Abstract
Early blight is an economically important foliar disease of processing and fresh market tomatoes. The aim of this multi-year field study was to investigate the impact of weekly foliar sprays of dried and aqueous formulations of Bacillus subtilis QST 713 (Serenade and Rhapsody), alone or as tank mixes with copper hydroxide, on early blight disease development and fruit yields of tomato. Field plots were established in London, Ontario during the 2008–2010 growing seasons and disease conditions were created artificially by inoculations with spore suspension of the early blight pathogen. The tank mixes of copper hydroxide and chlorothalonil were included as a standard spray treatment. Late blight also appeared naturally in the plots late in 2009 and 2010 field seasons and affected fruit in both years, but more severely in 2009. Spray treatments had variable effects on tomato early blight and fruit yields depending on the level of disease pressure in the inoculated plots. The weekly sprays of biofungicide alone did not consistently reduce disease severity on tomato foliage or incidence on fruit, but in a tank mix with copper hydroxide, both formulations consistently reduced foliar disease severity even when disease pressure was high in plots. Under high disease conditions, copper hydroxide alone or as a tank mix with chlorothalonil also consistently reduced disease severity on foliage. The mixture of two fungicides also consistently increased total fruit yields in all 3 years. Plots sprayed with copper hydroxide alone and in tank mixes with chlorothalonil and the dried formulation of biofungicide consistently had less late blight-infected fruit. The combined effects of biofungicides and copper hydroxide or other fungicides should be further investigated for consistent disease management and yield improvement.
Résumé
L’alternariose est une maladie foliaire de grande importance économique qui touche autant les tomates de transformation que les tomates fraiches destinées au marché. Le but de cette étude pluriannuelle menée sur le terrain était d’évaluer les effets d’applications foliaires hebdomadaires de préparations aqueuses et sèches du biofongicide Serenade (Bacillus subtilis QST 713), seul ou comme mélanges en réservoir avec de l’hydroxyde de cuivre, sur le développement de l’alternariose et le rendement des plants de tomate. De 2008 à 2010, durant les saisons de croissance, des parcelles ont été délimitées à London, en Ontario, et la maladie y a été introduite artificiellement en inoculant les plants avec une suspension de spores de l’agent pathogène. Les mélanges en réservoir d’hydroxyde de cuivre et de chlorthalonil constituaient un traitement par pulvérisation standard. Tard dans les saisons de 2009 et 2010, le mildiou est apparu naturellement dans les parcelles et a infecté les fruits, mais plus gravement en 2009. Les traitements par pulvérisation ont eu des effets variables sur l’alternariose de la tomate et le rendement des plants, selon le taux de pression de la maladie dans les parcelles inoculées. Les pulvérisations hebdomadaires de biofongicide utilisé seul n’ont pas invariablement réduit la gravité de la maladie sur le feuillage des plants ni son incidence sur les fruits, mais, dans les mélanges en réservoir avec l’hydroxyde de cuivre, les deux préparations ont invariablement réduit la gravité de la maladie sur le feuillage, et ce, même lorsque, dans les parcelles, la pression de la maladie était forte. Dans les cas de forte pression de la maladie, l’hydroxyde de cuivre utilisé seul, ou comme mélange en réservoir avec le chlorthalonil, a également réduit invariablement la gravité de la maladie sur le feuillage. Le mélange des deux fongicides a également accru invariablement le rendement total des plants durant les trois années. Les parcelles pulvérisées seulement avec l’hydroxyde de cuivre et en mélanges en réservoir avec le chlorthalonil et la préparation sèche de biofongicide ont invariablement produit moins de tomates infectées par le mildiou. Les effets combinés des biofongicides et de l’hydroxyde de cuivre ou d’autres fongicides devraient être davantage étudiés afin de gérer plus efficacement la maladie et d’améliorer le rendement.
Introduction
Tomato (Solanum lycopersicum Mill.) is an important vegetable crop that provides fresh, processing and other value-added products for consumers worldwide. In 2011, Canada had a total cultivated area of 6800 ha of field tomatoes, with an annual production of 0.5 million tonnes and a farm gate value of 80 million dollars (Statistics Canada 2012). More than 90% of these field tomatoes are produced in Southwestern Ontario, with a farm gate value greater than 70 million dollars (Statistics Canada 2012). In 2011, the farm gate value of greenhouse tomatoes was 496 million dollars and 59% of these greenhouse tomatoes were produced in Ontario (Statistics Canada 2012). Foliar diseases are one of the key factors limiting maximum production of both processing and fresh market tomatoes. Early blight, caused by Alternaria solani (Ellis & Martin) Sorauer, is one of the most common and serious foliar diseases of tomato and other solanaceous plants that can affect foliage of the plants throughout the growing season, causing severe defoliation of the tomato crop (Jones et al. Citation1991; Chaerani & Voorrips Citation2006).
