Abstract
Phytophthora infestans, a hemibiotrophic and heterothallic oomycete pathogen, attacks a wide variety of crop plants belonging to the Solanaceae family. In 2007, late blight symptoms were observed and confirmed on Physalis peruviana in fields during a late blight survey in Colombia. Subsequent to these observations, no further symptoms have been detected in the field. This prompted an interest in investigating the interaction between P. infestans and P. peruviana using histological and molecular assays, as well as characterizing defence reactions produced on P. peruviana leaves. During this interaction, callose depositions, accumulation of hydrogen peroxide, activation of pathogenesis-related proteins (β-1,3 glucanase and peroxidase), induction of the expression of genes associated with plant defences (glucanase A and carbonic anhydrase) as well as cell death, showed that P. peruviana has active defences against P. infestans strains present in Colombia.
Resumé
Phytophthora infestans, un pathogène hemibiotrophe est un oomycète hetérothallique qui attaque une grande variété de cultures qui appartiennent à la famille des solanacées. En 2007, des symptômes similaires à ceux du mildiou de la pomme de terre ont été observés en Colombie sur Physalis peruviana (groseille du Cap), lors d’une surveillance de la maladie. Dès lors, les symptômes n’ont plus été observés sur les champs. De ce fait, la conduite d’une recherche pour comprendre l’interaction entre P. infestans et P. peruviana s’est mise en place en utilisant des essais histologiques, moléculaires et biochimiques. Les dépositions de callose, l’accumulation de peroxyde d’hydrogène, l’activation des enzymes β-1,3 Glucanase et Peroxidase et l’induction de l’expression de gènes associés aux mécanismes de défense de la plante (glucanase A et anhydrase carbonique) ainsi que la mort cellulaire, ont montré que les souches du pathogène et P. peruviana sont incompatibles.
Introduction
Phytophthora infestans is the causal agent of late blight, a plant disease that causes multibillion dollar losses in potato and tomato crops worldwide (Vleeshouwers et al. Citation2000; Fry Citation2007, Citation2008; Runno-Paurson et al. Citation2010). This pathogen is also able to attack a wide range of plants within the Solanaceae family, including tobacco (Nicotiana benthamiana L.), petunia (Petunia × hybrida. hort. ex E.Vilm.), Calibrachoa (Calibrachoa Cerv., a hybrid cultivar) (Becktell et al. Citation2006), Andean exotic fruits such as Solanum betaceum, S. quitoense (Vargas et al. Citation2009) and S. muricatum (Adler et al. Citation2004), and wild relatives such as S. viarum (Cárdenas et al. Citation2011) and S. ochrantum (Chacón et al. Citation2006).
In 2007, Vargas et al. (Citation2007) observed P. infestans causing disease in a new host, Physalis peruviana (Peruvian groundcherry, Cape gooseberry), and reported the presence of the P. infestans A2 mating type for the first time in Colombia, isolated from P. peruviana leaves (Vargas et al. Citation2009). This herbaceous perennial is the second most exported fruit in Colombia after bananas (Espinosa et al. Citation2004) and today Colombia is the leading producer of P. peruviana in the world (Bonilla et al. Citation2008). There is great demand for this fruit mainly because of its attractive colour and pleasant flavour due to its high sugar content (Novoa et al. Citation2006). Production of P. peruviana in Colombia occurs year-round and 80% of the commercial production of this fruit is found in the region of Cundinamarca (Novoa et al. Citation2006).
Subsequent to the first observations of P. infestans causing disease on P. peruviana (Vargas et al. Citation2007, Citation2009), no further symptoms have been detected in the field. Loss of virulence has been reported in P. infestans since 1933 (Reddick & Crosier Citation1933). This phenomenon has repeatedly been documented in P. infestans (Jinks & Grindle Citation1963; Samen et al. Citation2003; Andrivon et al. Citation2011) and in P. sojae, where pathogenic variability during asexual reproduction can change the virulence structure of the pathogen (Chen et al. Citation2009). The lack of late blight symptoms on P. peruviana plants prompted an interest in investigating the interaction between P. infestans and P. peruviana. We hypothesize that the lack of symptoms observed in the field is caused by the recognition of the pathogen, leading to the activation of a defence response triggering the production of reactive oxygen species (ROS) and the induction of pathogenesis-related (PR) proteins. We used histological studies, molecular markers and biochemical assays to investigate our hypothesis.
Materials and methods
Plant growth conditions
Six-week-old P. peruviana ecotype Colombia plants and 4-week-old potato plants (Solanum tuberosum L. ‘Kennebec’) were used for the microscopic assays. Ecotype Colombia was the P. peruviana cultivar cultivated in 2007, the year of the first report of P. infestans on this host (Vargas et al. Citation2007). Both species of plants were grown in a greenhouse maintained between 25 and 30 ºC. Plants of P. peruviana were grown from seeds and potato plants were grown from tubers, both in 15-cm plastic pots (one plant per pot) containing a soil-less mix (Cornell mix) consisting of 1:2:2 (v/v/v) of perlite, peat, and vermiculite with 4 kg of dolomitic limestone, 0.33 kg nitrogen (N) and potassium (K2O), and 0.16 kg of phosphate (P2O5) per cubic metre of mix.. Natural light was supplemented with 400 W high pressure sodium lamps for 12 h per day. Plants were fertilized once or twice a week with liquid fertilizer containing 200–300 ppm nitrogen.
Inoculum production
Detached leaves were inoculated with P. infestans strains 1018 (A1 mating type isolated from S. tuberosum), 4022 (A1 mating type isolated from P. peruviana) both belonging to the clonal lineage EC-1, and 4084 (A2 mating type originally isolated from P. peruviana in 2007) belonging to the clonal lineage US-8. These strains were obtained from the collection of the Mycology and Plant Pathology Laboratory in the Biological Sciences Department at Universidad de los Andes in Bogotá, Colombia, and were previously characterized by Vargas et al. (Citation2009). Isolates were routinely grown on rye B agar (Caten & Jinks Citation1968) and maintained at 20 ºC. Sporangia from P. infestans were obtained by washing 2-week-old cultures grown on rye B agar with sterile distilled H2O (Goodwin et al. Citation1995). Sporangial concentration was measured using a hemocytometer and adjusted to 25 × 104 sporangia mL−1. After the concentration was adjusted, suspensions of each isolate were stored at 4 ºC for 2 h to induce zoospore formation. A drop (15 μL) of the zoospore suspension was placed on each side of the main vein of the P. peruviana leaves and potato leaflets.
