Abstract
Fusarium oxysporum f. sp. fragariae, the causal agent of Fusarium wilt in strawberry (Fragaria ananassa Duch.), is a serious threat to commercial production. To understand the interaction between strawberry and Fusarium oxysporum f. sp. fragariae, we transformed the pathogen with a green fluorescent protein as a labelled gene and Hyg as a selectable marker gene using the Agrobacterium tumefaciens-mediated transformation method. The transformants obtained were stable with strong and uniform fluorescence. A green fluorescent protein-tagged strain with similar phenotype and pathogenicity as the wild-type strain was used to study the infection process on strawberry. The observations indicated that the spores attached to the root surface and infected the roots from germ tubes, while the hyphae produced suction cup-like structures from the hyphal tips to infect the strawberry plants. Once inside, hyphal growth was observed mainly in the epidermal and cortical tissues with only a few hyphae detected in the vascular tissues after colonization.
Résumé
Fusarium oxysporum f. sp. fragariae, l’agent causal du flétrissement chez le fraisier (Fragaria ananassa Duch.), pose une menace réelle à la production commerciale. Afin de comprendre l’interaction entre le fraisier et F. oxysporum f. sp. fragariae, nous avons transformé l’agent pathogène en gène marqué avec une protéine fluorescente verte et Hyg en gène de sélection spécifique par la méthode de transformation fondée sur Agrobacterium tumefaciens. Les transformants obtenus étaient stables, émettant une fluorescence intense et uniforme. Une souche marquée avec une protéine fluorescente verte affichant un phénotype et une pathogénicité semblables à celles de la souche naturelle a été utilisée pour étudier le processus d’infection chez le fraisier. Les observations ont montré que les spores se fixaient à la surface des racines et que l’infection se propageait à ces dernières par des tubes germinatifs, tandis que les hyphes produisaient des structures semblables à des ventouses à partir des apex des hyphes pour infecter les fraisiers. Une fois à l’intérieur, il a été possible d’observer la croissance hyphale qui, après colonisation, se produisait principalement dans les tissus épidermiques et corticaux et marginalement dans les tissus vasculaires.
Introduction
Continuous cropping of strawberry (Fragaria ananassa Duch.) has been threatened by soil-borne fungal diseases. The wilt caused by Fusarium oxysporum f. sp. fragariae (Fof) causes severe damage and yield losses in strawberry (Fravel et al. Citation2003; Zhao et al. Citation2009; Gu et al. Citation2010). To date, Fof has been reported in the USA (Koike et al. Citation2009), China (Zhao et al. Citation2009), Spain (Arroyo et al. Citation2009) and Australia (Fang et al. Citation2011). Strawberry plants infected by F. oxysporum show necrosis on roots or leaves, plant stunting and wilting, fruit reduction, deterioration in quality, and plant death (Yang et al. Citation2008; Fang et al. Citation2011). Fusarium oxysporum can survive for many years in soil and dead plant tissues, which makes the disease difficult to control (Fravel et al. Citation2003). Growing resistant cultivars is deemed the most effective way to control F. oxysporum infection. However, only a few studies have been conducted on the mechanism of pathogenesis and on the identification or functional characterization of Fof virulence genes. This inadequate research effort has limited selection for resistance and development of new cultivars with resistance (Fravel et al. Citation2003).
Studies on the infection and colonization process of pathogens in plants could contribute to the development of new resistant cultivars (Ramírez-Suero et al. Citation2010; Fang et al. Citation2012a). The colonization process of Fof in strawberry has been studied by using histological staining techniques and scanning electron microscopy (Fang et al. Citation2012a). However, studies that investigate in situ colonization of strawberry by a green fluorescent protein (GFP)-labelled Fof strain have not been reported. GFP as a reporter protein has been widely used to study plant-fungal interactions. It does not require cofactors or substrates for protein activity. Furthermore, pathogens labelled with GFP can be investigated during the entire infection process without any treatment or manipulation of samples (Lagopodi et al. Citation2002; Zhang et al. Citation2012). GFP-labelled F. oxysporum strains have been obtained and used to study the infection process in other hosts, such as banana (Musa nana Lour.) (Li et al. Citation2011), carnation (Dianthus caryophyllus Linn.) (Sarrocco et al. Citation2007), melon (Cucumis melo Linn.) (Nonomura et al. Citation2003) and tomato (Solanum lycopersicum Miller) (Lagopodi et al. Citation2002). Therefore, GFP-labelled strains are ideal to visualize the in situ colonization of plants, and they facilitate the investigation of both pathogen infection and plant resistance.
