Abstract
Bananas are usually harvested at a green preclimacteric stage prior to sale. The preclimacteric phase is called green life (GL), which is defined as the number of days between harvesting and initiation of the natural ripening process. Sigatoka disease (SD) has previously been shown to influence the postharvest behaviour of banana fruit. This work was set out to determine whether there is a relationship between the level of SD severity and the extent of the effects on banana quality as characterized by several pomological traits, especially pulp colour. In an experiment conducted with fruits harvested at a constant physiological age, our results showed that SD had a major effect on the percentage of fruit ripening in the field and on fruit weight, but not on fruit diameter. We showed too that pulp colour was greatly affected by SD; when severity increased, pulp colour parameters were modified – L* (lightness) decreased, a* (axis red to green) and b* (axis yellow to blue) increased, giving a more greyish pulp colour, with less green and more yellow and red. These results may enable the development of a method for controlling fruit quality, by measuring pulp colour in the field, in order to reduce post-harvest losses.
Résumé
Les bananes sont généralement récoltées vertes, en phase pré-climactérique, avant la mise en marché et la commercialisation. Le temps de transport nécessaire entre la zone de production et les chambres où sont mûris des fruits est appelé durée de vie verte ou green life (GL), qui est défini par le nombre de jours entre la récolte et l’initiation naturelle des processus de maturation. Il a été montré que la Sigatoka Disease (SD) a une influence sur le comportement post récolte des fruits. Ce travail a pour objectif de déterminer s’il existe une relation entre le niveau de sévérité de la SD et des paramètres de qualité de la banane caractérisés par des caractéristiques pomologiques, notamment la couleur de la pulpe. Dans une expérimentation conduite avec des fruits récoltés à âge physiologique constant, nos résultats montrent que la SD a un effet très marqué sur le pourcentage de fruits mûrs au champ et sur le poids des fruits à la récolte, mais pas sur le calibre. Nous avons également montré que la couleur de la pulpe était fortement affectée par la SD ; quand l’intensité de la maladie augmente, les paramètres définissant la couleur de la pulpe sont modifiés – la valeur de L* (clarté) décroit ; les valeurs de a* (axe rouge – vert) et b* (axe jaune-bleu) augmentent, donnant un teint grisâtre à la couleur de la pulpe, avec un peu moins de teinte verte et plus de jaune et de rouge. Ces résultats nous permettent de proposer une méthode de contrôle de la qualité des fruits, par mesure au champ de la couleur de la pulpe, dans le but de réduire les pertes après-récoltes.
Introduction
Bananas are climacteric fruits that are harvested at a green preclimacteric stage prior to shipment at 13 °C, followed by ethylene treatment in commercial ripening rooms and marketing (Marriott Citation1980). The time between flowering and harvest, called the flowering-to-harvest period (FHP), varies under tropical conditions from 60 to 130 days for the Cavendish variety. In most commercial banana plantations, fruits are generally harvested when they reach a commercial grade (usually a fruit diameter of 34 mm). Another criterion used by banana growers to determine commercial maturity is an assessment of pulp colour (Wainwright & Hugues Citation1989). The time between harvest and the onset of natural ripening is called green life (Peacock & Blake Citation1970). Green life (GL) depends on the physiological age of the fruits, and is calculated by a daily thermal sum above a given threshold (Jullien et al. Citation2008).
Sigatoka disease (SD) is one of the main banana leaf diseases worldwide; it is caused by the pathogenic fungus Mycosphaerella musicola Leach. Heavy SD severity can lead to a considerable reduction in the banana photosynthetic leaf area. However, the greatest damage caused by this disease is its effect on banana fruit quality because high severity leads to early ripening fruit (Meredith Citation1970; Stover Citation1972; Wardlaw Citation1972), a reduction in green life (Ramsey et al. Citation1990; Chillet et al. Citation2009; Castelan et al. Citation2012) and alteration of flavour (astringency) (Barnell & Barnell Citation1945). This disease greatly influences banana exports. In the event of high severity in commercial plantations, banana growers usually observe the pulp colour of selected fruits, to try to see if ripening has been triggered in this sample. This empirical method is often used in industrial banana plantations (Deullin Citation1963).
The main objective of this work was to determine the effect of various levels of Sigatoka disease on banana quality, especially on pulp colour, and to assess whether the observation of the banana pulp colour is a good criterion for judging fruit exportability.
