Abstract
Stripe rust, caused by Puccinia striiformis f. sp. tritici, is one of the most destructive diseases of wheat. The disease is mainly controlled by growing resistant cultivars and applying fungicides when necessary. To determine potential yield loss and fungicide response, major cultivars grown in the US Pacific Northwest and susceptible checks were tested in complete split-block design experiments. From 2002 to 2012, stripe rust caused yield losses ranging from 18% to more than 90% and from 5% to more than 50% with an average of 44% and 33% on susceptible winter and spring wheat checks, respectively. Without fungicide application, the commercially grown cultivars with various levels of stripe rust resistance could reduce potential yield losses to 2–21%, with an average of 8% for winter wheat, and to 0–27% with an average of 13% for spring wheat. Significant or insignificant effects of fungicide applications on yield increase were determined for each cultivar, and the results were used to guide stripe rust management by selecting resistant cultivars to grow and appropriately applying fungicides under different levels of stripe rust epidemic. The results should be useful for developing wheat cultivars with high durable resistance, and for improving chemical control in order to minimize yield losses and maximize profits while protecting the environment by reducing use of fungicides.
Résumé
La rouille jaune, causée par Puccinia striiformis, f. sp. tritici, est une des maladies les plus destructrices du blé. Elle est principalement maîtrisée en semant des cultivars résistants et en appliquant des fongicides, au besoin. Afin de déterminer la perte de rendement possible et la réaction aux fongicides, les principaux cultivars semés dans la région du Nord-Ouest du Pacifique américain et des cultivars témoins réceptifs ont été testés au cours d’expériences menées sur des parcelles divisées. De 2002 à 2012, la rouille jaune a causé des pertes variant de 18 % à plus de 90 % et de 5 % à plus de 50 %, avec des pertes moyennes de 44 % et de 33 % sur les cultivars témoins réceptifs de blé d’hiver et de blé de printemps, respectivement. Sans applications de fongicides, les cultivars commerciaux affichant différents degrés de résistance à la rouille jaune pouvaient réduire la possibilité de pertes de rendement de 2 à 21 %, avec une moyenne de 8 % pour le blé d’hiver, et de 0 à 27 %, avec une moyenne de 13 % pour le blé de printemps. Les effets significatifs ou non significatifs des applications de fongicides sur l’accroissement des rendements ont été déterminés pour chaque cultivar et les résultats ont été utilisés pour orienter la gestion de la rouille jaune en sélectionnant les cultivars résistants à semer et en appliquant de façon appropriée les fongicides en fonction de la variabilité de l’ampleur des épidémies de rouille jaune. Les résultats devraient servir à développer des cultivars possédant une résistance durable élevée et pour améliorer la lutte chimique afin de minimiser les pertes de rendement et de maximiser les profits, et ce, tout en protégeant l’environnement en réduisant l’utilisation des fongicides.
Introduction
Stripe rust (yellow rust), caused by Puccinia striiformis Westend. f. sp. tritici Erikss., is one of the most destructive diseases of wheat (Triticum aestivum L.) in the US Pacific Northwest (PNW) due to the favourable climatic conditions and cropping systems (Chen Citation2005). In this region, the disease can cause significant damage almost every year. In fact, stripe rust caused yield losses from 3% to more than 90%, with a mean of 36% for winter wheat and 30% for spring wheat on susceptible varieties in experimental fields near Pullman, WA from 1975 to 2012 (Sharma-Poudyal & Chen Citation2011; Chen, unpublished data). The stripe rust epidemics in the PNW are generally classified as low (<20%), moderate (20–40%), severe (40–60%) and extremely severe (>60%) based on yield losses on susceptible cultivars in the experimental fields without fungicide application. During the period from 2001 to 2012, extremely severe epidemics occurred twice (2010 and 2011), severe epidemics occurred three times (2005, 2007 and 2012), moderate epidemics occurred six times (2001, 2002, 2003, 2004, 2006 and 2008), and low epidemics occurred once (2009). The control of stripe rust has mostly relied on planting resistant cultivars and applying foliar fungicides (Chen Citation2005, Citation2007, Citation2013).
Stripe rust resistance has been among the top priorities of wheat breeding programmes in the PNW since the early 1960s (Allan & Vogel Citation1961; Line & Chen Citation1995; Line Citation2002; Chen Citation2005, Citation2013). Both all-stage (AS) resistance and high-temperature adult-plant (HTAP) resistance have been used in developing cultivars with stripe rust resistance, with more emphasis placed on HTAP resistance because of its non-race specificity and durability (Line & Chen Citation1995; Line Citation2002; Chen Citation2005, Citation2013). Wheat cultivars developed and grown in this region commonly have a combination of genes for AS and HTAP resistance. When the genes for AS resistance in wheat cultivars are effective, they completely protect the cultivars from stripe rust damage even when the disease is very severe. However, when the genes for AS resistance are no longer effective due to the development of new races in the pathogen population, the cultivars either become completely susceptible if they do not have HTAP resistance or become less resistant if they have some level of HTAP resistance. Every year, the PNW grows more than 80 cultivars in all market classes of winter and spring wheat except for durum. Most of these cultivars have various levels of HTAP resistance. The overall level of resistance in the wheat cultivars made stripe rust damage insignificant in the years when the epidemic level was low or moderate, and reduced yield losses from potentially more than 90% to less than 20% in 2011, when stripe rust was extremely severe. Although the contribution of resistance in commercially grown cultivars is great, the 20% yield loss in an extremely severe year without fungicide applications would be huge for the region. While breeding programmes have been improving the overall level of stripe rust resistance, fungicide application will continue to be a necessary approach for further reducing yield losses in the foreseeable future.
The commercial application of fungicides for control of stripe rust in the PNW was started in 1981, which was the first large-scale fungicide application for control of diseases of field crops in the USA (Line Citation2002). Since then, chemical control has become a major component of the integrated management of stripe rust. Triadimefon (Bayleton) was the first fungicide successfully used to control stripe rust. New fungicides have been tested every year for their efficacy for control of stripe rust on both winter and spring wheat crops and the results have been published in Fungicide and Nematicide (F&N) Tests and Plant Disease Management Reports (Chen & Wood Citation2002a, Citation2002b, Citation2003a, Citation2003b, Citation2004, Citation2005a, Citation2005b, Citation2006a, Citation2006b, Citation2007a, Citation2007b, Citation2008a, Citation2008b, Citation2009a, Citation2009b, Citation2009c, Citation2010a, Citation2010b, Citation2011a, Citation2011b; Chen et al. Citation2012a, Citation2012b, Citation2013a, Citation2013b). The results of these tests have led to the registration of several fungicides and provided guidelines for use of fungicides. Currently, the following fungicides are labelled for control of stripe rust: Absolute (tebuconazole and trifloxystrobin), Alto Elite (cyproconazole), Evito (fluoxastrobin), Folicur (tebuconazole), Headline (strobilurin), Muscle (tebuconazole), Priaxor (fluxapyroxad and pyraclostrobin), Prosaro (prothioconazole and tebuconazole), Quadris (azoxystrobin), Quilt (propiconazole and azoxystrobin), Stratego (propiconazole and trifloxystrobin), Tilt (propiconazole) and Twinline (strobilurin and triazole). These labelled fungicides with different active ingredients provide choices for growers to use and may reduce selection pressure in the fungal pathogen to develop resistance to chemicals. In the fungicide efficacy studies, one or two highly susceptible cultivars are used, which are not commercially grown cultivars, and therefore, the results do not provide information on fungicide responses of commercially grown cultivars. Because of various levels of resistance in commercial cultivars, it is necessary to test individual cultivars for response to fungicide application and estimate their yield loss caused by stripe rust.
