Abstract
This review examines how changing fungicide usage in Canada is increasing the risk of fungicide insensitivity in pathogen populations, using examples from field, vegetable and horticultural crops. The current generation of systemic fungicides is selective, effective and has a reduced impact on the environment compared with older contact fungicides. However, many of these new fungicides also have an increased risk of fungicide insensitivity. On the Canadian prairies, a trend towards specialization of production within regions has resulted in limited cropping rotations, or even no rotation, in many fields, and yield targets are increasing. As a result, the frequency and extent of fungicide usage is increasing rapidly and insensitivity to certain fungicides has been identified in several crop pathogens. Case studies of fungicide insensitivity on Ascochyta rabiei on chickpea, Mycosphaerella pinodes on field pea, Fusarium sambucinum and Phytophthora infestans on potato, Pythium violae, P. sulcatum and other species on carrot, and Botrytis cinerea on grapes and berry crops are examined. Fungicide manufacturers have taken action to protect the efficacy of high-risk fungicides by formulating them with active compounds with a different mode of action. Also, producers have been advised to rotate fungicides with different modes of action to minimize the risk of insensitivity. Monitoring for fungicide insensitivity is central to early warning of an impending issue with insensitivity, for disease risk prediction and for avoiding disease control failures. In addition, information on insensitivity at the population level could be used to develop and implement insensitivity management programmes.
Résumé
À partir d’exemples provenant des grandes cultures, des cultures légumières et des cultures horticoles, ce compte rendu examine à quel point les changements dans l’utilisation des fongicides au Canada engendrent un risque accru d’insensibilité aux fongicides chez les populations d’agents pathogènes. La génération actuelle de fongicides systémiques est sélective ainsi qu’efficace et a réduit les effets néfastes des plus anciens fongicides de contacts sur l’environnement. Toutefois, plusieurs de ces nouveaux fongicides ont également accru le risque d’insensibilité à leur égard. Sur les Prairies canadiennes, une tendance à la spécialisation des productions dans certaines régions a concouru à limiter la rotation des cultures dans de nombreux champs, voire à l’éliminer, et les cibles de rendement augmentent. Par conséquent, la fréquence et l’ampleur de l’utilisation des fongicides s’accroissent rapidement et l’on a déjà signalé des cas d’insensibilité à certains fongicides chez plusieurs agents pathogènes. On examine donc des études de cas d’insensibilité aux fongicides chez Ascochyta rabiei sur le pois chiche, chez Mycosphaerella pinodes sur le petit pois, chez Fusarium sambucinum et Phytophthora infestans sur la pomme de terre, chez Pythium violae, P. sulcatum et d’autres espèces sur la carotte, et chez Botrytis cinerea sur le raisin et une variété de petits fruits. Les fabricants ont pris des mesures pour protéger l’efficacité des fongicides à haut risque en les préparant avec des composés actifs qui possèdent des modes d’action différents. De plus, on a conseillé aux producteurs d’alterner l’utilisation des fongicides possédant différents modes d’action afin de minimiser les risques d’insensibilité. La surveillance de l’insensibilité aux fongicides est essentielle à une alerte précoce relative à un problème imminent d’insensibilité, permettant de prédire un risque de maladies et d’éviter les échecs de lutte contre les maladies. En outre, on pourrait utiliser l’information sur l’insensibilité à l’échelle de la population pour élaborer et mettre en place des programmes de gestion de l’insensibilité.
Introduction
The purpose of this review is to examine how recent changes in fungicide usage in Canada are affecting the development of fungicide insensitivity in pathogen populations. Changes in usage pattern in the extensive production systems of field crops on the Canadian prairies have been particularly rapid in recent years, but examples are drawn from field, vegetable and horticultural crops.
Crop producers have been managing plant diseases since plants were domesticated. Fungicides were added to the arsenal of management strategies in the 1800s with the introduction of Bordeaux mixture. Insensitivity to fungicides was first detected in the 1970s (benzimidazol). In recent years, an increasing emphasis on food quality has made disease reduction via in-crop application of fungicides an integral component of the production system for many crops. At the same time, expansion of international trade and travel has increased the frequency of introduction of new plant pathogens/strains into Canada and production areas around the world. Climate change may also be contributing to changes in disease distribution and in the frequency of fungicide applications needed to protect a crop in a competitive marketplace. Finally, the importance of plant pathogens and pesticide residues in crops as a barrier to trade is increasing. As a major exporter of crops, changes that can potentially affect exports are of particular concern to the agricultural industry in Canada.
Contact fungicides such as mancozeb and chlorothalonil, which remain on the exterior of the plant, are generally older chemistries that have activity against a broad spectrum of plant pathogens. Systemic fungicides are taken up by the crop and transported within the plant to some degree. Systemic fungicides are, with a few exceptions, newer chemistries that are active against a narrower range of pathogens. Contact fungicides interfere with multiple metabolic pathways in the pathogen, while systemics generally affect a specific site in a specific pathway and so are less toxic to non-target organisms (many are classified as ‘reduced risk’ pesticides). Contact, broad-spectrum fungicides are generally applied at higher rates but are less expensive per application than the newer reduced-risk products. Also, contact fungicides are often less effective because they need to be in place before the pathogen arrives, so any plant tissue that is not thoroughly covered (e.g. a newly emerged leaf) is not protected (Brent & Hollomon Citation2007; Mueller et al. Citation2013).
The site-specific mode of action of systemic fungicides has a strong up-side, because their potential for a negative impact on health, safety and the environment is reduced. However, single-site-specific activity can also be an important drawback, because the pathogen may rapidly develop insensitivity to a specific active ingredient, or an entire class of actives. How quickly this occurs depends on the pattern of usage and the ability of the pathogen to develop insensitivity without sacrificing virulence and aggressiveness (FRAC Citation2011). There is no evidence of insensitivity to contact fungicides from field studies (Gisi & Sierotzki Citation2008), and so these fungicides have maintained their efficacy over time (Brent & Hollomon Citation2007).
One example of a class of active compounds with a high risk of pathogen insensitivity is strobilurin fungicides. Strobilurins bind at the Qo site of cytochrome b in the cytochrome bc1 enzyme complex (and so are known as Qo inhibitors, or QoIs) found in the mitochondrial membranes of fungi (Bartlett et al. Citation2002). The site-specific mode of action of these active compounds increases the potential for insensitivity to develop in pathogen populations (Koller Citation1991). Many fungal species are reported to have dramatically reduced levels of sensitivity to strobilurin fungicides associated with substitution of a single amino acid in the cytochrome b site (Chin et al. Citation2001; Pasche et al. Citation2005; Grasso et al. Citation2006). Insensitive isolates generally tolerate very high levels of fungicide (Ypema & Gold Citation1999; Avila-Adame & Koller Citation2003; Mondal et al. Citation2005), resulting in complete loss of disease control when these fungicides are used as solo products (Gisi et al. Citation2000).
The risk of a pathogen population developing insensitivity to a high-risk fungicide is a cumulative function of several key indicators: (i) genetic diversity of the pathogen; (ii) potential for rapid production and dispersal of pathogen propagules; and (iii) need for repeated application of the fungicide to provide disease management each year (Brent & Holloman Citation2007). Polycyclic pathogens generally produce propagules rapidly, and windborne spores ensure rapid dispersal. Production systems with large fields and low diversity (within crop and over time) generally require more frequent application of fungicide than systems with high diversity. Insensitivity in closely related species is another strong indicator of risk of insensitivity to that class of compounds. Additional information on these relationships is available at the FRAC website (FRAC Citation2011).
Fungicide efficacy can be substantially reduced by the development of insensitivity. Since the fungicide still effectively controls sensitive isolates, the frequency of insensitive isolates increases under the selection pressure imposed by each additional application, until the fungicide no longer controls the disease (Ma & Michailides Citation2005). There are two types of insensitivity: quantitative and qualitative. In quantitative insensitivity, the population becomes less sensitive to each subsequent application of the fungicide, but high rates of fungicide are still effective. In qualitative insensitivity, isolates are almost completely insensitive and control is no longer possible at label rates.
There can be a temptation to blame all instances of disease control failure on loss of fungicide efficacy caused by insensitivity. Factors that could contribute to disease control failure include inappropriate application timing or rate, poor crop coverage and wrong or deteriorated product. Misidentification of the pathogen/problem can also result in disease control failures that might resemble pathogen insensitivity. For example, repeated use of strobilurin fungicides to prevent early blight of potato (Solanum tuberosum L.) caused by Alternaria solani Sorauer has contributed to an increase in the prevalence of brown spot disease. Brown spot is caused by A. alternata (Fr.:Fr.) Keissl., which produces similar foliar symptoms to early blight, but is insensitive to strobilurins (Pasche et al. 2003).
