Abstract
Dollar spot, caused by Sclerotinia homoeocarpa, is one of the most economically devastating diseases of amenity turfgrasses worldwide. The pathogen is readily isolated from active lesions, but detection from seed, dormant host tissue and other plant debris that may serve as a source of primary inoculum is difficult. A semi-selective medium was developed to enhance isolation of S. homoeocarpa. Various fungicides used on turfgrass, the pH indicator dye bromophenol blue, and two pH levels, were assessed for their effects on, and in the case of bromophenol blue, response to growth of S. homoeocarpa and contaminant fungi frequently isolated from field and seed samples. Amendment of the medium to pH 4 promoted growth of S. homoeocarpa in the absence of fungicides and enhanced pathogen growth relative to contaminant isolates on medium amended with 0.1 μg mL−1 triticonazole or 5 μg mL−1 azoxystrobin. The growth rate of S. homoeocarpa on these three media, as determined by in vitro radial growth assays, was consistent for many isolates representing the majority of S. homoeocarpa vegetative compatibility groups. Isolation efficiency on the pH-amended media was tested against antibiotic-amended potato dextrose agar from field samples and artificially inoculated turfgrass seed. In both cases, the medium amended to pH 4 and containing 5 μg mL−1 azoxystrobin significantly decreased contaminant growth with no adverse effects on recovery of S. homoeocarpa in comparison with antibiotic-amended PDA. The enhanced specificity of this medium will be a useful tool for selective detection of S. homoeocarpa and identification of sources of initial inoculum for dollar spot development.
Résumé
La brûlure en plaques, causée par Sclerotinia homoeocarpa, est une des maladies du gazon d’agrément dont les répercussions économiques sont des plus importantes à l’échelle mondiale. L’agent pathogène est facilement isolé à partir de lésions actives, mais la détection à partir de semences, de tissu dormant et d’autres débris végétaux qui peuvent servir de source primaire d’inoculum n’est pas aussi simple. On a développé un milieu semi-sélectif pour mettre en relief l’isolement de S. homoeocarpa. Divers fongicides utilisés sur le gazon, l’indicateur de pH bleu de bromophénol et deux niveaux de pH ont été évalués quant à leurs effets sur S. homoeocarpa et, dans le cas du bleu de bromophénol, quant à la réaction par rapport à sa croissance ainsi qu’aux champignons contaminants fréquemment isolés d’échantillons de sol et de semences. L’amendement du milieu pour obtenir un pH de 4 a stimulé la croissance de S. homoeocarpa en l’absence de fongicides et a favorisé la croissance de l’agent pathogène par rapport aux isolats contaminants se développant sur le milieu amendé avec 0.1 µg mL−1 de triticonazole ou 5 µg mL−1 d’azoxystrobine. Le taux de croissance de S. homoeocarpa sur ces trois milieux, comme défini pas les biotests de croissance radiale in vitro, était uniforme pour plusieurs isolats représentant la majorité des groupes de compatibilité végétative de S. homoeocarpa. L’efficacité de l’isolement sur le milieu dont le pH avait été modifié a été comparée à celle de la gélose dextrosée à la pomme de terre, amendée avec des antibiotiques sur laquelle on avait placé des échantillons prélevés au champ et des semences de gazon inoculées artificiellement. Dans les deux cas, le milieu amendé dont le pH avait été ajusté à 4 et contenant 5 µg mL−1 d’azoxystrobine a réduit significativement la croissance des contaminants sans pour autant nuire au rétablissement de S. homoeocarpa, comparativement à la gélose dextrosée amendée avec des antibiotiques. La spécificité accrue de ce milieu s’avérera utile pour la détection sélective de S. homoeocarpa et l’identification des sources initiales d’inoculum impliqué dans le développement de la brûlure en plaques.
Introduction
Dollar spot, caused by the ascomycetous fungus Sclerotinia homoeocarpa (F.T. Bennett), is one of the most serious and widespread diseases of turfgrasses (Bennett, Citation1937; Walsh et al., Citation1999; Allen et al., Citation2005), as well as other economically important plants including switchgrass (Vu et al., Citation2011) and perennial peanut (Hoover & Kucharek, Citation1995). On individual blades of grass, the disease manifests as bleached, hourglass-shaped to oblong lesions with reddish-brown borders (Allen et al., Citation2005; Smiley et al., Citation2007). These symptoms vary between host species as well as between cultivars with varying susceptibility to S. homoeocarpa (Vincelli et al., Citation1997; Vincelli & Dixon, Citation2002; Bonos et al., Citation2004). Dollar spot earns its name from the sunken, straw-coloured, roughly silver dollar-sized infection centres that are typically present when the disease occurs on closely mown turfgrass swards, such as golf course putting greens or fairways (Vargas, Citation1994; Couch, Citation1995). Severe disease outbreaks result in the coalescence of individual foci into large blighted areas, which detract from both aesthetic and playing quality of the affected turf (Couch, Citation1995; Smiley et al., Citation2007). On less rigorously maintained turfgrass, including homelawns and playing fields, dollar spot symptoms typically include larger, more diffuse patches of blighted grass.
Chemical control is the most common and effective method for managing dollar spot (Smiley et al., Citation2007). However, increasingly stringent regulatory measures and concerns about economic and environmental costs have necessitated decreased dependence on fungicides for dollar spot management (Latin, Citation2011). Chemical control of dollar spot has also been complicated by development of S. homoeocarpa populations with resistance to benzimidazole (Warren et al., Citation1974, Citation1977; Burpee, Citation1997), dicarboximide (Detweiler et al., Citation1983), and sterol demethylation-inhibitor (DMI) fungicide classes (Warren et al., Citation1977; Golembiewski et al., Citation1995; Hsiang et al., Citation1997, Citation2007; Koch et al., Citation2009; Ok et al., Citation2011).