In the present study, we describe the transformation of Fof with a plasmid vector harbouring the GFP and hygromycin resistance (Hyg) genes via Agrobacterium tumefaciens-mediated transformation (ATMT). We investigated the infection and colonization of strawberry by a GFP-tagged mutant using fluorescence microscopy.
Materials and methods
Fungal strain and plasmid
The wild-type Fof was isolated from a diseased strawberry plant in 2012 from an experimental strawberry field of the Jiangsu Academy of Agricultural Sciences, Nanjing, China. The identity of this strain was confirmed by morphological and molecular identification. First, the strain was identified from morphology in culture and characteristic symptoms of disease. Then universal primers (ITS1, 5ʹ-TCCGTAGGTGAACCTGGGG-3ʹ and ITS4, 5ʹ-TCCTCCGCTTATTGATATGC-3ʹ) were used to amplify the DNA and the results of sequence alignment of the PCR product showed that the strain belonged to Fusarium oxysporum (data not shown). The isolate was stored in 20% glycerol at −70°C. The binary plasmid vector pCAMBIA 1300-GFP was kindly provided by Dr Yongfeng Liu (Jiangsu Academy of Agricultural Sciences). This vector contains the hygromycin B phosphotransferase gene (Hyg) as the selected marker gene driven by the Aspergillus nidulans tryptophan C (Trp C) promoter and the green fluorescent protein gene (GFP) as a reporter marker gene driven by the Trp C promoter (Chen et al. Citation2011). The plasmid was transferred into Agrobacterium tumefaciens strain AGL-1 using the freeze-thaw method (Wise et al. Citation2006).
Confirmation of the optimum concentration of hygromycin B and cefotaxime for transformation
To determine the optimum concentration of hygromycin B for transformation, 100 μL of spore suspension (1 × 107 conidia mL−1) obtained from new mycelium were placed on potato dextrose agar (PDA) that was supplemented with different concentrations of hygromycin B (Roche, Basel, Switzerland) (0, 10, 20, 30, 40, 50, 70, 80 and 100 mg L−1) and kept at 25°C. Mycelial plugs (5 mm in diameter) from the edge of freshly growing cultures of the wild type strain were also placed on PDA with different concentrations of hygromycin B. Each test was repeated three times. The growth of spores and mycelia was examined 7 days after inoculation. The above method was also used to determine the optimum concentration of cefotaxime (Sangon) (100, 200, 300, 400, 500, 600, 700 and 1000 mg L−1) for transformation.
Transformation
The method for Agrobacterium-mediated transformation of Fof was described by Chen et al. (Citation2011). The A. tumefaciens strain AGL-1 containing the binary vector was cultured in a lysogeny broth (1% tryptone, 1% NaCl and 0.5% yeast extract) supplemented with 50 mg L−1 kanamycin and 25 mg L−1 rifampicin (Sangon). The culture was shaken at 150 rpm and 28°C. After 24 h, the culture was centrifuged at 4000 rpm for 5 min. After discarding the supernatant, the Agrobacterium cells were diluted to OD600 = 0.2 using the induction medium (IM) (Bundock et al. Citation1995) and were grown for another 4 h at 28°C. Thereafter, they were mixed with equal volumes of Fof spore suspension at a concentration of 1 × 106 conidia mL−1. The spore suspension was grown in Czapek’s medium for 3–5 days at 25°C with shaking at 150 rpm. One hundred microlitres of the mixture was plated on a cellulose membrane (Sangon) which was placed on IM solid medium supplemented with 40 μg mL−1 acetosyringone (Sangon). The cellulose membrane was then transferred onto the selection medium (PDA supplemented with 70 mg L−1 hygromycin B and 500 mg L−1 cefotaxime) and cultivated at 25°C for 2 days in the dark. The hygromycin B-resistant colonies were isolated 7–10 days after incubation and placed on the selection media for additional incubation.