To determine yield losses caused by stripe rust and yield increase by fungicide application for individual cultivars, we conducted field experiments near Pullman, Washington with major winter and spring wheat cultivars grown in the PNW. The data have been used for stripe rust management based on the resistance levels of individual cultivars. This article summarizes the data and provides useful information to integrate cultivar resistance and fungicide application in control of stripe rust.
Materials and methods
Wheat cultivars
To determine yield loss caused by stripe rust and effect of fungicides on yield increase for major cultivars, experiments were conducted at field sites near Pullman, Washington every year from 2002 to 2012. For the winter wheat experiments, cultivars consisted of a susceptible check ‘PS 279’ and 23 commercially grown cultivars. PS 279 is a winter club wheat line developed by the USDA-ARS Wheat Genetics Unit with no known resistance to any races of P. striiformis f. sp. tritici identified so far in the USA. The other 23 cultivars were selected in each year based on their commercial acreage in the previous year in Washington State and also in Idaho and Oregon, with a few exceptions of newly released cultivars that had not been commercially grown previously. For the spring wheat experiments, 16 entries were used in each year, including a susceptible check (‘Fielder’ or ‘Lemhi’) and 15 commercially grown cultivars. Commercial cultivars were selected in the same way as for the winter wheat cultivars. In general, the selected cultivars accounted for 65–75% of the total wheat acreage in the previous year and the same year of the experiment in Washington State.
Experiment design and field management
A randomized split-block design was used for the field experiments with four replications. Fertilizer (Osmocote 14-14-14) was applied at 67.28 kg ha−1 at the time of cultivation for both winter and spring plots, and urea was applied at 51.58 kg ha−1 for winter plots in early spring. Winter wheat cultivars were planted starting in the third to fourth week of October and spring wheat cultivars were planted from the fourth week of April to the first week of May, depending upon the field moisture conditions and other field experiments. The plots were seeded in rows spaced 36 cm apart at 67.28 kg ha−1 with a drill planter. The plot dimension was 1.37 m width and about 4.57 to 5.49 m in length, and each plot was individually measured before harvest to calculate the area for each plot. Herbicides were applied in middle to late May for winter plots and early June for spring plots when wheat plants were at late tillering to early jointing stage (Zadoks 26–30). Different herbicides were used in different years depending upon major species of weeds and grasses.
Fungicide application and data collection
Fungicide Tilt 3.6E was applied in 150 L ha−1 at 307.30 gram ha−1 mixed with surfactant M-90 at 1% (v/v) using a 601C backpack sprayer with a CO2-pressurized spray boom at 18 psi having three operating 0.64 cm nozzles spaced 48.26 apart. The fungicide application dates and the crop stages varied from year to year, but generally were from boot to flowering stage. Stripe rust incidence or severity ranged from 1% to 10% with exceptions of over 10% in 2010 and 2011 influenced by weather conditions. To consider the effect of occasional planting problems affecting emergence on plot yield, the stand of plants in each plot was recorded as percentage of the ground area prior to fungicide application and used in the calculation of plot yield. Stripe rust severity (percentage of diseased foliage per plot) was assessed visually for each plot just before fungicide application and three or more times in a 1–2 week interval, depending upon winter or spring crops and growth stages. The final disease recording was done at the soft dough stage, except in the 2002 winter experiment, for which only one severity measurement was collected at the soft dough stage. Plots were harvested in early to middle August for winter wheat and in late August to middle September for spring wheat when plants were completely mature and kernels were naturally dry (about 3–5% kernel moisture). The grain yield from each plot was measured in grams and test weight of kernels was determined by measuring a pinto (0.55 L) of grain in grams.
Data analyses
Area under the disease progress curve (AUDPC) was calculated for each plot using the multiple sets of severity data as previously described (Chen & Line Citation1995; Lin & Chen Citation2007). Mean per cent reduction of AUDPC in the fungicide-sprayed plots in comparison with the non-fungicide plots was calculated as: [mean AUDPC (fungicide-applied plots) – mean AUDPC (non-fungicide-applied plots)]/mean AUDPC (non-fungicide-applied plots) × 100. Relative AUDPC (rAUDPC) was calculated as per cent of the mean AUDPC of the non-fungicide treated plots of the susceptible check. Both reduction of AUDPC and rAUDPC were used to assess the effect of fungicide application in control of stripe rust for each cultivar.
The mean yields of the non-fungicide-sprayed and sprayed plots were compared to determine the yield loss and percentage loss by stripe rust and yield increase by fungicide application for each cultivar.
Analysis of variance (ANOVA) was performed using the SAS statistical package (SAS Institute, Cary, NC) for comparing the AUDPC, test weight and yield data for fungicide-applied and non-applied plots for each crop experiment in each year. The least significant difference (LSD) value was recorded and used to determine whether the mean difference between the fungicide-applied and non-applied treatments was significant and rating wheat cultivars for each crop experiment in each year.
To rate cultivars by yield loss to indicate their relative vulnerability to stripe rust damage, relative yield loss (rYL) was calculated for each cultivar in each experiment as the percentage of its yield loss divided by the yield loss percentage of the susceptible check. The baseline of relative yield loss (rYL) for each experiment as the threshold of fungicide application was calculated using the formula: Baseline rYLI = LSD/grand mean of yield × 100/percentage yield loss of the susceptible check. A relative yield loss index (rYLI) for each cultivar was obtained using its rYL in relation to the base line rYL of the experiment. Taking the yield loss data of the 2012 winter wheat experiment as an example (), the LSD value was 664.08 (kg ha−1), the grand mean yield was 6909.61 (kg ha−1) and the yield loss (%) of the susceptible check was 57.5%, and then the baseline rYL (%) = 664.08/6909.61 × 100/57.50% = 16.71%. Based on the baseline rYL value, the cultivars were rated as 1 (rYLI) for a rYL less than 16.71% (1 × 16.71%), those as 2 from 16.71% to 33.42% (2 × 116.71%), those as 3 from 33.42% to 50.13% (3 × 16.71%), those as 4 from 50.13% to 66.84% (4 × 16.71%), those as 5 from 66.84% to 83.55% (5 × 16.71%) and those as 6 from 83.55% to 100% or higher (6 × 16.71%). Cultivars with rYLI values greater than 1 should be sprayed with fungicide to reduce the potential significant yield loss.
Because each crop experiment consisted of 24 or 16 cultivars with different levels of stripe rust resistance or susceptibility, which would increase variation for the disease, test weight and yield data, the data of each cultivar were also analysed separately to find the lowest value of LSD using P = 0.05 in the experiment.
Results
The summarized data showing AUDPC, yield, yield loss and increase in percentage, and test weight and significant levels of their comparison between fungicide-applied and non-applied treatments for each cultivar are maintained at the stripe rust website (http://striperust.wsu.edu/diseaseManagement/cultivar-resistance-yield-loss-data-stripe-rust.html). The data were sent to growers when they were completed in each year and used to provide guidance for selecting resistant cultivars to grow and making decisions of fungicide application for particular cultivars to reduce stripe rust damage in the following years. The following results are presented to summarize the study over an 11-year period.