There are also instances where disease control failures resulted from increased biological degradation of the active ingredient in soil, rather than a change in pathogen sensitivity. For example, enhanced microbial degradation following application of the carboximide fungicides vinclozolin and iprodione resulted in loss of control of soilborne pathogens such as Sclerotium cepivorum (Walker et al. Citation1986; Mitchell & Cain Citation1996). Enhanced degradation of metalaxyl has also been identified and related to failure to control cavity spot of carrot (Farrar et al. Citation2002; Hiltunen & White Citation2002).
Loss of sensitivity to a fungicide has often been assessed based on reduction in mycelium growth ( and ) or spore germination of the target fungus in the presence of a discriminant dose of the fungicide on agar media. However, these assessments are slow and time-consuming, and often represent an obstacle to processing large numbers of samples. Insensitivity to fungicide can also be monitored using conventional or real-time PCR to detect single nucleotide polymorphisms (SNPs) that indicate insertion, replacement or deletion of specific nucleic acids that are associated with fungicide insensitivity (Van Der Heyden et al. Citation2014). Use of these molecular tools has increased sampling capacity and effectiveness, and allowed research to be conducted on populations rather than isolates. Moreover, molecular assays can be used to identify the molecular basis for insensitivity at the SNP level to obtain genotypic patterns of insensitivity. This facilitates study of sub-population dynamics (Ma & Michailides Citation2005; Van Der Heyden et al. Citation2014). Information on the frequency of isolates carrying different SNPs in a fungal population is important because the SNPs may differ their impact on pathogen fitness and level of insensitivity (Veloukas et al. Citation2011; Billard et al. Citation2012).
Recent changes in fungicide usage
Research into crop diversification in the 1970s and 1980s identified several crops with potential for western Canada. The enthusiastic uptake of these new crops by growers resulted in canola and pulses (field pea, lentil and chickpea) becoming important components of the production system on the Canadian prairies, which had previously been dominated by cereals, forages and summer fallow. Diverse cropping rotations, supplemented with genetic disease resistance, became the main mechanism of disease management (Krupinsky et al. Citation2002), supplemented with occasional application of foliar fungicides (). The longer intervals between a particular crop that are possible in diverse cropping rotations have been shown to reduce the impact of many important field crop diseases in Canada (Bailey et al. Citation2000, Citation2001; Holm et al. Citation2006; Kutcher et al. Citation2013; Peng et al. Citation2014). However, a trend towards specialization of production within regions has resulted in short (or even no) rotation in many areas (Kutcher et al. Citation2013). Also, yield targets have increased following a number of years of high commodity prices, and fungicide usage has increased rapidly in response. From 1996 to 2006, the mean proportion of conventional crop land on the Canadian prairies that received at least one application of foliar fungicide increased from 7% to 11%. However, the proportion of the area treated with a fungicide increased to 23% in 2011, and this proportion is continuing to increase rapidly (T.K. Turkington, personal communication). In contrast, the mean proportion of agricultural land that received an application of foliar fungicide in Ontario increased from 8% in 1996 to 11% in 2006, which reflected a similar pattern to the prairies, but was only 17% in 2011 (). This indicates that the rapid increase in fungicide usage in Saskatchewan and Manitoba may not be occurring as quickly in Ontario.
Table 1. Changes in the proportion of the area under conventional field crop management1 that was treated with fungicide each year, based on Canadian Census of Agriculture figures.
In vegetable crops, disease pressure has steadily increased as intensive crop production has expanded. Increasing emphasis on quality has made disease management through application of fungicides critical in sustaining growth and delivering a competitive product. As a result, fungicides are applied frequently. Contact fungicides are often preferred in vegetable crops because they are (usually) less expensive and have a broad spectrum of activity. For some vegetable crops, contact fungicides are the only materials that were registered for use. However, many vegetable crops have a dense crop canopy or other growth habit that reduces access by the contact fungicides, so use of a systemic fungicide can provide more effective disease reduction.
In grape and berry crops, flowers are important sites for infection by Botrytis cinerea Pers.:Fr. (Jarvis Citation1977). After infection, the pathogen can remain quiescent for several weeks. Near harvest, when the sugar content of berries increases, B. cinerea rapidly invades mature fruits, causing complete loss of infected berries. As a result, fungicide applications are required during the flowering period and, to a lesser extent, near harvest. An application of fungicide is generally recommended for grapes at the beginning of flowering, 80% cap fall, and at bunch closure. Application for berry crops is recommended at the beginning of the flowering period (10% bloom), at full bloom (first green berries) and at the end the flowering period. During warm wet seasons, a fourth fungicide application could be required a few days before harvest. Growers are moving from reliance on multi-site fungicides to single-site fungicides. However, this increases the risk that fungicide insensitivity will result in failure of disease control.
Case Study 1. Fungicide insensitivity in pathogens of pulse crops
Ascochyta blight on chickpea
Chickpea (Cicer arietinum L.) is a high-value, drought-tolerant, nitrogen-fixing legume crop adapted to the driest region of the Canadian prairies (Chongo & Gossen Citation2001). Ascochyta blight, caused by Ascochyta rabiei (Pass.) Labrousse [teleomorph Didymella rabiei (Kovachevski) Arx], is the most important constraint to chickpea production in the region (Gan & McDonald Citation2002) and is important around the world (Nene & Reddy Citation1987). Management of ascochyta blight relies on partial crop resistance (Ahmed et al. Citation2006), crop rotation and tillage (Gossen & Miller Citation2004) and application of fungicides (Reddy & Singh Citation1990; Gan et al. Citation2006).
Strobilurin fungicides are highly effective against ascochyta blight and so were widely and repeatedly used when they first became available for use on chickpea in Canada in 2003. However, the pathogen was at high risk for developing insensitivity to strobilurins because it reproduces sexually by means of wind-borne ascospores (Armstrong et al. Citation2001), is genetically diverse (Chongo et al. Citation2004) and repeated applications of foliar fungicides are required to provide acceptable disease management each year (Reddy & Singh Citation1990; Gan et al. Citation2006). Also, insensitivity to a strobilurin (QoI) fungicide had already been reported in a related pathogen, Didymella bryoniae (Fuckel) Rehm (Olaya & Holm Citation2001; Stevenson et al. Citation2002). Fungicide insensitivity can be dispersed rapidly across large areas via airborne ascospores (Torianni et al. 2008), so the presence of airborne spores made the risk of rapid breakdown of disease management a regional issue rather than a local issue.
Insensitivity to QoI fungicides in A. rabiei was first reported from baseline studies (isolates that were never exposed to QoI fungicides) in western Canada in 2004 (Gossen & Anderson Citation2004) and a rapid shift to insensitivity to QoIs occurred across large areas of the Northern Great Plains in 2007 (Chang et al. Citation2007; Gossen, Chang et al. Citation2008; Wise et al. Citation2008, Citation2009; Thaher Citation2011). The life science industry in western Canada was informed immediately that populations of A. rabiei in the region were no longer sensitive to QoIs (Gossen, Chang et al. Citation2008, Citation2009). As a result, one company removed this use from their QoI label, and replaced their strobilurin fungicide with a dual-action fungicide for ascochyta blight management across North America. Extension and industry specialists passed the information to retailers and crop advisors in Canada and the northern USA. Most producers across the region changed their fungicide selections in response to this timely warning, and levels of ascochyta blight remained low to moderate in 2007–2012 (Bassendowski & Gossen Citation2011, Citation2012).
Mycosphaerella blight on field pea
Mycosphaerella blight, caused by Mycosphaerella pinodes (Berk. & Blox.) Vestergr (anamorph Ascochyta pinodes (Berk. & Blox.) Jones), is an important disease of field pea (Pisum sativum L.) in western Canada and around the world (Davidson & Ramsay Citation2000; Banniza & Vandenberg Citation2003; Bretag et al. Citation2006). Yield losses in Canada can be as high as 50% (Conner et al. Citation2007). Management of mycosphaerella blight requires repeated fungicide application each year (Beasse et al. Citation2000; Warkentin et al. Citation2000). QoI fungicides have excellent efficacy against this pathogen and so are widely used. Mycosphaerella pinodes was identified as being at a moderate to high risk of developing insensitivity to QoI fungicides because it is genetically diverse (Xue et al. Citation1998), is a polycyclic pathogen with windborne spores and populations of several closely related pathogens are insensitive to QoIs (Grasso et al. Citation2006; Keinath Citation2009).