Confirmation of transformants by PCR and fluorescence microscopy
The protocol described by Saitoh et al. (Citation2006) was used to extract DNA from Fof mycelia for PCR analysis. The transformed isolates were grown in Czapek’s medium for 3–5 days at 25°C and shaking at 150 rpm. Spores used for DNA extraction were collected by centrifuging for 5 min at 1000 rpm. GFP-specific primers GFP-F (5ʹ-ACGGCAAGCTGACCCTGAAG-3ʹ) and GFP-R (5ʹ-CTCGTCCATGCCGAGAGTGA-3ʹ) were used to confirm the GFP gene in the Fof genome by PCR, which would produce a 590 bp fragment. PCR consisted of a total volume of 15 μL containing 8.7 μL of ddH2O, 1.5 μL of buffer (10×), 1.25 μL of each of the dNTPs (2.5 mM), 1 μL of Mg2+ (25 mM), 0.2 μL of primer 1 (10 μM), 0.2 μL of primer 2 (10 μM), 0.15 μL of Taq polymerase (TaKaRa) and 2 μL of DNA (2 ng). The reaction conditions were: an initial denaturation step at 94°C for 4 min, followed by 30 cycles of denaturation at 94°C for 30 s, annealing at 58°C for 40 s, and elongation at 72°C for 40 s. The GFP expression in conidia/mycelia of transformants was examined using a fluorescence microscope (Nikon ECLIPSE 80i, Japan) equipped with filter blocks for GFP (450–490 nm excitation; 590 nm long pass emission).
Confirmation of stability of transformants
Five randomly selected transformants were subjected to a stability test. Following subculture (5 times) on PDA without hygromycin B, the transformants were cultured on PDA supplemented with 70 mg L−1 hygromycin B to investigate the colony morphogenesis and growth rate. The GFP expression was examined in all the five transformants.
Inoculum preparation and plant inoculation
The strawberry cultivar ‘Allstar’ used in this study was imported from Maryland, USA. It is susceptible to Fof (Zhang et al. Citation2004). Strawberry seeds were washed with 75% alcohol for 1 min, 1.5% sodium hypochlorite for 5 min, and sterile distilled water 5 times. Thereafter, they were planted in a paper cup filled with sterile vermiculite and kept under a 16 h light/8 h dark cycle at 25°C. Two-month-old plants were inoculated with the GFP-tagged Fof strain. A root-dip method as described by Fang et al. (Citation2012b) was used for inoculation.
A stable GFP-tagged transformant was grown in Czapek’s medium at 25°C with shaking at 150 rpm for 3–5 days. The culture was then filtered through four layers of medical gauze to remove the mycelium. The spore suspension used for inoculation was diluted with distilled water to a final concentration of 1 × 107 conidia mL−1. For root dipping, the plant was pulled out from the paper cup and the vermiculite was removed by extensive washing. These seedlings were dipped into the spore suspension for 2 min. After inoculation, the plants were put into half-strength MS liquid medium (Murashige & Skoog Citation1962) and placed in a growth chamber set at a 16 h light/8 h dark cycle at 25°C. The control seedlings were inoculated with distilled water by the same method.
Fluorescence microscopy observation
Following inoculation, the strawberry plants were examined for the infection process every day for the first 7 days after inoculation and every 2 days thereafter until the disease symptoms appeared. For the early stage observations, the roots were directly placed on a slide with a drop of water and covered with a glass cover slip. Cross-sections of roots and stems were manually obtained using a razor blade and were viewed using a fluorescence microscope to observe the interactions at later stages. Three plants were randomly selected for each examination.
Results
Transformation of Fof with the Hyg and GFP genes
The best concentrations of hygromycin B and cefotaxime were determined for transformation using two different methods. In the spore suspension experiment, no spores germinated at hygromycin B concentrations above 30 mg L−1, while in the mycelial plug experiment, the emergence of new hyphae was not observed above 50 mg L−1 of hygromycin B. To avoid false positive colonies, a concentration of 70 mg L−1 hygromycin B was determined as the optimal concentration of transformation in this study.
Isolates of Fof did not show significant differences in growth in response to varying concentrations of cefotaxime, indicating that cefotaxime does not influence the growth of Fof. We selected 500 mg L−1 cefotaxime as the optimal concentration to kill A. tumefaciens cells for transformation in this study.
