 30 ºC) and 12 h light regime. A 4-day-old mycelial plug (4-mm diameter) was taken from the edge of a colony and placed onto a new Petri dish (90 × 15 mm) with APDA. After 96 h of incubation, the perpendicular diameters of each colony were measured and averaged. Four repetitions were included per isolate and plug diameter was included in these measurements. The third degree polynomial model was adjusted to include mycelial growth and temperature data. The determination of optimum temperature was done by using the mathematical calculus operation, and the obtained values are one of the two solutions of Bhaskara equation (Khuri Citation2003). Confidence interval for optimum temperature was obtained using the Delta Method (Oehlert Citation1992). Optimum growth temperature and growth intervals were determined for each isolate using a linear regression of colony diameter with the statistical software R (version 2.15.1).
The same 75 isolates used to determine the optimum growth temperature were tested for pathogenicity on ripe pear fruit ‘Williams’. Sporulation pattern on pear fruit has been a recommended method to differentiate M. fructicola and M. laxa (Ogawa et al. Citation1995). A 4-mm diameter mycelial plug from a 4 day-old colony on APDA at 25 °C was inoculated on uninjured fruit. Three replicates were prepared per isolate and placed in individual plastic containers (12 cm diameter × 11 cm high). Lesion diameter was measured 4 days after inoculation in two perpendicular directions, including the size of the mycelial plug.
Analysis of variance was used to compare the pathogenicity between isolate and isolate group of similarity. Initially, residuals were evaluated in regards to their normality and 1/x transformed (Box & Cox Citation1964). Results with significant differences (P > 0.05) were compared using pairwise contrasts with false discovery rate (FDR) correction on P-values, for each single hypothesis. The statistical software R (version 2.15.1) was used. All experiments were performed twice.
Results
In total, 11 groups were identified (numbers in parentheses indicate the numbers in each group) based on macro-morphological characteristics described by the synoptic key as follows: group I (1), group II (1), group III (1), group IV (1), group V (3), group VI (2), group VII (46), group VIII (23), group IX (3), group X (9) and group XI (1) (see ). Three groups (I, II and III) were identified as M. laxa, two groups (IV and V) were identified as M. fructigena, and the last six groups (VI, VII, VII, IX, X, XI) were identified as M. fructicola (). The characteristic with the most variation within and among the groups was colony colour. The groups identified as M. laxa had common mycelium growth in distinct layers or ‘resetting’, showing lobed colony margins, and the absence of concentric sporulation rings. Characteristics such as sparse sporulation and absence of black arcs or rings were observed only on isolate 15 (group II) and were not found on isolates 2 and 37, which belong to groups I and III, respectively. As for the colony growth, group I had a medium rate, while groups II and III were slower. Results and colony morphology are shown in and (A1 and A2).
Fig. 1. Macro-morphological features for identification of Monilinia spp. isolates. Colonies after 10 days of incubation at 22 ºC and 12 h light regime on acidified potato dextrose agar. A, Monilinia laxa – isolate number 15. B, Monilinia fructicola – isolate number 50.C, Monilinia fructigena – isolate number 152. (1) upper colony surface, (2) underside of colony.
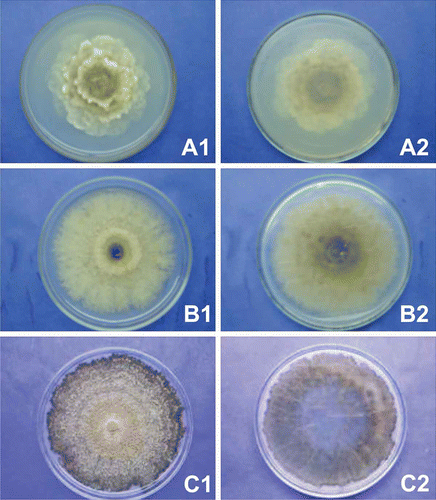
Table 1. Assignment of synoptic key letters to Monilinia isolates identification based in macro-morphological features.