Identifying the source of initial inoculum for dollar spot is critical for development of integrated, pathogen biology-based control methods that limit dependence on fungicides (Walsh et al., Citation1999). Research demonstrates that S. homoeocarpa does not thrive in soil without a source of organic matter; thus, it is unlikely that this fungus survives as a soil-inhabiting saprophyte (Wilson, Citation2011). Infected seed, dormant overwintering mycelia, and symptomless infections are all possible sources of S. homoeocarpa inoculum (Fenstermacher, Citation1980; Walsh et al., Citation1999). Attempts to isolate S. homoeocarpa from such tissues on antibiotic amended potato dextrose agar (PDA+++) are largely unsuccessful due to lack of medium specificity and the large number of contaminant fungi present on such samples. Consequently, the objectives of this study were to: (i) develop a semi-selective medium that inhibits the growth of common contaminant fungi while minimizing inhibitory effects on S. homoeocarpa; and (ii) compare the effectiveness of such media against PDA+++ for isolation of S. homoeocarpa and contaminant fungi from field and seed samples.
Materials and methods
Fungal strains
Four isolates of S. homoeocarpa and eight contaminant fungi obtained from field and seed samples were used for preliminary growth assays on trial media (). All isolates were obtained by plating turfgrass leaves or seed samples on Petri plates containing potato dextrose agar (PDA) amended with 50 mg L−1 each of the antibiotics chloramphenicol, streptomycin sulphate and tetracycline (PDA+++). Hyphal tips of putative S. homoeocarpa and contaminant colonies were excised and transferred to fresh PDA+++ to obtain pure cultures. When possible, isolates were identified to genus by sequencing of the ITS region with primers ITS1 and ITS2 (White et al., Citation1990); however, not all contaminant isolates were amenable to common DNA extraction methods and were left as ‘unidentified’. Additionally, not all contaminant fungi could be identified to genus by BLASTN searches of resulting sequences and were only identified to the lowest definite taxonomic group (Altschul et al., Citation1997). Sclerotinia homoeocarpa vegetative compatibility grouping (VCG) tester isolates for validation studies were obtained from Dr Lane Tredway (formerly at North Carolina State University), and were initially described by Viji et al. (Citation2004) (). All isolates were maintained by weekly transfer to fresh PDA and stock cultures were stored on filter paper discs at −80 °C.
Table 1. Fungal isolates used for medium evaluations.
Medium additives and preparation
Three antibiotics, five fungicides at varying concentrations, the pH indicator dye bromophenol blue, and 12.1N hydrochloric acid (HCl) were used as medium additives in this research (). The base medium PDA was prepared according to the manufacturer’s instructions and cooled to 50 °C prior to addition of amendments. All amendments were added to media on a 50 °C stir plate to ensure homogeneity and media were poured immediately after amendment addition to avoid settling. To decrease variability inherent in manual pouring, a 10 mL pipettor (Eppendorf, New York, NY, USA) was used to pipette 6.5 mL medium into each 60 × 15 mm Petri plate. Plates were allowed to solidify within a biological safety cabinet and then stored in the dark at 4 °C until used. To avoid breakdown of media additives, all plates were used within 5 days of pouring.
Table 2. Amendments added to experimental semi-selective media.
Mycelial growth assays on trial media
Agar plugs (6 mm diameter) were excised using a sterile cork borer from the advancing edge of 1-week-old fungal colonies of S. homoeocarpa and contaminant fungi. Plugs were placed in the centre of 60 × 15 mm Petri plates containing semi-selective media. Plates were inverted, arranged randomly, and incubated at room temperature and ambient lighting for 48 h. To prevent edge or other position effects, plates were arbitrarily rearranged 24 h after transfer. At 48 h post-transfer, radial growth was measured and per cent growth versus the standard medium PDA+++ was calculated using the formula:
[(Average colony diameter on trial media) / (Average colony diameter on PDA+++)] × 100%].
Each fungal isolate × medium combination was performed in triplicate and the experiment was repeated three times.
Acid production on bromophenol blue amended media
To test acid production as a positive indicator for S. homoeocarpa, 6 mm diameter agar plugs were excised from the advancing edge of 1-week old fungal colonies with a sterile cork borer and placed in the centre of 60 × 15 mm agar plates amended with 50 mg L−1 bromophenol blue as previously described (Steadman et al., Citation1994). Plates were sealed with Parafilm and incubated upside-down at room temperature and ambient lighting for 48 h. Yellow colour production was then rated as (0) for no colouration, (1) for weak colouration, and (2) for strong colouration. Due to the subjective nature of this rating scale, all ratings were performed by the same individual and all plates in a repetition were rated at the same time. Each fungal isolate × medium combination was performed in triplicate and the experiment was repeated three times.
Effect of decreased pH on fungal growth
HCl (12.1N) was added to cooled medium with a pipette (600 μL L−1) to achieve a desired medium pH of 4. The pH of control PDA was checked with pH strips and was consistently near 6. For fungicide-amended media, fungicides were added prior to HCl to prevent interference of pH with solubility. Mycelial growth of S. homoeocarpa and contaminant isolates was determined as described above. Each isolate × pH × fungicide interaction was performed in triplicate and the experiment was repeated three times.
Evaluation of semi-selective media for detection of S. homoeocarpa in naturally infected field samples
In the spring of 2012, a total of 50 soil cores were collected for use in validating semi-selective medium efficiency. Ten were from active dollar spot infection centres, 20 from asymptomatic turf, and 20 from dollar spot infection centres that had formed the previous autumn (overwintering samples). Fewer soil cores were selected from symptomatic infection centres because S. homoeocarpa is easily isolated from active lesions on PDA+++. For each sample, S. homoeocarpa isolation efficiency with and without surface disinfestation was determined.