Several hygromycin B resistant colonies emerged on the cellulose membrane 7–10 days after transformation. These colonies were then transferred onto fresh PDA plates containing hygromycin B and cefotaxime. They were regarded as transformants if there was additional growth (Mullins et al. Citation2001). Transformation efficiency was in the range of 100–300 hygromycin B resistant transformants per 1 × 106 conidia of Fof.
Detection of GFP by gene-specific PCR primers and fluorescence microscopy
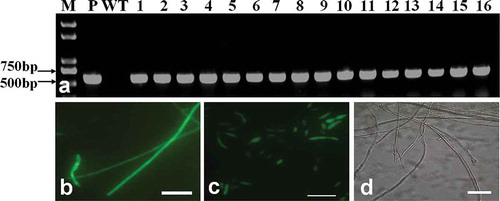
The genomic DNA of transformants was used as templates for PCR using the primers GFP-F and GFP-R. All of the randomly selected transformants produced a fragment with the expected length of 590 bp (). To detect the GFP expression, all the transformants were tested by fluorescence microscopy. The test results showed that a strong and uniform GFP signal could be observed from hyphae () and conidia () of the transformants. There was no detectible signal from the wild type ().
Fig. 1 (Colour online) PCR analysis and expression of green fluorescent protein from transformants of Fof. (a) M: DNA marker Trans 2K plus; P: Plasmid; WT: Fof wild type; 1–16: Fof transformants. (b) GFP expression in mycelium of transformants. (c) GFP expression in conidia of transformants. (d) No GFP expression observed in mycelia and conidia from wild-type Fof. Scale bars: b, c, d = 10 μm.
Detection of the stability of the transformants
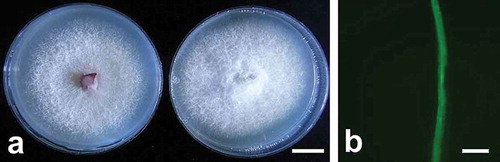
The stability of the transformants was tested by culturing them on PDA with 70 mg L−1 hygromycin B after subculturing on PDA without hygromycin B. The transformants grew normally as the wild type (). Moreover, the fluorescent signals in all of the transformants were still strong (). Therefore, we concluded that the obtained GFP-tagged transformants were stable regarding the Hyg and GFP expression. A stable transformant with morphology and pathogenicity similar to the wild type (data not shown) was used to study the infection of strawberry.
Fig. 2 (Colour online) Morphology and green fluorescent protein expression of selected Fof transformants. (a) Left: Fof wild type with 5 days after inoculation on PDA; Right: transformant with 5 days after inoculation on PDA containing 70 mg L−1 hygromycin B. (b) GFP expression in hyphae of transformants after subculturing 5 times. Scale bars: a = 1 cm; b = 10 μm.
Early stages of GFP-labelled isolate colonization of strawberry
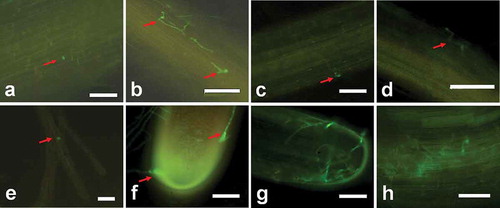
The roots of infected strawberry plants were used to examine the fungal development and colonization of the plant. At 24 h after inoculation, no discoloration could be visually detected on the roots. Fluorescence microscopic observation showed that the GFP-labelled Fof isolate could be observed, and the spores were attached to the root surface randomly (). At 36 h after inoculation, most spores had germinated with the emergence of a germ tube (). Germ tubes elongated and most likely infected the root through junctions of epidermal cells (, ). However, no appressorium was observed through the infection stages. We also noticed that a few spores attached to root hairs, but they did not cause infection (). At 48 h after inoculation, the hypha was attached to the root through suction cup-like structures originated from the hyphal apex ().