Stripe rust damage on susceptible checks
The disease- and yield-related data of the susceptible checks for winter wheat () and spring wheat () indicated the effects of different levels of stripe rust epidemics from 2002 to 2012. Because stripe rust started at different crop growth stages in different years (data not shown), the AUDPC values in these years were not comparable. However, the levels of stripe rust epidemics in these years can be determined by the percentages of yield losses in the non-fungicide sprayed plots compared with fungicide-sprayed plots. The yield losses of the susceptible winter wheat check PS 279 ranged from 18.30% in 2009 to 89.78% in 2011 (). In the 11 years, 1 year (2009) had low (less than 20%) yield loss; 5 years (2002, 2003, 2004, 2006 and 2008) had moderate yield loss (20–40%); 4 years (2005, 2007, 2010 and 2012) had severe loss (40–60%); and 1 year (2011) had extremely severe (more than 60% yield loss). For spring wheat, the yield losses ranged from 4.56% in 2008 to 50.63% in 2005 (). Of the 11 years, 2 years (2008 and 2009) had low loss; 5 years had moderate loss (2003, 2006, 2007, 2010 and 2012); and 4 years (2002, 2004, 2005 and 2011) had severe loss. When regression analysis was used to determine the correlation of the winter wheat yield losses with the spring wheat yield losses, they were significantly correlated (r = 0.62, P = 0.04) with a formula of Y = 12.80 + 0.46X, where Y = spring wheat yield loss and X = winter wheat yield loss of susceptible checks.
Table 1. Area under the disease progress curve (AUDPC), yield loss and test weight (TW) loss by stripe rust in plots of no fungicide and reduction of AUDPC and increases of yield and TW by fungicide application on the susceptible check variety of winter wheat (PS 279) in 2002–2012.
Table 2. Area under the disease progress curve (AUDPC), yield loss, and test weight (TW) loss by stripe rust in plots of no fungicide and reduction of AUDPC and increases of yield and TW by fungicide application on the susceptible check variety of spring wheat (Lemhi or Fielder) in 2002–2012.
Stripe rust reduces test weight, which is a major component of yield. The non-fungicide-sprayed plots of winter wheat susceptible check PS 279 had test weight reduced by 1.28% (2011) to 9.32% (2004) in all years, except 2002 and 2003, in which test weights were not measured (). In all of the tested years, except 2011, the test weight reductions were significant (P = 0.001 to 0.05). In contrast, the test weight reductions of susceptible spring wheat check (‘Lemhi’) were significant only in 4 (2005, 2006, 2007 and 2010) out of the tested 9 years (), and the reductions were generally lower than those of the winter wheat susceptible check.
Control effects of fungicide application on susceptible checks
The fungicide-sprayed plots showed effective disease control. In every year, the treated plots had significantly low (P = 0.001 to 0.05) AUDPC values compared with the winter or spring wheat susceptible checks. The reductions ranged from 42.13 to 100% (, ). Compared to the non-fungicide plots, the yield increases ranged from 22.4% in 2009 to 878.1% in 2011 for the winter wheat () and from 4.78 to 102.55% for the spring wheat checks (). The yield increases were significant in all 11 years for the winter wheat and in 9 of the 11 years for spring wheat (P = 0.001 to 0.05). The fungicide applications significantly increased test weight in all tested 9 years, except 2011, for the winter wheat; and in 4 of the 9 years for spring wheat (, ).
Mean rAUDPC values of commercially grown cultivars
Comparisons of mean rAUDPC values of commercially grown cultivars in non-fungicide-sprayed and fungicide-sprayed plots over the 11 years are shown in . The mean rAUDPC values ranged from 4.34% in 2002 to 35.34% in 2011, with an average of 18% for winter wheat (A), and ranged from 5.62% in 2007 to 60.86% in 2010, with an average of 31.68% for spring wheat (B) in the non-fungicide-applied plots. The mean rAUDPC values of the winter wheat and spring wheat crops had a moderate level of correlation and their regression formula is: Y = 6.22 + 0.37X, where X is the winter wheat rAUDPC and Y is the spring wheat rAUDPC (r = 0.68, P = 0.02).
Fig. 1 Mean relative area under the disease progress curve (rAUDPC) values and standard deviations of the tested commercially grown winter (A) and spring (B) wheat cultivars. A * indicates that the means of the non-fungicide-sprayed and fungicide-sprayed plots were significantly different (P < 0.05).
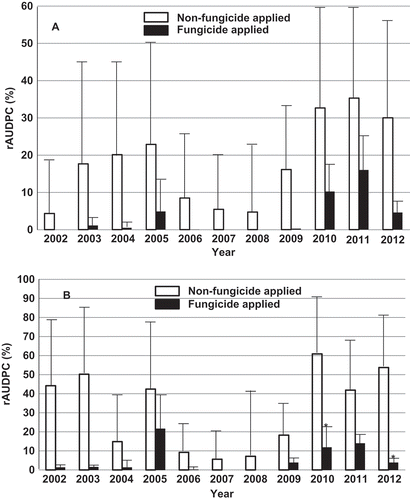
The high standard deviations of rAUDPC in the non-fungicide-sprayed plots indicated the great variation in resistance/susceptibility among commercially grown cultivars. Because of this, none of the 11 years for winter wheat and only 2 (2010 and 2012) of the 11 years for spring wheat showed significant reductions in rust severity by fungicide application compared with the non-fungicide application treatment, although the mean differences were large ().
Compared with the AUDPC values of the susceptible checks in the non-fungicide-sprayed plots, which were treated as 100% rAUDPC to calculated the rAUDPC values for their fungicide-sprayed plots and those of commercially grown cultivars, the various levels of resistance in the commercial cultivars were able to reduce stripe rust severity on average by 82% in winter wheat and by 68.32% in spring wheat.
Yield losses and increases in the tested commercially grown cultivars
As shown in for winter wheat and for spring wheat, the tested commercially grown cultivars were planted at average frequencies of 79% for winter wheat and 72.6% for spring wheat. Therefore, the tested cultivars had reasonably good representations of the wheat cultivars grown in each of the 11 years. When not sprayed with a fungicide, the winter wheat cultivars had a yearly mean yield ranging from 4232.09 to 8063.17 kg ha−1 with a grand mean of 6642.83 kg ha−1 (). In the plots sprayed with fungicide Tilt, the yearly yield means ranged from 4390.88 to 8437.94 kg ha−1 with a grand mean of 7176.38 kg ha−1. The yield differences ranged from 158.11 to 1338.25 kg ha−1 with a grand mean of 532.88 kg ha−1. The yield differences could be converted to yield losses, ranging from 2.18% in 2008 to 20.75% in 2011 with a grand mean of 8.47%. Similarly, if one-time fungicide application was used as in the applied plots, yield would be increased by up to 29.43% in 2011 with a grand mean of 10.85% for the 11 years.
Table 3. Mean yields, yield losses by stripe rust and yield increases by fungicide application for commercially grown winter wheat cultivars from 2002 to 2012.
The spring wheat experiments had similar results. The grand mean difference between fungicide-applied and non-applied plots was 577.96 kg ha−1, translated into 12.74% yield loss by stripe rust and 17.03% increase by fungicide application ().
Table 4. Mean yields, yield losses by stripe rust and yield increases by fungicide application for commercially grown spring wheat cultivars from 2002 to 2012.
The LSD value was used to determine whether the difference between the sprayed and non-sprayed means for each cultivar in each crop experiment was significant. These values ranged from 664.08 to 1404.86 with a grand mean of 950.71 kg ha−1 for winter wheat () and from 353.23 to 840.36 with a grand mean of 605.55 kg ha−1 for spring wheat (). When these LSD values were used to determine if the yield difference between sprayed and non-sprayed treatments was significant, only the means of 2012 were significantly different for winter wheat (). In contrast, five years (2002, 2003, 2005, 2010 and 2012) had significant differences between sprayed and non-sprayed plots for spring wheat ().