Materials and methods
Plant material
The experiment was conducted in Guadeloupe FWI (16°N, 62°W) with Musa acuminata banana plants (Sub-group Cavendish, AAA, ‘Grande Naine’). This banana cultivar is commonly used for commercial exportations and is highly susceptible to Sigatoka disease.
Evaluation of SD severity on banana plants
For each banana plant in the experiment, SD was quantified at flowering stage. The severity index (SI) was assessed according to the method developed by Stover (Citation1971) and later modified by Gauhl et al. (Citation1993). This method involves a visual estimation of the necrotic area per plant, scored on a scale of seven disease grades attributed to all plant leaves. Each leaf is scored on the following scale: 0 = no necrotic lesion; 1 = under 1% necrotic lesions; 2 = 1–5% necrotic lesions; 3 = 6–15% necrotic lesions; 4 = 16–33% necrotic lesions; 5 = 34–50% necrotic lesions; 6 = more than 50% necrotic lesions. The SI was calculated as SI = (∑scores/6 × NTL) × 100, where ∑scores is the sum of the scores for all the leaves of the banana plant and NTL is the total number of plant leaves.
Experimental design
A banana plot (1 ha) was selected in a commercial plantation located in Montbelley – Capesterre Belle Eau (annual rainfall 2 500 mm; andosol; 180 m elevation; annual mean temperature 25.5 °C). The experimental plot was a second crop cycle, with a density of 1 800 plants ha−1. This experimental plot did not receive any direct fungicide application for Sigatoka disease control for a period starting one month before the experiment up to the end of the trial. Nevertheless this plot was neighboured by another commercial plot that received fungicide applications through aerial applications. Then, the borders of the experimental plot were lightly sprayed by the resulting drift of aerial fungicide treatments which resulted in a gradually declining rate of disease control over the experimental plot and a consequent disease gradient.
For each replicate of the experiment, 40 banana plants homogeneous in height, circumference and bunch size were selected at the same horizontal flowering stage (identical stage of development). They were classified into four different treatments (4 × 10 plants) according to the severity of SD at flowering, evaluated by the Severity Index (Stover Citation1971). The experiment was repeated three times – in June, August and September 2008 (). The crop management strategies were similar and complied with normal commercial practices for all the banana plants. A coloured band was tied to each plant to mark the flowering date, bunches were covered with a blue polyethylene sleeve, and monthly fertilization and crop-protection treatments (apart from fungicide sprays) were applied. Bunches were harvested at a constant physiological age, calculated in degree day (dd), at 900 dd, according to the method described by Ganry and Chillet (Citation2008). A banana fruit was then cut from the third hand (median internal fruit) of each bunch, washed, and treated with thiabendazol (500 ppm) by dipping for 120 s.
Table 1. Initial plant classification level by Severity Index.
Calculation of fruit physiological age
Temperature probes (Gemini Data Loggers) were placed under a weather station shelter in the experimental plot. The average daily temperature was calculated on the basis of hourly temperatures. The physiological age of the fruit, expressed in dd, was calculated from the cumulative mean daily temperature sums at the 14 °C threshold during the flowering-to-harvest period (Ganry & Meyer Citation1975).
Measurement of pulp colour
Pulp colour was measured, at harvest, with a MINOLTA CR 200 Chroma Meter (Minolta, Roissy – France). The fruit was cut transversely and the measurement taken at the centre of the fruit. The results were recorded using the Hunter scale which includes the L*, a* and b*. The L * coordinate measures light intensity, the a* coordinate measures the colour from green (negative values) to red (positive values) and coordinate b* measures the colour blue (negative values) to yellow (positive values). The values of L*, a*, b* can be converted to the hue value (H = Arc tan (b/a) and saturation (C = (a2 + b2)1/2) (McGuire Citation1992).
Measurement of fruit green life and pomological parameters
GL was measured as described by Chillet et al. (Citation2008), but at 13 °C and 85% HR, to simulate maritime transport in containers. For each treatment, the percentage of ripe fruits in the field was also assessed as the number of bunches whose ripening was initiated on the plant before harvest at 900 dd, divided by the total number of bunches harvested (×100). The diameter and weight of each banana fruit was also measured.