Fig. 3 (Colour online) Early stages of the colonization of strawberry roots by GFP labelled Fof isolate. (a) Spore attached to the root surface (arrow) 24 h after inoculation. (b) Germinated conidia on the root surface with the emergence of a germ tube (arrows) 36 h after inoculation. (c) A conidium infecting the root through junctions of epidermal cells (arrow) 36 h after inoculation. (d) Hyphal growth along the junctions of epidermal cells (arrow) 48 h after inoculation. (e) Spore attached to root hair (arrow) 24 h after inoculation. (f) Hyphae are attached to the root tip via suction cup-like structures (arrow) 48 h after inoculation. (g) Colonization on the root tip surface by hyphae growths that are forming a complex network 3 days after inoculation. (h) Hyphae network formed on the root elongation surface 4 days after inoculation. Scale bars: a = 100 μm; b–h = 10 μm.
Three to four days after inoculation, a small number of plants were found to have light brown root tips which were wrapped by complex hyphal networks (). At this stage, most of the roots in the area of brown discoloration were surrounded by intricate hyphae (). The strawberry leaves were still healthy with no disease symptoms.
Advanced stages of GFP-labelled isolate colonization of strawberry
Five to six days after inoculation, hyphae were inside the root cells and became swollen ( and ). They grew longitudinally and laterally to form complex networks inside the epidermis of the root maturation zone (–), which was dark in colour with a reddish hue. The results of the examined cross-sections showed that the hyphae were mainly in the cortex with only a few hyphae in the vascular tissues (). At this stage, root tips were dark and brownish colour and some leaves appeared to be withered.
Fig. 4 (Colour online) Advanced stages of infection and colonization of strawberry roots and petioles by Fof isolate marked with GFP. (a) Hyphae were swollen inside the epidermal tissues 5 days after inoculation. (b) Hyphae forming an expanding network inside epidermal tissues 6 days after inoculation. (c) Intra- and inter-cellular spreading of hyphae in epidermis tissues 6 days after inoculation. (d) Intercellular growth of hyphae in the cortex (arrows) and cross-section of the primary root (arrow) 6 days after inoculation. (e) Spread of hyphae in epidermal tissues (arrow) in a cross-section of the primary root 12 days after inoculation. (f) Spread of hyphae in cortical tissues (arrow) in the cross-section of the primary root 12 days after inoculation. (g) No hyphae in vascular tissues seen in the cross-section of the primary root 12 days after inoculation. (h) No hyphae on the surface of the root tips 12 days after inoculation. Auto-fluorescence of the dead root can be seen. (i) Spread of hyphae in intra- and inter-cellular epidermal tissues of petioles 12 days after inoculation. (j, k, l) Intracellular growth of the pathogen (arrow) in the cortical tissues, intercellular (arrows) area of the epidermal cell layers, and cross-section of petiole 12 days after inoculation. Scale bars: a–l = 10 μm.
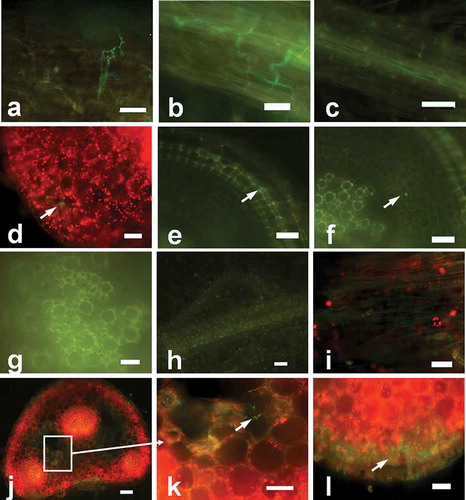
At 10–12 days after inoculation, two or three leaves of the plant infected by GFP-labelled isolate displayed typical symptoms of leaf blade edge wilting and reddish discoloration of the petiole at the ground level, while the other leaves were still healthy. At this stage, all primary and lateral root tissues were severely affected and showed blackish discoloration indicating decay (data not shown). Fluorescence microscopy showed that most of the growing hyphae spread in the epidermis, hyphae were seldom evident in the cortex, and there were no hyphae in vascular tissues of the primary root (–). Petioles with black necrotic lesions were subjected to further investigation. At this stage, no hyphae were observable on root surfaces and only auto-fluorescent spots from decayed roots could be detected (). Hyphae development and dense mycelial networks were observed inside the epidermal cells of the infected petiole (). Abundant hyphae were found in the epidermis and cortex of the infected petiole, and a few hyphae were present in the vascular tissues (–). More leaves started to wilt 15–16 days after inoculation, while a few leaves were completely withered at this stage.