As the experimental LSD values might mask real significant differences for individual cultivars as large variations were observed among tested cultivars, individual cultivars were analysed separately. Based on these analyses, we identified cultivars that had significantly different yield in comparison of their sprayed and non-sprayed plots, which were determined to be insignificant when the experimental LSD value was used. With the purpose of searching for the lowest difference in yield, but still significant at P = 0.05, we determined the lowest LSD value for each experiment. These values ranged from 212.61 to 941.96 with a grand mean of 518.75 kg ha−1 for the winter wheat experiments () and from 174.26 to 610.93 with a grand mean of 364.00 kg ha−1 for the spring wheat experiments (). These grand means were about a half of their respective grand means based on all cultivars in each experiment.
Control of stripe rust based on resistance/susceptibility of individual cultivars
The most important objective of this study over the past 11 years was to determine if fungicide application is needed for individual cultivars with different levels of resistance under different levels of disease pressure.
Excluding the susceptible checks, 71 winter wheat and 60 spring wheat cultivars/breeding lines were tested in 1–10 years depending upon their release year and acreage in each year. The number of years in tests and mean and standard deviations of rAUDPC, rYL, significance of fungicide application (SFA), and rating based on rYL for each cultivar/breeding line are given in Supplement Table 1 for winter wheat and Supplement Table 2 for spring wheat. The mean acreages indicated the popularity of the cultivars, and the standard deviations indicated their acreage changes. The acreage data together with the number of years in testing should indicate whether the cultivars were lasting or short-lived with exceptions of newly released ones. Detailed data were provided to growers each year and are maintained in our stripe rust website (http://striperust.wsu.edu). For example, winter wheat cultivars ‘Eltan’ and ‘Madsen’ were tested in 10 years and they had a mean percentage of cultivation over the 10 years of 22.76 ± 6.76% and 11.97 ± 7.55%, respectively. They were among the most popular winter wheat cultivars over the last decade. Similarly, ‘Alpowa’, tested in 11 years, and ‘Louise’, tested in 9 years, accounted for 20.42 ± 17.21% and 18.37 ± 14.07% of the total spring wheat acreage. They were among the most popular spring wheat cultivars.
The data of rAUDPC, rYL, SFA and cultivar rating by rYL presented in the supplementary tables allowed the conduction of regression analyses to determine the correlations among the disease-related parameters. The regression formulae are presented in . The correlations between the tested parameters were all significant in both winter and spring wheat tests, with only one exception between rYL and SFA in spring wheat. The significance of the formulae indicated that the value of the independent variable could be used to predict the value of the dependent variable. The results also showed that the disease severity data measured as rAUDPC in comparison with the susceptible check can be well translated into yield loss, which in turn can be used to rate cultivars for their response to stripe rust and for making a decision as to whether fungicide application is appropriate or not.
Table 5. Regression analyses of relative AUDPC (rAUDPC) with relative yield loss (rYL), significance of fungicide application (SFA) and rating by rYL.
The mean ratings of the winter wheat cultivars by rYLI ranged from 1.0 to 5.0 and those of the spring wheat cultivars ranged from 1.0 to 3.09. Cultivars with a rating of 1 did not have significant differences in yield between non-fungicide sprayed and fungicide sprayed plots based on the LSD value of the experiment. In contrast, a greater-than-1 rating indicated that stripe rust significantly reduced yield. Based on the ratings, the 72 winter wheat cultivars, including the susceptible check PS 279, could be classified into three groups (Supplement Table 1). The first group, consisting of 12 cultivars, had ratings greater than 2, showing that stripe rust caused significant yield losses and the fungicide application significantly reduced yield losses. Therefore, it was necessary to spray fungicide in the years when they were tested. The second group of 22 cultivars, including the most popular cultivar ‘Eltan’, had a rating greater than 1 but smaller than 2, with SD values of 0.33–0.84. The data showed that stripe rust caused significant yield loss in some, but not all, of the tested years, and therefore, fungicide application is needed only in the years when stripe rust caused significant damage. The remaining 38 cultivars including ‘Madsen’ formed the third group as they all had a rating of 1. Because the yields between fungicide-applied and not-applied plots were not significant, they could have adequate resistance without using fungicides.
Similarly, the 61 spring wheat cultivars\breeding lines were also classified into three groups. The first susceptible group consisting of 11 cultivars received ratings greater than 2 as they showed significant yield differences in fungicide-sprayed plots compared with non-sprayed plots. The second group of 13 cultivars received ratings between 1 and 2 with various SD values (0.35–0.53) as stripe rust caused significant yield losses in some, but not all of the tested years. The remaining 33 cultivars/breeding lines had a rating of 1 as their yield losses were not significant in the test years.
Effect of fungicide application on yield without significant stripe rust
Both yield decreases and increases were observed for some cultivars in the fungicide-sprayed plots compared with non-sprayed plots without significant stripe rust throughout the tests in both winter and spring wheat from 2002 to 2012. To determine if fungicide application had any effect on yield without significant stripe rust, the data of yield increase for cultivars without significant stripe rust were extracted from the data of each year and compared for the decrease and increase groups. Over the 11 years, 51 winter wheat cultivar tests had yield decreases, with a mean of 2.47%, and 58 had increases, with a mean of 3.39%, and the mean difference was not significant (P = 0.20) (). This result indicated that fungicide application did not have a significant effect on yield change if stripe rust was not a problem on wheat cultivars. In contrast, yield decrease of spring wheat was observed in 15 cultivar tests while increases were observed in 35 cultivar tests, in which stripe rust was not significant (). The means of decrease and increase, −1.26 and 5.86%, respectively, were significantly different (P = 0.01).
Table 6. Decrease and increase of grain yield by fungicide application for cultivars without significant stripe rust.
Discussion
Although some cultural practices can reduce stripe rust damage, stripe rust is mainly controlled by growing resistant cultivars and applying fungicides (Chen Citation2005). The present study focused on determining if fungicide application is appropriate for major commercially grown cultivars with various levels of resistance. In addition to the data that were used to guide appropriate disease management on a yearly basis, the study demonstrates the importance of stripe rust, contribution of resistance, and effect of fungicides.
Because of favourable weather conditions and growing both winter and spring wheat crops, stripe rust is one of the most important diseases of wheat in the PNW (Line Citation2002; Chen Citation2005). Using susceptible checks, the present study showed that stripe rust could cause from 18% to more than 90% yield losses, with an average of 44% on winter wheat, and from 5% to more than 50% yield losses, with an average of 32.8%, on spring wheat crops without fungicide application during the 11-year period from 2002 to 2012. In general, these potential yield loss data were higher than those recorded from 1975 to 2001, during which potential yield losses ranged from 6% in 1986 to 86% in 1981, with an average of 36% on susceptible winter wheat, and from 3% in 1997 to 48% in 1999, with an average of 27% on susceptible spring wheat cultivars (Sharma-Poudyal & Chen Citation2011; Sharma-Poudyal & Chen, unpublished data). The more frequent severe epidemics were due to the changes in weather conditions (Sharma-Poudyal & Chen Citation2011), race compositions (Chen et al. Citation2010; Wan & Chen Citation2012), and cultivars. Some of the changes will be discussed later related to cultivar resistance and crops of winter and spring wheat. Nevertheless, the yield loss data of both historical studies and the present study show that stripe rust can cause substantial damage whenever susceptible cultivars are grown in the PNW.