To determine isolation efficiency, 12 grass blades were removed from a single core using sterile forceps. Three blades were then plated on each of the four previously mentioned media in a circular pattern with at least 10 mm between each blade to ensure that none overlapped. This process was repeated for each soil core collected. Plates were sealed with Parafilm, inverted and incubated at room temperature for 48 h. At 48 h, total fungal colony forming units (CFUs) per plate and number of putative S. homoeocarpa colonies per plate were determined. Putative S. homoeocarpa colonies were subcultured to obtain pure cultures and to confirm identity.
After the initial plating of grass blades, the same cores were immersed in a 0.06% sodium hypochlorite (10% Clorox) solution for 15 s and the shoots were gently rubbed, then rinsed in distilled water twice for 30 s each time. Samples were then allowed to dry on sterile paper towels and the entire leaf blade plating process was repeated as described above.
Evaluation of potential semi-selective media for detection of S. homoeocarpa in artificially infested creeping bentgrass seed
Five grams of commercially available creeping bentgrass ‘Penncross’ seed was mixed in a 50 mL beaker with 2.5 mL of deionized water. The beaker was sealed with a double layer of aluminium foil and sterilized on a dry cycle at 121 °C and 15 psi for 30 min. The sterilized seed was allowed to cool overnight and infested the following day with two agar plugs taken from the advancing edge of a 5-day-old colony of S. homoeocarpa isolate 2F92-1. The beaker was sealed with Parafilm and incubated at room temperature and ambient lighting with daily shaking to disperse inoculum and prevent clumping. After 3 weeks, an arbitrary sample of approximately 100 seeds was plated on PDA+++ to determine colonization. The remaining infested seeds were placed on sterile paper towels and allowed to dry overnight in a biological safety hood. One gram of infested seed was then mixed with 9 g of untreated ‘Penncross’ seed in a 1:9 ratio of infested to non-infested seed and this was conducted four times to provide four replicate samples. Seed mixtures were stored in 50 mL conical tubes (BD Falcon, San Jose, CA) at room temperature with ambient lighting.
Two days after mixing infested and non-infested seed, 400 seeds were collected from the mixtures and distributed evenly among the four media (10 Petri plates of each medium with 10 seeds on each plate). Seeds were plated individually and spaced so that none touched. Plates were sealed with Parafilm and incubated upside-down at room temperature and ambient lighting for 5 days. The proportion of seed with S. homoeocarpa recovery was recorded after 24 h, and after 5 days the number of colonies of contaminant fungi per plate was determined. This entire experiment was repeated 9, 13 and 14 days after the initial seed mixtures were prepared, for a total of four experimental replications.
Statistical analysis
All experimental data were analysed using the generalized linear mixed model (GLIMMIX) procedure in SAS Version 9.3 (SAS Institute, Cary, NC). Model fitting criteria and studentized residual plots were assessed to determine the best model for each dataset. Generally, isolate and media were treated as fixed effects and experimental replicate (block) was set as a random effect. Replicate was not included as a random effect when model fitting criteria indicated a negligible effect on variance components.
Mycelial growth assays were best fit by a lognormal distribution. Pre-planned single degree of freedom contrast statements were used to make meaningful comparisons between groups of interest. These included: S. homoeocarpa versus contaminant isolates on trial semi-selective media, growth on media at pH 6 versus pH 4, and growth of Wisconsin S. homoeocarpa isolates versus VCG tester isolates on pH-amended media. Yellow colouration on bromophenol blue-amended media was best fit by a normal distribution and differences between S. homoeocarpa and contaminant isolates were again evaluated with pre-planned contrast statements.
The seed and field sample validation experiments both consisted of count data and were best fit by a Poisson and negative binomial distribution, respectively. Dunnett’s test was used to compare each candidate semi-selective medium against the control PDA+++ for isolation frequency of both S. homoeocarpa and contaminants. For field samples, the analysis was sliced by sample type to allow for comparison of candidate semi-selective media against PDA+++ only within the same sample type.
Results
Effect of fungicide amendments
Mycelial growth assays were conducted on media containing five fungicides at varying concentrations, for a total of 13 fungicide-amended media tested in the absence of other additives. The average colony diameter and growth relative to the standard isolation medium PDA+++ were compared for four isolates of S. homoeocarpa and eight contaminant isolates (). In general, S. homoeocarpa and contaminant isolates had similar colony diameter and growth relative to PDA+++ on fungicide-amended media. Greater average colony diameter and relative growth of S. homoeocarpa when compared with contaminant fungi were the criteria used to select potential semi-selective media. None of the initial media tested met these criteria.
Table 3. Relative growth of S. homoeocarpa and contaminant isolates on fungicide-amended media.
Even at the lowest concentrations of PCNB and thiophanate methyl, large differences were noted in growth of the four S. homoeocarpa isolates. Isolates S10 and OJN9 grew nearly as well on media amended with 10 μg mL−1 thiophanate methyl as on the standard isolation medium, PDA+++. Conversely, growth of isolates 2F92-1 and Heath1A was roughly 10% of growth on PDA+++. Medium containing 5 μg mL−1 of PCNB severely limited or prevented growth of all S. homoeocarpa isolates except Heath1A. Due to the variability in S. homoeocarpa growth responses to these media, they were not considered for selective isolation of S. homoeocarpa and were not included in pH or BB trials.