Discussion
The transformation of different formae speciales of F. oxysporum with the GFP gene has been previously reported, such as for F. oxysporum f. sp. lycopersici (Olivain et al. Citation2006), F. oxysporum f. sp. cubense (Li et al. Citation2011), F. oxysporum f. sp. melonis (Nonomura et al. Citation2003), and F. oxysporum f. sp. dianthi (Sarrocco et al. Citation2007). However, there is no previous report on the transformation of Fof. Here, we report the expression of the GFP gene in Fof. The transformation method used in this study was previously described as highly efficient for transformation of Verticillium species (Chen et al. Citation2011). Data from the present study indicate that the efficiency of the transformation of Fof was 0.01–0.03%, which is comparable with that previously reported for F. oxysporum (Mullins et al. Citation2001).
The strawberry root colonization processes by Fof have been previously studied using SEM and histological staining techniques (Fang et al. Citation2012a). In this study, we used fluorescence microscopy to examine the colonization of strawberry by the Fof isolate expressing GFP. Using a GFP-labelled Fof strain enables superior observations of the entire infection process in vivo. The method also reflects more realistic invasion process of Fof that requires no additional staining. We used the same inoculation method reported by Fang et al. (Citation2012a) in order to compare the differences and similarities in infection. Our study showed that infection of strawberry by Fof manifested features that were different from those previously reported using SEM. The major difference is that we found only a few hyphae in the vascular tissue of the susceptible strawberry while Fang et al. (Citation2012a) indicated that this phenomenon only occurred in resistant plants.
Lagopodi et al. (Citation2002) demonstrated that infection and colonization of tomato by GFP-labelled F. oxysporum was mainly initiated at the root hairs. In contrast, we found that conidia and hyphae from the GFP-labelled Fof isolate could attach to the root hairs of strawberry but could not cause infection. Our result corroborates a previous study in banana infected by F. oxysporum f. sp. cubense (Yin et al. Citation2011). Moreover, spores could directly penetrate strawberry by elongated germ tubes (Olivain & Alabouvette Citation1999; Fang et al. Citation2012a), while hyphae could infect strawberry through special suction cup-like structures. These structures emerged from the hyphal tip and have not been reported in previous studies. Each branch of hyphae could produce a suction cup to penetrate into the root. We also found that the germ tubes most likely penetrate strawberry through the junctions of epidermal cells, which is in agreement with a previous study on Fof infection of strawberry using SEM (Fang et al. Citation2012a). However, Olivain & Alabouvette (Citation1999) observed that F. oxysporum penetrated tomato through growing hyphae rather than germ tubes. These differences could be explained by host-specific infection modes utilized by formae speciales of F. oxysporum on different hosts (Yin et al. Citation2011), although the pathogens belong to the same species.
Fang et al. (Citation2012a) found that the hyphae of Fof could rapidly colonize vascular tissues of susceptible strawberry plants only after penetrating the epidermal layer, while in resistant strawberry plants the hyphae were restricted to the cortex. The accumulation of phenolic substances in the hypodermal layer and invasion by a large number of hyphae that block the vascular tissue caused the susceptible strawberry plants to wither and die. The strawberry cultivar used in this study was Fof-susceptible. However, only a few hyphae were found in vascular tissues in our study and most of the hyphae appeared in the cortex. The differences may be related to plant genotypes and fungal isolates or to the different techniques used in observation.
There are no preferential sites for infection by several formae speciales of F. oxysporum (Bhalla et al. Citation1992; Olivain & Alabouvette Citation1999), which is in agreement with our study on Fof. We observed that Fof randomly adhered to the root tips of strawberry as well as to the root elongation zone. This indicated that F. oxysporum strains could be present everywhere on the root tissues, which is in agreement with the previous studies on F. oxysporum colonization of strawberry (Fang et al. Citation2012a) and tomato (Olivain & Alabouvette Citation1999).