Isolate groups identified as M. fructigena presented the most colony homogeneity. Isolate groups IV (isolate 7) and V (isolates 71, 152 and 179) were characterized by a fast growth rate (> 80 mm), abundant sporulation, and the presence of concentric rings. The lobed colony margin, rosetting, and black arcs or rings were not observed ( and – C1 and C2). Three different colourations were observed among isolates of M. fructicola groups – grey (VIII), yellow (VI, VII and IX) and cream/white (X and XI). For the colony growth rate, isolates were characterized as fast (groups VI, VII, VIII and X) and medium (groups IX and XI). Abundant sporulation was observed for all isolate groups, except those of group VI (isolates 84 and 89) and XI (isolate 50) which had sparse sporulation. The absence of sporulation concentric rings, lobed colony margins, rosetting and black arcs or rings were commonly observed for all M. fructicola groups ( and – B1 and B2).
Amplification using a common reverse and three species-specific primers produced a 444-bp PCR product for isolates identified based on their macro-morphology as M. laxa (isolates 2, 15 and 37), M. fructigena (isolates 7 and 152), and M. fructicola (isolates 100, 101, 157 and 159) indicating they were positive for M. fructicola species, as indicated in .
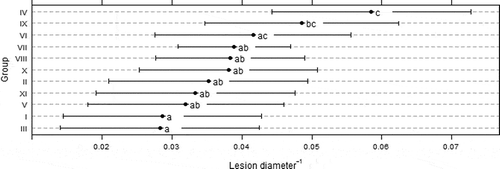
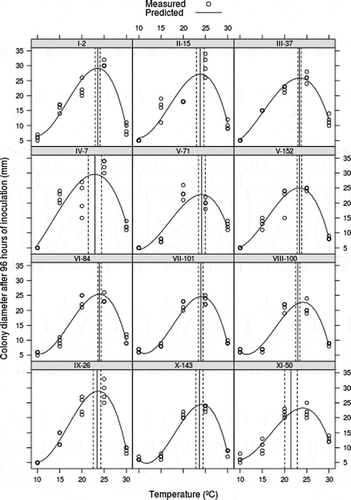
The optimum growth for all isolates was at 23.7 °C, and ranged from 20.5 °C to 24.5 °C without significant differences between isolates groups (P = 0.93) (). The curves of isolate growth for the five temperatures are presented in . The pathogenicity test revealed no statistical differences in lesion growth of isolates belonging to the same macro-morphological group (P = 0.69). However, group IV (M. fructigena) had the slowest lesion rate with significant differences (P = 0.001) compared with other groups, with exception of groups VI (P = 0.074), IX (P = 0.326) and X (P = 0.145) ().
Fig. 2. Growth interval and optimum growth temperature for Monilinia spp. separated in groups based on macro-morphological characteristics and on the cubic model adjusted. Mycelial growth was assessed after 4 days of growth at 10 °C, 15 ° C, 20 ° C, and 25 ° C with 12 h light regime. Arabic and Roman numerals indicate isolates and macro-morphological groups, respectively.
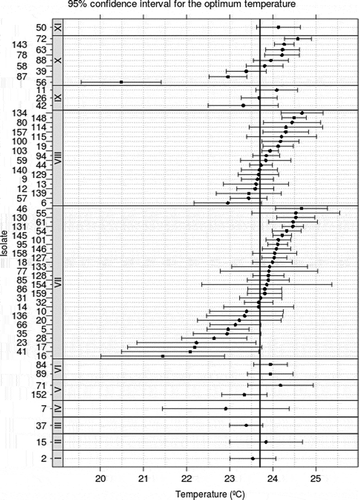
Fig. 3. Growth interval and optimum growth temperature for Monilinia spp. separated by isolates and based on the cubic model adjusted. Mycelial growth was assessed after 4 days of growth at 10 ° C, 15 ° C, 20 ° C, and 25 ° C with 12 h light regime. Arabic and Roman numerals indicate isolates and macro-morphological groups, respectively.