Based on this risk assessment, isolates of M. pinodes were collected from across the Northern Great Plains and compared with a baseline group with no previous contact with QoIs. All of the baseline isolates were sensitive to QoIs. Of the 324 new isolates assessed, all of the isolates collected from the USA were sensitive but 19 isolates from Canada were insensitive. The mean EC50 of the insensitive isolates was 180 mg L−1, corresponding to 65× the label rate (Bowness Citation2013). Extension specialists and the life science industry in Canada were advised of the potential problem (Bowness et al. Citation2011, Citation2012). As a result, recommendations have generally changed from single-action QoI fungicides to dual-action fungicides, which are less prone to loss of sensitivity. Fortunately, QoIs are not used as intensively on field pea as on chickpea, so a rapid shift to insensitivity is not anticipated.
Other pathogens of concern
Almost all of the research on fungicide insensitivity on field crops on the Canadian prairies has focused on pathogens of pulse crops, but there is an old example of fungicide insensitivity on alfalfa (Medicago sativum L.) grown for seed (Gossen et al. Citation2001). Pulse crops represent a relatively small component of the production area in the region, even though they are grown on millions of hectares each year. Canola (Brassica napus L.) has become the largest crop in the region, surpassing wheat. The level of fungicide insensitivity in important pathogens such as Leptosphaeria maculans (Desmaz.) Ces. & De Not. (blackleg) on canola and Fusarium graminearum Schwabe (teleomorph Gibberella zeae (Schw.) Petch) (fusarium head blight) of wheat is not known. The rapid rate of increase in fungicide usage on field crops in the region indicates that insensitivity may quickly become an issue in these pathogens, if indeed it is not already resulting in losses in disease control.
Case Study 2. Fungicide insensitivity in pathogens of potato
Fusarium dry rot
Fungicide insensitivity is not a new issue in disease management in potato crops. For example, treatment of seed pieces with the benzimidazole fungicide thiophanate-methyl (TPM) and pre-storage applications of thiabendazole (TBZ) fungicide initially provided effective reduction in silver scurf caused by Helminthosporium solani Durieu & Mont. However, these fungicides were quickly rendered ineffective by fungicide insensitivity (Kawchuk et al. Citation1994). Benzimidazoles bind to the tubulin molecule to inhibit microtubule assembly and cell division. Although TPM does not belong structurally to the benzimidazoles, it acts via the alkyl benzimidazole carbamate, carbendazim. Insensitivity often occurs between actives, such as TBZ and carbendazim, which have related structures and a similar mechanism of action.
Dry rot of potato tubers associated with infection by Fusarium spp. can cause severe losses in tuber quality and yield in Canada. One of the pathogens that causes dry rot, F. sambucinum Fuckel (teleomorph Gibberella pulicaris), also produces trichothecene mycotoxins (Kawchuk et al. Citation2002). Benzimidazole fungicides were initially used to manage dry rot, with TBZ as a post-harvest treatment and TPM as a seed treatment (Holley & Kawchuk Citation1996), which is the same pattern of use that led to development of insensitivity to silver scurf.
Pathogen insensitivity to benzimidazoles was initially observed as a result of substantial increases in the incidence of dry rot and the loss of entire potato storages. Insensitivity to TBZ was found in F. sambucinum (), which is the dominant pathogen causing potato dry rot in Canada, but was rare in other Fusarium spp. on potato. The EC50 values for benzimidazole-insensitive isolates of F. sambucinum ranged between 34 and 71 mg L−1 for TBZ and > 2500 mg L−1 for TPM (Kawchuk et al. Citation1994; Holley & Kawchuk Citation1996). Production practices were quickly changed to avoid the use of TPM seed treatments. Also, the use of TBZ as a post-harvest treatment was limited to badly bruised tubers that were to be stored for more than several months and post-harvest TBZ was recommended only for farms experiencing dry rot caused by sensitive F. sambucinum or other Fusarium spp.
To characterize the benzimidazole insensitivity in F. sambucinum, homokaryons were derived from single ascospores from crosses of TBZ-insensitive with sensitive isolates. The β-tubulin gene was amplified from these homokaryons using degenerate oligonucleotides synthesized to conserved sequences of the β-tubulin gene derived from related fungi. An open reading frame containing three putative introns and a deduced amino acid sequence of 446 amino acids exhibited a high level of homology to the β-tubulin gene of other fungi. Although nucleotide and amino acid differences were observed in the β-tubulin gene, none could be linked to TBZ insensitivity (Kawchuk et al. Citation2002). Linkage analysis confirmed that, unlike many other plant pathogens, the isolated β-tubulin gene was single copy and was not linked to TBZ insensitivity in F. sambucinum. The mechanism of benzimidazole insensitivity in F. sambucinum remains unknown.
Insensitivity to TBZ was expected to persist in the F. sambucinum population even after application of TBZ was discontinued, because insensitive isolates have similar growth rates and virulence/aggressiveness as sensitive isolates. Current management recommendations are that fungicides with different modes of action should be applied in succession to minimize exposure to a single active ingredient. Also, these fungicides should be used in combination with a contact fungicide whenever possible. In addition, management of dry rot should include production and storage practices that minimize the incidence of dry rot, e.g. preventing tuber damage, which is required for infection by Fusarium spp. (Lynch et al. Citation2003).
Late blight
Late blight caused by Phytophthora infestans Mont. (de Bary) is an important disease of potato worldwide, and can also infect tomato and other members of the Solanaceae. Repeated fungicide application over the growing season is required to reduce losses, but worldwide annual losses are still estimated to be in the billions of dollars (Kawchuk et al. Citation2014).
Genotypes of P. infestans have shown a propensity for developing insensitivity to certain site-specific fungicides (Hu et al. Citation2012; Kalischuk et al. Citation2012; Wijekoon et al. 2013). Prior to 1993, the phenylamide fungicide mefenoxam (the R-enantiomer of metalaxyl) was effective against late blight. Mefenoxam inhibits sporulation and mycelium growth inside host tissues by inhibiting RNA polymerase-1, so mutations that change the affinity of this enzyme can result in insensitivity to this fungicide (Davidse et al. Citation1983). In 1993, the mefenoxam-insensitive genotypes US-7 and US-8 displaced sensitive isolates (Goodwin et al. Citation1998) and US-8 has continued to predominate in many production areas.
The genotypes US-8 and US-20 are consistently insensitive to mefenoxam, while genotypes US-22, US-23 and US-24 were initially highly sensitive to mefenoxam (). However, recent studies have demonstrated mefenoxam insensitivity among these previously sensitive genotypes (Hu et al. Citation2012; Kalischuk et al. Citation2012; Wijekoon et al. 2013). Recent epidemics of late blight in Canada and the USA have occurred in large part as the result of insensitivity to mefenoxam and long-distance movement of P. infestans on infected plant material (Hu et al. Citation2012; Kalischuk et al. Citation2012; Fry et al. Citation2013; Wijekoon et al. 2013).
Many new systemic fungicides with activity against late blight have been identified. Fungicides with different modes of action are being used in rotation with, or co-applied with, contact fungicides such as chlorothalonil or mancozeb. Use of mefonoxam has been reduced, and co-application of mefenoxam with a contact fungicide has become a standard practice. This change in usage pattern has lowered the selection pressure favouring insensitive strains and slowed the development of insensitivity, and has been associated with the recurrence of mefenoxam-sensitive strains (Grünwald & Flier Citation2005).
The latest generation of fungicides represent promising components in an integrated management strategy for late blight (Lobato et al. Citation2008). For example, phosphites stimulate plant defence responses, are active against oomycetes in vivo, and have a low potential impact on the environment. These fungicides have important implications for late blight management at a time when fungicide insensitivity in P. infestans is rapidly increasing (Peters et al. Citation2014).
Case Study 3. Fungicide insensitivity in cavity spot on carrot
Cavity spot is an important root disease of carrot in many parts of the world. The disease is characterized by superficial, elliptical brown lesions on the root surface, which may also be associated with vertical cracks. The presence of more than two or three small lesions makes the carrot crop visually unacceptable for the fresh market. The lesions are also a problem on processing carrots because they are not easily removed in the peeling process.