The strawberry petioles that showed disease symptoms under a fluorescence microscope were tested for the advanced stage of infection. The results showed that most growing hyphae appeared in the cortex of the petioles; only a few hyphae were observed in vascular tissues. Moročko-Bičevska & Fatehi (Citation2010) reported a similar pattern in the infection of strawberry by Gnomonia fragariae. When F. oxysporum f. sp. fragariae infected a susceptible strawberry genotype, hyphae were found from the epidermal layer into the cortex of the root and onwards into vascular tissues. However, in a resistant strawberry genotype, hyphae were restricted to the epidermal layer and the pathogen could not enter the vascular tissues (Fang et al. Citation2012a). In our microscopic observations, hyphae were found mainly in the epidermal and cortical tissues with a few hyphae detected in the vascular tissues after colonization. It could be hypothesized that the different infection responses may be due to different host genotypes. This GFP-labelled F. oxysporum f. sp. fragariae strain can be useful to study infection differences in further studies.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 31371930), Independent Innovation of Agricultural Sciences in Jiangsu Province (No. cx (12)5022), the National Science and Technology Major Project for Transgenic Breeding (No. 2014ZX08005-001B) and the Natural Science Foundation of Jiangsu Province (BK20131336).
References
- Arroyo FT, Llergo Y, Aguado A, Romero F. 2009. First report of Fusarium wilt caused by Fusarium oxysporum on strawberry in Spain. Plant Dis. 93:323–323. doi:10.1094/PDIS-93-3-0323B
- Bhalla MK, Nozzolillo C, Schneider EF. 1992. Observations on the responses of lentil root cells to hyphae of Fusarium oxysporum. J Phytopathol. 135:335–341. doi:10.1111/j.1439-0434.1992.tb04319.x
- Bundock P, Den Dulk-Ras A, Beijersbergen A, Hooykaas PJ. 1995. Trans-kingdom T-DNA transfer from Agrobacterium tumefaciens to Saccharomyces cerevisiae. Embo J. 14:3206–3214.
- Chen TZ, Yuan HB, Yang YW, Liu AM, Zhang BL. 2011. Optimization of Agrobacterium mediated transformation of Verticillium dahliae. Cotton Sci. 23:507–514.
- Fang XL, Kuo J, You MP, Finnegan PM, Barbetti MJ. 2012a. Comparative root colonisation of strawberry cultivars Camarosa and Festival by Fusarium oxysporum f. sp. fragariae. Plant Soil. 358:75–89. doi:10.1007/s11104-012-1205-8
- Fang XL, Phillips D, Li H, Sivasithamparam K, Barbetti MJ. 2011. Severity of crown and root diseases of strawberry and associated fungal and oomycete pathogens in Western Australia. Australas Plant Pathol. 40:109–119. doi:10.1007/s13313-010-0019-5
- Fang XL, Phillips D, Verheyen G, Li H, Sivasithamparam K, Barbetti MJ. 2012b. Yields and resistance of strawberry cultivars to crown and root diseases in the field, and cultivar responses to pathogens under controlled environment conditions. Phytopathol Mediter. 51:69–84.
- Fravel D, Olivain C, Alabouvette C. 2003. Fusarium oxysporum and its biocontrol. New Phytol. 157:493–502. doi:10.1046/j.1469-8137.2003.00700.x
- Gu CB, Shi XB, Jiang LL, Wang KY, Lin CH, Duan HM. 2010. Resistance of Fusarium oxysporum f. sp. fragariae to carbendazim and the biological characters of carbendazim-resistant strain. Acta Phytophyl Sin. 37:266–272.