Pathogenicity tests
Six-week-old P. peruviana plants ecotype Colombia were grown in a greenhouse under natural sunlight conditions in plastic bags with soil supplemented with fertilizer containing nitrogen, phosphorus and potassium (8-8-8) and irrigated every 2 days. In preliminary experiments, all isolates had been inoculated individually on yellow potato (S. tuberosum group phureja) leaflets and on P. peruviana leaves, giving essentially the same result on each host, respectively (see Results). Thus in subsequent experiments (molecular and biochemical assays), inoculations were done with mixtures of all three isolates. Plants were randomly selected and sorted into two groups of 30 plants each. Two days prior to inoculation, plants were moved to an inoculation chamber, with a photoperiod of 12 h. One group of plants was inoculated with a mixture of P. infestans isolates belonging to the clonal lineage EC-1 (A1 mating type) that included three strains, two isolated in 2007 [one from S. tuberosum (strain 1018)], and the other from P. peruviana (strain 4022) (Vargas et al. Citation2009) and a third isolated from S. tuberosum in 2009, strain Z3-2. The second group of plants was inoculated with sterile dH2O (mock-inoculated control).
Phytophthora infestans sporangia were obtained as mentioned above and the sporangial suspension was stored at 4 ºC for 2 h to induce zoospore release. The whole plant was sprayed until run-off, as previously described (Smart et al. Citation1998). Leaves from zoospore-inoculated and mock-inoculated treatments were sampled at different time points post-inoculation to perform the different assays. Yellow potato (Solanum tuberosum group phureja) plants inoculated with the same zoospore suspension were used as positive controls.
Microscopic observations
Detached P. peruviana leaves and potato leaflets were submerged in a Tween 20 solution (0.03%), then washed with dH2O and placed, abaxial side up, inside plastic Petri dishes containing water agar. Household maize starch (0.025 g mL−1) was added to the inoculum to visualize the deposition of inoculum drops, which upon drying left white marks due to the starchy residue (Colon et al. Citation1993). A drop (15 μL) of the zoospore suspension was placed on each side of the main vein of the P. peruviana leaves and potato leaflets. Potato leaflets inoculated with P. infestans were used as a positive control. Leaves inoculated with 15 µL of sterile dH2O that contained 0.025 g mL−1 of household maize starch were used as negative controls. All leaves were incubated at 15 ºC with a photoperiod of 16 h.
Leaf discs of about 1 cm2 (including the inoculum drop) were cut from the leaflet using a razor blade and leaf discs were placed inside a 12-well cell culture plate (one leaf disc per well). Leaf discs were subjected to trypan blue staining/clearing and aniline blue fluorescent staining (see below). Tissue was harvested at 1 to 7, 10, 12 and 15 days post-inoculation. For each time period, four P. peruviana and two potato leaf discs were selected for each of the three staining methods applied. The entire procedure was repeated three times. All images were obtained using a Zeiss Axio Imager A1 microscope (Zeiss, USA), equipped with a Zeiss AxioCam MRc colour video camera, and ZEISS AXIOVS40 4.6.3.0 software.
Trypan blue staining and trypan blue staining/clearing method
Leaves were stained following the procedure described by Wilson & Coffey (Citation1980). Trypan blue clearing was used to distinguish between dead and living cells. For clearing, leaves were first transferred into chloral hydrate solution (250 g per 100 mL of dH2O), placed under vacuum for 2 min and then incubated (still in the chloral hydrate solution) at 40 ºC for 1–5 days until they turned light blue in colour as previously described (Wilson & Coffey Citation1980; Colon et al. Citation1993; Smart et al. Citation2003). Physalis peruviana leaves and potato leaflets were then mounted in 60% glycerol (v/v) and were observed through a Zeiss Filter set 09 (product number 488,009–9901-000), which has a broad-pass excitation filter of 450–490 nm, an FT beam splitter at 510 nm, and a long-pass emission filter of 515+ nm.
Aniline blue fluorescent staining
For callose detection, leaves were stained using the method described by Adam & Somerville (Citation1996) with the following modifications. To de-stain chlorophyll, leaves were vacuum-infiltrated with 1.5 mL of alcoholic lactophenol (phenol: glycerol: lactic acid: water: ethanol = 1:1:1:1:8, v/v). Before staining with K2HPO4 (pH 9.5) containing 0.01% aniline blue, leaves were submerged in toluidine blue O (TBO) (0.05% TBO, 0.1 M sodium acetate pH 4.4) and left shaking at low speed for 20 min. Leaves were mounted in 25% glycerol and observed under epifluorescent illumination (365 nm excitation filter, 395 nm FT beam splitter and a BP emission filter 445/50 nm).
Electron microscopy
Inoculated leaves were observed directly by low-vacuum electron microscopy with a JEOL JSM 6490-LV scanning electron microscope at 1, 2, 3 and 4 days post-inoculation. Mock-inoculated leaves were also observed at these time periods.
Molecular and biochemical assays
Histochemical detection of H2O2
As mentioned above, for the biochemical assays, whole plants were inoculated and leaves were detached at different time points and used as needed. To detect hydrogen peroxide (H2O2), leaves were detached from inoculated and mock-inoculated plants at 12, 24, 48, 72 and 96 h after inoculation (hai). The detection of H2O2 in P. peruviana leaves was carried out using an in situ staining, where 3,3 diaminobenzidine (DAB) (Sigma-Aldrich Inc., St. Louis, MO) was used as a substrate according to the methods described by Orozco-Cardenas & Ryan (Citation1999). Three inoculated and three mock-inoculated leaves were stained and photographed per time point. The whole procedure was repeated three times.