As demonstrated in the present study, fungicide application is effective for control of stripe rust. Compared with non-sprayed plots, fungicide application reduced AUDPC by 83.89% on average, increased yield by 146.92% and increased test weight by 5.0% on the susceptible winter wheat check. On susceptible spring wheat checks, the AUDPC reduction and yield and test weight increases were 80.18%, 55.66% and 1.56%, respectively. In this study, Tilt was used throughout the study as it is effective, has been most used by growers, and has become the standard fungicide to control stripe rust in the PNW. Only one time of application was used in the 11-year experiments to keep the fungicide treatment relatively consistent, which was adequate for most years as indicated by more than 80% AUDPC reduction in 8 out of the 11 years for winter wheat and 7 of the 11 years for spring wheat. In 2005, 2010 and 2011, the one-time fungicide application did not provide adequate control as the rust seasons were unusually long and damage was more severe. As shown in our separate fungicide tests, in which one or more applications of various fungicides and timings were used, two or three fungicide applications were needed to provide adequate control (Chen & Wood Citation2006a, Citation2006b Citation2011a, Citation2011b; Chen et al. Citation2012a, Citation2012b). In fact, two to three applications of fungicides were used in many commercial fields in 2010 and 2011 in the PNW. In Washington State alone, fungicide application saved 13.7 million bushels of wheat grain (about $96 million value) at the cost of about $27 million in 2010. In 2011, the stripe rust potential damage was even greater (more than 90% yield loss on susceptible winter wheat cultivars), fungicide application saved 19 million bushels (about $136 million value) at the cost of about $28 million. The input and output ratio for fungicide control was about 1:5 on winter wheat. In the same year, the fungicide control cost about $12.3 million, which saved 5.6 million bushels of grain (about $39.4 million value), at about a 1:3 ratio of input to output for spring wheat (Chen, unpublished data).
Timing of fungicide application is important for effective and economical control of stripe rust as the start and duration of disease development can vary a lot due to the great variations in the weather conditions (Chen Citation2005; Sharma-Poudyal & Chen Citation2011). It depends highly upon how early stripe rust starts and how long the disease continues developing. In the PNW, winter wheat is planted from middle August to late October and harvested from middle July to middle August; and spring wheat is planted from late February to May and harvested from September to October. The long growing season of the wheat crops, together with the climate favourable for both summer and winter survival, provides ideal conditions for the stripe rust pathogen to carry over from crop to crop, from season to season, and from area to area in the PNW and beyond the region. During a growing season, stripe rust almost consistently starts from infections in the autumn. The amount and level of autumn infection depend upon the inoculum produced in the previous crop season (especially the spring crop) and moisture in the autumn. How well the pathogen can survive winter is dependent on the winter temperatures and/or snow cover (Sharma-Poudyal & Chen Citation2011). More winter survival will lead to a quick start of the disease in the spring. Under cool and wet late spring and summer conditions, the disease will develop and spread quickly and last long. The longer the rust season, the more yield losses occur. In the 2009–2010 and 2010–2011 crop seasons, for example, the start of stripe rust in the 2010 spring was about normal, but the cool and moist conditions from May to July allowed stripe rust to develop quickly and last very long. The low temperature and high-moisture conditions also delayed crop maturity, which allowed abundant urediniospores to be produced in late 2010 summer. The unusually high moisture in September and October caused the winter wheat crop to emerge and grow quickly and also allowed the stripe rust pathogen to heavily infect the early planted winter wheat crop. By early November, stripe rust foci of several metres in diameter were observed in fields of the Horse Heaven Hills region in south-central Washington and infection was easily found further north. For the fields without observed sporulation in November in eastern Washington, infection should have occurred. The long period of snow cover, especially during the cold periods of the 2010–2011 winter, allowed the rust to survive very well. When sampled in mid-January to February, rust was present. The rust started developing very early and fungicide application began in March. Coupled with cool and moist conditions in May to early July, which was also not good for HTAP resistance to express, the long-lasting stripe rust season caused over 90% yield loss on susceptible checks and substantial yield losses on winter wheat crops, the highest on record; and was also one of the highest yield losses for the spring crop. In 2011, many fields were sprayed two or three times.
In the stripe rust situation like the autumn of 2010 and the spring of 2011 for winter wheat, seed treatment with fungicides could be beneficial. Old chemicals like Oxathiin and triadimenol (Baytan) were used as seed treatments to reduce seedling infection (Hardison Citation1975; Rokotondradona & Line Citation1984; Scott & Line Citation1985). However, several current and new chemicals tested under both greenhouse and field conditions did not provide good control (Chen et al. Citation2013c, Citation2013d, Citation2013e). Chemicals with a much longer duration of effectiveness are needed for seed treatments to control stripe rust. Appropriate delay of planting date can reduce autumn infection for winter wheat and shorten the rust season for spring wheat. However, the planting delay should not lead to yield reduction as planting timing can be critical for good emergence to have a uniform crop in dry-land areas.
As shown in the present study, foliar application of fungicides is important for control of stripe rust. One to three applications after the winter season should provide adequate control, depending upon the duration of the rust season, the duration of fungicide effectiveness, and the types and levels of cultivar resistance. For moderately susceptible and susceptible cultivars, application of foliar fungicides is needed almost every year in the PNW. One-time application may be adequate in one year but not another, depending upon the rust season. If the rust pathogen has survived the winter well, as indicated by observations in the early planted fields in early spring, the first application of foliar fungicide is better in a mixture of a fungicide with herbicide at the herbicide application time, which will reduce rust infection by killing the rust mycelia in the infected leaf tissue and save the application cost. However, if the rust survival is very low and rust does not start to infect or develop until more than 3 weeks later, the use of fungicides at the herbicide application is unnecessary. Then, the first and possibly only application should be used when stripe rust is just starting to develop (before 10% incidence or severity). Depending upon fungicide and the duration of the rust season, a second and third application may be needed if rust re-starts developing 4–6 weeks after the previous application. Taking Tilt as an example, it provides good control for about a month. To maximize yield increase, it is important to cover the flag leaves and protect the grain-filling period. Because most currently registered fungicides cannot be used after flowering time, it is important to make decisions whether to apply fungicides during the flowering time. Because our fungicide tests over many years did not show good control for one-half or reduced rates, and problems were caused by using one-half or reduced rates in the past, we generally recommend using a full rate for each application of fungicides.
The recent reduction in the costs of several fungicides has tended to encourage growers to use excessive applications. Some over-simplified schemes of fungicide applications just based on crop stages without checking disease situations could increase the unnecessary use of chemicals. Moreover, the controversial hypothesis of ‘vigour effect’ and ‘yield increase’ of fungicides is sometimes used to promote fungicide use without disease occurrence. The present study did not support this hypothesis at least for winter wheat. For spring wheat, fungicide application appeared to increase yield, but such increase may be due to the fact that the fungicide application might control other diseases like leaf rust. However, this needs further studies. To reduce excessive use of fungicides in order to reduce management cost and potential environment hazards, it is important to use fungicides only when necessary to reduce disease pressure and maximize profit.
Growing resistant cultivars is the most effective approach to control stripe rust without additional cost to growers and potential harmful effects to the environment, to people on and around farmlands, and to improve food safety to consumers. Developing resistant cultivars has been one of the top priorities in the wheat breeding programmes in the PNW since early 1960s, and the overall resistance level and durability in wheat cultivars have been continually improved (Allan & Vogel Citation1961; Line Citation2002; Chen Citation2005, Citation2013). As shown in the present study, all winter wheat cultivars had rAUDPC and yield losses less than the susceptible check, except ‘Hatton’, ‘Moreland’ and ‘Gaines’, which are no longer grown since 2005 (Supplement Table 1). Similarly, most spring wheat cultivars also have various levels of resistance. Growing these cultivars with various levels of resistance reduced yield losses from the potential 43.71% to 8.47% on average (, ). Similarly, the commercially grown spring wheat cultivars were collectively able to reduce yield losses from potentially 32.8 to 12.74% on average over the 11 years (, ). Based on the data obtained in the present study, resistance in commercial cultivars saved wheat growers in Washington State alone more than $500 million a year on average. The overall level of resistance in wheat cultivars is adequate to protect wheat crops without fungicide application when stripe rust epidemic levels are low, as in 2008 and 2009. However, the level is not good enough when the disease is severe and extremely severe as in 2010 and 2011, under which wheat crops could suffer up to 20% yield loss. Breeding programmes have been making great efforts to improve the overall level of resistance by developing new cultivars with durable but high levels of resistance.
