Abstract
Fusarium head blight (FHB) of wheat, caused principally by Fusarium graminearum in Canada, results in accumulation of mycotoxins in the grain. The fungus produces deoxynivalenol (DON) and its acetylated forms 3-ADON or 15-ADON. Increasing numbers of F. graminearum isolates collected in Manitoba between 1998 and 2004 were of the 3-ADON chemotype, whereas prior to 1998, the 15-ADON chemotype was considered the only significant cause of FHB in North America. Between 2008 and 2012, a series of studies were conducted at Carman (2008, 2009) and Glenlea, Manitoba (2008–2012) to monitor the ratio of 3-ADON to 15-ADON chemotypes recovered after wheat plots were artificially inoculated, usually in a 1:1 ratio of 3-ADON:15-ADON isolates of F. graminearum. Additional studies were conducted under controlled conditions. In 2008, the majority of isolates recovered from both Carman and Glenlea were of the 3-ADON chemotype. In subsequent years, however, the ratio was closer to 1:1 or sometimes with a slight predominance of 15-ADON isolates. Under controlled conditions, recovery of 15-ADON isolates predominated at lower incubation temperatures (18–22°C) whereas at 28°C, 3-ADON isolates were more prevalent. At moderate temperature (24°C), results were mixed – seven samples had a 1:1 ratio, three had higher recovery of 3-ADON isolates, and two had higher recovery of 15-ADON. However, an examination of weather variables over the 5-year period of the field studies showed no correlation between recovery of chemotype and temperature or precipitation.
Résumé
La brûlure de l’épi (FHB) du blé, causée principalement au Canada par Fusarium graminearum, engendre des accumulations de mycotoxines dans les grains. Le champignon produit du déoxynivalénol (DON) et ses formes acétylées 3-ADON ou 15-ADON. De plus en plus d’isolats de F. graminearum collectés au Manitoba de 1998 à 2004 appartenaient au chimiotype du 3-ADON alors que, avant 1998, le chimiotype du 15-ADON était considéré comme la seule cause importante de FHB en Amérique du Nord. De 2008 à 2012, une série d’études ont été menées à Carman (2008, 2009) et Glenlea (2008–2012), au Manitoba, pour surveiller la proportion récupérée des chimiotypes du 3-ADON et du 15-ADON après que les parcelles de blé avaient été inoculées artificiellement, le rapport des isolats de F. graminearum produisant du 3-ADON et du 15-ADON étant habituellement de 1:1. Des études supplémentaires ont été menées dans des conditions contrôlées. En 2008, la majorité des isolats récupérés à Carman et à Glenlea appartenaient au chimiotype du 3-ADON. Toutefois, au cours des années suivantes, le rapport était plus près de 1:1 ou parfois affichait une légère prédominance des isolats produisant du 15-ADON. Dans des conditions contrôlées, les isolats récupérés appartenant au chimiotype du 15-ADON prédominaient à des températures d’incubation plus basses (18 à 22°C) tandis que, à 28°C, les isolats appartenant au chimiotype du 3-ADON étaient plus nombreux. À 24°C, température considérée comme modérée, les résultats variaient—sept échantillons affichaient un rapport de1:1, trois affichaient un rapport plus élevé de récupération des isolats produisant du 3-ADON et deux, un rapport plus élevé d’isolats produisant du 15-ADON. Toutefois, un examen des variables météorologiques des cinq années qu’ont duré les études en champ a montré qu’il n’y avait aucune corrélation entre la récupération des chimiotypes et la température ou les précipitations.
Introduction
Fusarium head blight (FHB) is one of the most serious diseases of cereals on the Canadian prairies. The principal causal agent in Manitoba is Fusarium graminearum (Schwabe), which in addition to causing head blight, also results in mycotoxin contamination of the grain, reducing its suitability for most purposes, including milling, malting, food and animal feed (Gilbert & Tekauz, Citation2000). The main mycotoxin produced is the trichothecene deoxynivalenol (DON) but different isolates of F. graminearum can also produce 3-acetyldeoxynivalenol (3-ADON), 15-ADON or nivalenol (NIV). Isolates which produce DON/3-ADON and DON/15-ADON have been reported to occur in Canada, and those producing NIV only rarely (R. Clear, personal communication).