Specificity of bromophenol blue for detection of S. homoeocarpa
The pH indicator dye BB was added to media containing 5, 10 and 20 μg mL−1 of azoxystrobin (A 5, 10, 20). The purpose of this dye was to visualize a reduction of medium pH in response to oxalic acid production by S. homoeocarpa (Steadman et al., Citation1994; Venu et al., Citation2009). All isolates were rated at 48 h for the production of yellow colouration in the medium and average pigmentation was compared between S. homoeocarpa and contaminant isolates (Table S1). On bromophenol blue-amended medium with 5 or 20 μg mL−1 azoxystrobin, contaminant isolates produced more yellow pigmentation than S. homoeocarpa isolates. In medium containing 10 μg mL−1 azoxystrobin, an orthogonal contrast comparing S. homoeocarpa isolates to contaminants indicated that S. homoeocarpa produced significantly more pigmentation than contaminants; however, this was the result of very strong medium acidification by some S. homoeocarpa isolates and negligible colour change by others (Table S2).
Effect of medium pH
PDA+++pH 4 had a strong positive effect on S. homoeocarpa growth relative to PDA+++ (a). Growth of all contaminant isolates, with the exception of 24FR, was suppressed on PDA+++pH 4 and was significantly less than that of the four S. homoeocarpa isolates. Growth of 24FR on PDA+++pH 4 was similar to that on PDA+++. Both the average colony diameter (41.14 mm) and growth relative to PDA+++ (138.3%) of S. homoeocarpa isolates were greater than those of contaminants (17.82 mm and 70.92%, respectively) on PDA+++pH 4 (Table S3).
Fig. 1 Effect of medium pH-amendment on growth of S. homoeocarpa and contaminant isolates. On the x-axis, ‘Sh’ indicates the combined mean colony diameter of the four Wisconsin S. homoeocarpa isolates; ‘Contam’ indicates the combined mean colony diameter of the eight common contaminant fungi. Columns represent mean colony diameter (mm) of S. homoeocarpa and contaminant isolates with (white) and without (black) addition of HCl to medium for (a) PDA+++, (b) T01, (c) T1, (d) P01, (e) P1, (f) A5, (g) A10 and (h) A20, respectively. Errors bars represent one standard error of the mean. Two-sided independent t-tests were used to compare colony diameter between the two media and significance is indicated as follows: * P < 0.05, ** P < 0.01 and *** P < 0.001.
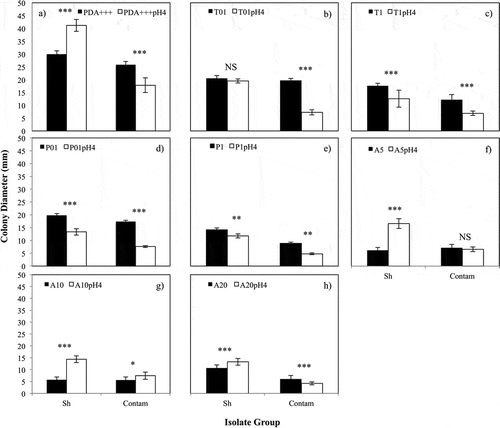
Growth of S. homoeocarpa isolates on A5pH 4 was greater than that on A5 (two-way independent t-test, P < 0.0001; f). Contaminant isolates grew equally well on both A5 and A5pH 4 media. Direct comparison between S. homoeocarpa and contaminant isolate growth on A5pH 4 revealed significantly greater colony diameter and growth relative to PDA+++ for S. homoeocarpa than for contaminant isolates (Table S3). Colony diameter and relative growth of S. homoeocarpa remained higher than that of contaminants on pH 4 azoxystrobin-amended media with 10 and 20 μg mL−1 azoxystrobin, although both were decreased by the additional active ingredient (Table S3). Additionally, contaminant isolates also showed increased growth on A10pH 4 compared with A10 (g). This pH-mediated growth benefit was not observed for contaminant isolates at A20pH 4 (h).
The DMI-fungicides triticonazole (T) and propiconazole (P) suppressed the growth of both S. homoeocarpa and contaminant isolates in acidified media relative to media containing the same concentration of active ingredient at neutral pH (b–e). There was no growth reduction in S. homoeocarpa isolates on T1pH 4 medium relative to T1 medium. Contaminant growth was strongly suppressed on T1pH 4 relative to T1 (b). Consequently, both S. homoeocarpa growth and growth relative to PDA+++ were greater than that of contaminants on T1pH 4 (Table S3). Sclerotinia homoeocarpa colony diameter was greater than that of contaminants on T1pH 4 and P1pH 4 but growth relative to PDA+++ was not different or only slightly better on these two media, respectively.
Growth of S. homoeocarpa VCG tester isolates on candidate semi-selective media
Candidate semi-selective media were chosen from those media for which colony diameter and growth relative to PDA+++ were both significantly greater for S. homoeocarpa than for contaminant isolates. Media meeting these criteria included PDA+++pH 4, T1pH 4 and azoxystrobin-amended media at pH 4 for all concentrations evaluated. Of the three azoxystrobin-amended media, A5pH 4 was selected as a candidate semi-selective medium because the largest differences between S. homoeocarpa and contaminant isolate colony diameter and relative growth were observed on this medium.
A total of 12 VCG tester isolates from groupings A through F, as well as three isolates of undefined VCG, were included in growth assays on candidate semi-selective media (Table S4). Pre-planned single degree of freedom contrasts were used to compare colony diameter between Wisconsin S. homoeocarpa isolates used for initial growth assays and VCG tester isolates. No statistically significant differences were detected between colony diameter of Wisconsin S. homoeocarpa isolates and VCG tester isolates on PDA+++ as well as the three candidate semi-selective media (). Similarly, no differences in growth were detected when representative isolates from different VCGs were compared (P > 0.05; data not shown). Growth of all S. homoeocarpa isolates was greater on PDA+++pH 4 than on PDA+++.
Table 4. Growth of Wisconsin S. homoeocarpa and vegetative compatibility grouping tester isolates on candidate semi-selective media.