The first and most noticeable symptoms appear on the lower or older leaves as tiny dark brown spots soon after fruit set (Kucharek Citation2000). These spots enlarge in diameter and develop concentric or target-like rings often surrounded by a yellow halo. Similar spots may also develop on fruits and stems. The infected fruit may either drop before harvest or may not be marketable. The pathogen can survive in infected leaf or stem tissues left in the field, or can be carried on seed. Infection occurs in warm, humid weather with heavy dews or rain (Rotem Citation1994; Kemmitt Citation2002). Alternaria spores can be carried to healthy plants by air currents, wind-blown soil, splashing rain, irrigation water and moving machinery. Early blight can be more severe when plants are stressed by poor nutrition, drought or other diseases and pests (Rowe et al. Citation1995).
Management of foliar diseases has always been challenging in both organic and conventional tomato production systems. In conventional production systems, tomato early blight is almost exclusively managed by intensive fungicide spray programmes with or without a disease forecasting system (Madden et al. Citation1978; Brammall Citation1993; Keinath et al. Citation1996; Dillard et al. Citation1997; Mills et al. Citation2002; Cowgill et al. Citation2005). Several effective fungicides have been registered for use against this disease on several hosts (Bartlett et al. Citation2002). However, the use of fungicides is not always considered a long-term and most ideal solution for managing diseases. Fungicides can be expensive, and there are exposure risks, residue problems and other health and environmental hazards as well. On the other hand, fungicide use in organic production systems is very limited. The need for alternative and reduced-risk disease management strategies is ever growing. The development of microbial-based fungicides as alternative and safe options of managing plant diseases has received considerable attention during the past two decades (Bélanger Citation2006; Pérez-García et al. Citation2011).
The efforts in developing microbial-based disease control products have led to registration of several biofungicides for commercial use in Canada. Strain QST 713 of Bacillus subtilis is available in a wettable powder or dried formulation (QRD 141 Serenade® MAX) and also as an aqueous suspension (QRD 145 Serenade® ASO or Rhapsody®). Both formulations have been recently registered in Canada (under Health Canada registration numbers 28548 and 28626 for Serenade MAX and Serenade ASO, respectively) for use on various crops to control foliar diseases such as Botrytis blight, early blight, downy mildew, fire blight, powdery mildew and white mould. Recently, the biofungicide B. subtilis QST 713 has also been assayed for the control of clubroot on canola (Lahlali et al. Citation2013). However, a multi-year study showing the impact of this biofungicide on the development of early blight of tomato under field conditions has not been undertaken.
The objective of this study was to investigate the effects of dried and aqueous formulations of Serenade biofungicide B. subtilis QST 713 alone or in combination with copper hydroxide as foliar sprays on reducing development of early blight on tomato foliage and fruit and on increasing yield of field tomatoes.
Materials and methods
Tomato seedlings, fungal culture and biofungicide
Seedlings of processing tomato cultivar ‘H 9478’ were used in the 2008 and 2009 field experiments whereas the cultivar ‘H 9909’ was used in the 2010 field experiment (‘H 9478’ was no longer available and replaced by ‘H 9909’). These transplants produced in commercial greenhouses in soilless planting mix in plug trays were provided by H. J. Heinz (Leamington, ON). Seedlings from the greenhouse were kept outdoors under spruce trees to be hardened-off for 4–6 days prior to planting them in the field. The fungal culture of Alternaria solani isolate # 2242 was obtained from Dr Jim Traquair, Agriculture and Agri-Food Canada, London, ON. This isolate of the pathogen was originally isolated from early blight-infected tomato plant in 2004 by Dr W. R. Jarvis, Agriculture and Agri-Food Canada, Harrow, ON. The culture was stored on sterile paper discs with desiccant at −20 °C, and for this study it was cultured and maintained on V8 agar or PDA plates during the course of each field experiment. The aqueous and dried formulations of Serenade biofungicide B. subtilis QST 713 were provided by AgraQuest Inc, Davis, CA.
Field experiments
Field experiments were conducted during the 2008, 2009 and 2010 growing season at the Agriculture and Agri-Food Canada research farm in London, ON on an alkaline loam soil (pH 8.0) with a moderate level (~2%) of organic matter. Tomato seedlings were planted on 29 May 2008, 4 June 2009 and 31 May 2010 with a single-row planter 0.45 m apart in single 6.75-m long rows on 1.2-m centres. Treatments were arranged in a randomized complete block design in each of four replicate plots. Each treatment row was separated by border rows on both sides. Plots were fertilized with a standard fertilizer regime following the recommended guidelines for field tomatoes (OMAF Citation2005). Plants were fertilized with the plant starter Plant Prod® (10–52–10; Plant Products Co. Ltd., Brampton, ON) during planting; each plant received approximately 150–180 mL of 0.5% starter fertilizer.