Prior to 1994, DON/15-ADON isolates caused FHB in North America (Miller et al., Citation1983; Abramson et al., Citation1993) but between 1998 and 2004, an increase in prevalence of the 3-ADON chemotype was documented (Gale et al., Citation2007; Ward et al., Citation2008). Field and laboratory studies show that the DON/3-ADON isolates are sometimes more aggressive, causing more severe disease symptoms (Puri & Zhong, Citation2010), as well as higher levels of DON accumulation (Gilbert et al., Citation2010; Puri & Zhong, Citation2010; von der Ohe et al., Citation2010). Moreover, emerging 15-ADON populations in Canada also appear to be more aggressive, resulting in higher levels of Fusarium-damaged kernels (FDK) and higher DON accumulation (Foroud et al., Citation2012).
The reason for the rapid increase in the DON/3-ADON F. graminearum population in Canada is not understood, but may result from a transcontinental introduction (Ward et al., Citation2008). This conclusion is supported by the fact that the 3-ADON populations examined from eastern and western Canada and from the northern states of North Dakota and Minnesota all have a greater similarity with an Italian population, first described by Gale et al. (Citation2007), than to the 15-ADON populations in North America (Ward et al., Citation2008). The possibility of long-distance dispersal is also supported by the collection of F. graminearum spores in the mesoboundary layer of the atmosphere, up to 320 m above the earth’s surface, in seasons and locations where no inoculum would be produced (Schmale et al., Citation2012). Between four and 36 colonies of Fusarium were obtained over four sampling plates from 11 collections. Interestingly, among 11 isolates analysed for trichothecenes, most produced DON/15-ADON, but one isolate collected at 324 m above the earth’s surface produced DON/3-ADON, and one isolate produced NIV. The latter two are rare chemotypes in the study location. The authors describe the potential of air masses sandwiched between repelling atmospheric transport barriers to carry spores over distances of hundreds of kilometres (Schmale et al., Citation2012).
The FHB disease nurseries located in Carman and Glenlea, Manitoba have been inoculated with a mixture of 3- and 15-ADON F. graminearum isolates since the discovery that the prevalence of 3-ADON isolates was increasing. The same isolates used to inoculate the nurseries were also used in other experiments at the University of Manitoba and the Cereal Research Centre in Winnipeg. The objectives of this study were to examine: (i) the recovery of chemotypes from these inoculated nurseries and experimental plots, and from experiments conducted under controlled conditions, during 2008–2012; and (ii) to determine how consistently one or the other chemotype was recovered and if there was a competitive advantage to 3-ADON isolates which might explain, in part, the rapid increase in prevalence of the latter.
Materials and methods
Field studies (2008–2009) and pathogen recovery – Carman, MB
Five spring wheat genotypes were grown at the Ian N. Morrison Research Farm, University of Manitoba Research Farm, Carman, MB in 2008 and 2009. The genotypes represented a range of reactions to FHB: ‘93FHB37’ (resistant [R]), ‘5602HR’ (moderately resistant [MR]), ‘AC Barrie’ (intermediate [I]), and ‘CDC Teal’ and ‘Superb’ (susceptible [S]). Plants were inoculated during 2008 and 2009 with two 3-ADON isolates (M9-04-6 and M6-04-4, ARS Culture Collection NRRL numbers 37480 and 37456, respectively) and two 15-ADON isolates (M1-04-1 and M8-04-3, NRRL 37424 and NRRL 37466, respectively) which were individually increased in aerated carboxy methylcellulose (CMC). The spore concentration was determined using a hemacytometer, and the isolates were mixed to achieve a 1:1:1:1 ratio. Inoculum (or water as a control) was applied to plots using a CO2-powered backpack sprayer at a concentration of 5 × 104 macronidia mL−1 and a rate of 40–50 mL m−1 row each time (Gilbert & Woods, Citation2006). Mist irrigation was supplied to foster disease development. After harvest, infested kernels were surface-sterilized with 0.3% sodium hypochlorite solution for 1 min, aseptically dried in a laminar-flow hood, and placed on potato dextrose agar (PDA, Difco Labs., Detroit, MI). The isolates were incubated under cool white light at 22 –24 °C for 7 days, after which 56 monosporic cultures were established on PDA originating from each wheat genotype. A polymerase chain reaction (PCR) assay was used to identify the chemotype of each isolate – DON/3-ADON or DON/15-ADON – as described below. The single-spore isolations, DNA extraction and PCR were carried out at the Grain Research Laboratory, Canadian Grain Commission in Winnipeg, MB.