Cavity spot is a disease complex caused by several species of Pythium. Worldwide, P. violae Chesters & Hickman and P. sulcatum Pratt & Mitchell are the most common species, but P. sylvaticum Campbell & Hendrix and several others are also commonly isolated (Hiltunen & White Citation2002). Identification of the pathogens associated with cavity spot in a region is important because species differ in host range and also vary in sensitivity to metalaxyl fungicide (Hiltunen & White Citation2002). For example, P. sulcatum was less susceptible to metalaxyl than P. violae (White Citation1988). In Ontario, P. sulcatum, P. irregulare, P. violae and P. ultimum are the species most commonly isolated from cavity spot lesions (McDonald Citation1994; M. Tesfaendrias, personal communication). In Quebec, P. sulcatum and P. sylvaticum are most common, but P. macrosporum Vaartaja & van der Plaats-Niterink and several other species are also present (Allain-Boule et al. Citation2004).
The recommended cultural practices for management of cavity spot include avoiding highly infested fields and selecting carrot cultivars that are less susceptible to the disease. Most commercial cultivars are susceptible (Lu et al. Citation2012; Saude et al. Citation2014) and crop rotation is generally not effective (Hiltunen & White Citation2002; Lu et al. Citation2012). Application of the fungicide metalaxyl or its R-enantiomer, mefanoxam, is the standard practice for managing cavity spot. The fungicide is applied at seeding or early in the growing season in Canada, but timing of application varies in different regions of the world (Hiltunen & White Citation2002).
There have been many reports of partial or complete failure of metalaxyl to control cavity spot. One source of failure in disease management may be that metalaxyl/mefenoxam is not effective against the Pythium spp. that predominates at the site. For example, P. sulcatum is less sensitive to metalaxyl than P. violae in the UK. A pre-plant soil test was developed to identify the Pythium spp. present in a field, so that growers could assess their risk of cavity spot (Hiltunen & White Citation2002). Also, Pythium isolates from diseased carrots in Michigan and California were evaluated for sensitivity to mefanoxam and two new fungicides (zoxamide and fluopicolide) with activity against Oomycetes. These new fungicides were not registered for use on carrots, so pathogen populations had not been exposed to these actives. Lack of activity against an isolate would therefore be a characteristic of the pathogen species, rather than the result of development of insensitivity from selection pressure. All of the species were sensitive to zoxamide and most were sensitive to mefanoxam. Each isolate of P. ultimum, P. sylvaticum and P. violae was sensitive to fluopicolide, but P. dissocticum, P. irregulare and P. sulcatum were not sensitive (Lu et al. Citation2012). Similarly, isolates of P. sulcatum, P. sylvaticum and P. macrosporum from Quebec were sensitive to metalaxyl and zoxamide, but the isolates of P. macrosporum showed a wide variation in sensitivity to zoxamide (Martinez et al. Citation2005). This indicates that a fungicide might be highly effective at one production site, but much less effective at another site, based on differences in the species that are dominant at the sites. Also, species that are not sensitive to a fungicide would be expected to proliferate in a particular field or region with repeated application of that fungicide. It is important to note, however, that the complex interactions that affect resource utilization and microbial ecology in soil may act as a counter-balance to this selection pressure, ensuring that the proportions of pathogenic Pythium spp. at a site change slowly in many situations.
Some failures of metalaxyl/mefanoxam to control cavity spot of carrot result from reduced persistence of the fungicide caused by enhanced microbial degradation of the active ingredient. For example, enhanced degradation of metalaxyl was identified in a soil that had been treated with metalaxyl six times over 14 years (Hiltunen & White Citation2002). Rapid degradation of mefenoxam was also observed in fields in California that had received repeated applications of the fungicide compared with fields where no fungicide had been applied (Farrar et al. Citation2002). In both instances, the pathogen population remained sensitive to metalaxyl, so the breakdown in cavity spot management was not related to insensitivity to the fungicide. Instead, the active compound was degraded so rapidly in soil that it was no longer effective at normal rates of application (Fararr et al. 2002; Hiltunen & White Citation2002). Enhanced degradation may be a major cause of disease control failures for these products.
Finally, fungicide insensitivity may also cause failures in control of cavity spot. In the study of isolates from Michigan and California, a few isolates of P. irregulare were highly insensitive to mefanoxam and some isolates of P. intermedium and P. sylvaticum had intermediate insensitivity. One of 116 isolates of P. violae had intermediate insensitivity to mefanoxam (Lu et al. Citation2012). However, an extensive study in England found no P. violae isolates that were insensitive to metalaxyl (Hiltunen & White Citation2002).
Several authors have noted the need for alternatives to metalaxyl to protect carrots from cavity spot (Hiltunen & White Citation2002; Martinez et al. Citation2005; Lu et al. Citation2012). The report that several Pythium spp. isolated from carrots in the USA were insensitive to fluopicolide, which is not registered for this use, represents a concern because it indicates that the efficacy of this active ingredient may not be durable. There is also evidence that repeated exposure to zoxamide (also not registered on carrots) can select for insensitivity in some Pythium spp. When isolates were grown on media amended with zoxamide and transferred 11 times, three isolates of P. sulatum developed some insensitivity and one isolate of P. sylvaticum developed a high level of insensitivity (> 900×) (Martinez et al. Citation2005). Interestingly, zoxamide effectively reduced cavity spot in an inoculated greenhouse trial with carrots grown in soil-less mix, but metalaxyl had no effect (Martinez et al. Citation2005). This lack of activity of metalaxyl was not related to insensitivity or enhanced degradation, and raised questions about other factors that affect efficacy, such as the fungicide binding to the organic matter in the peat-based growing medium (Martinez et al. Citation2005).
Currently, none of the genes associated with fungicide sensitivity in Pythium spp. have been identified and there is no information about how insensitivity affects pathogenic fitness. Thus, cultural methods for management of cavity spot, such as use of resistant cultivars, should be practiced where available. Also, banding of the fungicide over the row, to treat only a portion of the entire field, may reduce the rate of development of both fungicide insensitivity and enhanced degradation. Biological controls or products that enhance host resistance could also play a role in protecting carrots from cavity spot, but there has been no progress in these approaches to date.
Case Study 4. Fungicide insensitivity in Botrytis cinerea on grapes and berry crops
Fungicides that specifically target Botrytis spp. make up 10% of the world market in fungicides, with application to wine and table grapes representing 50% of this market segment (Dean et al. Citation2012). In Canada, both multi-site inhibitors and single-site fungicides are registered for management of botrytis diseases in stone fruits, grapes and berry crops. Multi-site inhibitors, such as chlorothalonil and dithiocarbamate, generally act by blocking enzymes related to respiration (Leroux et al. Citation2010). They are only effective if applied prior to infection (Van der Heyden et al. Citation2012). The single-site fungicides have a broader window of efficacy but have a moderate to high risk of development of insensitivity. Strobilurins and carboxamides affect mitochondrial respiration by inhibiting the activity of mitochondrial complex ІІІ and ІІ, respectively (Leroux et al. Citation2010). Dicarboximides affect osmoregulation by inducing lipid peroxidation, membrane destruction and over-production of reactive oxygen species. Sterol biosynthesis inhibitors affect C-4 demethylation during ergosterol biosynthesis (Leroux et al. Citation2010).
For the most part, information about fungicide insensitivity is acquired through surveys conducted at various spatial scales (farm, region, province or country). As with any survey, the precision of fungicide insensitivity estimation increases with increasing number of samples assessed (Van Der Heyden et al. Citation2014).
Inventory of phenotypic insensitivity
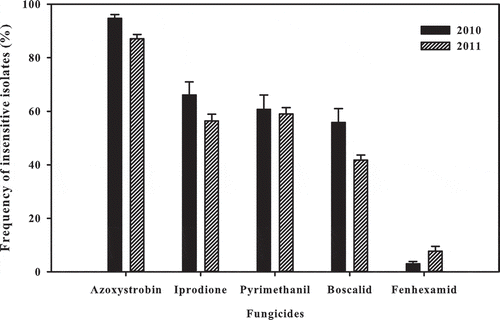
In 2010 and 2011, a total of 230 samples were collected in 18 vineyards in the province of Quebec. Spores from 10 infected berries were collected from each vineyard. Each berry was selected because it carried a single sporulating colony of B. cinerea, and the spores were collected using individual dry BD-BBL culture swabs (Fisher Scientific, Withby, ON). For each fungicide (azoxystrobin, iprodione, pyrimethanil, boscalid, fenhexamid), a discriminant dose was added to the media following Leroux et al. (Citation2010). The assays were conducted on 1.3% agar media, except for boscalid, where the assays were conducted on PS media (agar plus 4 g of sodium succinate, 2 g of K2HPO4 and 2 g of KH2PO4). For each sample, 0.3 mL of a spore suspension was spread over the surface of the agar medium and incubated at 19 °C in the dark for 24 h. The proportion of germinated spores was calculated based on assessment of 100–200 spores per treatment. Only spores with a germ tube at least twice the average length of non-germinated spores were considered to have germinated.