Determination of the activity of pathogenesis-related proteins
We assayed activity of three pathogenesis-related proteins: β-1,3 glucanase (GLU), peroxidase (POX) and phenylalanine ammonia-lyase (PAL). Whole P. peruviana plants were inoculated or treated with water (mock-inoculated) and then leaves were detached at 0, 6, 12, 24, 48, 72, 96, 144, 216 and 288 hai. Leaves were frozen in liquid N2, ground to a fine powder, and stored at −80 °C until needed.
β-1,3 glucanase activity was assayed following the method described by Thangavelu et al. (Citation2003) measuring the production of glucose from the β-1,3 glucan laminarin (Sigma-Aldrich) at 500 nm. The concentration of glucose in the assays was calculated using a standard curve with known concentrations of glucose starting at 1 mg mL−1 and four 1/1 serial dilutions. Peroxidase activity was assayed colorimetrically using guaiacol as a substrate, following the method described by Małolepsza (Citation2006). Changes in optical densities were determined by measuring absorbance at 480 nm for 4 minutes at 1-minute intervals. Phenylalanine ammonia-lyase activity was assayed using L-phenylalanine as a substrate and measuring the amount of trans-cinnamic acid produced, according to the method described by Thangavelu et al. (Citation2003).
Total protein concentrations of all the extracts used for enzymatic activities were determined as performed by Bradford (Citation1976), using bovine serum albumin (BSA) as standard (Sigma-Aldrich). Enzymatic activity was calculated as the product formed in each reaction divided by the total protein concentration of each extract (μM product mg−1 of total protein).
Effects of treatment on the activity of the three proteins studied were analysed using R (R Development Core Team Citation2008). An independent-sample t-test was conducted to determine if the activity of β-1,3 glucanase, peroxidase and phenylalanine ammonia-lyase differed between treatments (inoculated and mock-inoculated) for each time period.
Real-time PCR expression analysis
Whole P. peruviana plants were inoculated or treated with water (mock-inoculated) and then leaves (from each of the two treatments) were harvested at 24, 48, 72 and 144 hai. Leaves were flash frozen in liquid N2 and stored at −80 °C. Three biological replicates were performed for the whole experiment.
RNA extraction was performed using the ConcertTM Plant RNA Reagent Kit from Invitrogen (Carlsbad, CA) and the resultant nucleic acid extract was treated with DNase (Sigma-Aldrich) following the manufacturer’s instructions. Quality of the RNA was assessed by electrophoresis on an RNA formaldehyde denaturing gel. RNA was quantified using a NanoDrop ND-1000 (Thermo Fisher Scientific, Wilmington, DE) and the concentration of each sample was adjusted to approximately 1000 ng μL−1. cDNA synthesis was performed using the iScript Select cDNA synthesis kit (BIORAD, Hercules, CA) following the manufacturer’s instructions.
The expression of three genes, acidic glucanase (GluA), carbonic anhydrase (CA) and glutathione reductase (GT), associated with plant defence, was investigated using real-time PCR. Primer 3 (Rozen & Skaletsky Citation2000) was used to design the primers: 5ʹ-AAGAATGGCAAGAAGGAGCA-3ʹ and 5ʹ-GCTTGCTACGACTCCTGGTC-3ʹ for GLUA; 5ʹ-CTGGCCCTTTGCTAGTTCAC-3ʹ and 5ʹ-GAAATGGCAACCGAATCCTA-3ʹ for CA; 5ʹ-AGGGAAGGGTGATAGGTCCA-3ʹ and 5ʹ-GCACGCTTCGGTAACTCTTC-3ʹ for GT; and 5ʹ-ACCACTGGTGGTTTTGAAGC-3ʹ and 5ʹ-ACGACCAACAGGGACAGTTC-3ʹ for elongation factor (EF). The latter gene was used as a constitutive expressed endogenous control. RNA isolated from mock-inoculated plants was used as the calibrator.
For the qRT-PCR the 2-Step SsoFast EvaGreen Supermix Kit was employed (BIORAD) and the PCR was run in an iCycler iQ5Tm (BIORAD). PCR consisted of one initial denaturation cycle at 95 °C for 30 s followed by 45 cycles of a two-step procedure: 5 s at 95 °C, and a final step of 10 s at 57.7 °C. After this, a melting curve was performed adding an extra step of 55–95 °C increasing 0.5 °C every 10 s.
All assays were carried out in triplicate and included two non-template controls for each pair of primers tested. One plate corresponded to each biological replicate and three biological replicates were evaluated. Results were analysed in the iCycler iQ5TM program and relative expression was calculated using REST 2009 Software (Pfaffl et al. Citation2002).
Evaluation of susceptibility to P. infestans in P. peruviana Colombian germplasm
Leaves from 54 accessions of P. peruviana were obtained from the germplasm bank at the Faculty of Agronomy of Universidad Nacional de Colombia in Bogotá (Herrera et al. Citation2012) (Supplementary Table 1). Plants were grown in a greenhouse under natural sunlight conditions and leaves were taken from the middle height of 6-week-old plants. Additionally, the Colombian commercial variety (ecotype Colombia) of P. peruviana was obtained from two different plant nurseries, both located in the department of Cundinamarca, Colombia (Semigar LTDA and Universidad Tadeo Lozano). Fully expanded P. peruviana leaves (from the germplasm and the nurseries) were placed abaxial side up on moistened absorbent paper in glass Petri plates (15 × 100 mm). These were then inoculated independently with P. infestans strains 1018, 4022 and 4084, by placing two drops of 15 μL of zoospore suspension (prepared as described above) on each side of the main vein of each leaf. Leaves inoculated with sterile distilled water were used as negative controls.