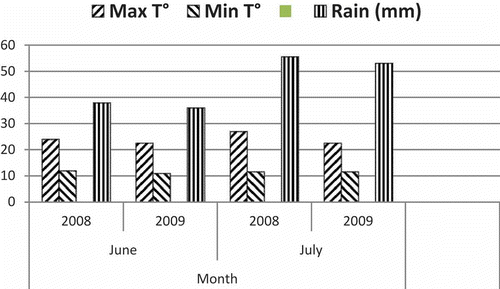
The average monthly precipitation and maximum and minimum temperatures were obtained for Carman, MB for the months of July and August 2008–2009 from a weather station.
Field studies (2008–2012)– Glenlea, MB
Six spring wheat genotypes were planted in the FHB nursery at Glenlea, MB from 2008 to 2012. The checks were ‘93FHB37’ (R), ‘5602HR’ (MR), ‘Snowbird’ (I), ‘AC Vista’ (S), ‘Roblin’ (S) and ‘AC Morse’ (S). Plots were spray-inoculated using standard protocols (Gilbert & Woods, Citation2006). Briefly, inoculum was applied to plots at anthesis and again 2–3 days later using a CO2-powered backpack sprayer. In 2008, the isolates were increased together in CMC in the same flasks. From 2009 to 2012, the isolates were increased individually and mixed in a 1:1 ratio. In 2008 and 2009, plants were inoculated with the same mix of F. graminearum isolates as used in the previous study described above. There was always an equal number of 3- and 15-ADON isolates, but in 2010 and 2011, more current isolates were used to inoculate the nursery. The isolates were M7-07-1 and M9-07-1 (3-ADON, NRRL 52008 and 52068, respectively) and M1-07-2 and M3-07-02 (15-ADON, NRRL 47847 and 47904, respectively). In 2012, the two isolates that sporulated well and consistently in culture were used: M7-07-1 (3-ADON) and M1-07-2 (15-ADON). Irrigation was supplied for 30 min following inoculation to foster disease development. Each year after harvest, 100 kernels of each genotype were plated on PDA and up to 40 F. graminearum isolates recovered from the kernels for each genotype. Single-spore cultures were established from these isolates and allowed to grow for a week under the same conditions described above and PCR used to identify the chemotype of each isolate as described below. In 2008, PCR was carried out at the facilities of the Grain Research Laboratory, Canadian Grain Commission in Winnipeg, MB. Subsequently, chemotyping was conducted at the Cereal Research Centre, Winnipeg, MB using the same protocols.
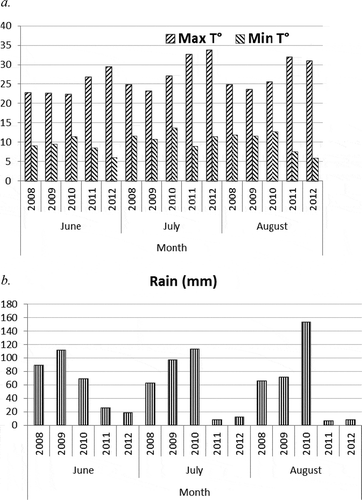
The average monthly precipitation and maximum and minimum temperatures were gathered for Morris, MB (36 km from Glenlea) for the months of June, July and August 2008–2012.
Controlled environment inoculation
A walk-in growth room at the Department of Plant Science and the greenhouse facility at the Crop Technology Centre, University of Manitoba were used in 2008–2009. Wheat ‘CDC Teal’ plants were grown in Sunshine LA4 soilless mix (Sun Gro, Horticulture Canada, AB, 158 L) containing 150 g N-P-K, 0.7 g chelated zinc (14%), 2 g fritted trace elements, 1.5 g chelated iron (13.2%), and 100 g calcium carbonate. Plants were grown in 15 cm diameter pots with a light cycle of 16 h day/8 h night. In the greenhouse, the temperature settings were 18–22 °C day and 14–18 °C night, and in the growth room 21 °C day and 19 °C night. Five treatment mixtures of the same 3-ADON and 15-ADON isolates of F. graminearum used in the field experiments, plus a water control () were used. Tween 20 ® was added to the inoculum or water control at 1 mL L−1. Inoculum of each isolate was increased on PDA as described above. Two opposite spikelets on each spike were inoculated with 10 µL of inoculum at anthesis using a micropipette. The inoculated heads were covered with glassine bags for 48 h. A completely randomized design was used with five replications per treatment. At maturity, the seed was harvested, surface-sterilized using the protocol described above for the field experiments, and plated onto PDA from which single-spore cultures of F. graminearum were generated for chemotyping by PCR. The single spore isolations, DNA extraction and PCR were carried out at the facilities of the Department of Plant Science, University of Manitoba.