The incidence of fungicide insensitivity was relatively constant over the 2 years (). When calculated across years, 91% of isolates were insensitive to azoxystrobin, 61% to iprodione, 60% to pyrimethanil, 49% to boscalid and 5% to fenhexamid. The most important result was that the level of fungicide insensitivity in B. cinerea isolates in Québec vineyards was >40% for most of the fungicides tested. Despite the high level of insensitivity identified in these studies, the relationship between insensitivity in isolates and breakdown of disease management in the field is not clear. Research to identify thresholds for disease control failures is needed.
Fig. 1 (Colour online) Isolates of Fusarium sambucinum recovered from diseased potatoes and grown on potato dextrose agar plates supplemented with 10 mg L−1 thiabendazole to assess fungicide sensitivity. Isolates sensitive (A) or insensitive (B) to benzimidazole fungicides were recovered in similar ratios over a 20-year period.
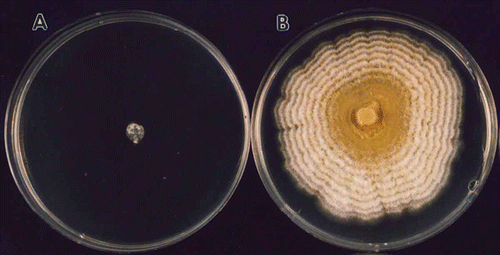
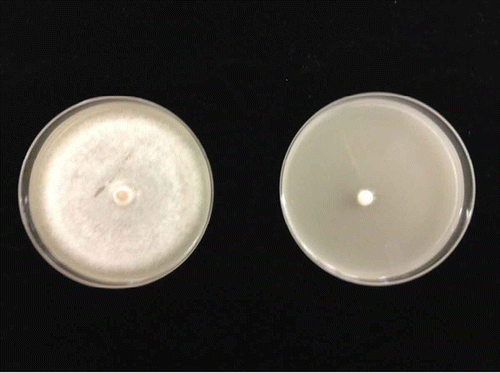
Fig. 2 (Colour online) Isolates of Phytophthora infestans genotype US-23 grown on V8-potato dextrose medium supplemented with 5 mg L−1metalaxyl. Almost all of the Canadian isolates of P. infestans genotype US-23 were sensitive to metalaxyl (right) in 2010, but had been replaced by insensitive isolates (left) by 2013v.
Inventory of genotypic insensitivity
Isolates of B. cinerea were collected from vineyards and berry fields in Nova Scotia, Québec, Ontario and British Columbia in 2012 as described previously. Each swab was used to inoculate solid media amended with novobiocin (100 μg mL−1) and tetracycline (50 μg mL−1) for purification and conservation. DNA extraction was performed using a commercial isolation kit (MP Biomedicals, Solon, OH). Isolates that were insensitive to the fungicides assessed in the phenotypic screening were identified with RFLP-PCR and PIRA-PCR assays using published primers (Veloukas et al. Citation2011). The detection of mutation F412wt-I-S-V related to fenhexamid insensitivity was performed using an ASPPAA assay (Billard et al. Citation2012).
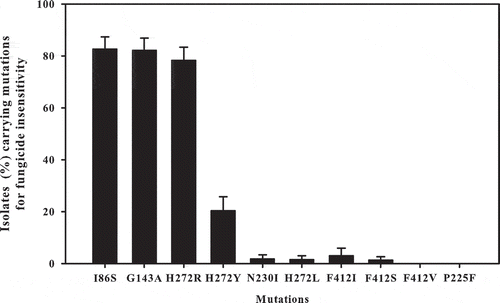
The result of the genotypic survey was similar to that from the phenotypic assessments. The frequency of isolates carrying mutations related to fungicide insensitivity was 82% for insensitivity to azoxystrobin (mutation G143A), 83% to iprodione (I86S), 80% to boscalid (H272R, H272Y, H272L, N230I, P225F), and 3% to fenhexamid (F412I, F412S, F412V) ().
Fig. 4 Proportion of single nucleotide polymorphisms (SNPs) associated with specific fungicide insensitivity in Botrytis cinerea isolates collected from vineyards across Quebec in 2012. Capped lines indicate standard error.
Overall, the proportion of B. cinerea isolates carrying mutations related to fungicide insensitivity was 71% to azoxystrobin, 57% to iprodione, 44% to boscalid mutation H272R, 20% to boscalid H272Y, 1% to boscalid N230I, 3% to boscalid H272L, 0.1% to boscalid P225F and 4% to fenhexamid (). The pattern of insensitivity was generally similar among the provinces, except that the proportion of isolates carrying mutation G143A for azoxystrobin was higher in British Columbia and Ontario (94%) than in Quebec and Nova Scotia (66%) and the proportion of isolates carrying mutation I86S for iprodione was higher in Ontario (80%) than in the other provinces (49–64%). This pattern was similar across the four crops surveyed (cherry, grape, strawberry and raspberry), with a higher proportion of insensitivity in cherry and grapes ().
Fig. 5 Proportion of single nucleotide polymorphisms (SNPs) associated with specific fungicide insensitivity in Botrytis cinerea isolates collected from cherry, grape, strawberry and raspberry fields in Nova Scotia, Quebec, Ontario and British Columbia in 2012. Capped lines indicate standard error.
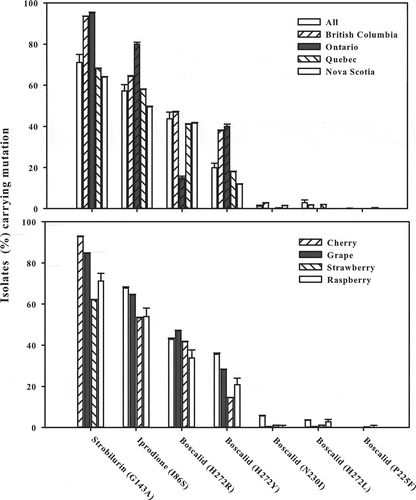
Recent developments in molecular detection of fungicide insensitivity have enhanced the surveillance and monitoring capacities of researchers and crop advisors. Estimates of insensitivity levels provided by classical phenotypic assays were generally similar to those obtained with PCR-based assays of specific SNPs in B. cinerea. The benefit of using molecular tools to test for fungicide insensitivity is not only to provide insight on the specificity of a polymorphism towards a particular insensitivity phenotype, but also to provide a more precise characterization in terms of fitness and the practical implications of insensitivity. For example, each of the five known SNPs for insensitivity to boscalid were found in each province. Mutation H272R was by far the most frequent (44% of isolates), followed by H272Y (20%). The three other mutations occurred at a low frequency. The value of the insensitivity factor associated with the two dominant SNPs is low to intermediate, while the other SNPs confer a much higher degree of insensitivity (Veloukas et al. Citation2011; Yin et al. Citation2011). Field insensitivity is influenced by the proportion of insensitive individuals carrying SNPs associated with a low, intermediate or high insensitivity factor, so the proportion of specific SNPs in the population has a very important practical significance for field insensitivity. Interpreting the results of insensitivity assays based on the frequency of each SNP may lead to a more accurate estimation of the potential impact of this insensitivity on practical disease management.
Summary and conclusions
In this review, insensitivity to fungicides was shown to be an important issue in crop production across Canada. Also, the need for management of insensitivity via rotating or tank mixing products with different modes of action, monitoring pathogen populations for insensitivity, and identifying alternatives to fungicide application, was a consistent factor across crop types and regions.
Case Study 1 demonstrated that fungicide usage has increased rapidly on the Canadian prairies in response to widespread use of short rotations, in combination with high yield targets for field crops. This represents a very substantial change in the production system. Also, many of the newer single-site-of-action fungicides have a moderate to high risk of fungicide insensitivity, unlike the older contact active compounds, which have a very low risk of insensitivity. As a result of increased usage and increased risk, insensitivity to certain fungicides has already been identified in several pathogen populations and other fungicides are at risk. Manufacturers have moved to protect the efficacy of several high-risk fungicides by formulating them together with active compounds that have a different mode of action. Another approach is for producers to rotate fungicides with different modes of action to minimize the risk of development of insensitivity.