Results
Typical late blight symptoms were observed for all P. infestans isolates on potato when inoculated individually or as a mixture (Supplementary ). During the course of the experiments no macroscopic symptoms of late blight on P. peruviana were detected for any of the P. infestans isolates tested (Supplementary ). Indirect and direct germination of sporangia on inoculated leaves were observed microscopically on P. peruviana leaves (, and ). On potato, infection and sporulation were common (c) while in P. peruviana, although all of the P. infestans isolates produced appressoria (), sporulation was never observed. Any references made henceforth to the growth of the pathogen on P. peruviana leaves are strictly in reference to the growth of the initial inoculum.
Fig. 1 Inoculated leaves with the presence of sporangia, zoospores and/or germinating sporangia when observed directly with a scanning electron microscope. From the top left image to the bottom right one, the growth of Phytophthora infestans sporangia at different hours after inoculation (hai) can be observed. The presence of sporangia and encysted zoospores could be seen at 24 hai followed by the direct germination of zoosporangia at 48 hai and the mycelial growth of the initial inoculum at 72 and 96 hai. Strain 4084, isolated from P. peruviana in 2007, is shown in this figure.
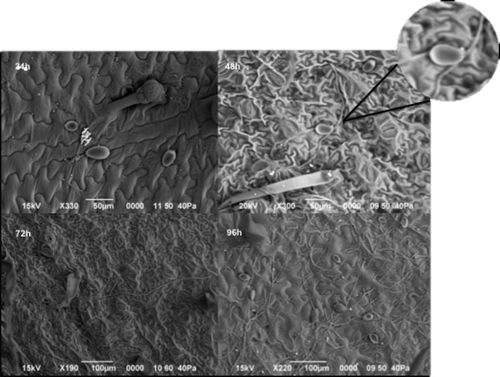
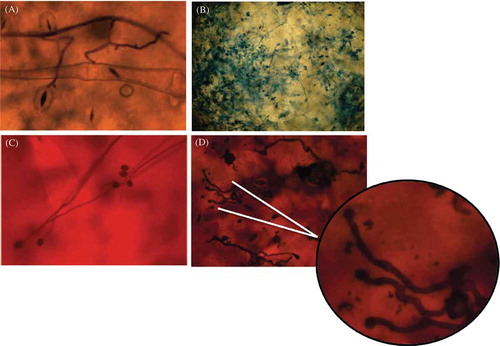
Fig. 2 (Colour online) Development of Phytophthora infestans at a microscopic level on inoculated P. peruviana leaves and potato leaflets. (A) Sporangial germination on infected P. peruviana leaves. Abundant mycelial growth was observed at 48 hai. (B) Formation of new zoosporangia was not seen on P. peruviana, in contrast to what was observed on potato leaflets. (C, D) Appressoria formation observed on P. peruviana leaves. Strain 4084, originally isolated from P. peruviana, was used for (A, B) and strain 2400 originally isolated from potato was used for (C, D).
Evidence for callose depositions, cell death and hydrogen peroxide accumulation
An increase in callose deposition was observed in inoculated P. peruviana leaves (a) when compared with the mock-inoculated ones (). Callose depositions were also observed in the interfaces between epidermal cells where the pathogen was present ( and ). This cell wall appositional material was never observed in mock-inoculated leaves. Epidermal cells showed thickened cell walls and auto-fluorescence under UV light, characteristic of the hypersensitive response (). These characteristics were associated with the location of the pathogen ().
Fig. 3 (Colour online) Callose depositions in the interfaces between epidermal cells are related to the presence of the pathogen. (A) Callose depositions observed as blue fluorescence on a P. peruviana leaf at 48 hai. (B) Mock-inoculated P. peruviana leaf. (C) The presence of the pathogen (white arrows) was evident on inoculated P. peruviana leaves after staining with aniline blue and observed through bright field microscopy. (D) Callose depositions were observed in the interfaces between epidermal cells when observed under epifluorescent illumination. (C) and (D) correspond to the same field photographed under bright field or (D) fluorescence microscopy. Arrows indicate the presence of the pathogen. Strain 4084, originally isolated from P. peruviana, was used for (A) and strain 2400, originally isolated from potato, was used for (C) and (D).
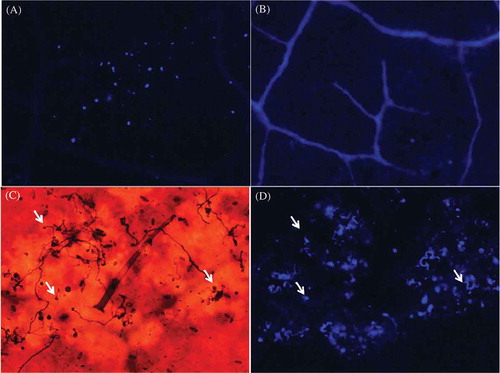
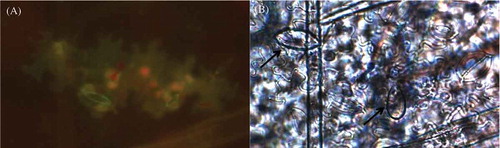
Fig. 4 (Colour online) Auto-fluorescence of cells expressing the hypersensitive response to Phytophthora infestans following the trypan blue clearing method. (A) Hypersensitive response observed as auto-fluorescence of the cells was mostly associated with the presence of sporangia and/or mycelia when detected through bright field microscopy. (B) Arrows indicate sporangia present near the affected cells. Strain 4084, originally isolated from P. peruviana, is shown on this figure.
The differential accumulation of hydrogen peroxide between inoculated and mock-inoculated treatments was first observed at 48 hai by the appearance of dark brown areas in inoculated leaves (). Differences between inoculated and mock-inoculated treatments could still be detected at 72 hai (data not shown). At 96 hai the presence of hydrogen peroxide in inoculated leaves did not differ from that observed in the mock-inoculated treatment (data not shown).