Pathogen inoculation
Plants were inoculated with spores (conidia) of A. solani produced on PDA or V-8 agar plates. For inoculum production, the fungus culture was started off on a PDA plate to establish a growing culture. A total of 100 PDA and V-8 agar plates were inoculated with the fungal plugs taken from the growing edge of 24 h old plates. Spore production was induced by incubating the V-8 agar or PDA plates in a growth chamber under diurnal UV light (clear UV C bulbs) on a 12 h light and 12 h dark cycle for 2 weeks (Fourtouni et al. Citation1998). Conidia were harvested once the growing edge reached the edge of the plates and colonies were brown in colour. Plates were flooded with 5 mL of sterile water containing 1% Tween 80 and spores were gently dislodged with a spatula. The resulting liquid was then pipetted off into a sterile beaker. This process was repeated until all plates were harvested and the resulting liquid was pooled together. Liquid was then run through several layers of fine mesh cloth to separate mycelia from spores and the mesh was washed with sterile water to recover as many spores as possible. Spore concentration was estimated using a haemocytometer and adjusted with sterile water. Spore suspension was then sprayed using a hand-held compressed-air sprayer (RL Flo-Master®; capacity 7.6 L; Root-Lowell Manufacturing Co., Lowell, MI) onto tomato plants in the field once the first flowers were setting on the tomato plants. Inoculations were made either directly onto the treatment or border plots or both, based on the availability of spore concentration, weather conditions and planting date. In 2008, plots were inoculated twice, first on 25 June (both border and treatment plots) with 2 × 103 spores mL−1 and then again on 24 July (treatment plots only) with 1 × 104 spores mL−1. In 2009, the border plots were inoculated with 1 × 104 spores mL−1 on 26 June and disease was allowed to spread naturally to the treatment plots. Due to low spore production on plates for the 2010 field trial, the treatment plots were inoculated directly very late on 13 July with 1 × 103 spores mL−1.
Treatment application
Tomato plants were sprayed at 7–10 day intervals with solutions of various treatments starting in late June (prior to inoculating the plots) and ending in early August. Treatments were applied using a hand-held compressed-air sprayer at 30 ± 5 psi with an adjustable cone nozzle. In each season, a total of 7 (2008 and 2010) or 8 (2009) sprays were applied. At each spray, approximately 15–25 mL of solution was sprayed onto each plant depending on the plant age. Control plots were sprayed with water. The dried formulation (Serenade MAX, 1 kg ha−1) and aqueous suspension (Serenade ASO or Rhapsody, 4 L ha−1) of biofungicide B. subtilis QST713 and copper hydroxide fungicide (Kocide 2000, 1.1 kg ha−1) were sprayed alone or as tank mixes (Serenade MAX, 1 kg ha−1 and Kocide 2000, 1.1 kg ha−1; Serenade ASO, 4 L ha−1 and Kocide 2000, 1.1 kg ha−1; and Kocide 2000, 1.1 kg ha−1 and chlorothalonil or Bravo 500, 1.1 L ha−1).
Disease evaluations and harvesting
After inoculations, plants were scouted for symptom development and the disease progression was followed by weekly ratings of the plants for foliar disease severity. All plants in a treatment plot were rated for foliar disease severity six times using a modified Horsfall and Barratt (Citation1945) disease rating scale (1 = no disease to 12 = 100% disease). The disease severity data were also converted to area under the disease progressive curve (AUDPC) using the midpoints of each per cent disease rating (Shaner & Finney Citation1977; Campbell & Madden Citation1990). All fruits from 10 plants in the middle of each plot were harvested during the last week of August. Fruits were analysed for the incidence of bacterial spot, early blight, anthracnose and late blight. A tomato fruit free of any disease symptoms was sorted as healthy fruit. All fruit from a plot was weighed to determine total fruit yield. Tomato fruit was also analysed for disease severity by counting the number of lesions on each fruit.
Data analysis
Data were tested for normality and, if required, transformed to normalize before analysis. However, actual means were presented for clarity. Field data from each year were analysed separately and subjected to analysis of variance using MINITAB statistical software version 13.0 (Minitab Inc., State College, PA), and if P values indicated a significant difference (P < 0.05), means were separated by Fisher’s protected least significant different (LSD) test.