The commercially grown wheat cultivars have different levels of resistance, and the various levels of resistance are all-stage resistance, HTAP resistance or a combination of both types of resistance in individual cultivars. The characteristics, advantages and drawbacks of all-stage and HTAP resistance have been previously discussed (Line & Chen Citation1995; Line Citation2002; Chen Citation2005, Citation2007, Citation2013). Most cultivars tested in the present study have HTAP resistance or combination of HTAP and race-specific resistance. Some of the cultivars have adequate HTAP resistance that is durable. For example, soft white common winter wheat cultivar ‘Madsen’, which has been widely grown since its release in 1988, did not have significant yield loss in all tested 10 years. Because HTAP resistance in ‘Madsen’ is durable and fungicide application is not needed, it has become a standard reference for developing new wheat cultivars with durable and high levels of resistance to stripe rust. Using a cultivar with a high level of resistance does not guarantee obtaining derivative cultivars with high resistance. For example, the imidazoline (IMI) herbicide-resistant winter wheat cultivar ORCF 102, which was developed from ‘Madsen’, does not retain all stripe rust resistance genes from ‘Madsen’. In the present study, ORCF 102 had more than 50% rAUDPC and over 40% rYL in 2011 and 2012, while ‘Madsen’ had only 5–8% rAUDPC and 0–4% rYL in the 2 years (data not shown). Because of its herbicide resistance and high yield potential, ORCF 102 has become popular in recent years. By acreage, winter wheat ‘Eltan’, which was released in 1990, is the most commonly grown cultivar. It was highly resistant to stripe rust in the 1990s and early 2000s, but gradually become moderately resistant to moderately susceptible as its all-stage resistance was no longer effective against new races of the pathogen. It had 35–38% rAUDPC and 27–36% rYL. Although its HTAP resistance is still effective, the level is not adequate to completely protect the crop without using fungicides. The change of major cultivars from highly resistant ‘Madsen’ to reduced resistance ‘Eltan’ and ORCF 102 in the last decade, especially in recent years, was one of the reasons for the increased frequency of severe stripe rust epidemics. This emphasizes that stripe rust should not be ignored or its priority should not be reduced when trying to quickly use new technology for other traits. The change of spring wheat cultivars such as ‘Hank’, ‘Jefferson’, ‘Nick’, ‘Tara 2002’ and ‘WPB 926’ from highly resistant to highly susceptible over the last decade added disease pressure. It is reassuring to see that these cultivars have been reduced in acreage. ‘Louise’ has replaced ‘Alpowa’ to become the most widely grown spring wheat cultivar. Both cultivars have HTAP resistance (Lin & Chen Citation2007; Carter et al. Citation2009), but resistance in ‘Louise’ is higher than in ‘Alpowa’. ‘Alpowa’ was rated as 1 (fungicide is not needed) for 6 years and as 2 (fungicide is needed) for 5 years. In contrast, ‘Louise’ was rated as 1 for all tested 9 years. Together with our germplasm screening studies, the present study has proven the durability of HTAP resistance in many commercially grown wheat cultivars.
As shown by the present study, HTAP resistance is still a key for developing stripe rust resistant cultivars for sustainable control of the disease (Chen Citation2013). High levels of durable resistance can be achieved by using individual or combinations of genes or quantitative trait loci (QTL) with major effects for HTAP resistance and combining genes/QTL for HTAP resistance with effective all-stage resistance. Numerous genes with molecular markers for HTAP and effective all-stage resistance have been identified, and improved resistant germplasm lines have been developed for diversifying resistance genes used in breeding programmes (Chen et al. Citation2003; Yan et al. Citation2003; Uauy et al. Citation2005; Lin & Chen Citation2007, Citation2008, Citation2009; Santra et al. Citation2008; Carter et al. Citation2009; Fu et al. Citation2009; Murphy et al. Citation2009; Li et al. 2010; Fang et al. Citation2011; Chen et al. Citation2012; Ren et al. Citation2012; Wang et al. Citation2012; Zhao et al. Citation2012; Chen Citation2013; Christopher et al. Citation2013a, Citation2013b; Xu et al. Citation2013; Lu et al. Citation2014; Zhou et al. Citation2014). A large number of advanced breeding lines have been developed. For example, among the 79 entries of the 2012 PNW winter wheat variety nursery tested under natural infection in multiple field locations and against selected predominant races in seedling and adult-plant stages, 39 (49.4%) entries showed high levels and 24 (30.4%) showed moderate levels of HTAP resistance, plus 9 (11.3%) were highly resistant in all seedling and adult-plant tests. From the 108 winter wheat cultivars/lines of the 2013 nursery, we identified 55 (50.9%) entries with high level and 17 (15.7%) with moderate levels of HTAP resistance, plus 22 with resistance in all tests. Among the 55 entries with high level of HTAP resistance, 32 also had all-stage resistance to some of the tested races and 21 just had HTAP resistance. Releasing some of the breeding lines should improve the overall level of stripe rust resistance in the coming years.
To make stripe rust management more effective and economical, it is important to integrate cultivar resistance with cultural practices and fungicide application. Early planting is not a problem for cultivars with effective all-stage resistance, but can be a problem in early stage infection for susceptible cultivars and those with only HTAP resistance, which can carry inoculum from season to season as discussed above. Appropriate delay of planting such cultivars can reduce autumn infection for winter wheat and shorten the disease season for spring wheat. Even among HTAP resistant cultivars, variations exist as some resistance genes are more dependent on plant stage while others are more sensitive to temperature (Chen Citation2013). In general, the higher the level of HTAP resistance, the earlier in the growth stage is resistance able to express. Similarly, reducing irrigation is relevant to reducing disease severity on susceptible cultivars and those with partial HTAP resistance. For chemical control, it is relatively easy to make a decision for spraying fungicides on a susceptible crop and not on a completely resistant crop. However, it can be a challenge for making a decision for applying or not applying a fungicide on cultivars with incomplete resistance. The disease and yield loss data obtained in the present study were provided to growers every year to help them make decisions. Based on our prediction models (Sharma-Poudyal & Chen Citation2011), forecasts were provided to growers on potential yield losses of susceptible cultivars. As the crop season progresses, recommendations were made for applying or not applying fungicides to groups of cultivars classified as resistant, moderately resistant, moderately susceptible, and susceptible. Such recommendations may still be too general and a threshold may be helpful.
To establish a threshold for making decisions on fungicide application on specific cultivars, we analysed the disease and yield loss data as rAUDPC and rYL related to those of the susceptible checks. The rYL data were highly correlated to the rAUDPC data. Because the LSD value can be used to determine if the means of fungicide-applied and non-applied treatments are significantly different or not, the LSD value was used to rate cultivars based on the rYL values. The mean and standard deviation of the ratings could be used to make decisions on use of fungicides. For example, if a cultivar received a consistent rating of 1, fungicide application should be unnecessary. If a cultivar had a consistent rating of 2 or above, fungicide should be used. If a cultivar was rated 1 for some years but 2 or higher for others, fungicides may or may not be used depending upon races and disease pressure. As a general guide, the 11-year mean of 14% rYL for winter wheat () and 9% rYL for spring wheat () may be used as thresholds for making decisions on fungicide application. However, because the entire experimental field is relatively large, the variations among cultivars could mask significant differences between sprayed and non-sprayed plots for individual cultivars, so the data of the individual cultivars were also analysed separately. From these analyses, we determined the smallest LSD based on individual cultivars for each experiment (, ). The mean of these LSD values is 7.71% for winter wheat and 5.41% for spring wheat. These values can be used as rYL thresholds for making decisions to enhance grain production with necessary fungicide application. This management strategy is based on ‘historical’ data or at best on the data of the previous year, and the predicted epidemic levels. To take into consideration unexpected issues such as sudden change from resistant to susceptible reactions, growers should be advised to check individual fields at the critical time points as mentioned above for real-time information. Appropriate decisions on fungicide applications should also consider presence of weeds, insects and other foliar diseases for integrated pest management.