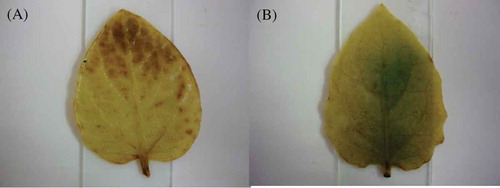
Fig. 5 (Colour online) Accumulation of hydrogen peroxide in P. peruviana leaves 48 h after inoculation with Phytophthora infestans and detected with diaminobenzidine. Hydrogen peroxide produces a dark pigment with diaminobenzidine. (A) P. peruviana leaf challenged with P. infestans. (B) Mock-inoculated P. peruviana leaf.
Activity of pathogenesis-related proteins
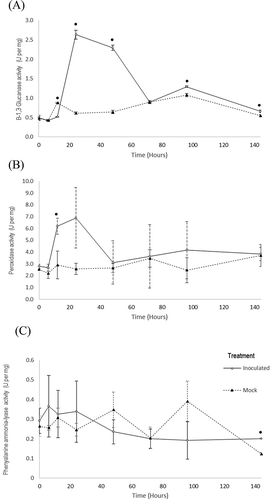
The activity of three pathogenesis-related proteins was assessed on P. peruviana leaves. An increase in the activity of β 1,3 glucanase (GLU) in inoculated P. peruviana leaves was observed at time points of 24, 48, 96 and 144 hai (P < 0.0001) (a). Significantly higher levels of POX were observed only at 12 hai on inoculated P. peruviana leaves when compared with mock-inoculated ones (P = 0.01) (). The activity of PAL, a key enzyme of the phenylpropanoid metabolism, was also measured. For PAL, a statistically significant difference between treatments was only observed at 144 hai (P = 0.01) ().
Fig. 6 Protein activity assays of three pathogenesis related proteins under controlled experimental conditions in P. peruviana leaves inoculated with Phytophthora infestans. (A) β-13 glucanase, (B) Peroxidase and (C) Phenylalanine ammonia lyase. Bars represent the standard deviation. The asterisk symbol (*) represents significant differences between the relative expressions of the genes with respect to mock-inoculated leaves. * (P < 0.05).
Gene expression of defence pathways and the antioxidative system
As observed in the enzymatic activity assays, expression of acidic glucanase (GluA) was highest at 24 and 48 hai on inoculated P. peruviana leaves when compared with mock-inoculated ones. Inoculated plants showed a significant increase in gene expression at all time points studied in comparison with the expression levels of mock-inoculated plants (P < 0.05) ( a). GluA showed a 10-fold increase in its expression at 24 hai followed by a decrease in expression that continued until the end of the experiment at 144 hai.
Fig. 7 Relative expression over time of genes involved in SA-dependent responses and genes induced by oxidative stress. (A) Acidic glucanase (GluA), (B) Carbonic anhydrase (CA) and (C) Glutathione reductase (GT). Numbers on the y-axis represent the change in relative expression compared with the expression on mock-inoculated leaves. Bars represent the expression of each gene studied. Bars represent the standard deviation. The asterisk symbol (*) represents significant differences between the relative expressions of the genes with respect to mock-inoculated leaves. * P < 0.05; ** P < 0.001.
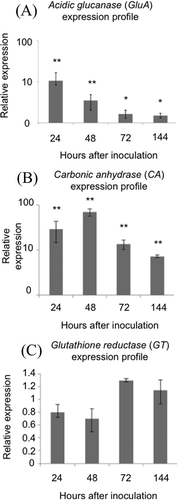
To further investigate the oxidative response of P. peruviana during its interaction with P. infestans, we studied the expression of carbonic anhydrase (CA) and glutathione reductase (GT). The plastidic CA gene experienced the largest change in expression among the genes tested. The expression of CA was significantly enhanced during the course of the experiment (P < 0.001), with highest expression at 48 hai and lowest at the final time point assayed (144 hai) (). For GT, no significant differences were observed in the relative gene expression of inoculated plants compared with the mock-inoculated treatment at any of the time points included in our experiment ().
Evaluation of susceptibility to P. infestans in P. peruviana Colombian germplasm
To test our hypothesis that P. peruviana cultivars grown in Colombia express defence responses against P. infestans strains, we screened wild and cultivated P. peruviana leaves for late blight symptoms. All 54 accessions as well as the commercial variety of P. peruviana showed no macroscopic symptoms after being inoculated with the P. infestans zoospore suspension. Yellow potato (S. tuberosum group phureja) was susceptible to this same inoculum in all tests.
Discussion
The unexpected absence of late blight symptoms on P. peruviana plants in the field subsequent to its initial observation led us to investigate and report the responses triggered by P. infestans in P. peruviana leaves. We demonstrated that the P. peruviana ecotype used in this study, which is the most commonly grown ecotype in Colombia, showed plant defence responses against some P. infestans strains.
All results confirmed that the populations of P. peruviana in Colombia are incompatible when challenged with P. infestans. Depositions of (1,3) β-glucan (callose) were more abundant in inoculated leaves versus mock-inoculated ones, implying an active defence response. As observed by Vleeshouwers et al. (Citation2000), callose depositions appeared to be mainly associated with the occurrence of the hypersensitive response (HR) in the tissue. These depositions accumulated distinctively at the walls of epidermal cells that were in direct contact with the pathogen, perhaps blocking intercellular metabolite exchange (Scharte et al. Citation2005). These pathogen-induced cell wall fortifications have also been previously shown in the interaction between the resistant Solanum nigrum and P. infestans (Colon et al. Citation1993). In this study, we observed limited penetration and mycelial development of P. infestans, as well as necrosis of invaded and adjacent cells. Similar results were obtained by Colon et al. (Citation1993) when studying the interaction between the highly resistant S. nigrum and P. infestans.
The hypersensitive response is one of the main defence responses associated with all forms of resistance against P. infestans (Vleeshouwers et al. Citation2000). Production of reactive oxygen species has been proposed as a marker of HR in plant defence reactions (Orozco-Cardenas & Ryan Citation1999) and it is well known as an indicator for successful recognition of plant pathogens (Torres & Dangl Citation2005). In our study, we observed a strong accumulation of ROS in hypersensitive response type lesions 48 hai.