Minimizing the need for fungicide application through crop rotation, utilization of genetic resistance and use of agronomic packages that optimize crop growth will also reduce the risk of fungicide insensitivity. However, monitoring for insensitivity is crucial to identifying the onset of insensitivity, its distribution and evolution, and to identify factors that influence insensitivity. As such, monitoring is an essential first step in the design and implementation of management strategies to minimize insensitivity.
Case Study 2 described situations that represent the status quo for fungicide sensitivity of vegetable crops in Canada. Fungicide usage is high in many vegetable crops, where visual quality is a critical component in a competitive market. These are crops where producers are aware that the normal patterns of fungicide usage can result in development of fungicide insensitivity, and new fungicides are routinely being sought to replace classes of fungicides when their efficacy is lost to insensitivity. For most vegetable crops, rotation of fungicides together with crop rotation generally provides effective disease management, without the need for rapid response to changes in pathogen insensitivity within a growing season.
In Case Study 3, many of the reported instances of breakdown in management of cavity spot on carrots are likely caused by incorrect identification of the causal agent and/or enhanced degradation of the fungicide. However, insensitivity also contributed to management failures in some situations, and rotation of fungicides with different modes of action is currently not an option. Cavity spot on carrot appears to be a system where use of molecular tools for rapid identification of the pathogen(s) at a site, combined with assessment of the presence of mutations for insensitivity, could be used to reduce the risk of management failures.
Case Study 4 of fungicide insensitivity in vineyards and berry crops represents a situation where insensitivity has developed against many of the most common fungicides. Molecular assessments are being used to make disease management using fungicides possible. A very high proportion of the production area receives repeated application of fungicide throughout the growing season, and there is no opportunity to use crop rotation to reduce disease pressure on the crop. Information about the status of fungicide insensitivity is used for fungicide selection and to assess the need to implement insensitivity management strategies. Researchers and crop advisors are focusing on techniques to screen for fungicide insensitivity in real time, to provide producers with recommendations for selecting effective fungicides for their situation and on how best to manage insensitivity.
Management of fungicide insensitivity is important for growers and agribusiness because it affects yield, stability of production and economic sustainability. Information on the level of insensitivity is essential for making informed disease-management decisions. Management of fungicide insensitivity is also indirectly important to consumers because it can affect quality and product availability. Fungicide insensitivity has been an important issue for agriculture in Canada for many years, but its importance is increasing as fungicide usage becomes the norm in field crops, as it is already for vegetable and horticultural crops. Monitoring high-risk pathogens of field crops to identify the development of insensitivity is clearly required, before this change in production practice results in large-scale breakdown of disease control.
Acknowledgements
The authors thank the many colleagues who provided isolates and diseased specimens for our studies on fungicide insensitivity. We also thank the Agriculture Development Fund of Saskatchewan, Saskatchewan Pulse Growers, Cavendish Farms, Conagra Limited Lamb-Weston Division, Potato Growers of Alberta, the Conseil pour le développement de l’agriculture du Québec, Canadian Agricultural Adaptation Program and AAFC for financial support of this research. Finally, thanks to the Canadian Phytopathological Society for supporting the symposium that formed the basis of this invited review.
References
- Ahmed HU, Chang KF, Hwang SF, Gossen BD, Howard RJ, Warkentin TD. 2006. Components of disease resistance in desi and kabuli chickpea varieties against ascochyta blight. Plant Pathol J. 5:336–342. doi:10.3923/ppj.2006.336.342
- Allain-Boulé N, Lévesque CA, Martinez C, Bélanger RR, Tweddell RJ. 2004. Identification of Pythium species associated with cavity-spot lesions on carrots in eastern Quebec. Can J Plant Pathol. 26:365–370. doi:10.1080/07060660409507154
- Armstrong CL, Chongo G, Gossen BD, Duczek LJ. 2001. Mating type distribution and incidence of the teleomorph of Ascochyta rabiei (Didymella rabiei) in Canada. Can J Plant Pathol. 23:110–113. doi:10.1080/07060660109506917
- Avila-Adame C, Koller W. 2003. Impact of alternative respiration and target-site mutations on responses of germinating conidia of Magnaporthe grisea to Qo-inhibiting fungicides. Pest Manage Sci. 59:303–309. doi:10.1002/ps.638
- Bailey KL, Gossen BD, Lafond GP, Watson PR, Derksen DA. 2001. Effect of tillage and crop rotation on root and foliar diseases of wheat and pea in Saskatchewan from 1991 to 1998: univariate and multivariate analyses. Can J Plant Sci. 81:789–803. doi:10.4141/P00-152
- Bailey KL, Johnston AM, Kutcher HR, Gossen BD, Morrall RAA. 2000. Managing crop losses from foliar diseases with fungicides, rotation, and tillage in the Saskatchewan Parkland. Can J Plant Sci. 80:169–175. doi:10.4141/P99-069
- Banniza S, Vandenberg A. 2003. The influence of plant injury on development of Mycosphaerella pinodes in field pea. Can J Plant Pathol. 25:304–311. doi:10.1080/07060660309507083
- Bartlett DW, Clough JM, Godwin JR, Hall AA, Hamer M, Parr-Dobrzanski B. 2002. The strobilurin fungicides. Pest Manage Sci. 58:649–662. doi:10.1002/ps.520
- Bassendowski KA, Gossen BD. 2011. Ascochyta blight on chickpea in Saskatchewan, 2009 and 2010. Can Plant Dis Surv. 91:127.
- Bassendowski KA, Gossen BD. 2012. Ascochyta blight on chickpea in Saskatchewan, 2011. Can. Plant Dis. Surv. 92:133.
- Beasse C, Ney B, Tivoli B. 2000. A simple model of pea (Pisum sativum) growth affected by Mycosphaerella pinodes. Plant Pathol. 49:187–200. doi:10.1046/j.1365-3059.2000_t01-1-00432.x
- Billard A, Laval V, Fillinger S, Leroux P, Lachaise H, Beffa R, Debieu D. 2012. The allele-specific probe and primer amplification assay, a new real-time PCR method for fine quantification of single-nucleotide polymorphisms in pooled DNA. Appl Env Microbiol. 78:1063–1068. doi:10.1128/AEM.06957-11
- Bowness R. 2013. Sensitivity of Mycosphaerella pinodes to pyraclostrobin and optimizing fungicide application in field pea [MSc dissertation]. Edmonton (AB): University of Alberta.
- Bowness R, Chang KF, Gossen BD, Goswami RS, Hwang SF, Willenborg C, Strelkov SE. 2011. Initial insensitivity response of Mycosphaerella pinodes isolates to pyraclostrobin fungicide. In Proceedings of the Plant Pathology Society of Alberta, 2011 November 7− 9, Edmonton (AB); p. 19.
- Bowness R, Chang KF, Gossen BD, Goswami RS, Hwang SF, Willenborg C, Strelkov SE. 2012. Insensitivity to pyraclostrobin fungicide in Mycosphaerella pinodes on the Northern Great Plains. Proceedings of the Canadian Pulse Research Workshop, 2012 November 6–9, Niagara Falls; p. 30.
- Brent KJ, Hollomon DW. 2007. Fungicide resistance: the assessment of risk. [Online]. Fungicide Resistance Action Committee, Monograph 2, Global Crop Protection Federation. Available from: http://www.frac.info/frac/index.htm.