Table 1. Treatments for controlled environment study inoculating plants with mixtures of 3-acetyldeoxynivalenol (ADON) and 15-ADON chemotypes of Fusarium graminearum.
Studies under controlled conditions at different temperatures
Growth cabinets at the Cereal Research Centre, Winnipeg, were used to grow the six genotypes used in the FHB Nursery at Glenlea, MB. Plants were grown in 15 cm fibre pots in a mix of Sunshine soilless Mix 5 (Sun Gro, Horticulture Canada, AB, Canada) and soil (50:50) with a 16 h light/8 h dark photoperiod at a constant 16°C. When flag leaves emerged on the earliest maturing genotype, temperatures in the growth cabinets were increased to one of 20, 24, or 28 °C. Spikes were inoculated with the same mix of isolates used in the FHB nursery in a 1:1:1:1 ratio. The first experiment used 2009 field isolates and the repetition used 2010 isolates. At anthesis, spikes were both point- and spray-inoculated with the mixed inoculum and covered with glassine bags for 48 hours (Gilbert & Woods, Citation2006). At maturity, spikes were harvested, hand-threshed, and kernels plated on PDA to generate monosporic cultures as for the field study described above. The same protocol for chemotyping isolates was used as outlined below.
Protocol for chemotyping isolates
DNA was extracted and isolates were identified as either 3- or 15-ADON using a modification of the PCR method of Ward et al. (Citation2008). The TRI12 multiplex assay and PCR primers 12CON, 12NF, 12-15F and 12-3F were used. The TRI12 multiplex produced amplicons of approximately 840 bp, 670 bp and 410 bp with isolates of NIV, 15-ADON and 3-ADON chemotypes, respectively. For the 670 bp 15-ADON band, the forward primer was 5′- CATGAGCATGGTGATGTC-3′, and the reverse primer was 5′-TACAGCGGTCGCAACTTC-3′. For the 410 bp 3-ADON band, the forward primer was 5′- CATGAGCATGGTGATGTC-3′ and the reverse primer was 5′- CTTTGGCAAGCCCGTGCA-3′. The PCR master mix recipe for multiplex PCR to determine the chemotype of F. graminearum isolates consisted of the following: 14.3 uL DDH2O, 2.5 uL AmpliTaq buffer II, 2 uL dNTPs (2 mM), 2 uL Ampli MgCl2 (25 mM), 0.5 uL 12CON primer (10 uM), 0.5 uL 12NF primer (10 uM), 0.5 uL 12-15F (10 uM), 0.5 uL 12-3F (10 uM), 0.2 uL Ampli Taq. The total master mix volume was 23 uL plus 2 uL DNA for a total reaction volume of 25 uL. For the controlled environment studies, 11.3 uL of DDH2O and 5 uL DNA were used. The thermocycler program for the PCR reaction was: denaturation at 94 °C for 2 min, followed by 30 cycles of 94 °C for 30 s, 52 °C for 30 s and 72 °C for 1 min, followed by a final extension at 72 °C for 7 min, and then the sample was held at 20 °C until removed from the thermocycler.
Statistical analysis
Chi-squared tests were used to determine if recovery of 3- and 15-ADON isolates from kernels from inoculated plots and experiments conformed to a 1:1 ratio.
Results
Field studies (2008–2009) and pathogen recovery – Carman, MB
Two of the five wheat genotypes tested in Carman resulted in higher recovery of 3-ADON isolates of F. graminearum. Significantly more 3-ADON isolates were recovered from ‘AC Barrie’ in both years, while the results for ‘93FHB37’ were significant only in 2008. Recovery of chemotypes from the remaining cultivars was not significantly different from a 1:1 ratio, although the overall values showed that more 3-ADON isolates were recovered than 15-ADON isolates in both years (). The weather for June and July of 2008 and 2009 at Carman was similar, with slightly warmer temperatures and more precipitation in 2008 than 2009 ().
Table 2. Recovery of 3-acetyldeoxynivalenol (ADON) and 15-ADON isolates of Fusarium graminearum from grain harvested from plants after inoculation with mixtures of chemotypes in a 1:1 ratio in different environments: Carman field plots 2008, Carman field plots 2009, and Greenhouse, and Growth Room experiments with ‘CDC Teal’ wheat.