Data analysis
Seven analyses of variance followed by Newman-Keuls tests (at the 5% significant level) and linear regression were performed to compare the different severity levels using the XLStat software package (2010). To compare the percentage of ripe fruit by ANOVA, it was necessary to transform the data into arcsine (% Ripe Fruit).
Results
Effects of SD on fruit ripening in the field and GL
presents the percentage of ripe fruits on the plant observed at harvest time at the physiological age of 900 dd for each disease level. For the lower disease levels (1 and 2), there was no ripe fruit at the harvest stage. For the higher disease levels, the percentages of ripe fruit at harvest time increased to 33% and 70% for disease levels 3 and 4, respectively.
Table 2. Percentage of ripe fruits in the field before harvest time and green life (GL).
also shows the mean GL value for disease level. As SI increased, the GL value correspondingly decreased. The statistical analysis showed that levels 1, 2 and 3 were different (at the 5% significance level). Bananas with the highest SD levels (levels 3 and 4) had GL values under 17 days; there was no significant difference between levels 3 and 4.
Effects of SD on fruit quality characteristics
Fruit quality characteristics (weight and diameter) are shown in . It was apparent that regardless of the SI level, there were no significant differences in fruit diameter (P = 0.084). The weight was affected by the SI levels, and bananas with a higher SI level (level 4) had statistically lighter fruit (138.98 g) than bananas of level 1 and level 2 (175.91 and 161.78 g) (P = 0.001).
Table 3. Pomological parameters (weight, diameter) of fruits harvested at 900 degrees days from plants differentially infested by Sigatoka disease.
Pulp colour values (L*, a*, b*) are shown in . It was found that the higher the level of disease severity, the more the L* values decreased (from 87.65 to 83.98 from level 1 to level 4). The fruit pulp was slightly darker when plants were severely infected. The a* value also varied significantly (from −1.95 to 0.76 between level 1 to level 4), indicating a loss of green colour and an increase in red tints. The b* value also varied from 31.26 to 39.13 from level 1 to level 4, showing a tendency towards a yellow pulp colour. Given these strong variations in a* and b*, the saturation (C) and hue (H) values also changed between severity levels 1 and 4 (C from 31.02 to 39.43, and H from –1.48 to 1.53, data not shown).
Table 4. Pulp colour (L*, a*, b*) of fruits harvested at 900 degrees days from plants differentially infested by Sigatoka disease.
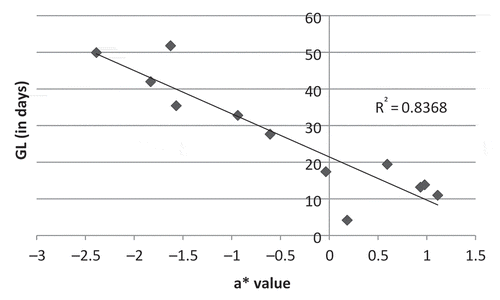
shows the linear relationship between SI and one pulp colour parameter (a*). The coefficient of correlation (r = 0.88; P = 0.00) shows that these two parameters were strongly linked. shows the relation between GL and the same pulp colour parameter (a*). The coefficient of correlation was high (r = −0.92; P <0.0001).
Fig. 1. Pulp colour (a*) depending on Severity Index (SI) value of fruits harvested at a constant physiological age (900 dd) according to the method described by Ganry and Chillet (Citation2008). The SI is calculated from the method of Stover (Citation1971).
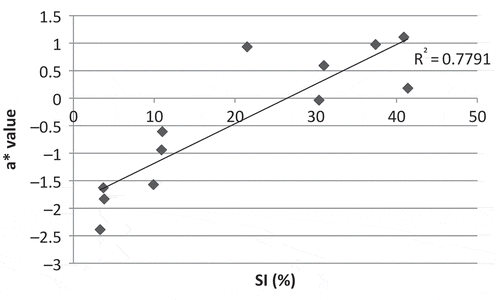
Fig. 2. Green life (GL in days) depending on pulp colour (a*) of fruits harvested at a constant physiological age (900 dd) according to the method described by Ganry and Chillet (Citation2008).