Isolation from naturally infected field samples
The three candidate semi-selective media were compared with PDA+++ for their isolation efficiency from naturally infected or asymptomatic field samples both with and without surface disinfestation. A total of 50 samples [10 from symptomatic turf, 20 from asymptomatic turf and 20 from dollar spot infection centres formed the previous autumn (overwintering samples)] were used in this study. In general, isolation frequency was very low, with the exception of symptomatic samples (). Dunnett’s test with PDA+++ as the control group revealed no differences in S. homoeocarpa isolation efficiency, regardless of sample type or treatment. Overall, the highest S. homoeocarpa isolations were made on the A5pH 4 medium and the fewest were made on PDA+++. Additionally, S. homoeocarpa was not recovered from surface disinfested samples on PDA+++ ().
Table 5. Proportion of field samples positive for S. homoeocarpa on standard and candidate semi-selective media.
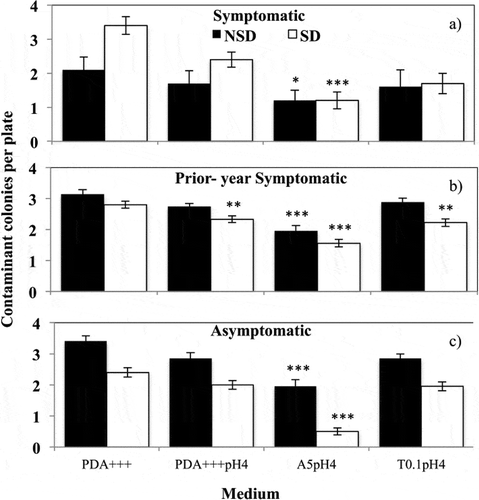
The number of contaminant fungi isolated per sample on candidate semi-selective media was also compared with the standard PDA+++ with Dunnett’s test. Fewer contaminant colonies were isolated on A5pH 4 than on PDA+++ for all samples types, regardless of surface disinfestation (a–). PDA+++pH 4 and T1pH 4 performed better than PDA+++ only on the prior-year symptomatic samples that had been surface-disinfested (a).
Fig. 2 Isolation of contaminant fungi from field samples on candidate semi-selective media. ‘NSD’ (black columns) represents non-surface disinfested samples and ‘SD’ (white columns) represents surface disinfested samples. Each column represents the mean number of contaminant fungal colonies isolated per plate from (a) symptomatic (N = 10), (b) prior-year symptomatic (N = 20), and (c) asymptomatic field samples (N = 20) either before (black) or after (white) surface disinfestation. Errors bars represent one standard error of the mean. Dunnett’s test used to compare contaminant isolations between the control medium, PDA+++ the two media and each candidate semi-selective medium. Significance is indicated as follows: * P < 0.05, ** P < 0.01 and *** P < 0.001.
Isolation from artificially infested creeping bentgrass seed
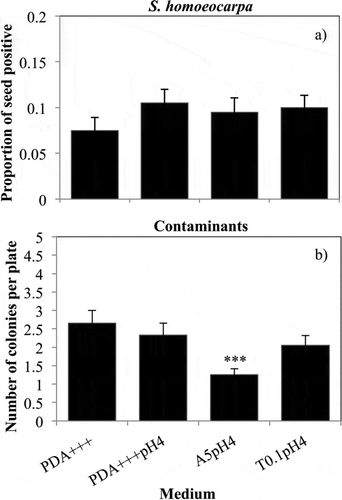
Candidate semi-selective media did not significantly increase isolation of S. homoeocarpa from artificially infected seed, although isolation of S. homoeocarpa was slightly greater on all semi-selective media than on PDA+++ (a). Isolation of S. homoeocarpa from infested seed was closer to the starting ratio of 1 : 9 (infected : untreated) seeds on candidate semi-selective media than on PDA+++. Of the three candidate semi-selective media, only A5pH 4 significantly decreased isolation of contaminant fungi when compared with PDA+++ (b). The average number of contaminants per plate was reduced from 2.65 on PDA+++ to 1.25 on A5pH 4.
Fig. 3 Isolation of S. homoeocarpa and contaminant fungi from inoculated seed samples. (a) The mean proportion of seeds out of 100 positive for S. homoeocarpa. (b) The mean number of contaminant fungal colonies isolated per plate (N = 10). Columns represent the mean of four replicated experiments treated as blocks in statistical analyses. Dunnett’s test used to compare contaminant isolations between the control medium, PDA+++ the two media and each candidate semi-selective medium. Significance is indicated as follows: * P < 0.05, ** P < 0.01 and *** P < 0.001.
Discussion
Both in planta overwintering (Fenstermacher, Citation1980) and introduction of S. homoeocarpa on contaminated seed have been hypothesized as possible sources of initial inoculum for epidemics of dollar spot on turfgrasses. Isolation of S. homoeocarpa from such samples, however, is difficult due to the large number of contaminant fungi living on or in the plant material, as well as decreased activity of S. homoeocarpa in asymptomatic plant tissues. Molecular detection methods using S. homoeocarpa-specific PCR primers have recently become available, but do not confirm viability of the pathogen and may not be sensitive enough to detect minute amounts of S. homoeocarpa DNA (Abd-Elmagid et al., Citation2013). Consequently, a medium that can select for S. homoeocarpa is essential for determining the contribution of infected seed, pathogen overwintering and latent periods in dollar spot epidemics. The A5pH 4 medium developed in this study, which significantly decreases contaminant growth without affecting growth of S. homoeocarpa, will help with isolations from these difficult samples and contribute to understanding of dollar spot epidemiology.