Results
Effect on early blight development on foliage
During the 2008 field trial, tomato plots had to be re-inoculated in late July and as a result early blight developed late but progressed quickly on foliage. The aqueous suspension of the biofungicide B. subtilis QST713 (Serenade ASO or Rhapsody) and copper hydroxide (Kocide 2000) reduced the severity of early blight on tomato foliage (by 37.6 and 40.4% based on AUDPC value and 59.4 and 42.7% based on final disease severity rating, respectively) as compared with the control treatment (). The dried formulation (Serenade Max) of the biofungicide alone did not reduce the severity of early blight on tomato foliage, but when used in a tank mix with copper hydroxide, it slowed down the progression of the disease (with a 38.1% reduction in severity based on AUDPC value and 42.7% based on final disease severity rating) as compared with the control treatment (). A tank mix of copper hydroxide and chlorothalonil (Bravo) reduced the early blight severity on tomato foliage (by 53.3% based on AUDPC value and 59.4% based on final disease severity rating) as compared with the control treatment ().
Table 1. Effect of weekly foliar sprays of dried (Serenade) or aqueous (Rhapsody) formulations of biofungicide Bacillus subtilis QST 713 and copper hydroxide (Kocide) on foliar disease severity of early blight severity on tomato foliage during 2008–2010 field trials.
In the 2009 field trial, all treatments except the Serenade ASO formulation of the biofungicide reduced early blight severity on tomato foliage (). Copper hydroxide alone or in a tank mix with chlorothalonil reduced disease severity on foliage (by 33.5% based on AUDPC value and 51.3% based on final disease severity rating) as compared with the control treatment (). Disease pressure was very low on all inoculated tomato plots during the 2010 field trial, and no treatment differences for disease reduction were recorded.
Effect on early blight development on fruit
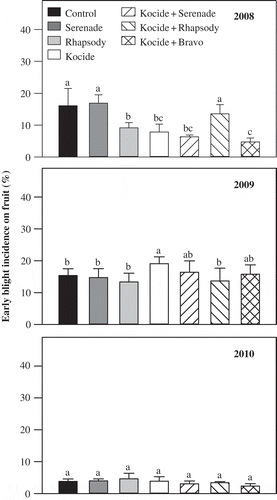
In the 2008 field trial, the weekly foliar sprays of the ASO formulation of Serenade and copper hydroxide reduced the percentage of early blight-infected fruit by 45.6 and 50.9%, respectively, as compared with the control treatment (). The dried formulation of the biofungicide alone did not have any impact on the occurrence of early blight on fruit, but in a tank mix with copper hydroxide, it reduced the percentage of early blight-infected tomato fruit by 60.9% as compared with the control treatment (). A tank mix of copper hydroxide and chlorothalonil was the most effective treatment that reduced the percentage of early blight-infected tomato fruit by more than 70% as compared with the control treatment (). In the 2009 field trial, late blight also appeared naturally on the tomato plots and severely affected the fruit. There were no treatment differences in terms of reduction in the percentage of early blight-infected fruit (). In the 2010 field trial, disease pressure was low and as a result fewer symptoms appeared on tomato fruit. Late blight also appeared on the plots very late in the season.
Fig. 1 Effect of weekly foliar sprays of dried (Serenade) and aqueous (Rhapsody) formulations of biofungicide Bacillus subtilis QST 713 and copper hydroxide (Kocide 2000) alone or in combination on early blight incidence on tomato fruit during 2008 to 2010 field trials. A tank mix of copper hydroxide (Kocide 2000) and chlorothalonil (Bravo 500) was used as a standard treatment. Error bars are standard error of the mean from four replicate plots. Treatments within a year with a common letter are not significantly different (P < 0.05).
Effect on late blight on fruit
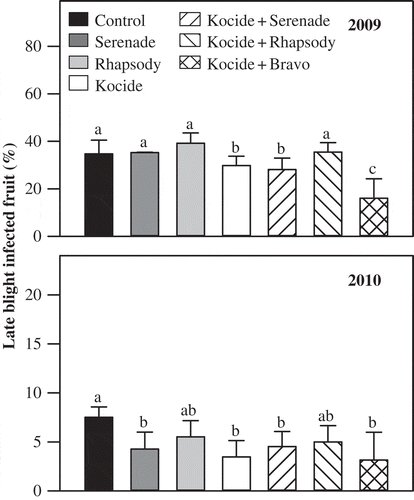
Late during the 2009 and 2010 field seasons, late blight (Phytophthora infestans) developed naturally on the tomato foliage and fruit but its effect was more pronounced on fruit in both years. Late blight was more severe in 2009 than in 2010, and more than 34% of the fruit harvested from control plots was late blight-infected (). Tomato fruit harvested from plots sprayed with a tank mix of copper hydroxide and chlorothalonil consistently had lower late blight-infected fruit, with a decrease of 53.6% in 2009 and 58.7% in 2010 in the percentage of infected fruit as compared with the fruit harvested from control plots (). A reduction in the percentage of late blight-infected fruit was also noticed in both years with the treatments copper hydroxide alone (14.1% reduction in 2009 and 53.3% in 2010) and a tank mix of copper hydroxide and the dried formulation of Serenade biofungicide (19% reduction in 2009 and 40% in 2010) ().