Discussion
The macro-morphological identification made with the synoptic key proposed by Lane (Citation2002) indicates M. laxa, M. fructigena and M. fructicola as the causal agents of brown rot in Paraná, Brazil. However, the molecular identification contradicted the synoptic key results. The molecular study showed that all isolates tested from Paraná to be M. fructicola. In accordance with our results, Malvárez et al. Citation(2001) observed a wide morphological variation among 312 Monilinia isolates recovered in Uruguay, that later were identified as M. fructicola using the same species-specific primers with the multiplex PCR method. Côté and Tardif et al. Citation(2004) also demonstrated that the use of the multiplex PCR method based on species-specific primers was a reliable tool for Monilinia species identification. In the Brazilian State of São Paulo, Souza et al. (Citation2008) reported the occurrence of M. laxa in one of 17 tested isolates, and showed that the same PCR method used in the present study was able to differentiate Monilinia species causing brown rot in stone fruits.
Difficulties were encountered during the macro-morphological identification of atypical colonies, making this method unreliable for identifying isolates to species in this study. In the case of colony colour, considered by Lane (Citation2002) to be accurate for species identification, only one out of six groups of M. fructicola possessed grey colouration. Although the grey colour has been described previously for this species by Byrde and Willetts (Citation1977), Batra (Citation1991), De Cal and Melgarejo (Citation1999) have reported variation in colour (including grey, buff and white colouration) for M. fructicola isolates from a worldwide collection.
The characterization for colony growth revealed M. fructicola as predominantly fast growing (above 80 mm diameter), although groups IX and XI revealed medium growth (ranging between 70 and 80 mm in diameter). All isolates with slow growth rate (under 70 mm diameter) were classified as M. laxa. This slow growth, according to Lane (Citation2002), is common to M. laxa and can also occur within M. fructigena, but not in M. fructicola isolates. Despite the similar results that were obtained in the present study and the study by Lane (Citation2002), large variation between and within species compromises species identification. In other studies, van Leeuwen and van Kesteren (Citation1998) considered, among other characteristics, colony growth rate was sufficient to identify Monilinia species. It was also shown that M. fructicola always attained higher growth rates when compared with growth of M. fructigena and M. laxa, confirming results obtained by Batra (Citation1991), De Cal and Melgarejo (Citation1999), Hu et al. (Citation2011).
Abundant sporulation was observed in four out of six M. fructicola groups, all M. fructigena groups, and two out of three M. laxa groups. Similar results were observed by Lane (Citation2002) except for M. laxa, which always produced a sparse pattern of sporulation. Batra (Citation1991) has described the sparse sporulation of M. laxa colonies grown on potato dextrose agar at 10–12 °C. Tamm and Flückiger (Citation1993) studied the temperature influence on M. laxa sporulation and observed sparse sporulation at temperatures above 20 °C and a significant increase in conidial production at lower temperatures. Abundant sporulation for all three Monilinia species was observed by van Leeuwen and van Kesteren (Citation1998) under a light/dark regime. Contradicting results obtained by Lane (Citation2002), concentric rings of spores were absent in isolates identified as M. fructicola in Paraná State, and were only present in M. fructigena. De Cal and Melgarejo (Citation1999) found the concentric rings in isolates of M. fructicola from an international collection, while van Leeuwen et al. (Citation2002) observed the presence of concentric rings on Japanese isolates of fast-growing M. fructigena isolates.
The lobed colony margin seems to be an important feature to differentiate M. laxa from M. fructicola and M. fructigena. The Paraná isolates showed similar results to those found by Lane (Citation2002). Hu et al. (Citation2011) stated that the presence of lobed margins differentiates M. laxa and M. mumecola from all other Monilinia species. However, De Cal and Melgarejo (Citation1999) had also noted lobed and smooth margins present in M. fructicola and M. fructigena when allowed to grow under long-wave UV light. In our study, there was a predominance of isolates without rosettes and associated arcs. These were only found in M. laxa from groups I and II. For group II of M. laxa, rosettes were found but not associated with arcs. These results confirm observations made by Lane (Citation2002), whereas black arcs were not seen for M. fructicola and M. fructigena, and only one out of nine M. laxa failed to develop black arcs.