Field studies (2008–2012)– Glenlea, MB
When analysed by year, the ratio of 3-ADON to 15-ADON isolates was significantly different from a 1:1 ratio in 2008 and 2011 (); in 2008 3-ADON isolates comprised 79% of the recovered F. graminearum, while in 2011 just 43% of the recovered isolates were of the 3-ADON chemotype. In all other years, the ratio was not different from 1:1 (). When examined by genotype, the recovery of 3-ADON isolates was significantly higher for the resistant genotype ‘93FHB37’ (). A similar trend was seen for ‘5602 HR’ (MR) and ‘Roblin’ (S), but data across years was heterogeneous. No differences from a 1:1 ratio were found for ‘AC Snowbird’ (I), ‘AC Morse’ (S), or ‘AC Vista’ (S) ().
Fig. 2 Recovery of 3-acetyldeoxynivalenol (ADON) and 15-ADON isolates of Fusarium graminearum from Glenlea MB, fusarium head blight nurseries inoculated with both chemotypes from 2008 to 2012. Results for 2008 and 2011 did not fit a 1:1 ratio.
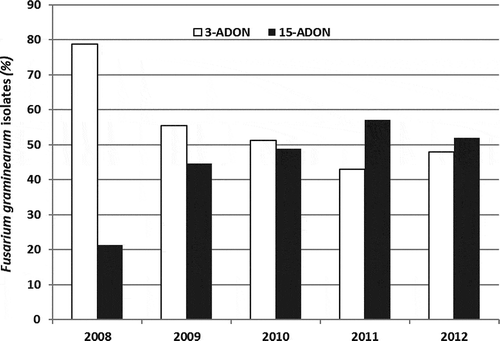
Table 3. Recovery of 3-acetyl deoxynivalenol (ADON) and 15-ADON isolates of Fusarium graminearum recovered from wheat kernels in inoculated fusarium head blight nurseries at Glenlea, Manitoba from 2008–2012.
An examination of weather variables throughout the five seasons showed no consistent pattern that might account for the sudden change from prevalence of 3-ADON isolates to almost equal, or higher numbers of 15-ADON isolates recovered between 2008 and the succeeding years (, b). In 2009, conditions were cooler than in 2008 and 2010, but 2011 and 2012 were hot, dry years. There was higher precipitation in 2010 than in 2008 and 2009, and 2011 and 2012 were extremely dry. Minimum temperatures were mostly above 10°C from 2008 to 2010, but rarely reached 10°C in 2011 and 2012 (, b). There were no conditions that were consistently present during the five growing seasons which could explain the prevalence of either 3-ADON or 15-ADON isolates, or a 1:1 ratio of recovery of the chemotypes.
Controlled environment inoculation
Under controlled conditions at temperatures ranging from 14 to 22 °C, the recovery of 15-ADON isolates predominated. Seven of the 10 samples resulted in higher recovery of 15-ADON isolates, while the remainder showed no significant deviation from a 1:1 ratio (). Only Treatment Mixture 2 resulted in recovery of chemotypes which were not significantly different from a 1:1 ratio in the greenhouse trial (). The trial in the growth room resulted in recovery of isolates in a 1:1 ratio for Treatment Mixtures 1, 2 and 5, but a significantly different ratio for Treatment Mixtures 1 and 4 was observed, in which recovery of the 15-ADON chemotype was more prevalent (). Under these conditions, the overall values for both experiments showed high recovery of 15-ADON isolates.
Studies under controlled conditions at different temperatures
When plants were incubated at 20 °C after inoculation with F. graminearum, the ratio of 3-ADON:15-ADON isolates recovered was significantly different from a 1:1 ratio in 7 of 11 of the samples, with 15-ADON isolates more prevalent (). At 28 °C, the ratio reversed and recovery of 3-ADON isolates was significantly higher than recovery of 15-ADON isolates in 5 of 11 samples. At 24 °C, the results were mixed. Of the 12 samples, five did not fit a 1:1 ratio, but 3/5 of the samples produced more 3-ADON isolates and 2/5 more 15-ADON isolates ().
Table 4. Recovery of 3-acetyldeoxynivalenol (ADON) and 15-ADON isolates of Fusarium graminearum after incubation of inoculated wheat plants at 20, 24 and 28 °C.