Discussion
The objective of this work was to determine the effect of Sigatoka disease on banana quality attributes, and particularly on pulp colour as an indicator of early ripening. In this experiment, SD severity had no effect on fruit diameter. Fruits from the most severely diseased plants showed equivalent diameters to those from less infested plants. This result differs from the effects of other cropping stresses, including water stress and anoxia, on banana fruit growth (Hegde & Srinivas Citation1989; Goenaga & Irizarry Citation1998; Chillet et al. Citation2006). This means that even when SD pressure is very high, photosynthetic activity is sufficient to allow a nearly normal fruit growth. However, fruits at disease level 3 and 4 weighed less than fruits at levels 1 and 2. This result is similar to those obtained by Ramsey et al. (Citation1990), where the authors noted an effect of SD on bunch weight when the plants had fewer than five viable leaves at the harvest stage. Therefore, fruits at level 3 and 4 displayed the same diameter as those at levels 1 and 2 but had a lower weight, which probably indicates a lower-density fruit. It is thus possible that fruit filling was affected by SD, including the formation of starch grains.
In this study, we show that SD substantially affected fruit GL, and that there was a relationship between the colour of the pulp and the GL. The effect of SD on fruit pulp colour at the harvesting stage had already been observed, but never proven. Indeed, Wardlaw (Citation1939) had already noted the appearance of a salmon-coloured fruit pulp from heavily infected plants. Similarly, Barnell and Barnell (Citation1945) noted the same salmon colour in the pulp of fruits from infected plants, and also that fruits from infected plants had high tannin contents. In this experiment, we showed that all parameters characterizing the pulp colour were correlated with the level of SD. The higher the level of the disease, the higher the probability that fruit had lost its clarity (lower L*); the redness (positive a*) appeared and the loss of green and yellow was reinforced (increased b*). This red colour may result from the synthesis of anthocyanins resulting from foliar stress induced by SD, and internal information transmission mechanisms involving plant hormones such as abscisic acid (ABA). In fact, ABA plays a major role in plant responses to stress (Zhang et al. Citation2006), especially in pathogen infection (Dixon et al. Citation1994; Chaiker-Scott Citation1999). Furthermore, it has been shown that ABA is responsible for stimulating anthocyanin synthesis (Gagné et al. Citation2011). Experiments on ‘Crimson seedless’ grapes showed that application of ABA stimulated anthocyanin synthesis, and led to a decrease in L*, with darker and redder fruit colouration (Peppi et al. Citation2008). The salmon colour of the fruit pulp might therefore result from a chain of events involving anthocyanin synthesis. This hypothesis must be clearly demonstrated to be validated, and our results do not allow a conclusion on the mechanisms involved.
In this study, we have also shown that the percentage of ripe fruit in the field increased when the disease severity rose. It was at zero for levels 1 and 2 and reached 70% for level 4. Although fruit ripening before harvest had already been reported in banana plots with SD (Meredith Citation1970), we showed in this experiment that the higher the disease level, the more ripe bananas in the field.
In conclusion, these results are very interesting for harvest management in SD-infested areas in order to improve empirical observations that are presently performed routinely and that may lead to improper decisions. Indeed, it is very easy to measure pulp colour at harvest at a production site by chromametry or by using a calculation chart. Colour measurement, including a*, can help to easily select bunches from a highly infected plot. In fact, fruit with positive a* values are fruits that will have a very low GL. These bunches are therefore unexportable because ripening may be triggered naturally during storage and transport to the export areas. However, all bunches with a* values below −1 are quite able to withstand the storage and transportation periods. However, this must be verified by experiments carried out in conditions of production and commercialization, with transportation and storage. Use of this method in production could enable objective selection of fruits suitable for export.
Acknowledgements
The authors wish to thank the banana grower, Mr Louis Dormoy, for his collaboration in this project. They equally wish to thank the dynamic team of the CIRAD in Guadeloupe (Marc Dorel, Jean Michel Risède, Didier M’Béguié-A-M’Béguié, Pierre Yves Teycheney and Christophe Jenny) for their assistance in this project.
References
- Barnell HR, Barnell E. 1945. Studies in tropical fruits. XVI. The distribution of tannins within the banana and the change in their conditions and amount during ripening. Ann Bot. 33:77–99.
- Castelan FP, Saraiva LA, Lange F, De Lapeyre De Bellaire L, Cordenunsi BR, Chillet M. 2012. Effects of black leaf streak disease and sigatoka disease on fruit quality and maturation process of bananas produced in the subtropical conditions of southern Brazil. Crop Prot. 35:127–131.