- Bretag TW, Keane PJ, Price TV. 2006. The epidemiology and control of ascochyta blight in field peas: a review. Austr J Agric Res. 57:883–902. doi:10.1071/AR05222
- Chang KF, Ahmed HU, Hwang SF, Gossen BD, Strelkov SE, Blade SF, Turnbull GD. 2007. Sensitivity of field populations of Ascochyta rabiei to chlorothalonil, mancozeb, and pyraclostrobin fungicides, and effects of strobilurin fungicides on the progress of ascochyta blight of chickpea. Can J Plant Sci. 87:937–944. doi:10.4141/CJPS07019
- Chin KM, Chavaillaz D, Kaesbohrer M, Staub T, Felsenstein FG. 2001. Characterizing resistance risk of Erysiphe graminis f. sp. tritici to strobilurins. Crop Prot. 20:87–96. doi:10.1016/S0261-2194(00)00059-4
- Chongo G, Gossen BD. 2001. Effect of plant age on resistance to Ascochyta rabiei in chickpea. Can J Plant Pathol. 23:358–363. doi:10.1080/07060660109506956
- Chongo G, Gossen BD, Buchwaldt L, Adhikari T, Rimmer SR. 2004. Genetic diversity of Ascochyta rabiei in Canada. Plant Dis. 88:4–10. doi:10.1094/PDIS.2004.88.1.4
- Conner RL, Hwang SF, Woods SM, Chang KF, Bing DJ, Su H, McAndrew DW, Yager LM, Yager LM. 2007. Influence of agronomic traits on the expression of tissue-specific resistance to mycosphaerella blight in field pea. Can J Plant Sci. 87:157–165. doi:10.4141/P05-213
- Davidse LC, Hofman AE, Velthuis GCM. 1983. Specific interference of metalaxyl with endogenous RNA polymerase activity in isolated nuclei from Phytophthora megasperma f. sp. medicaginis. Exp Mycol. 7:344–361. doi:10.1016/0147-5975(83)90019-1
- Davidson JA, Ramsey MD. 2000. Pea yield decline syndrome in South Australia: the role of diseases and the impact of agronomic practices. Austr J Agric Res. 51:347–354. doi:10.1071/AR99111
- Dean R, Van Kan JAL, Pretorius ZA, Hammond-Kosack KE, Di Pietro A, Spanu PD, Rudd JJ, Dickman M, Kahmann R, Ellis J, Foster GD. 2012. The top 10 fungal pathogens in molecular plant pathology. Mol Plant Pathol. 13:414–430. doi:10.1111/j.1364-3703.2011.00783.x
- Farrar J, Nunez J, Davis RM. 2002. Repeated soil applications of fungicide reduce activity against cavity spot in carrots. Calif Agric. 56:76–79. doi:10.3733/ca.v056n02p76
- FRAC. 2011. Pathogens with field resistance towards QoI fungicides. [On-line]. Available from: http://www.frac.info/frac/index.htm. [accessed December 2011].
- Fry WE, McGrath MT, Seaman A, Zitter TA, McLeod A, Danies G, Small I, Myers K, Everts K, Gevens A, et al. 2013. The 2009 late blight pandemic in the eastern United States - causes and results. Plant Dis. 97:296–306. doi:10.1094/PDIS-08-12-0791-FE
- Gan YT, McDonald CL. 2002. Severity of ascochyta blight vs. leaf type of chickpea. In Proceedings of the 4th Canadian Pulse Crop Research Workshop, 2002 Dec., Edmonton (AB); p 270–272.
- Gan YT, Siddique KHM, MacLeod WJ, Jayakumar P. 2006. Management options for minimizing the damage by ascochyta blight (Ascochyta rabiei) in chickpea (Cicer arietinum L.). Field Crops Res. 97:121–134. doi:10.1016/j.fcr.2005.10.002
- Gisi U, Chin KM, Knapova G, Farber KR, Mohr U, Parisi S, Sierotzki H, Steinfeld U. 2000. Recent developments in elucidating modes of resistance to phenylamide, DMI and strobilurin fungicides. Crop Prot. 19:863–872. doi:10.1016/S0261-2194(00)00114-9
- Gisi U, Sierotzki H. 2008. Fungicide modes of action and resistance in downy mildews. Eur J Plant Pathol. 122:157–167. doi:10.1007/s10658-008-9290-5
- Goodwin SB, Smart CD, Sandrock RW, Deahl KL, Punja ZK, Fry WE. 1998. Genetic change within populations of Phytophthora infestans in the United States and Canada during 1994 to 1996: role of migration and recombination. Phytopathology. 88:939–949. doi:10.1094/PHYTO.1998.88.9.939
- Gossen BD, Anderson KL. 2004. First report of resistance to strobilurin fungicides in Didymella rabiei. Can J Plant Pathol. 26:411. [abstr.].
- Gossen BD, Chang KF, Hwang SF, McDonald MR. 2008. Change in sensitivity of Ascochyta rabiei to strobilurin fungicides in Saskatchewan, 2002–2006. Can J Plant Pathol. 30:384–385.
- Gossen BD, Hwang SF, Thaher NH, McDonald MR. 2009. Insensitivity of Ascochyta rabiei to strobilurin fungicide in Canada, 2007. Can J Plant Pathol. 31:486. [abstr.].
- Gossen BD, Miller PR. 2004. Survival of Ascochyta rabiei in chickpea residue on the Canadian prairies. Can J Plant Pathol. 26:142–147. doi:10.1080/07060660409507125
- Gossen BD, Peng G, Wolf TM, McDonald MR. 2008. Improving spray retention to enhance the efficacy of foliar-applied disease and pest management products in field and row crops. Can J Plant Pathol. 30:505–516. doi:10.1080/07060660809507550
- Gossen BD, Rimmer SR, Holley JD. 2001. First report of resistance to benomyl fungicide in Sclerotinia sclerotiorum. Plant Dis. 85:1206. doi:10.1094/PDIS.2001.85.11.1206C
- Grasso V, Palermo S, Sierotzki H, Garibaldi A, Gisi U. 2006. Cytochrome b gene structure and consequences for resistance to Qo inhibitor fungicides in plant pathogens. Pest Manage Sci. 62:465–472. doi:10.1002/ps.1236
- Grünwald NJ, Flier WG. 2005. The biology of Phytophthora infestans at its center of origin. Ann Rev Phytopathol. 43:171–190. doi:10.1146/annurev.phyto.43.040204.135906
- Hiltunen LH, White JG. 2002. Cavity spot of carrot (Daucus carota). Ann Appl Biol. 141:201–223. doi:10.1111/j.1744-7348.2002.tb00213.x
- Holley JD, Kawchuk LM. 1996. Distribution of thiabendazole and thiophanate-methyl resistant strains of Helminthosporium solani and Fusarium sambucinum in Alberta potato storages. Can Plant Dis Surv. 76:21–27.
- Holm FA, Zentner RP, Thomas AG, Sapsford K, Légère A, Gossen BD, Olfert O, Leeson JY. 2006. Agronomic and economic responses to integrated weed management systems and fungicide in a wheat-canola-barley-pea rotation. Can J Plant Sci. 86:1281–1295. doi:10.4141/P05-165
- Hu CH, Perez FG, Donahoo R, McLeod A, Myers K, Ivors K, Secor G, Roberts PD, Deahl KL, Fry WE, Ristaino JB. 2012. Recent genotypes of Phytophthora infestans in the eastern United States reveal clonal populations and reappearance of mefenoxam sensitivity. Plant Dis. 96:1323–1330. doi:10.1094/PDIS-03-11-0156-RE
- Jarvis WR. 1977. Botryotinia and Botryis species: taxonomy physiology and pathogenicity. Ottawa (ON): Canada Department of Agriculture.
- Kalischuk ML, Al-Mughrabi KI, Peters RD, Howard RJ, Platt HW, Kawchuk LM. 2012. Genetic composition of Phytophthora infestans in Canada reveals migration and increased diversity. Plant Dis. 96:1729–1735. doi:10.1094/PDIS-10-11-0859-RE
- Kawchuk LM, Holley JD, Lynch DR, Clear RM. 1994. Resistance to thiabendazole and thiophanate-methyl in Canadian isolates of Fusarium sambucinum and Helminthosporium solani. Am Potato J. 71:185–192. doi:10.1007/BF02849053
- Kawchuk LM, Hutchison LJ, Verhaeghe CA, Lynch DR, Bains PS, Holley JD. 2002. Isolation of the β-tubulin gene and characterization of thiabendazole resistance in Gibberella pulicaris. Can J Plant Pathol. 24:233–238. doi:10.1080/07060660309507001
- Kawchuk LM, Hwang YT, Wijekoon C, Kalischuk M, Johnson D, Howard R, Prüfer D. 2014. Evolution and management of the Irish potato famine pathogen Phytophthora infestans in Canada and the United States. Am J Potato Res. 91. (In press).
- Keinath A. 2009. Sensitivity to azoxystrobin in Didymella bryoniae isolates collected before and after field use of strobilurin fungicides. Pest Manag Sci. 65:1090–1096. doi:10.1002/ps.1797
- Koller W. 1991. Fungicide resistance in plant pathogens. In: Pimentel D, editor. CRC handbook of pest management in agriculture. 2nd ed. Vol. 2. Boca Raton (FL): CRC Press; p. 679–720.