Fig. 2 Effect of weekly foliar sprays of dried (Serenade) and aqueous (Rhapsody) formulations of biofungicide Bacillus subtilis QST 713 and copper hydroxide (Kocide 2000) alone or in combination on the percentage of late blight-infected tomato fruit during 2009 and 2010 field trials. A tank mix of copper hydroxide (Kocide 2000) and chlorothalonil (Bravo 500) was used as a standard treatment. Error bars are standard error of the mean from four replicate plots. Treatments within a year with a common letter are not significantly different (P < 0.05).
Effect on yield of field-grown tomatoes
In the 2008 field trial, the weekly sprays of copper hydroxide alone and a tank mix of copper hydroxide with the aqueous (Serenade) or dried (Rhapsody) formulation of Serenade biofungicide or chlorothalonil improved yields of healthy tomato fruit by 88.5, 43.6, 129.9 or 117.2%, respectively, as compared with the weekly water sprays (control treatment) (). In the 2009 field trial, only the tank mix of copper hydroxide and chlorothalonil significantly increased the yield of healthy fruit by 151.1% as compared with the control treatment (). In the 2010 field trial, most of the fruit was healthy in all tomato plots due to low disease pressure. However, a tank mix of copper hydroxide with the aqueous and dried formulation of Serenade biofungicide significantly increased healthy tomato fruit by 12.8 and 12.1% as compared with the control treatment ().
Table 2. Effect of weekly foliar sprays of dried (Serenade) or aqueous (Rhapsody) formulations of biofungicide Bacillus subtilis QST 713 and copper hydroxide (Kocide) on healthy and total fruit yields of tomato during 2008–2010 field trials.
A tank mix of copper hydroxide with the dried formulation of Serenade biofungicide and chlorothalonil significantly increased total fruit yield by 42.7 and 35.9%, respectively, in the 2008 field trial (). In the 2009 field trial, only the tank mix of copper hydroxide and chlorothalonil significantly increased total fruit yield by 80.7% (). Fruit yields were comparatively higher in 2010, and weekly foliar sprays of copper hydroxide in a tank mix with the aqueous or dried formulation of Serenade biofungicide and chlorothalonil significantly increased total fruit yield over the control treatment ().
Discussion
This multi-year field study was undertaken to investigate the impact of weekly foliar sprays of dried and aqueous formulations of Serenade biofungicide containing B. subtilis QST 713, alone or as tank mixes with copper hydroxide, on the development and spread of early blight disease on tomato foliage or fruit and on fruit yields. The tank mixes of copper hydroxide and chlorothalonil are routinely used by tomato and other vegetable growers as a standard practice to manage foliar diseases, including early blight and bacterial spot. In this study, the tank mixes of these two fungicides were used as one of the spray treatments as a positive control for comparing treatment effects on tomato early blight and fruit yields.
We found that various foliar treatments had variable effects on the development and progression of early blight on tomato foliage and fruit as well as on fruit yields, depending on the level of disease pressure in the inoculated plots. During the first and second years of the study, early blight pressure was high in the inoculated plots and the disease developed and progressed well on tomato foliage and fruit. The aqueous formulation of the biofungicide B. subtilis QST 713 reduced foliar disease severity during the first year, whereas the dried formulation was effective in slowing the disease progression during the second year. Therefore, the biofungicide alone did not consistently reduce disease severity. A tank mix of both formulations of the biofungicide with copper hydroxide consistently reduced foliar disease severity when disease pressure was high. However, foliar sprays of Serenade alone or combined with fixed copper did not provide adequate early blight control in field trials in New York (Zitter et al. Citation2005). In this study, copper hydroxide alone or as a tank mix with chlorothalonil also consistently reduced disease severity on foliage. Rogers and Wszelaki (Citation2012) also reported a lower severity of early blight on tomato plots sprayed with copper sulphate.