Many semi-selective media for phytopathogenic fungi rely on specific morphological characteristics to aid in identification of the target organism. These include traits such as profuse sporulation (Stanosz & Stanosz, Citation2002; Chen et al., Citation2010), microsclerotial production (Kabir et al., Citation2004) or pigmentation (Tsao, Citation1970; Steadman et al., Citation1994). North American isolates of S. homoeocarpa, as well as most isolates worldwide, do not produce spores, fruiting bodies or true survival structures (Orshinsky & Boland, Citation2011) and the white, aerial mycelia initially produced by S. homoeocarpa in culture are indistinguishable from those of many common saprophytic fungi, as well as other plant pathogens. The formation of substratal stroma in culture medium is fairly exclusive to S. homoeocarpa but may not form for weeks and can be difficult to detect in the presence of many competing fungi. Consequently, the primary goal of a semi-selective medium for S. homoeocarpa was to significantly reduce growth of contaminant fungi.
All fungicides tested in this study had a negative effect on the growth of S. homoeocarpa, despite the fact that resistance or insensitivity has been reported for many classes of fungicides (Warren et al., Citation1977; Golembiewski et al., Citation1995; Burpee, Citation1997; Jo et al., Citation2006; Benedetto & Hsiang, Citation2009; Koch et al., Citation2009; Ok et al., Citation2011). The exception was PCNB, which was included in this study as a result of its efficacy in semi-selective medium for isolation of S. sclerotiorum (Steadman et al., Citation1994). Sclerotinia homoeocarpa isolates differed significantly in their responses to PCNB and thiophanate methyl; thus, these fungicides would not be useful in a semi-selective medium for S. homoeocarpa because only resistant populations would be selected. While thiophanate methyl resistance has been well documented in S. homoeocarpa populations (Jo et al., Citation2008; Koch et al., Citation2009), PCNB resistance has not been reported for turfgrass pathogens (Latin, Citation2011). Resistance to PCNB has been documented in other ascomycete plant pathogens, including Botrytis cinerea and Sclerotinia sclerotiorum, as well as in Alternaria sp. and Magnaporthe oryzae (Steel & Nair, Citation1993; Bradley et al., Citation2006; Luo et al., Citation2012). Recently, Luo et al. (Citation2012) demonstrated that an osmosensitive signal transduction pathway present in many of these fungi governs resistance to both PCNB and dicarboximides. Whether the reduced sensitivity to PCNB in S. homoeocarpa isolate Heath1A in our in vitro assays correlates with practical resistance to this fungicide in the field or with cross-resistance to dicarboximide fungicides is unknown, but additional studies with this isolate may be of interest.
All S. homoeocarpa isolates grew similarly on media amended with various concentrations of azoxystrobin, triticonazole and propiconazole. This indicated that any of these fungicides could be a potential additive in a semi-selective medium for S. homoeocarpa because they would not preferentially select for specific populations. Fungicide-amended media can run the risk of preferentially selecting for insensitive populations, particularly with DMI fungicides, for which substantial differences in S. homoeocarpa sensitivity are common (Latin, Citation2011). By screening isolates with three different concentrations of the DMI fungicides propiconazole and triticonazole in our initial medium development, we were able to select fungicide concentrations well below those at which differences in S. homoeocarpa isolate sensitivity were detected (Table S1) and also below previously established threshold values for resistance (Miller et al., Citation2002; Jo et al., Citation2006). Fungicide amendments alone, however, did not produce significant differences in growth between S. homoeocarpa and common contaminant fungi. Additionally, bromophenol blue was determined to be an unnecessary media additive due to variability in acid production between S. homoeocarpa isolates and media acidification by some of the contaminant fungi tested.
Decreasing media pH with the addition of HCl was the most important amendment for selection of S. homoeocarpa and inhibition of contaminant fungi. Increased growth of S. homoeocarpa on PDA at pH 4 has been demonstrated previously (Venu et al., Citation2009) and is in agreement with our results for growth of S. homoeocarpa isolates on PDA+++pH 4 relative to PDA+++. Since S. homoeocarpa produces oxalic acid (Venu et al., Citation2009), it can acidify its own environment to create conditions suitable for growth. Various acids, including tannic, gallic, lactic and polygalacturonic, have been used in semi-selective media for other phytopathogenic fungi (Tsao, Citation1970; Stanosz & Stanosz, Citation2002; Blodgett et al., Citation2003; Kabir et al., Citation2004). In addition to promoting growth of specific target species over competitors, organic acids are frequently selected for addition to selective media due to the specific morphological changes they produce in the target organism or promotion of sporulation (Tsao, Citation1970; Stanosz & Stanosz, Citation2002).
This study revealed that there was a complex interplay between medium pH, fungicide amendment and growth of S. homoeocarpa. Of particular interest is the increase in S. homoeocarpa growth on acidified azoxystrobin-amended media relative to azoxystrobin-amended media at neutral pH (). This could be a result of decreased fungicide solubility; however, fungicide amendments were added to media first to avoid this possibility and prior research indicates that solubility of azoxystrobin is not pH dependent (von Stackelberg, Citation2012). Previous research shows that, in certain cases, azoxystrobin application can exacerbate epidemics of dollar spot in the field (Benedetto & Hsiang, Citation2009). In general, this disease enhancement has been attributed to effects on competing microbes or on soil and foliar microbial communities. The present data indicate a possible role of environmental pH as well. Further studies are needed to determine the mechanism by which decreased pH allows S. homoeocarpa to grow better in the presence of azoxystrobin.