A protein activity assay of three PR proteins (GLU, POX and PAL) was performed in order to test our hypothesis that PR proteins are activated when P. peruviana plants are challenged with P. infestans. We found that two of the three enzymes tested, GLU and POX, showed an early increased activity in P. peruviana plants challenged with P. infestans when compared with mock-inoculated ones. Plant β-1,3-glucanases show a broad range of biological functions both in normal physiological processes as well as in the defence response against pathogens (Liu et al. Citation2013). It has been proposed that a target for this hydrolytic enzyme are β-1,3 glucans, one of the main cell wall components of Phytophthora species (Schroder et al. Citation1992; Kim & Hwang Citation1997). However, these enzymes may also degrade plant-derived callose deposits and their induction can even enhance susceptibility to viruses (Iglesias & Meins Citation2000). Furthermore, plant β-1,3-glucanases have been shown to negatively affect the callose deposition in Arabidopsis challenged by the fungus Leptosphaeria maculans (Oide et al. Citation2013). The regulation of callose synthesis or the differential subcellular localization of members of the β-1,3-glucanases protein family may explain the ambiguity of the role of GLU in defence (Voigt & Somerville Citation2009). Undoubtedly more studies are needed to understand the role of GLU in different plant–pathogen interactions.
An increase in the activity of POX was observed only at 12 hai. Peroxidase activity positively correlates with defence to pathogens (Peng & Kuc Citation1992; Joseph et al. Citation1998; Thangavelu et al. Citation2003). Furthermore, POX activity has been implicated in ROS generation as well as ROS removal (Wojtaszek Citation1997; Małolepsza Citation2006). Our combined results, showing the increase in ROS generation as well as an increase in POX activity, suggest that both mechanisms are most likely a part of the P. peruviana defence responses against P. infestans. These defence mechanisms might have contributed to the restriction of invasion of P. infestans in P. peruviana leaves.
The upregulation of the plastid gene for the CA at all time periods studied indicates that there is an increase in the oxidative pathway when P. peruviana plants are challenged with P. infestans. Previous studies have reported CA upregulation at early time points during incompatible interactions between P. infestans and potato or tobacco (Nicotiana benthamiana), contrasting with downregulation during the compatible interactions between the same hosts (Slaymaker et al. Citation2002; Restrepo et al. Citation2005). The plastidic CA seems to have a key role in the activation of HR in resistant genotypes of potato challenged with P. infestans (Restrepo et al. Citation2005). The CA is located in the stroma of the chloroplast and has been shown to facilitate the supply of CO2 to Rubisco by maintaining equilibrium between HCO and CO2 within the chloroplast. It has also been demonstrated to bind to salicylic acid (SA) (Slaymaker et al. Citation2002), but it seems that these two functions are independent. Further research is needed to understand how the CA is involved in the regulation of ROS levels inside the chloroplast and in which ways SA interacts with CA in the resistance response.
Due to the cellular location of the reactions performed by the genes tested in this study, our results agree with studies performed in other hosts that identify the chloroplast as an important location in the plant defence response against pathogens such as P. infestans (Matsumura et al. Citation2003). It is well known that disease development downregulates photosynthesis-related genes and alters the photosynthetic electron transport (Schnabel et al. Citation1998; Restrepo et al. Citation2005). However, the mechanism through which the photosynthetic apparatus is turned down remains to be elucidated. Further investigation is needed to understand the way in which the chloroplast is affected by different sources of stress.
We have shown that P. peruviana activates typical host defence mechanisms against the P. infestans strains studied. The observation of very limited germination of the pathogen, restricted to growth of the initial inoculum, in addition to the detectable defence responses such as callose depositions, accumulation of hydrogen peroxide, cell death, activation of pathogenesis-related proteins and expression of genes associated with plant defence, supports our conclusions that the P. peruviana genotype used for this study recognizes the pathogen and induces defence responses.
The reason(s) for changes in disease reaction from 2007 remains unknown. Three possible explanations are: (i) loss of pathogenicity of the strains during storage, (ii) a change in the genetic composition of the host given that in Colombia, no breeding programmes for P. peruviana exist and growers make their own selection of planting material from the previous cycle, thus changing the genetic composition of what they know as ecotype Colombia by selecting for resistant genotypes; and (iii) an environmental factor operating in 2007 that we have not been able to identify and replicate. The first explanation seems improbable because none of the strains lost pathogenicity to potato. The possibility exists that P. peruviana may have changed over time or that environmental conditions were unique in 2007.
Supplemental data
Supplemental data for this article can be accessed here: http://dx.doi.org/10.1080/07060661.2014.975157
Supplemental Figures
Download PDF (125.6 KB)Acknowledgements
This work was supported by the Faculty of Science at Universidad de los Andes. We thank the Biological Science Department for the scholarship given to G. D. and C. A. We are grateful to the LAZOEA laboratory directed by Emilio Realpe and the EVODEVO laboratory directed by Federico Brown, especially to Marcela Bolaños. We also thank the Instituto Amazónico de Investigaciones Cientificas SINCHI as well as María Isabel Chacón from Universidad Nacional de Colombia for providing access to leaves from 54 different accessions of Physalis peruviana. We further thank Paola Zuluaga for her assistance and Gregory J. Buda for his help and advice in microscopy.
References
- Adam L, Somerville SC. 1996. Genetic characterization of five powdery mildew disease resistance loci in Arabidopsis thaliana. Plant J. 9:341–356. doi:10.1046/j.1365-313X.1996.09030341.x
- Adler NE, Erselius LJ, Chacón MG, Flier WG, Ordoñez ME, Kroon LP, Forbes GA. 2004. Genetic diversity of Phytophthora infestans sensu lato in Ecuador provides new insight into the origin of this important plant pathogen. Phytopathology. 94:154–162. doi:10.1094/PHYTO.2004.94.2.154
- Andrivon D, Avendaño-Córcoles J, Cameron AM, Carnegie SF, Cooke LR, Corbière R, Detourné D, Dowley LJ, Evans D, Forisekova K, et al.. 2011. Stability and variability of virulence of Phytophthora infestans assessed in a ring test across European laboratories. Plant Pathol. 60:556–565. doi:10.1111/j.1365-3059.2010.02392.x
- Becktell MC, Smart CD, Haney CH, Fry WE. 2006. Host-pathogen interactions between Phytophthora infestans and the solanaceous hosts Calibrachoa x hybridus, Petunia x hybrida and Nicotiana benthamiana. Plant Dis. 90:24–32. doi:10.1094/PD-90-0024
- Bonilla ML, Espinosa K, Posso AM, Vásquez HD, Muñoz JE. 2008. Morphological characterization of 24 accessions of cape gooseberry from the national university campus Palmira’s gemplasm bank. Acta Agron. 57:101–108.