Supplemental Data
Supplement Table 1 shows the number of years in test and mean and standard deviation (SD) of grown acreage, relative area under the disease progress curve (rAUDPC), relative yield loss (rYL), significance of fungicide application (SFA), and rating by rYL for winter wheat cultivars tested in 2002 to 2012. Supplement Table 2 shows the similar data for spring wheat cultivars.
Supplement Table 2
Download MS Word (35.5 KB)Supplement Table 1
Download MS Word (38.6 KB)Acknowledgements
This research was supported by the US Department of Agriculture, Agricultural Research Service (Project No. 5348-22000-015-00D) and Washington Wheat Commission (Project No. 13C-3061-3923). The author would like to thank David Wood, Yumei Liu, Kent Evans, John Garner and numerous temporary workers for planting and managing the experiment fields and collecting data.
References
- Allan RE, Vogel OA. 1961. Stripe rust resistance of Suwon 92 and its relationship to several morphological characteristics in wheat. Plant Dis Rep. 45:778–779.
- Carter AH, Chen XM, Garland-Campbell K, Kidwell KK. 2009. Identifying QTL for high-temperature adult-plant resistance to stripe rust (Puccinia striiformis f. sp. tritici) in the spring wheat (Triticum aestivum L.) cultivar ‘Louise’. Theor Appl Genet. 119: 1119–1128. doi:10.1007/s00122-009-1114-2
- Chen JL, Chu CG, Souza EJ, Guttieri MJ, Chen XM, Xu S, Hole D, Zemetra R. 2012. Genome-wide identification of QTL conferring high-temperature adult-plant (HTAP) resistance to stripe rust (Puccinia striiformis f. sp. tritici) in wheat. Mol Breed. 29:791–800. doi:10.1007/s11032-011-9590-x
- Chen XM. 2005. Epidemiology and control of stripe rust [Puccinia striiformis f. sp. tritici] on wheat. Can J Plant Pathol. 27:314–337. doi:10.1080/07060660509507230
- Chen XM. 2007. Challenges and solutions for stripe rust control in the United States. Aust J Agric Res. 58:648–655. doi:10.1071/AR07045
- Chen XM. 2013. Review Article: High-Temperature Adult-Plant Resistance, Key for Sustainable Control of Stripe Rust. Am J Plant Sci. 4:608–627. doi:10.4236/ajps.2013.43080
- Chen XM, Evans CK, Garner JP. 2012a. Control of stripe rust of winter wheat with foliar fungicides, 2011. Plant Dis Manag Rep. 6:CF032.
- Chen XM, Evans CK, Garner JP. 2012b. Control of stripe rust of spring wheat with foliar fungicides, 2011. Plant Dis Manag Rep. 6:CF031.
- Chen XM, Evans CK, Garner JP, Liu YM. 2013a. Control of stripe rust of spring wheat with foliar fungicides, 2012. Plant Dis Manag Rep. 7:CF031.
- Chen XM, Evans CK, Garner JP, Liu YM. 2013b. Control of stripe rust of winter wheat with foliar fungicides, 2012. Plant Dis Manag Rep. 7:CF032.
- Chen XM, Evans CK, Garner JP, Liu YM. 2013c. Evaluation of chemical seed treatments for control of stripe rust in wheat under controlled conditions. Plant Dis Manag Rep. 7:ST003.
- Chen XM, Evans CK, Garner JP, Liu YM. 2013d. Evaluation of chemical seed treatments for control of stripe rust in spring wheat, 2012. Plant Dis Manag Rep. 7:ST012.
- Chen XM, Evans CK, Garner JP, Liu YM. 2013e. Evaluation of chemical seed treatments for control of stripe rust in winter wheat, 2012. Plant Dis Manag Rep. 7:ST013.
- Chen XM, Line RF. 1995. Gene action in wheat cultivars for durable, high-temperature, adult-plant resistance and interaction with race-specific, seedling resistance to Puccinia striiformis. Phytopathology. 85:567–572. doi:10.1094/Phyto-85-567
- Chen XM, Penman L, Wan AM, Cheng P. 2010. Virulence races of Puccinia striiformis f. sp. tritici in 2006 and 2007 and development of wheat stripe rust and distributions, dynamics, and evolutionary relationships of races from 2000 to 2007 in the United States. Can J Plant Pathol. 32:315–333. doi:10.1080/07060661.2010.499271
- Chen XM, Soria MA, Yan GP, Sun J, Dubcovsky J. 2003. Development of sequence tagged site and cleaved amplified polymorphic sequence markers for wheat stripe rust resistance gene Yr5. Crop Sci. 43:2058–2064. doi:10.2135/cropsci2003.2058
- Chen XM, Wood DA. 2002a. Control of stripe rust of spring barley with foliar fungicides, 2001. F&N Tests. 57:CF01.
- Chen XM, Wood DA. 2002b. Control of stripe rust of spring wheat with foliar fungicides, 2001. F&N Tests. 57:CF03.
- Chen XM, Wood DA. 2003a. Control of stripe rust of spring barley with foliar fungicides, 2002. F&N Tests. 58:CF003.
- Chen XM, Wood DA. 2003b. Control of stripe rust of spring wheat with foliar fungicides, 2002. F&N Tests. 58:CF004.
- Chen XM, Wood DA. 2004. Control of stripe rust of spring wheat with foliar fungicides, 2003. F&N Tests. 59:CF022.
- Chen XM, Wood DA. 2005a. Control of stripe rust of winter wheat with foliar fungicides, 2004. F&N Tests. 60:CF027.
- Chen XM, Wood DA. 2005b. Control of stripe rust of spring wheat with foliar fungicides, 2004. F&N Tests. 60:CF021.
- Chen XM, Wood DA. 2006a. Control of stripe rust of spring wheat with foliar fungicides, 2005. F&N Tests. 61:CF022.
- Chen XM, Wood DA. 2006b. Control of stripe rust of winter wheat with foliar fungicides, 2005. F&N Tests. 61:CF023.
- Chen XM, Wood DA. 2007a. Control of stripe rust of winter wheat with foliar fungicides, 2006. Plant Dis Manag Rep. 1:FC108.
- Chen XM, Wood DA. 2007b. Control of stripe rust of spring wheat with foliar fungicides, 2006. Plant Dis Manag Rep. 1:FC109.
- Chen XM, Wood DA. 2008b. Control of stripe rust of winter wheat with foliar fungicides, 2007. Plant Dis Manag Rep. 2:CF015.
- Chen XM, Wood DA. 2008a. Control of stripe rust of spring wheat with foliar fungicides, 2007. Plant Dis Manag Rep. 2:CF014.
- Chen XM, Wood DA. 2009a. Control of stripe rust of winter wheat with foliar fungicides, 2008. Plant Dis Manag Rep. 3:CF038.
- Chen XM, Wood DA. 2009b. Control of stripe rust of spring wheat with foliar fungicides, 2008. Plant Dis Manag Rep. 3:CF039.
- Chen XM, Wood DA. 2009c. Testing fungicide Evito for control of stripe rust on spring wheat, 2008. Plant Dis Manag Rep. 3:CF040.