In validation experiments with both naturally infected field samples and artificially infected seed, semi-selective media did not perform significantly better than the current standard isolation medium PDA+++. This result was not unexpected. It is well-known that S. homoeocarpa is readily isolated from symptomatic tissues without selective agents, as demonstrated by our high isolation rates from symptomatic field samples and artificially infected seed for all media tested. Semi-selective media for S. homoeocarpa are needed for isolation from more difficult sample types, such as seed and asymptomatic tissues, where the pathogen may linger but is not easily isolated from due to the presence of saprophytic fungi. These events are so rare that it is difficult to generate a sample size large enough to detect significant differences between media types. Indeed, a power analysis from the field dataset indicated that roughly 200 samples of each type would be needed to detect a difference between S. homoeocarpa isolation rates on A5pH 4 and PDA+++, although more recovery from the surface disinfested and non-surface disinfested samples were seen on A5pH4 in validation studies. Similarly, natural infection of turfgrass seed with S. homoeocarpa is so low (<1 : 1000; Rioux, Citation2014) that an impractical number of naturally infected seeds would need to be plated to detect a statistically significant difference between media. Nevertheless, S. homoeocarpa has only been isolated from naturally infected turfgrass seeds on semi-selective media (Rioux Citation2014).
A5pH 4 performed better than PDA+++ at reducing growth of contaminants in all samples tested. The other candidate semi-selective media were not as successful at reducing contaminant isolation and would not lead to significant improvement in S. homoeocarpa isolation efficiency. A5pH 4 suppressed contaminant isolations in both field and seed samples, which may have very different saprophyte fungal populations, indicating that it will be a useful medium for selective isolation of S. homoeocarpa from a range of sample types.
Supplemental Tables
Download MS Word (62.5 KB)Acknowledgements
The authors would like to thank Jeanette Shultz for technical assistance and Patricia McManus for critical review of previous versions of the manuscript. RR was supported by the John and Flora Berbee Wisconsin Distinguished Turfgrass Graduate Fellowship.
References
- Abd-Elmagid A, Garrido P, Hunger R, Lyles JL, Mansfield MA, Gugino BK, Smith DL, Melouk HA, Garzon CD. 2013. Discriminatory simplex and multiplex PCR for four species of the genus Sclerotinia. J Microbiol Meth. 92:293–300. doi:10.1016/j.mimet.2012.12.020
- Allen TW, Martinez A, Burpee LL. 2005. Dollar spot of turfgrass. Plant Health Instruct. doi:10.1094/PHI-I-2005-0217-02
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402. doi:10.1093/nar/25.17.3389
- Benedetto D, Hsiang T. 2009. Effect of azoxystrobin on dollar spot disease development in creeping bentgrass and Kentucky bluegrass. Int Turf Soc Res J. 11:151–163.
- Bennett FT. 1937. Dollar spot disease of turf and its causal organism Sclerotinia homoeocarpa n. sp. Ann Appl Biol. 24:236–257. doi:10.1111/j.1744-7348.1937.tb05032.x
- Blodgett JT, Bonello P, Stanosz GR. 2003. An effective medium for isolating Sphaeropsis sapinea from asymptomatic pines. Forest Pathol. 33:395–404. doi:10.1046/j.1437-4781.2003.00342.x
- Bonos SA, Casler MD, Meyer WA. 2004. Plant responses and characteristics associated with dollar spot resistance in creeping bentgrass. Crop Sci. 44:1763–1769. doi:10.2135/cropsci2004.1763
- Bradley CA, Lamey HA, Endres GJ, Henson RA, Hanson BK, McKay KR, Halvorson M, LeGare DG, Porter PM. 2006. Efficacy of fungicides for control of Sclerotinia stem rot of canola. Plant Dis. 90:1129–1134. doi:10.1094/PD-90-1129
- Burpee LL. 1997. Control of dollar spot of creeping bentgrass caused by an isolate of Sclerotinia homoeocarpa resistant to benzimidazole and demethylation-inhibitor fungicides. Plant Dis. 81:1259–1263. doi:10.1094/PDIS.1997.81.11.1259
- Chen M, Chung W, Huang H, Huang J. 2010. Development of a semi-selective medium for detection of Mycosphaerella pinodes in soil, plant debris and seed. Can J Plant Pathol. 32:342–350. doi:10.1080/07060661.2010.499267
- Couch HB. 1995. Disease of turfgrasses. 3rd ed. Malibar (FL): Krieger Publishers.
- Detweiler AR, Vargas JM, Danneberger TK. 1983. Resistance of Sclerotinia homoeocarpa to iprodione and benomyl. Plant Dis. 67:627–632. doi:10.1094/PD-67-627
- Fenstermacher JM. 1980. Certain features of dollar spot disease and its causal organism, Sclerotinia homoeocarpa. In: Joyner BG, Larsen PO, editors. Advances in turfgrass pathology. Duluth (MN): Harcourt Brace Jovanovich; pp. 49–53.
- Golembiewski RC, Vargas JM, Jones AL, Detweiler AR. 1995. Detection of demethylation inhibitor (DMI) resistance in Sclerotinia homoeocarpa populations. Plant Dis. 79:491–493. doi:10.1094/PD-79-0491
- Hoover RJ, Kucharek TA. 1995. First report of a leaf spot on perennial peanut caused by Sclerotinia homoeocarpa. Plant Dis. 79:1249. doi:10.1094/PD-79-1249A
- Hsiang T, Liao A, Benedetto D. 2007. Sensitivity of Sclerotinia homoeocarpa to demethylation-inhibiting fungicides in Ontario, Canada, after a decade of use. Plant Pathol. 56:500–507. doi:10.1111/j.1365-3059.2007.01573.x
- Hsiang T, Yang L, Barton W. 1997. Baseline sensitivity and cross-resistance to demethylation-inhibiting fungicides by Ontario isolates of Sclerotinia homoeocarpa. Eur J Plant Pathol. 103:409–416. doi:10.1023/A:1008671321231
- Jo Y, Chang S, Boehm M, Jung G. 2008. Rapid development of fungicide resistance by Sclerotinia homoeocarpa on turfgrass.