During the third year of the study, the disease pressure in the inoculated plots was very low and as a result very little disease developed on tomato foliage or fruit. Most of the fruit harvested from these plots was healthy. Under these conditions of low disease development, none of the spray treatments affected foliar disease severity on tomato foliage or fruit, but there was a slight increase in the percentage of healthy fruit with copper hydroxide alone or as a tank mix with biofungicide B. subtilis QST 713. In one of three Southwestern Ontario locations where disease development was not adequate, Poysa et al. (Citation1993) also reported no effect of fungicide sprays on tomato foliar disease severity. The development of early blight symptoms on tomato plants is affected by inoculum concentration, leaf wetness duration, plant age and host susceptibility (Vloutoglou & Kalogerakis Citation2000). It is possible that one or more of these factors might have been responsible for less disease development on tomato foliage or fruit in the inoculated plots in our study.
Late in the season during the 2009 and 2010 field trials, late blight also appeared naturally on tomato plots and affected fruit in both years. The impact of late blight on fruit was more severe during the 2009 season as compared with the 2010 season and most of the diseased fruit was due to late blight infection. It is possible that late blight occurrence may have affected the results of spray treatments on early blight on fruit. Based on the evaluation of late blight-infected fruit at harvest, we found that tomato plots sprayed with copper hydroxide alone and as tank mixes with chlorothalonil or the dried formulation of biofungicide B. subtilis QST 713 consistently had less late blight-infected fruit. Tomato fruit harvested from the plots receiving weekly foliar sprays of the dried formulation of Serenade biofungicide also had less late blight-infected fruit during the 2010 field season when late blight attack was not severe.
The mode of action of B. subtilis QST 713 or its effect on the early blight pathogen was not investigated in this study. However, this antagonistic strain of B. subtilis can colonize the leaf surface and compete with the pathogen for nutrients and space and can physically prevent attachment and penetration by the pathogen. This endophytic bacterium produces lipopeptide metabolites such as iturins and surfactins (Kinsella et al. Citation2009) that cause permeability changes in the fungal membrane (Patel et al. Citation2011) leading to pathogen disintegration. Physical prevention of the pathogen or direct mode of action of biofungicide is only possible when the pathogen is in contact with biofungicide on the plant surface. Induction of plant resistance can be another possible mode of action of Serenade biofungicide (Lahlali et al. Citation2013). The strain OTPB1 of B. subtilis has been specifically shown to enhance systemic resistance in tomato seedlings against the early blight pathogen through induction of growth hormones and defence enzymes (Chowdappa et al. Citation2013). Due to these multiple modes of actions, it is less likely that application of this biofungicide would lead to resistance development in pathogens.
The use of protectant fungicides such as chlorothalonil is important for reducing fungal foliar disease severity, maintaining fruit quality, and prolonging fruit ripening; but fungicide sprays may not always have positive effects on fruit yields (Brammall Citation1993; Poysa et al. Citation1993; Louws et al. Citation1996; Byrne et al. Citation1997). Under natural conditions of disease development, foliar sprays of chlorothalonil fungicide significantly reduced foliar disease severity at two of the three locations in Southwestern Ontario (Poysa et al. Citation1993). However, fungicide sprays in that study did not increase total and marketable yield of tomatoes (Poysa et al. Citation1993). In field trials in New York and New Jersey, fungicide sprays resulted in a reduction in foliar fungal disease severity and an increase in usable yield of processing tomatoes (Dillard et al. Citation1997). In this study, tomato plots sprayed with a tank mix of copper hydroxide and chlorothalonil consistently showed higher fruit yields in all 3 years.
Fungicides may be effective in reducing foliar diseases but accumulation of their residues in fresh and processed food products (Lukens & Ou Citation1976; Patterson et al. Citation2001) is a real concern. Attempts have been made to reduce fungicide residues on tomato fruit by decreasing the fungicide rates (Precheur et al. Citation1992; Louws et al. Citation1996). Weather-based forecast systems can also reduce fungicide residues by decreasing the number of sprays (Madden et al. Citation1978; Pennypacker et al. Citation1983; Pitblado Citation1988; Gleason et al. Citation1995). Similarly, integration of various disease management options (Bauske et al. Citation1998; Zitter et al. Citation2005) can also lead to lower residues of fungicides. For instance, combined application of biofungicides and chemical fungicides can be an important integrated strategy (Jacobsen et al. Citation2004) for managing foliar diseases and lowering fungicide residues in food products. An effective combination of microbial-based biofungicides and copper-based fungicides can offer a disease management strategy for foliar diseases in organic production systems.