- Chen XM, Wood DA. 2010a. Control of stripe rust of spring wheat with foliar fungicides, 2009. Plant Dis Manag Rep. 4:CF002.
- Chen XM, Wood DA. 2010b. Control of stripe rust of winter wheat with foliar fungicides, 2009. Plant Dis Manag Rep. 4:CF003.
- Chen XM, Wood DA. 2011a. Control of stripe rust of spring wheat with foliar fungicides, 2010. Plant Dis Manag Rep. 5:CF003.
- Chen XM, Wood DA. 2011b. Control of stripe rust of winter wheat with foliar fungicides, 2010. Plant Dis Manag Rep. 5:CF004.
- Christopher MD, Liu SY, Hall MD, Marshall DS, Fountain MO, Johnson JW, Milus EA, Garland-Campbell KA, Chen XM, Griffey CA, McIntosh RA.. 2013a. Identification and mapping of adult plant stripe rust resistance in soft red winter wheat cultivar ‘USG 3555’. Plant Breed. 132: 53–60. doi:10.1111/pbr.12015
- Christopher MD, Liu SY, Hall MD, Marshall DS, Fountain MO, Johnson JW, Milus EA, Garland-Campbell KA, Chen XM, Griffey CA. 2013b. Identification and mapping of adult plant stripe rust resistance in soft red winter wheat VA00W-38. Crop Sci. 53:871–879. doi:10.2135/cropsci2012.02.0086
- Fang TL, Campbell KG, Liu ZY, Chen XM, Wan AM, Li S, Liu ZJ, Cao S, Chen Y, Bowden RL, et al. 2011. Stripe rust resistance in the wheat cultivar jagger is due to Yr17 and a novel resistance gene. Crop Sci. 51:2455–2465. doi:10.2135/cropsci2011.03.0161
- Fu DL, Uauy C, Distelfeld A, Blechl A, Epstein L, Chen XM, Sela H, Fahima T, Dubcovsky J. 2009. A kinase-START gene confers temperature-dependent resistance to wheat stripe rust. Science. 323:1357–1360. doi:10.1126/science.1166289
- Hardison JR. 1975. Control of Puccinia striiformis by two systemic fungicides, Bay MEB 6447 and BAS 31702 F. Plant Dis Rep. 59:652–655.
- Li Q, Chen XM, Wang MN, Jing JX. 2011. Yr45, a new wheat gene for stripe rust resistance on the long arm of chromosome 3D. Theor Appl Genet. 122:189–197. doi:10.1007/s00122-010-1435-1
- Lin F, Chen XM. 2007. Genetics and molecular mapping of genes for race-specific all-stage resistance and non-race specific high-temperature adult-plant resistance to stripe rust in spring wheat cultivar Alpowa. Theor Appl Genet. 114:1277–1287. doi:10.1007/s00122-007-0518-0
- Lin F, Chen XM. 2008. Molecular mapping of genes for race-specific overall resistance to stripe rust in wheat cultivar Express. Theor Appl Genet. 116:797–806. doi:10.1007/s00122-008-0713-7
- Lin F, Chen XM. 2009. Quantitative trait loci for non-race-specific, high-temperature adult-plant resistance to stripe rust in wheat cultivar Express. Theor Appl Genet. 118:631–642. doi:10.1007/s00122-008-0894-0
- Line RF. 2002. Stripe rust of wheat and barley in North America: a retrospective historical review. Annu Rev Phytopathol. 40:75–118. doi:10.1146/annurev.phyto.40.020102.111645
- Line RF, Chen XM. 1995. Successes in breeding for and managing durable resistance to wheat rusts. Plant Dis. 79:1254–1255.
- Lu Y, Wang MN, Chen XM, See D, Chao SM, Jing JX. 2014. Mapping of Yr62 and a small effect QTL for high-temperature adult-plant resistance to stripe rust in spring wheat PI 192252. Theor. Appl. Genet. DOI:. doi:10.1007/s00122-014-2312-0
- Murphy LR, Santra D, Kidwell K, Yan GP, Chen XM, Campbell KG. 2009. Linkage maps of wheat stripe rust resistance genes Yr5 and Yr15 for use in marker assisted selection. Crop Sci. 49:1786–1790. doi:10.2135/cropsci2008.10.0621
- Rakotondradona R, Line RF. 1984. Control of stripe rust and leaf rust of wheat with seed treatments and effects of treatments on the host. Plan Dis. 68:112–117. doi:10.1094/PD-69-112
- Ren RS, Wang MN, Chen XM, Zhang ZJ. 2012. Characterization and molecular mapping of Yr52 for high-temperature adult-plant resistance to stripe rust in spring wheat germplasm PI 183527. Theor Appl Genet. 125:847–857. doi:10.1007/s00122-012-1877-8
- Santra DK, Chen XM, Santra M, Campbell KG, Kidwell KK. 2008. Identification and mapping QTL for high-temperature adult-plant resistance to stripe rust in winter wheat (Triticum aestivum L.) cultivar ‘Stephens’. Theor Appl Genet. 117: 793–802. doi:10.1007/s00122-008-0820-5
- Scott RB, Line RF. 1985. Control of stripe rust and leaf rust of wheat with triadimetol seed treatments and triadimefon foliar sprays. Phytopathology. 75:1383–1384.
- Sharma-Poudyal D, Chen XM. 2011. Models for predicting potential yield loss of wheat caused by stripe rust in the U. S. Pacific Northwest. Phytopathology. 101:544–554. doi:10.1094/PHYTO-08-10-0215
- Uauy C, Brevis JC, Chen XM, Khan IA, Jackson LF, Chicaiza O, Distelfeld A, Fahima T, Dubcovsky J. 2005. High-temperature adult plant (HTAP) stripe rust resistance gene Yr36 from Triticum turgidum ssp. dicoccoides is closely linked to the grain protein content locus Gpc-B1. Theor Appl Genet. 112:97–105. doi:10.1007/s00122-005-0109-x
- Wan AM, Chen XM. 2012. Virulence, frequency, and distribution of races of Puccinia striiformis f. sp. tritici and P. striiformis f. sp. hordei identified in the United States in 2008 and 2009. Plant Dis. 96:67–74. doi:10.1094/PDIS-02-11-0119
- Wang MN, Chen XM, Xu LS, Cheng P, Bockelman HE. 2012. Registration of 70 common spring wheat germplasm lines resistant to stripe rust. J Plant Regist. 6:104–110. doi:10.3198/jpr2011.05.0261crg
- Xu LS, Wang MN, Cheng P, Kang ZS, Hulbert SH, Chen XM. 2013. Molecular mapping of Yr53, a new gene for stripe rust resistance in durum wheat accession PI 480148 and its transfer to common wheat. Theor Appl Genet. 126:523–533. doi:10.1007/s00122-012-1998-0
- Yan GP, Chen XM, Line RF, Wellings CR. 2003. Resistance gene analog polymorphism markers co-segregating with the Yr5 gene for resistance to wheat stripe rust. Theor Appl Genet. 106:636–643.
- Zhao L, Feng J, Zhang CY, Xu XD, Chen XM, Su Q, Miao Q, Xu SC, Lin F. 2012. The dissection and SSR mapping of a high-temperature adult-plant stripe rust resistance gene in American spring wheat cultivar Alturas. Eur J Plant Pathol. 134:281–288. doi:10.1007/s10658-012-9987-3
- Zhou XL, Wang MN, Chen XM, Lu Y, Kang ZS, Jing JX. 2014. Identification of Yr59 conferring high-temperature adult-plant resistance to stripe rust in wheat germplasm PI 178759. Theor Appl Genet. 127:935–945. doi:10.1007/s00122-014-2269-z