Physiological parameters evaluated in two experiments for pathogenicity and optimum temperature do not support the segregation of Monilinia isolates recovered in Paraná into groups established by the synoptic key usage. The mean lesion growth on mature pear fruit was similar among all tested groups. Similar to our results, no significant differences were found by van Leeuwen et al. (Citation2002) when they tried to distinguish M. fructigena populations from Europe and Japan for identification of the new species, M. polystroma, whose identity was later confirmed using other methods.
The mean lesion values, measured in pear fruit inoculation experiments, are also contrary to those provided by the synoptic key. The smaller lesions observed in group IV did not follow the tendencies observed for the macro-morphological characterization of isolates grown on APDA. While the synoptic key indicated a possible segregation of M. laxa from M. fructicola and M. fructigena, the fruit inoculation results showed a different pattern. Group IV, macro-morphologically identified as M. fructigena, had smaller fruit lesions than groups identified as M. fructicola and M. laxa. When Hu et al. (Citation2011) evaluated four Monilinia species for colony and lesion growth rates on PDA and peach fruit, respectively, fastest colony growth rate was observed for M. fructicola on PDA and for M. laxa in inoculated pears.
The optimum growth temperature for Paraná isolates was 23.7 °C, without significant differences among studied groups. Favourable growth temperatures were extensively reported for Monilinia species. Byrde and Willetts (Citation1977) had described 25 °C as the optimum growth temperature and similar temperatures were found by Biggs and Northover (Citation1988), Tamm and Flückiger (Citation1993), reporting optimum range of 22.5 up to 25 °C for M. fructicola and M. laxa. Xu et al. (Citation2001) reported a graduated increase in growth rate for M. fructigena when temperatures approached 25 °C. This was also confirmed by May De Mio et al. (Citation2004), Moreira and May De Mio (Citation2007) in experiments with M. fructicola. In general, temperatures above and below the optimum delay but do not inhibit mycelial growth.
In summary, the only causal agent of brown rot disease in Paraná is M. fructicola, and the macro-morphological synoptic keys proposed by Lane (Citation2002) are insufficient for species identification. The protocol for the synoptic keys consumes time and materials, while only two of seven characteristics seem to provide accurate identification. Temperature curves and pathogenicity tests were also not useful for discriminating differences between isolate groups. Molecular based methods such as PCR using species-specific primers (Côté & Tardif et al. Citation2004) are reliable and provide timely identification for applied research. Although the obtained results demonstrate that macro-morphological characters are insufficient for an accurate species identification, the broad sampling of Monilinia isolates from southern States where stone fruits are produced documents morphological differences among isolates of the same Monilinia sp. observed in Brazil.
Acknowledgements
We thank the Capes/Reuni doctoral fellowship. This material is based upon work supported by grants no. 479041/2010-5 Universal/CNPq.
References
- Adaskaveg JE, Schnabel G, Förster H. 2008. Disease of peach caused by fungi and fungal-like organisms: biology, epidemiology and management. In: Layne DR, Bassi D, editor. The peach - botany, production and uses. Wallingford (UK): CAB International; p. 352–406.
- Andrade PFS. 2011. Análise da conjuntura agropecuária safra 2012/ 2013. Curitiba (Brazil): Secretaria da Agricultura e do Abastecimento do Estado do Paraná. October 2012.
- Banks JN, Rizvi RH, Lane CR, Hughes KJD, Cook RTA. 1997. Development of monoclonal antibodies for the detection and identification of Monilinia spp. causing brown rot of stone and pome fruit. In: Dehne HW, Adam G, Diekmann M, Frahm J, Mauler-Machnik A, Van Halteren P, editor. Diagnosis and identification of plant pathogens. Amsterdam (NL): Kluwer Academy; p. 391–393.
- Batra LR. 1991. World species of Monilinia (Fungi): their ecology, biosystematics and control (n° 16). Berlin-Stuttgart (DE): Gebruder Borntraeger Verlagsbuchhandlung.
- Biggs AR, Northover J. 1988. Influence of temperature and wetness duration on infection of peach and sweet cherry fruits by Monilinia fructicola. Phytopathology. 78:1352–1356.
- Box GEP, Cox DR. 1964. An analysis of transformations. J Royal Stat. 26:211–252.