In conclusion, various spray treatments had variable effects on early blight and fruit yield depending on the level of disease pressure in tomato plots. Foliar sprays of Serenade biofungicide B. subtilis QST 713 alone did not consistently reduce disease severity on tomato foliage or incidence on fruit, but in tank mixes with copper hydroxide, both the dried and aqueous formulations of biofungicide consistently reduced foliar disease severity when disease pressure was high. Copper hydroxide alone or as a tank mix with chlorothalonil also consistently reduced disease severity on foliage when disease pressure was high. Tomato plots sprayed weekly with copper hydroxide alone and in tank mixes with chlorothalonil or the dried formulation of B. subtilis QST 713 consistently showed less late blight-infected fruit. The combined effects of biofungicides and copper hydroxide or other fungicides should be further investigated for consistent disease management and yield improvement in conventional as well as organic production systems.
Acknowledgements
Technical support was provided by Igor Lalin, Salaheddin Khabbaz, Albert Asztalos, Bruce McPherson and several summer students during 2008–2010. This research was partly funded by research grants from AgraQuest Inc., Davis, CA and Agriculture and Agri-Food Canada.
References
- Bartlett DW, Clough JM, Godwin JR, Hall AA, Hamer M, Parr-Dobrzanski B. 2002. The strobilurin fungicides. Pest Manag Sci. 58:649–662. doi:10.1002/ps.520
- Bauske EM, Zehnder GM, Sikora EJ, Kemble J. 1998. Southeastern tomato growers adopt integrated pest management. HortTechnol. 8:40–44.
- Bélanger RR. 2006. Controlling diseases without fungicides: a new chemical warfare. Can J Plant Pathol. 28:S233–238. doi:10.1080/07060660609507380
- Brammall RA. 1993. Effect of foliar fungicide treatment on early blight and yield of fresh market tomato in Ontario. Plant Dis. 77:484–488. doi:10.1094/PD-77-0484
- Byrne JM, Hausbeck MK, Latin RX. 1997. Efficacy and economics of management strategies to control anthracnose fruit rot in processing tomatoes in the Midwest. Plant Dis. 81:1167–1172. doi:10.1094/PDIS.1997.81.10.1167
- Campbell CL, Madden LV. 1990. Introduction to plant disease epidemiology. New York: John Wiley & Sons, Inc.
- Chaerani R, Voorrips RE. 2006. Tomato early blight (Alternaria solani): the pathogen, genetics, and breeding for resistance. J Gen Plant Pathol. 72:335–347. doi:10.1007/s10327-006-0299-3
- Chowdappa P, Mohan Kumar SP, Jyothi Lakshmi M, Upreti KK. 2013. Growth stimulation and induction of systemic resistance in tomato against early and late blight by Bacillus subtilis OTPB1 or Trichoderma harzianum OTPB3. Biol Control. 65:109–117. doi:10.1016/j.biocontrol.2012.11.009
- Cowgill WP Jr, Maletta MH, Manning T, Tietjen WH, Johnston SA, Nitzsche PJ. 2005. Early blight forecasting systems: evaluation, modification, and validation for use in fresh-market tomato production in northern New Jersey. HortScience. 40:85–93.
- Dillard HR, Johnston SA, Cobb AC, Hamilton GH. 1997. An assessment of fungicide benefits for the control of fungal diseases of processing tomatoes in New York and New Jersey. Plant Dis. 81:677–681. doi:10.1094/PDIS.1997.81.6.677
- Fourtouni A, Menetas Y, Christias C. 1998. Effect of UV-B radiation on growth, pigmentation and spore production in the phytopathogenic fungus Alternaria solani. Can J Bot. 76:2093–2099.
- Gleason ML, Macnab AA, Pitblado RE, Ricker MD, East DA, Latin RX. 1995. Disease warning systems for processing tomatoes in eastern North America: are we there yet? Plant Dis. 79:113–121. doi:10.1094/PD-79-0113
- Horsfall JG, Barratt RW. 1945. An improved grading system for measuring plant diseases. Phytopathology. 35:655.
- Jacobsen BJ, Zidack NK, Larson BJ. 2004. The role of Bacillus-based biological control agents in integrated pest management systems: plant diseases. Phytopathology. 94:1272–1275. doi:10.1094/PHYTO.2004.94.11.1272
- Jones JB, Jones JP, Stall RE, Zitter TA. 1991. Infectious diseases: diseases caused by fungi. Compendium of tomato diseases. St. Paul, MN: The American Phytopathological Society. 9–25.
- Keinath AP, Dubose VB, Rathnell PJ. 1996. Efficacy and economics of three fungicide application schedules for early blight control and yield of fresh-market tomato. Plant Dis. 80:1277–1282. doi:10.1094/PD-80-1277
- Kemmitt G. 2002. Early blight of potato and tomato. The Plant Health Instructor. doi:10.1094/PHI-I-2002-0809-01
- Kinsella K, Schulthess CP, Morris TF, Stuart JD. 2009. Rapid quantification of Bacillus subtilis antibiotics in the rhizosphere. Soil Biol Biochem. 41:374–379. doi:10.1016/j.soilbio.2008.11.019
- Kucharek T. 2000. Early blight on tomatoes and potatoes. Plant Pathology Fact sheet PP–7. Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida, Gainsville, FL, USA; pp.4.