Discussion
All values that deviated from a 1:1 ratio of 3- to 15-ADON isolates from the Carman field plots showed higher recovery of 3-ADON isolates in both 2008 and 2009. Over the five years of data from the Glenlea FHB nurseries, the majority of values (9 of 11) that were significantly different from a 1:1 ratio were for higher recovery of 3-ADON isolates. However, 19 of 30 values were not significantly different from a 1:1 ratio for the two chemotypes. In the 2008 Glenlea FHB nursery, a significantly higher proportion of the isolates were of the 3-ADON chemotype from all six genotypes, but the isolates used for inoculum that year were mixed and increased together in CMC. It is therefore difficult to conclude what proportion of the spores in the inoculum were of 3-ADON or 15-ADON chemotype in 2008. In an earlier study, 3-ADON isolates were found to have a higher fecundity and produced more conidia under controlled conditions on potato dextrose agar (Ward et al., Citation2008). However, on V8 agar, 15-ADON isolates produced greater numbers of conidia than 3-ADON isolates, indicating that a fundamental difference exists between the two chemotypes (R. Clear, unpublished data).
High numbers of 3-ADON isolates were consistently recovered from cultivar ‘AC Barrie’ and resistant line ‘93FHB37’. The latter showed a significant deviation from a 1:1 ratio at Carman in 2008 in favour of the 3-ADON chemotype. In 2009, the same trend was seen, although the difference was not significant. However, in the 5 years of the Glenlea nursery, the overall pooled Chi-squared value (data not shown) showed that 3-ADON isolates were recovered in a ratio that deviated significantly from 1:1, again in favour of 3-ADON isolates.
In the Carman field study, only ‘AC Barrie’ had a 3-ADON:15-ADON ratio that was significantly different from 1:1 over both 2008 and 2009. Guo et al. (Citation2008) examined the population distribution of 15-ADON and 3-ADON chemotypes across Manitoba and conducted 291 isolations to determine the chemotype distribution at different locations, including the average across locations and of cultivars across locations. The average over all cultivars tested was 66% 15-ADON, but ‘AC Barrie’ had the largest fraction of 3-ADON isolates recovered with an average of 62%. Between 1998 and 2006, ‘AC Barrie’ was the most widely grown wheat cultivar across the prairies, accounting for 38% and 36% in 2001 and 2002, respectively (Preston et al., Citation2002) and remained as one of the top three cultivars grown in 2006 and 2007. While there is a substantial body of evidence that resistance to FHB in wheat is not race specific (Eeuwijk et al., Citation1995; Mesterházy et al., Citation1999) neither of these studies examined the outcome of in planta competition between F. graminearum chemotypes. It is possible that ‘AC Barrie’ is predisposed to infection by 3-ADON isolates over 15-ADON isolates, although further studies would have to be conducted before any conclusions could be drawn.
A separate study also showed high recovery of isolates of the 3-ADON chemotype in 2008 (Clear et al., Citation2013). A barley nursery was inoculated with corn kernels infested with both 3-ADON and 15-ADON isolates of F. graminearum. The isolates recovered from grain harvested in different parts of the nursery showed a gradient from the east with a high percentage of 3-ADON isolates and high DON levels (67%, 46 ppm, respectively) to the west where 15-ADON isolates predominated and where DON levels in the grain were lower (84%, 13 ppm, respectively). In the following 2 years, however, the number of 3-ADON isolates that were recovered was much lower, accounting for just 18% and 13% of the F. graminearum isolates, respectively. As 2008 was the first year a 3-ADON isolate was introduced into the inoculum mix in that nursery, the origin of the 3-ADON component must have been either the infested grain spawn, or aerial sources of inoculum.
The significance of the inoculum applied to a nursery relative to recovery of the same isolates may not be as informative as we had anticipated. Using characterized isolates of F. graminearum, several studies have shown that there was a rapid reduction in recovery of the isolates as the distance from a point source of inoculum increased. When head blight gradients were measured from a point source of inoculum, using either an aqueous suspension of macroconidia, or F. graminearum-infested corn as a source of ascospores, seed infection declined to 10% of the maximum within 5–22 m from the focal centre in ascospore-inoculated plots, and within 5 m in a plot inoculated with macroconidia (Fernando et al., Citation1997). Other studies, in which characterized isolates were used and which could be recognized upon recovery using amplified fragment length polymorphism, also showed limited dispersal from a point source of inoculum (Keller et al., Citation2010, Citation2011). At 3 m from the point source, only 2% of the incidence of spike infection was attributable to the released clones, indicating that in spite of inoculation, infection in a nursery was probably increased with incoming aerial inoculum. Since the long-distance dispersal of spores was previously demonstrated and isolates may originate from many kilometres away (Schmale et al., Citation2012), this can confound the results from the current study.