- Byrde RJW, Willetts HJ. 1977. The brown rot fungi of fruit: their biology and control. 1st ed. Oxford (UK): Pergamon Press.
- Côté MJ, Prud’homme M, Meldrum AJ, Tardif MC. 2004. Variations in sequence and occurrence of SSU rDNA group I introns in Monilinia fructicola isolates. Mycology. 96:240–248.
- Côté MJ, Tardif MC, Meldrum AJ. 2004. Identification of Monilinia fructigena, M. fructicola, M. laxa and Monilia polystroma on inoculated and naturally infected fruit using multiplex PCR. Plant Dis. 88:1219–1225.
- De Cal A, Melgarejo P. 1999. Effects of long-wave UV light on Monilinia growth and identification of species. Plant Dis. 83:62–65.
- Fachinello JC, Silva CAP, Sperandio C, Rodrigues AC, Strelow EZ. 2000. Resistência de porta-enxertos para pessegueiro e ameixeira aos nematóides causadores de galhas (Meloidogyne spp). Ciência Rural. 30:69–72.
- Förster H, Adaskaveg JE. 2000. Early brown rot infections in sweet cherry fruit are detected by Monilinia-specific DNA primers. Phytopathology. 90:171–178.
- Fulton CE, Brown AE. 1997. Use of SSU rDNA group-I intron to distinguish Monilinia fructicola from M. laxa and M. fructigena. Microbiol Lett. 157:307–312.
- Hu MJ, Cox KD, Schnabel G, Luo CX. 2011. Monilinia species causing brown rot on peach in China. PloS One. 6:e24990. doi:10.1371/journal.pone.0024990.
- Khuri A. 2003. Advanced calculus with applications in statistics. 2nd ed. Hoboken US: Wiley-Interscience.
- Lane CR. 2002. A synoptic key for differentiation of Monilinia fructicola, M. fructigena and M. laxa, based on examination of cultural characters. OEPP/EPPO Bull. 32:489–493.
- Málvarez G, Rodrígues A, Aguilar C, Silvera E, Mondino P. 2001. Identificación de especies de Monilinia spp., en aislamientos obtenidos de Prunus spp. por PCR con primers específicos. Agrociencia. 5:48–53.
- May De Mio LL, Garrido L, Ueno B. 2004. Doenças de Fruteiras de Caroço. In: Monteiro LB, May De Mio LL, Serrat BM, Motta AC, Cuquel FL, editors. Fruteiras de Caroço uma Visão Ecológica. Curitiba (BR): UFPR; 169–185.
- Moreira LM, May De Mio LL. 2007. Metodologia para detecção de infecções latentes de Monilinia fructicola em frutas de caroço. Ciência Rural. 37:628–633.
- Oehlert GW. 1992. A note on the delta method. Am Stat. 46:27–29.
- Ogawa JM, Zehr EI, Bird GW, Ritchie DF, Uriu K, Uyemoto JK. 1995. Compendium of stone fruit diseases. St. Paul (US): APS Press.
- Souza DC, Fazza AC, Camargo LA, May De Mio LL, Angeli SS, Amorin L. 2008. First report of Monilinia laxa causing brown rot on peaches in Brazil. Phytopathology. 98:S148. [Abstr].
- Tamm L, Flückiger W. 1993. Influence of temperature and moisture on growth, spore production, and conidial germination of Monilinia laxa. Phytopathology. 83:1321–1326.
- van Leeuwen GCM, Baayen RP, Holb IJ, Jeger MJ. 2002. Distinction of the Asiatic brown rot fungus Monilinia polystroma sp. nov. from M. fructigena. Mycol Res. 106:444–451.
- van Leeuwen GCM, van Kesteren HA. 1998. Deliniation of three brown rot fungi of fruit crop (Monilinia spp.) on the basis of quantitative characteristics. Can J Bot. 76:2042–2050.
- Xu XM, Guerin L, Robinson JD. 2001. Effects of temperature and relative humidity on conidial germination and viability, colonization and sporulation of Monilinia fructigena. Plant Pathol. 50:561–568.