- Lahlali R, Peng G, Gossen BD, Mcgregor L, Yu FQ, Hynes RK, Hwang SF, Mcdonald MR, Boyetchko SM. 2013. Evidence that the biofungicide Serenade (Bacillus subtilis) suppresses clubroot on canola via antibiosis and induced host resistance. Phytopathology. 103:245–254. doi:10.1094/PHYTO-06-12-0123-R
- Louws FJ, Hausbeck MK, Kelly JF, Stephens CT. 1996. Impact of reduced fungicide and tillage on foliar blight, fruit rot, and yield of processing tomatoes. Plant Dis. 80:1251–1256. doi:10.1094/PD-80-1251
- Lukens RJ, Ou SH. 1976. Chlorothalonil residues on field tomatoes and protection against Alternaria solani. Phytopathology. 66:1018–1022. doi:10.1094/Phyto-66-1018
- Madden L, Pennypacker SP, Macnab AA. 1978. FAST, a forecast system for Alternaria solani on tomato. Phytopathology. 68:1354–1358. doi:10.1094/Phyto-68-1354
- Mills DJ, Coffman CB, Teasdale JR, Everts KL, Abdul-Baki AA, Lydon J, Anderson JD. 2002. Foliar disease in fresh-market tomato grown in differing bed strategies and fungicide spray programs. Plant Dis. 86:955–959. doi:10.1094/PDIS.2002.86.9.955
- OMAF. 2005. Vegetable Production Recommendations 2004–2005. Ontario Ministry of Agriculture and Food publication 363, pp. 201.
- Patel H, Tscheka C, Edwards K, Karlsson G, Heerklotz H. 2011. All-or-none membrane permeabilization by fengycin-type lipopeptides from Bacillus subtilis QST713. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1808:2000–2008. doi:10.1016/j.bbamem.2011.04.008
- Patterson JM, Nokes SE, Bennett MA, Riedel RE. 2001. Evaluation of residual chlorothalonil levels on processing tomato foliage using the TOM-CAST spray program. Appl Eng Agric. 17:445–448. doi:10.13031/2013.6459
- Pennypacker SP, Madden LV, Macnab AA. 1983. Validation of an early blight forecasting system for tomatoes. Plant Dis. 67:287–289. doi:10.1094/PD-67-287
- Pérez-García A, Romero D, de Vicente A. 2011. Plant protection and growth stimulation by microorganisms: biotechnological applications of Bacilli in agriculture. Cur Opin Biotechnol. 22:187–193. doi:10.1016/j.copbio.2010.12.003
- Pitblado RE. 1988. Development of a weather-timed fungicide spray program for field tomatoes. Can J Plant Pathol. 10:371.
- Poysa V, Brammall RA, Pitblado RE. 1993. Effects of foliar fungicide sprays on disease and yield of processing tomatoes in Ontario. Can J Plant Sci. 73:1209–1215. doi:10.4141/cjps93-160
- Precheur RJ, Riedel RM, Bennett MA, Wiese KL, Dudek J. 1992. Management of fungicide residues on processing tomatoes. Plant Dis. 76:700–702. doi:10.1094/PD-76-0700
- Rogers MA, Wszelaki AL. 2012. Influence of high tunnel production and planting date on yield, growth, and early blight development on organically grown heirloom and hybrid tomato. HortTechnol. 22:452–462.
- Rotem J. 1994. The genus Alternaria: biology, epidemiology and pathogenicity. St. Paul, MN: American Phytopathological Society.
- Rowe RC, Miller SA, Riedel RM. 1995. Early blight of potato and tomato. The Ohio State University Extension Fact sheet HYG-3101-95. pp 2.
- Shaner G, Finney RE. 1977. The effect of nitrogen fertilization on the expression of slow-mildewing resistance in Knox wheat. Phytopathology. 67:1051–1056. doi:10.1094/Phyto-67-1051
- Vloutoglou I, Kalogerakis SN. 2000. Effects of inoculum concentration, wetness duration and plant age on development of early blight (Alternaria solani) and on shedding of leaves in tomato plants. Plant Pathol. 49:339–345. doi:10.1046/j.1365-3059.2000.00462.x
- Zitter TA, Drennan JL, Mutschler MA, Kim M-J. 2005. Control of early blight of tomato with genetic resistance and conventional and biological sprays. Acta Hort. 695:181–190.