When tested under controlled conditions at temperatures between 14 °C and 22 °C, all values that deviated significantly from a 1:1 ratio showed a higher recovery of 15-ADON isolates. At 28 °C, all significant deviations from a 1:1 ratio were for recovery of 3-ADON isolates. These data suggested that temperature per se might affect the ability of the different chemotypes to out-compete each other. Under controlled conditions, higher temperatures appeared to shift the ratio to higher recovery of 3-ADON isolates, but temperature was not a major factor in this study. Field temperatures in 2008 and 2010 were similar, while 2009 was marginally cooler and 2011 and 2012 were hot (). There was less rain in July 2008 with a high recovery of 3-ADON isolates (). There was more rain in July 2009 and 2010 and a relatively higher recovery of 15-ADON isolates. However, there was little rain and high temperatures in 2011and 2012 when an equal or higher ratio of 15-ADON isolates were recovered.
The shift from occurrence of mainly 15-ADON isolates to a higher proportion of 3-ADON isolates is not unique to Canada. A recent study reported an ~11-fold (38%) increase in 3-ADON isolates of F. graminearum from barley in the Midwest of the USA in 2008, compared with older collections from 1997 to 2000 (3.5%) (Burlakoti et al., Citation2011). In particular, northern and central counties in North Dakota had higher levels of 3-ADON isolates. This finding agrees with an earlier study (Gale et al., Citation2007), who found that 5.1% of 587 samples collected in 1999 and 2000, mostly from wheat, but some from barley, were of the 3-ADON chemotype. Twenty-five of these 3-ADON isolates were collected from Minnesota and five from North Dakota.
There was little consistency in recovery of chemotypes applied to field nursery plots in this study, although the prevalence of 3-ADON isolates was confirmed in three of seven environments (Carman 2008, 2009 and Glenlea 2008–2012). 15-ADON isolates predominated in just 1 year and the 3 other years yielded a 1:1 ratio. The recovery of chemotypes from cultivars or lines was not consistent either. It is possible that 3-ADON isolates had some advantage in ‘AC Barrie’ and ‘93FHB37’, but there was no consistency in the prevalence of the different chemotypes on the other cultivars.
Over the last several years, 3-ADON isolates have become the major chemotype isolated from Manitoba, the Maritimes, and increasingly from Saskatchewan and Quebec (R. Clear, unpublished data). This was accompanied by the dominance of the 3-ADON toxin in commercial samples from the same areas. Therefore, it appears that the proportion of 3-ADON isolates has increased in proportion to the 15-ADON isolates. The field trials reported here did not provide data from which to draw conclusions on the cause of this change. It would seem that other unidentified factors are contributing, in whole or in part, to the dominance of the 3-ADON isolates in commercial fields.
References
- Abramson, D., Clear, R.M., & Smith, D.M. (1993). Trichothecene production by Fusarium spp. isolated from Manitoba grain. Can. J. Plant Pathol., 15, 147–152.
- Burlakoti, R.R., Neate, S.M., Adhikari, T.B., Gyawali, S., Salas, B., Steffenson, B.J., & Schwarz, P.B. (2011). Trichothecene profiling and population genetic analysis of Gibberella zeae from barley in North Dakota and Minnesota. Phytopathology, 101, 687–695.
- Clear, R.M., Tucker, J.R., Gaba, D., Patrick, S.K., Lee, S.J., Demeke, T., Tittlemier, S.A., Legge, W.G., & Gräfenhan, T. (2013). Deoxynivalenol levels and chemotype frequency in barley cultivars inoculated with two chemotypes of Fusarium graminearum. Can. J. Plant Pathol., 35, 37–45.
- Eeuwijk, F.A., Mesterhazy, A., Kling, C.I., Ruckenbauer, P., Saur, L., Bürstmayr, H., Lemmens, M., Keizer, L.C.P., Maurin, N., & Snijders, C.H.A. (1995). Assessing non-specificity of resistance in wheat to head blight caused by inoculation with European strains of Fusarium culmorum, F. graminearum and F. nivale using a multiplicative model for interaction. Theor. Appl. Genet., 90, 221–228.
- Fernando, W.G.D., Paulitz, T.C., Seaman, W.L., Dutilleul, P., & Miller, J.D. (1997). Head blight gradients caused by Gibberella zeae from area sources of inoculum in wheat field plots. Phytopathology, 87, 414–421.