- Chaiker-Scott L. 1999. Environmental significance of anthocyanins in plant stress response. Phytochem Photobiol. 70:1–9.
- Chillet M, Abadie C, Hubert O, Chilin-Charles Y, De Lapeyre de Bellaire L. 2009. Sigatoka disease reduces the greenlife of bananas. Crop Prot. 28:41–45.
- Chillet M, De Lapeyre de Bellaire L, Hubert O, Mbéguié-A-Mbéguié D. 2008. Measurement of banana green life. Fruits. 63:125–127.
- Chillet M, Hubert O, Rives MJ, De Lapeyre de Bellaire L. 2006. Effects of the physiological age of bananas on their susceptibility to Colletotrichum musae. Plant Dis. 90:1181–1185.
- Deullin R. 1963. Mesure de la couleur de la pulpe de la banane en phase préclimactérique. Fruits. 18:23–26.
- Dixon RA, Harrison MJ, Lamb CJ. 1994. Early events in the activation of plant defense responses. Ann Rev Phytopath. 32:479–501.
- Gagné S, Cluzet S, Merillon JM, Geny L. 2011. ABA initiates anthocyanin production in grape cell cultures. J. Plant Growth Reg. 30:1–10.
- Ganry J, Chillet M. 2008. Methodology to forecast the harvest date of banana bunches. Fruits. 63:371–373.
- Ganry J, Meyer JP. 1975. Recherche d’une loi d’action de la température sur la croissance des fruits du bananier. Fruits. 30:375–392.
- Gauhl F, Pasberg-Gauhl F, Vuylsteke D, Ortiz R. 1993. Multilocational evaluation of black sigatoka resistance in banana and plantain. IITA Research Guide no. 47. Ibadan (Nigeria): IITA; pp. 5–57.
- Goenaga R, Irizarry H. 1998. Yield of banana grown with supplemental drip irrigation on an ultisol. Exp Agric. 34:439–448.
- Hegde DM, Srinivas K. 1989. Effect of soil moisture stress on fruit growth and nutrient accumulation in banana cultivar ‘Robusta. Fruits. 44:135–138.
- Jullien A, Chillet M, Malezieux E. 2008. Preharvest growth and development determine postharvest green life of fruit in Musa (Musa sp. AAA group cv Grande Naine (Cavendish subgroup)). J Hort Sci Biotech. 83:506–512.
- Marriott J. 1980. Bananas - physiology and biochemistry of storage and ripening for optimum quality. Crit Rev Food Sci Nutr. 13:41–88.
- Mc Guire RG. 1992. Reporting of objective color measurements. Hortscience. 27:1254–1255.
- Meredith DS. 1970. Banana leaf spot disease (Sigatoka) caused by Mycosphaerella musicola Leach. Commonwealth Mycological Institute, Kew, England. Phytopathol Pap. 11:147.
- Peacock BC, Blake JR. 1970. Some effects of non-damaging temperatures on the life and respiratory behaviour of bananas. Q J Agric Anim Sci. 27:147–168.
- Peppi MC, Walker MA, Fidelibus MW. 2008. Application of abscisic acid rapidly upregulated UFGT gene expression and improved color of black berries. Vitis. 47:11–14.
- Ramsey MD, Danielles JW, Anderson VJ. 1990. Effects of Sigatoka leaf spot (Mycospherella musicola Leach) on fruit yield, Field ripening and greenlife of bananas in North Queensland. Sci Hort. 41:305–313.
- Stover RH. 1971. A proposed international scale for estimating intensity of banana leaf spot. Trop Agric (Trinidad). 48:185–196.
- Stover RH. 1972. Banana, plantain and abaca disease. Farnham Royal: Commonwealth Agricultural Bureaux; p. 318.
- Wainwright H, Hugues P. 1989. Objectives measurement of banana pulp colour. Int J Food Sci Techn. 24:553–558.
- Wardlaw CW. 1939. Cercospora leaf spot of banana. Nature. 144:11–14.
- Wardlaw CW. 1972. Banana disease including plantain and abaca. London: Longman.
- Zhang J, Jia W, Yang J, Ismail AM. 2006. Role of ABA in integrating plant response to drought and salt stresses. Field Crop Res. 97:111–119.