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 72:248–254. doi:10.1016/0003-2697(76)90527-3
- Cárdenas ME, Medina E, Tabima J, Vargas A, Lopera C, Bernal A, Restrepo S. 2011. First report of Phytophthora infestans causing late blight on Solanum viarum in Colombia. Plant Dis. 95:875. doi:10.1094/PDIS-11-10-0853
- Caten CE, Jinks JL. 1968. Spontaneous variability of single isolates of Phytophthora infestans. I. Cultural variation. Can J Bot. 46:329–348. doi:10.1139/b68-055
- Chacón MG, Adler NE, Jarrin F, Flier WG, Gessler C, Forbes GA. 2006. Genetic structure of the population of Phytophthora infestans attacking Solanum ochranthum in the highlands of Ecuador. Eur J Plant Pathol. 115:235–245. doi:10.1007/s10658-006-9012-9
- Chen C-Q, Huang -L-L, Buchenauer H, Zhao H-Y, Zuo Y-H, Kang Z-S. 2009. Diversity among single zoospores isolates derived from single-zoosporangia of Phytophthora sojae Kauf. and Gerd. J Phytopathol. 157:181–187. doi:10.1111/j.1439-0434.2008.01462.x
- Colon IT, Eijlander R, Budding DJ, Ijzendoorn MT, Pieters MMJ, Hoogendoorn J. 1993. Resistance to potato late blight (Phytophthora infestans (Mont.) de Bary) in Solanum nigrum, S. villosum and their sexual hybrids with S. tuberosum and S. demissum. Euphytica. 66:55–64. doi:10.1007/BF00023508
- Espinosa K, Bonilla ML, Muñoz JE, Posso AM, Vásquez HD. 2004. Colección, caracterización fenotípica y molecular de poblaciones de uchuva Physalis peruviana. Facultad de ciencias agropecuarias. 2:72–78.
- Fry W. 2007. The canon of potato science: 10. Late blight and early blight. Potato Res. 50:243–245. doi:10.1007/s11540-008-9046-9
- Fry W. 2008. Phytophthora infestans: the plant (and R gene) destroyer. Mol Plant Pathol. 9:385–402. doi:10.1111/j.1364-3703.2007.00465.x
- Goodwin SB, Sujkowski LS, Fry WE. 1995. Rapid evolution of pathogenicity within clonal lineages of the potato late blight disease fungus. Phytopathology. 85:669–676. doi:10.1094/Phyto-85-669
- Herrera AM, Fischer G, Chacón MG. 2012. Agronomical evaluation of cape gooseberries (Physalis peruviana l.) from central and North-Eastern Colombia. Agronomía Colombiana. 30:15–24.
- Iglesias VA, Meins Jr F. 2000. Movement of plant viruses is delayed in a beta-1,3-glucanase-deficient mutant showing a reduced plasmodesmatal size exclusion limit and enhanced callose deposition. Plant J. 21:157–166. doi:10.1046/j.1365-313x.2000.00658.x
- Jinks JL, Grindle M. 1963. Changes induced by training in Phytophthora infestans. Heredity. 18:245–264. doi:10.1038/hdy.1963.29
- Joseph L, Koon T, Man WS. 1998. Antifungal effects of hydrogen peroxide and peroxidase on spore germination and mycelial growth of Pseudocercospora species. Botany. 76:2119–2124.
- Kim YJ, Hwang BK. 1997. Isolation of a basic 34 kilodalton β-1,3-glucanase with inhibitory activity against Phytophthora capsici from pepper stems. Physiol Mol Plant Pathol. 50:103–115. doi:10.1006/pmpp.1996.0073
- Liu DQ, He X, Li WX, Chen CY, Ge F. 2013. A β-1,3-glucanase gene expressed in fruit of Pyrus pyrifolia enhances resistance to several pathogenic fungi in transgenic tobacco. Eur J Plant Pathol. 135:265–277. doi:10.1007/s10658-012-0083-5
- Małolepsza U. 2006. Induction of disease resistance by acibenzolar-s-methyl and o-hydroxyethylorutin against Botrytis cinerea in tomato plants. Crop Prot. 25:956–962. doi:10.1016/j.cropro.2005.12.009
- Matsumura H, Reich S, Ito A, Saitoh H, Kamoun S, Winter P, Kahl G, Reuter M, Kruger DH, Terauchi R. 2003. Gene expression analysis of plant host-pathogen interactions by Supersage. Proc Natl Acad Sci USA. 100:15718–15723. doi:10.1073/pnas.2536670100
- Novoa RH, Bojacá M, Galvis JA, Fischer G. 2006. La madurez del fruto y el secado del cáliz influyen en el comportamiento poscosecha de la uchuva (Physalis peruviana) almacenada a 12°C. Agronomía Colombiana. 24:77–86.
- Oide S, Bejai S, Staal J, Guan N, Kaliff M, Dixelius C. 2013. A novel role of pr2 in abscisic acid (aba) mediated, pathogen induced callose deposition in Arabidopsis thaliana. New Phytol. 200:1187–1199. doi:10.1111/nph.12436
- Orozco-Cardenas M, Ryan CA. 1999. Hydrogen peroxide is generated systemically in plant leaves by wounding and systemin via the octadecanoid pathway. Proc Natl Acad Sci USA. 96:6553–6557. doi:10.1073/pnas.96.11.6553
- Peng JLM, Koon TT, Man WS. 1998. Antifungal effects of hydrogen peroxide and peroxidase on spore germination and mycelial growth of Pseudocercospora species. Can J Bot. 76:2119–2124.