- Foroud, N.A., McCormick, S.P., MacMillan, T., Badea, A., Kendra, D.F., Ellis, B.E., & Eudes, F. (2012). Greenhouse studies reveal increased aggressiveness of emergent Canadian Fusarium graminearum chemotypes in wheat. Plant Dis., 96, 1271–1279.
- Gale, L.R., Ward, T.J., Balmas, V., & Kistler, H.C. (2007). Population subdivision of Fusarium graminearum sensu stricto in the upper midwestern United States. Phytopathology, 97, 1434–1439.
- Gilbert, J., Clear, R.M., Ward, T.J., Gaba, D., Tekauz, A., Turkington, T.K., Woods, S.M., Nowicki, T., & O‘Donnell, K. (2010). Relative aggressiveness and production of 3- or 15-acetyl deoxynivalenol and deoxynivalenol by Fusarium graminearum in spring wheat. Can. J. Plant Pathol., 32, 146–152.
- Gilbert, J., & Tekauz, A. (2000). Recent developments in research on fusarium head blight of wheat in Canada. Can. J. Plant Pathol., 22, 1–8.
- Gilbert, J., & Woods, S.M. (2006). Strategies and considerations for multi-location FHB screening nurseries. In T. Ban, J.M. Lewis and E.E. Phipps (Eds.). The Global Fusarium Initiative for International Collaboration: A Strategic Planning Workshop (pp. 93–102). El Batan, Mexico, D.F: CIMMYT.
- Guo, X.W., Fernando, W.G.D., & Seow-Brock, H.Y. (2008). Population structure, chemotype diversity, and potential chemotype shifting of Fusarium graminearum in wheat fields of Manitoba. Plant Dis., 92, 756–762.
- Keller, M.D., Thomason, W.E., & Schmale, I.I.I.D.G. (2011). The spread of a released clone of Gibberella zeae from different amounts of infested corn residue. Plant Dis., 95, 1458–1464.
- Keller, M.D., Waxman, K.D., Bergstrom, G.C., & Schmale, I.I.I.D.G. (2010). Local distance of wheat spike infection by released clones of Gibberella zeae disseminated from infested corn residue. Plant Dis., 94, 1151–1155.
- Mesterházy, A., Bartók, T., Mirocha, C.G., & Komoróczy, R. (1999). Nature of wheat resistance to Fusarium head blight and the role of deoxynivalenol for breeding. Plant Breed., 118, 97–110.
- Miller, J.D., Taylor, A., & Greenhalgh, R. (1983). Production of deoxynivalenol and related compounds in liquid culture by Fusarium graminearum. Can. J. Microbiol., 29, 1171–1178.
- Preston, K.R., Hatcher, D.W., & Marchylo, B.A. (2002). Quality of western Canadian wheat 2002. Canadian Grain Commission. Available from http://publications.gc.ca/site/eng/418225/publication.html [accessed 2 January 2014].
- Puri, K.D., & Zhong, S. (2010). The 3ADON population of Fusarium graminearum found in North Dakota is more aggressive and produces a higher level of DON than the prevalent 15ADON population in spring wheat. Phytopathology, 100, 1007–1014.
- Schmale, I.I.I.D.G., Ross, S.D., Fetters, T.L., Tallapragada, P., Wood-Jones, A.K., & Dingus, B. (2012). Isolates of Fusarium graminearum collected 40–320 meters above ground level cause Fusarium head blight in wheat and produce trichothecene mycotoxins. Aerobiologia, 28, 1–11.
- von der Ohe, C., Gauthier, V., Tamburic-Ilincic, L., Brule-Babel, A., Fernando, W.G.D., Clear, R., Ward, T.J., & Miedaner, T. (2010). A comparison of aggressiveness and deoxynivalenol production between Canadian Fusarium graminearum isolates with 3-acetyl and 15-acetyldeoxynivalenol chemotypes in field-grown spring wheat. Eur. J. Plant Pathol., 127, 407–417.
- Ward, T.J., Clear, R.M., Rooney, A.P., O‘Donnell, K., Gaba, D., Patrick, S., Starkey, D.E., Gilbert, J., Geiser, D.M., & Nowicki, T.W. (2008). An adaptive evolutionary shift in Fusarium head blight pathogen populations is driving the rapid spread of more toxigenic Fusarium graminearum in North America. Fungal Genet. Biol., 45, 473–484.