- Krupinsky JM, Bailey KL, McMullen MP, Gossen BD, Turkington TK. 2002. Managing plant disease risk in diversified cropping systems. Agron J. 94:198–209. doi:10.2134/agronj2002.0198
- Kutcher HR, Brandt SA, Smith EG, Ulrich D, Malhi SS, Johnston AM. 2013. Blackleg disease of canola mitigated by resistant cultivars and four-year crop rotations in western Canada. Can J Plant Pathol. 35:209–221. doi:10.1080/07060661.2013.775600
- Leroux P, Gredt M, Leroch M, Walker A-S. 2010. Exploring mechanisms of resistance to respiratory inhibitors in field strains of Botrytis cinerea, the causal agent of gray mold. Appl Environ Microbiol. 76:6615–6630. doi:10.1128/AEM.00931-10
- Lobato MC, Olivieri FP, González Altamiranda EA, Wolski EA, Daleo GR, Caldiz DO, Andreu AB. 2008. Phosphite compounds reduce disease severity in potato seed tubers and foliage. Eur J Plant Pathol. 122:349–358. doi:10.1007/s10658-008-9299-9
- Lu XH, Davis RM, Livingston S, Nunez J, Hao JJ. 2012. Fungicide Sensitivity of Pythium spp. Associated with Cavity Spot of Carrot in California and Michigan. Plant Dis. 96:384–388. doi:10.1094/PDIS-07-11-0562
- Lynch DR, Kawchuk LM, Chen Q, Kokko M. 2003. Resistance to Fusarium sambucinum in wild and cultivated Solanum species. Am J Potato Res. 80:353–358. doi:10.1007/BF02854246
- Ma Z, Michailides TJ. 2005. Advances in understanding molecular mechanisms of fungicide resistance and molecular detection of resistant genotypes in phytopathogenic fungi. Crop Prot. 24:853–863. doi:10.1016/j.cropro.2005.01.011
- Martinez C, Lévesque CA, Bélanger RR, Tweddell RJ. 2005. Evaluation of fungicides for the control of carrot cavity spot. Pest Manag Sci. 61:767–771. doi:10.1002/ps.1055
- McDonald MR. 1994. Cavity spot of carrot (Pythium spp.): etiology, epidemiology and control [dissertation]. Guelph (ON): University of Guelph.
- Mitchell JA, Cain RB. 1996. Rapid onset of the accelerated degradation of dicarboximide fungicides in a UK soil with a long history of agrochemical exclusion. Pestic Sci. 48:1–11. doi:10.1002/(SICI)1096-9063(199609)48:1<1::AID-PS445>3.0.CO;2-Y
- Mondal SN, Bhatia A, Shilts T, Timmer LW. 2005. Baseline sensitivities of fungal pathogens of fruit and foliage of citrus to azoxystrobin, pyraclostrobin, and fenbuconazole. Plant Dis. 89:1186–1194. doi:10.1094/PD-89-1186
- Mueller DS, Wise KA, Dufault NS, Bradley CA, Chilvers MI. 2013. Fungicides for field crops. St. Paul, (MN): APS Press.
- Nene YL, Reddy MV. 1987. Chickpea diseases and their control. In: Saxena MC, Singh KB, editors. The chickpea. Wallingford (UK): CAB Intern; p. 233–370.
- Olaya G, Holm A. 2001. Sensitivity of Didymella bryoniae isolates to azoxystrobin. Phytopathology. 91:S67. [abstr.].
- Pasche JS, Piche LM, Gudmestad NC. 2005. Effect of the F129L mutation in Alternaria solani on fungicides affecting mitochondrial respiration. Plant Dis. 89:269–278. doi:10.1094/PD-89-0269
- Pasche JS, Wharam CM, Gudmestad NC. 2004. Shift in sensitivity of Alternaria solani in response to QoI fungicides. Plant Dis. 88:181–187. doi:10.1094/PDIS.2004.88.2.181
- Peng G, Lahlali R, Hwang SF, Pageau D, Hynes RK, McDonald MR, Gossen BD, Strelkov SE. 2014. Crop rotation, cultivar resistance, and fungicides/biofungicides for managing clubroot (Plasmodiophora brassicae) on canola. Can J Plant Pathol. 36(sup1):99–112. doi:10.1080/07060661.2013.860398
- Peters RD, Al-Mughrabi KI, Kalischuk ML, Dobinson KF, Conn KL, Alkher H, Islam MR, Daayf F, Lynn J, Bizimungu B, De Koeyer D, Lévesque CA, Kawchuk LM. 2014. Characterization of Phytophthora infestans population diversity in Canada reveals increased migration and genotype recombination. Can J Plant Pathol. 36:73–82. doi:10.1080/07060661.2014.892900
- Reddy MV, Singh KB. 1990. Relationship between ascochyta blight severity and yield loss in chickpea and identification of resistant lines. Phytopathol Medit. 24:32–38.
- Saude C, Simon PW, McDonald MR. 2014. Incidence and severity of cavity spot of carrot as affected by pigmentation temperature and rainfall. Plant Dis. (in press). doi:10.1094/PDIS-10-13-1021-RE
- Statistics Canada. 2011. Canadian Census of Agriculture 2011. Available from: http://www.statcan.gc.ca/pub/95-640-x/2012002-eng.htm.
- Stevenson KL, Langston DB, Seebold KW. 2002. Resistance to azoxystrobin in the gummy stem blight pathogen in Georgia. Phytopathology. 92:S79. [abstr.]
- Thaher N. 2011. Fungicide insensitivity in Ascochyta Rabiei in Saskatchewan [MSc dissertation]. Guelph (ON): University of Guelph.
- Torriani SFF, Brunner PC, McDonald BA, Sierotzki H. 2009. QoI resistance emerged independently at least 4 times in European populations of Mycosphaerella graminicola. Pest Manag Sci. 65:155–162. doi:10.1002/ps.1662
- Van Der Heyden H, Carisse O, Brodeur L. 2012. Comparison of monitoring based indicators for initiating fungicide spray programs to control botrytis leaf blight of onion. Crop Prot. 33:21–28. doi:10.1016/j.cropro.2011.11.008
- Van der Heyden H, Dutilleul P, Brodeur L, Carisse O. 2014. Spatial distribution of single-nucleotide polymorphisms related to fungicide resistance and implications for sampling. Phytopathology. (in press). doi:10.1094/PHYTO-03-13-0085-R
- Veloukas T, Leroch M, Hahn M, Karaoglanidis GS. 2011. Detection and molecular characterization of boscalid-resistant Botrytis cinerea isolates from strawberry. Plant Dis. 95:1302–1307. doi:10.1094/PDIS-04-11-0317
- Walker A, Brown PA, Entwistle AR. 1986. Enhanced degradation of iprodione and vinclozolin in soil. Pestic Sci. 17:183–193. doi:10.1002/ps.2780170216
- Warkentin TD, Xue AG, McAndrew DW. 2000. Effect of mancozeb on the control of mycosphaerella blight of field pea. Can J Plant Sci. 80:403–406. doi:10.4141/P99-085
- White JG. 1988. Studies on the biology and control of cavity spot of carrots. Ann Appl Biol. 113:259–268. doi:10.1111/j.1744-7348.1988.tb03302.x
- Wijekoon CP, Peters RD, Al-Mughrabi KI, Kawchuk LM. 2014. First report of late blight caused by Phytophthora infestans clonal lineage US-23 on tomato and potato in Atlantic Canada. Plant Dis. 98:426. doi:10.1094/PDIS-08-13-0807-PDN
- Wise KA, Bradley CA, Pasche JS, Gudmestad NC. 2009. Resistance to QoI fungicides in Ascochyta rabiei from chickpea in the Northern Great Plains. Plant Dis. 93:528–536. doi:10.1094/PDIS-93-5-0528
- Wise KA, Bradley CA, Pasche JS, Gudmestad NC, Dugan FM, Chen W. 2008. Baseline sensitivity of Ascochyta rabiei to azoxystrobin, pyraclostrobin, and boscalid. Plant Dis. 92:295–300. doi:10.1094/PDIS-92-2-0295
- Xue AG, Warkentin TD, Gossen BD, Burnett PA, Vandenberg A, Rashid KY. 1998. Pathogenic variation of western Canadian isolates of Mycosphaerella pinodes on selected Pisum sativum genotypes. Can J Plant Pathol. 20:189–193. doi:10.1080/07060669809500426
- Yin YN, Kim YK, Xiao CL. 2011. Molecular characterization of boscalid resistance in field isolates of Botrytis cinerea from apple. Phytopathology. 101:986–995. doi:10.1094/PHYTO-01-11-0016
- Ypema HL, Gold RE. 1999. Kresoxim - methyl: modification of a naturally occurring compound to produce a new fungicide. Plant Dis. 83:4–19. doi:10.1094/PDIS.1999.83.1.4