- Peng M, Kuc J. 1992. Peroxidase-generated hydrogen peroxide as a source of antifungal activity in vitro and on tobacco leaf disks. Phytopathology. 82:696–699. doi:10.1094/Phyto-82-696
- Pfaffl MW, Horgan GW, Dempfle L. 2002. Relative expression software tool (REST(C)) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucl Acids Res. 30:36e–36. doi:10.1093/nar/30.9.e36
- R development core team. 2008. R: A language and environment for statistical computing. r foundation for statistical computing. Vienna, Austria: Institute for Statistics and Mathematics of WU (Wirtschaftsuniversität Wien). ISBN 3-900051-07-0, url http://www.r-project.org.).
- Reddick D, Crosier W. 1933. Biological specialization in Phytophthora infestans. Am Pot J. 10:129–134. doi:10.1007/BF02884488
- Restrepo S, Myers K, Del Pozo O, Martin GB, Hart AL, Buell CR, Fry WE, Smart CD. 2005. Gene profiling of a compatible interaction between Phytophthora infestans and Solanum tuberosum suggests a role for carbonic anhydrase. Mol Plant-Microbe Interact. 18:913–922. doi:10.1094/MPMI-18-0913
- Rozen S, Skaletsky H. 2000. Primer3 on the www for general users and for biologist programmers. Meth Mol Biol. 132:365–386.
- Runno-Paurson E, Fry WE, Remmel T, Mänd M, Myers KL. 2010. Phenotypic and genotypic characterization of Estonian isolates of Phytophthora infestans in 2004-2007. J Plant Pathol. 92:375–384.
- Samen A-E, Secor GA, Gudmestad NC. 2003. Variability in virulence among asexual progenies of Phytophthora infestans. Phytopathology. 93:293–304. doi:10.1094/PHYTO.2003.93.3.293
- Scharte J, Schon H, Weis E. 2005. Photosynthesis and carbohydrate metabolism in tobacco leaves during an incompatible interaction with Phytophthora nicotianae. Plant Cell Environ. 28:1421–1435. doi:10.1111/j.1365-3040.2005.01380.x
- Schnabel G, Strittmatter G, Noga G. 1998. Changes in photosynthetic electron transport in potato cultivars with different field resistance after infection with Phytophthora infestans. J Phytopathol. 146:205–210. doi:10.1111/j.1439-0434.1998.tb04681.x
- Schroder M, Hahlbrock K, Kombrink E. 1992. Temporal and spatial patterns of 1, 3-beta-glucanase and chitinase induction in potato leaves infected by Phytophthora infestans. Plant J. 2:161–172. doi:10.1111/j.1365-313X.1992.00161.x
- Slaymaker DH, Navarre DA, Clark D, Del Pozo O, Martin GB, Klessig DF. 2002. The tobacco salicylic acid-binding protein 3 (sabp3) is the chloroplast carbonic anhydrase, which exhibits antioxidant activity and plays a role in the hypersensitive defense response. Proc Natl Acad Sci USA. 99:11640–11645. doi:10.1073/pnas.182427699
- Smart CD, Myers KL, Restrepo S, Martin GB, Fry WE. 2003. Partial resistance of tomato to Phytophthora infestans is not dependent upon ethylene, jasmonic acid, or salicylic acid signaling pathways. Mol Plant-Microbe Interact. 16:141–148. doi:10.1094/MPMI.2003.16.2.141
- Smart CD, Willmann MR, Mayton H, Mizubuti ESG, Sandrock RW, Muldoon AE, Fry WE. 1998. Self-fertility in two clonal lineages of Phytophthora infestans. Fungal Genet Biol. 25:134–142. doi:10.1006/fgbi.1998.1099
- Thangavelu R, Palaniswami A, Doraiswamy S, Velazhahan R. 2003. The effect of Pseudomonas fluorescens and Fusarium oxysporum f. sp. cubense on induction of defense enzymes and phenolics in banana. Biol Plant. 46:107–112. doi:10.1023/A:1022374520121
- Torres M, Dangl J. 2005. Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr Opin Plant Biol. 8:397–403. doi:10.1016/j.pbi.2005.05.014
- Vargas AM, Correa A, Lozano DC, González A, Bernal AJ, Restrepo S. 2007. First report of late blight caused by Phytophthora infestans on Cape gooseberry (Physalis peruviana) in Colombia. Plant Dis. 91:464. doi:10.1094/PDIS-91-4-0464B
- Vargas AM, Quesada Ocampo LM, Céspedes MC, Carreño N, González A, Rojas A, Zuluaga AP, Myers K, Fry WE, Jiménez P, et al. 2009. Characterization of Phytophthora infestans populations in Colombia: First report of the A2 mating type. Phytopathology. 99:82–88. doi:10.1094/PHYTO-99-1-0082
- Vleeshouwers VGA, Van Dooijeweert W, Govers F, Kamoun S, Colon LT. 2000. The hypersensitive response is associated with host and nonhost resistance to Phytophthora infestans. Planta. 210:853–864. doi:10.1007/s004250050690
- Voigt CA, Somerville SC. 2009. Callose in biotic stress (pathogenesis): biology, biochemistry and molecular biology of callose in plant defence: Callose deposition and turnover in plant–pathogen interactions. In: Bacic A, Fincher GB, Stone BA, editors. Chemistry, biochemistry, and biology of 1-3 beta glucans and related polysaccharides. London, NY: Academic Press.
- Wilson UE, Coffey MD. 1980. Cytological evaluation of general resistance to Phytophthora infestans in potato foliage. Ann Bot. 45:81–90.
- Wojtaszek P. 1997. Oxidative burst: An early plant response to pathogen infection. Biochem J. 322:681–692.