- Jo Y, Niver AL, Rimelspach JW, Boehm MJ. 2006. Fungicide sensitivity of Sclerotinia homoeocarpa from golf courses in Ohio. Plant Dis. 90:807–813. doi:10.1094/PD-90-0807
- Kabir Z, Bhat RG, Subbarao KV. 2004. Comparison of media for recovery of Verticillium dahliae from soil. Plant Dis. 88:49–55. doi:10.1094/PDIS.2004.88.1.49
- Koch PL, Grau CR, Jo Y, Jung G. 2009. Thiophanate-methyl and propiconazole sensitivity in Sclerotinia homoeocarpa populations from golf courses in Wisconsin and Massachusetts. Plant Dis. 93:100–105. doi:10.1094/PDIS-93-1-0100
- Latin R. 2011. A practical guide to turfgrass fungicides. St. Paul (MN): APS Press; pp. 157–167.
- Luo Y, Yang J, Zhu M, Liu C, Li H, Lu Z, Pan W, Zhan Z, Bi W, Zhang K. 2012. The group III two-component histidine kinase AlHK1 is involved in fungicides resistance, osmosensitivity, spore production and impacts negatively pathogenicity in Alternaria longipes. Curr Microbiol. 64:449–456. doi:10.1007/s00284-012-0093-8
- Miller G, Stevenson K, Burpee L. 2002. Sensitivity of Sclerotinia homoeocarpa isolates to propiconazole and impact on control of dollar spot. Plant Dis. 86:1240–1246. doi:10.1094/PDIS.2002.86.11.1240
- Ok C, Popko JT, Campbell-Nelson K, Jung G. 2011. In vitro assessment of Sclerotinia homoeocarpa resistance to fungicides and plant growth regulators. Plant Dis. 95:51–56. doi:10.1094/PDIS-02-10-0098
- Orshinsky AM, Boland GJ. 2011. Ophiostoma mitovirus 3a, ascorbic acid, glutathione, and photoperiod affect the development of stromata and apothecia by Sclerotinia homoeocarpa. Can J Microbiol. 57:398–407. doi:10.1139/w11-019
- Rioux RA. 2014. New insights on the biology and host-pathogen interactions of the dollar spot pathogen Sclerotinia homoeocarpa. [dissertation]. Madison (WI): University of Wisconsin-Madison.
- Smiley RW, Dernoeden RH, Clarke BB. 2007. Compendium of turfgrass diseases. 3rd ed. St. Paul (MN): APS Press; pp. 22–24.
- Stanosz JC, Stanosz GR. 2002. A medium to enhance identification of Septoria musiva from poplar cankers. For Pathol. 32:145–152. doi:10.1046/j.1439-0329.2002.00278.x
- Steadman JR, Marcinkowska J, Rutledge S.. 1994. A semi-selective medium for isolation of Sclerotinia sclerotiorum. Can J Plant Pathol. 16:68–70. doi:10.1080/07060669409500791
- Steel C, Nair N. 1993. The physiological basis of resistance to the dicarboximide fungicide iprodione in Botrytis cinerea. Pestic Biochem Physiol. 47:60–68. doi:10.1006/pest.1993.1063
- Tsao PH. 1970. Selective media for isolation of pathogenic fungi. Annu Rev Phytopathol. 8:157–186. doi:10.1146/annurev.py.08.090170.001105
- Vargas JM. 1994. Management of turfgrass diseases. 2nd ed. Boca Raton (FL): Lew Publ.
- Venu RC, Beaulieu RA, Graham TL, Medina AM, Boehm MJ. 2009. Dollar spot fungus S. homoecarpa produces oxalic acid. Int Turf Res J. 11:263–270.
- Viji G, Uddin W, O’Neill NR, Mischke S, Saunders JA. 2004. Genetic diversity of Sclerotinia homoeocarpa isolates from turfgrasses from various regions in North America. Plant Dis. 88:1269–1276. doi:10.1094/PDIS.2004.88.11.1269
- Vincelli P, Dixon E. 2002. Resistance to QoI (Strobilurin-like) fungicides in isolates of Pyricularia grisea from perennial ryegrass. Plant Dis. 86:235–240. doi:10.1094/PDIS.2002.86.3.235
- Vincelli P, Doney JC, Powell AJ. 1997. Variation among creeping bentgrass cultivars in recovery from epidemics of dollar spot. Plant Dis. 81:99–102. doi:10.1094/PDIS.1997.81.1.99
- Von Stackelberg K. 2012. A systematic review of the evidence for adverse human health effects from exposure to azoxystrobin in the environment. [Internet]. [revised 2012 Mar; cited 2013 Sep 19]. Available from: http://ehrf.info/wp-content/uploads/2012/03/Azoxy-Systematic-Review.pdf
- Vu AL, Gwinn KD, Ownley BH. 2011. First report of dollar spot caused by Sclerotinia homoeocarpa on switchgrass in the United States. Plant Dis. 95:1585. doi:10.1094/PDIS-04-11-0332
- Walsh B, Ikeda SS, Boland GJ. 1999. Biology and management of dollar spot (Sclerotinia homoeocarpa), an important disease of turfgrass. HortSci. 34:13–21.
- Warren CG, Sanders PL, Cole H, Duich JM. 1977. Relative fitness of benzimidazole- and cadmium-tolerant populations of Sclerotinia homoeocarpa in the absence and presence of fungicides. Phytopathology. 77:704–708. doi:10.1094/Phyto-67-704
- Warren CG, Sanders PL, Cole H. 1974. Sclerotinia homoeocarpa tolerance to benzimidazole configuration fungicides. Phytopathology. 64:1139–1142. doi:10.1094/Phyto-64-1139
- White TJ, Bruns T, Lee S, Taylor JW. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand, DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. New York (NY): Academic Press, Inc; pp. 315–322.
- Wilson CM. 2011. Changing the paradigm of dollar spot biology and management [dissertation]. Madison (WI): University of Wisconsin-Madison.