- Koike ST, Kirkpatrick SC, Gordon TR. 2009. Fusarium wilt of strawberry caused by Fusarium oxysporum in California. Plant Dis. 93:1077–1077. doi:10.1094/PDIS-93-10-1077A
- Lagopodi AL, Ram AF, Lamers GE, Punt PJ, van den Hondel CA, Lugtenberg BJ, Bloemberg GV. 2002. Novel aspects of tomato root colonization and infection by Fusarium oxysporum f. sp. radicis-lycopersici revealed by confocal laser scanning microscopic analysis using the green fluorescent protein as a marker. Mol Plant-Microbe Interact. 15:172–179. doi:10.1094/MPMI.2002.15.2.172
- Li CY, Chen S, Zuo CW, Sun QM, Ye Q, Yi GJ, Huang BZ. 2011. The use of GFP-transformed isolates to study infection of banana with Fusarium oxysporum f. sp. cubense race 4. Eur J Plant Pathol. 131:327–340. doi:10.1007/s10658-011-9811-5
- Moročko-Bičevska I, Fatehi J. 2010. Infection and colonization of strawberry by Gnomonia fragariae strain expressing green fluorescent protein. Eur J Plant Pathol. 129:567–577. doi:10.1007/s10658-010-9720-z
- Mullins E, Chen X, Romaine P, Raina R, Geiser D, Kang S. 2001. Agrobacterium-mediated transformation of Fusarium oxysporum: an efficient tool for insertional mutagenesis and gene transfer. Phytopathology. 91:173–180. doi:10.1094/PHYTO.2001.91.2.173
- Murashige T, Skoog F. 1962. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 15:473–497. doi:10.1111/j.1399-3054.1962.tb08052.x
- Nonomura T, Tajima H, Kitagawa Y, Sekiya N, Shitomi K, Tanaka M, Maeda K, Matsuda Y, Toyoda H. 2003. Distinguishable staining with neutral red for GFP-marked and GFP-nonmarked Fusarium oxysporum strains simultaneously colonizing root surfaces. J Gen Plant Pathol. 69:45–48. doi:10.1007/s10327-002-0018-7
- Olivain C, Alabouvette C. 1999. Process of tomato root colonization by a pathogenic strain of Fusarium oxysporum f. sp. lycopersici in comparison with a non-pathogenic strain. New Phytol. 141:497–510. doi:10.1046/j.1469-8137.1999.00365.x
- Olivain C, Humbert C, Nahalkova J, Fatehi J, L’Haridon F, Alabouvette C. 2006. Colonization of tomato root by pathogenic and nonpathogenic Fusarium oxysporum strains inoculated together and separately into the soil. Appl Environ Microbiol. 72:1523–1531. doi:10.1128/AEM.72.2.1523-1531.2006
- Ramírez-Suero M, Khanshour A, Martinez Y, Rickauer M. 2010. A study on the susceptibility of the model legume plant Medicago truncatula to the soil-borne pathogen Fusarium oxysporum. Eur J Plant Pathol. 126:517–530. doi:10.1007/s10658-009-9560-x
- Saitoh K, Togashi K, Arie T, Teraoka T. 2006. A simple method for a mini-preparation of fungal DNA. J Gen Plant Pathol. 72:348–350. doi:10.1007/s10327-006-0300-1
- Sarrocco S, Falaschi N, Vergara M, Nicoletti F, Vannacci G. 2007. Use of Fusarium oxysporum f. sp. dianthi transformed with marker genes to follow colonization of carnation roots. J Plant Pathol. 89:47–54.
- Wise AA, Liu ZY, Binns AN. 2006. Three methods for the introduction of foreign DNA into Agrobacterium. Meth Mol Biol. 343:43–53.
- Yang HQ, Wang KY, Fan K, Lin CH, Duan HM, Yuan XL. 2008. Biological characteristics of strawberry Fusarium wilt and inhibitory effects of seven fungicides. Acta Phytophyl Sin. 35:169–174.
- Yin XM, Xu B, Zheng X, Zeng HC, Ma WH, Wang JB, Li HL, Jin ZQ. 2011. Histological observation of banana root infected by Fusarium oxysporum f. sp. cubense. Acta Phytopathol Sin. 41:570–575.
- Zhang WW, Jiang TF, Cui X, Qi FJ, Jian GL. 2012. Colonization in cotton plants by a green fluorescent protein labelled strain of Verticillium dahliae. Eur J Plant Pathol. 135:867–876. doi:10.1007/s10658-012-0131-1
- Zhang ZH, Liu Y, Du GD, Dai HY. 2004. Leaf disc assay for identification of resistance of strawberry to powdery mildew and screening of fungicides. Acta Horticult Sin. 31:505–507.
- Zhao XS, Zhen WC, Qi YZ, Liu XJ, Yin BZ. 2009. Coordinated effects of root autotoxic substances and Fusarium oxysporum Schl. f. sp. fragariae on the growth and replant disease of strawberry. Front Agric China. 3:34–39. doi:10.1007/s11703-009-0006-1
