Abstract
Verticillium wilt is caused mainly by Verticillium dahliae Kleb. and is a constraint for growers of many economic crops and ornamental plant species. Disease control necessitates the use of multiple methods usually coordinated as part of a disease management strategy. Unlike foliar diseases, much information is lacking for knowledge-based strategies to be developed for managing root infection. Revealing the mechanisms of interaction between the pathogen and its hosts would be a first step towards achieving such a goal. More effort needs to be directed towards understanding how the pathogen infects and colonizes its hosts, and how plants respond to these invaders. This review describes several of the efforts to elucidate the interactions of V. dahliae with its hosts, and raises some of the key points that need to be addressed in future work on this important disease.
Résumé
La flétrissure verticillienne est principalement causée par Verticillium dahliae Kleb. et constitue une entrave à la production de nombreuses plantes agricoles et ornementales de grande importance économique. La lutte contre cette flétrissure nécessite l’utilisation de diverses méthodes habituellement coordonnées dans le cadre d’une stratégie de gestion de la maladie. À la différence des maladies foliaires, l’information permettant de développer des stratégies fondées sur la connaissance visant à maîtriser l’infection racinaire fait défaut. La mise au jour des mécanismes qui gèrent les interactions entre l’agent pathogène et ses hôtes serait un premier pas dans la bonne direction. En conséquence, des efforts supplémentaires doivent être consentis à la compréhension de l’infection et de la colonisation des plantes hôtes par l’agent pathogène ainsi que de la réaction de ces plantes à leurs envahisseurs. Cette revue décrit plusieurs des efforts déployés pour expliquer les interactions entre V. dahliae et ses plantes hôtes, et souligne certains points essentiels qui doivent être pris en considération au cours des travaux futurs effectués sur cette maladie très importante.
Introduction
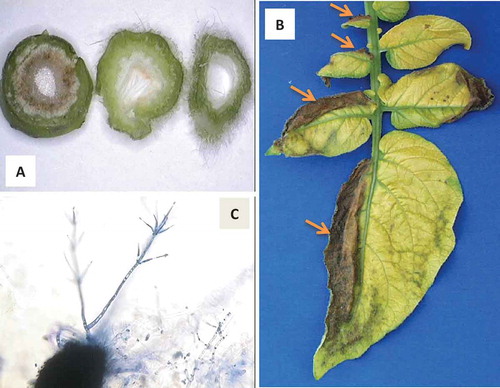
Verticillium wilt is an economically important disease affecting hundreds of dicotyledonous plant species worldwide, not only in field crops, vegetables and fruits, but also ornamental plants (Woolliams Citation1966; Krikun & Bernier Citation1987; Pegg & Brady Citation2002). Fungal pathogens in the genus Verticillium, which includes different species that infect plants, also infect insects, nematodes, arachnids and even other fungi (Bidochka et al. Citation1999). Six Verticillium species cause diseases in plants: V. nigrescens Pethybr., V. nubilum Pethybr., V. tricorpus Isaac., V. theobromae (Turc.) Mas. & Hughes, V. dahliae Kleb., and V. albo-atrum Reinke & Berth. (Pegg & Brady Citation2002). The latter two are the most devastating (Barbara & Clewes Citation2003). In some cases, these species cause plant diseases in conjunction with other pathogens, such as in the case of early dying syndrome of potatoes, where not only V. dahliae but other fungi, bacteria and nematodes can be involved (Rowe & Powelson Citation2002). Symptoms of verticillium wilt include chlorosis, necrosis, stunting, vascular discolouration (), and wilting. Sometimes, symptoms are first visible on one side of the leaves or leaflets ().
Fig. 1 (Colour online) (A) Cross-sections of a sunflower stem showing vascular discolouration in the lower stem section (left) as opposed to upper stem (middle and right) after inoculation with Verticillium dahliae; (B) Chlorosis and necrosis on a potato leaf, with necrosis more prominent on one side of each of the affected leaflets -arrows-; (C) Microsclerotium (in black) and mycelium showing the characteristic branching of Verticillium.
Protecting crops from wilts caused by V. dahliae is challenging due to many factors (Johnson & Dung Citation2010), with the soilborne nature of the fungus being a major constraint. As opposed to foliar fungal pathogens, which can be managed with repeated fungicide applications, soilborne pathogens reside deep in the soil, and cannot be targeted specifically using any of the current chemical application techniques. Chemicals with a broad activity range have been used to reduce Verticillium propagules in the soil through soil fumigation (Powelson & Rowe Citation1993), but such a practice presents several disadvantages: it is increasingly expensive and therefore less and less affordable by growers; it results in negative effects on the beneficial soil flora, making further fumigation a requirement in the following years in order to keep pathogenic colonizers away; it increases the risk of pathogen resistance development to the chemical; and above all, it can pose negative effects on the health of humans and the environment.
Cultural practices can also be used to manage wilts caused by soilborne pathogens. For example, rotations do help growers of many economically important crops in mitigating the impact of soilborne diseases (Xiao et al. Citation1998; Peters et al. Citation2003). However, such a practice is not always efficient in reducing the negative impact of verticillium wilts, because V. dahliae produces long-lived microsclerotia (), which may survive in soils for more than a decade (Wilhelm Citation1955) and invade hosts as soon as they become available in their vicinity. Other practices to manage verticillium wilts previously tested include solarization (Tjamos & Paplomatas Citation1988) and the use of biological control agents, including beneficial bacteria, and plant extracts (Uppal et al. Citation2007, Citation2008), or soil amendments (Molina et al. Citation2014). An ideal management tool would be the availability of plant cultivars with high levels of resistance to this disease, but these are lacking in many cultivated crop species. Defining resistance and susceptibility versus tolerance should be revisited, as classical interpretations of tolerance have been challenged with the advent of new techniques (Robb Citation2007). As in any other host-pathogen interaction, understanding the mechanisms by which the pathogen inflicts disease to its host, and by which the host either resists or succumbs, both represent an important starting point if sustainable disease control strategies are to be developed.
Understanding the interaction between V. dahliae and its host species
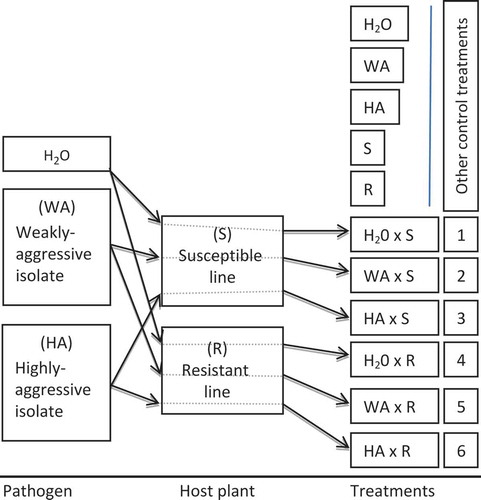
Investigating the intricacies of any host-pathogen interaction requires models that are best suited for their analysis and valid interpretation. For this purpose, some studies were aimed at identifying the most relevant components in a host-pathogen interaction (Daayf et al. Citation1998). Selecting the isolates, the host genotypes and environmental conditions that are relevant for such studies, and taking the time to devise a model provides more confidence in further interpretation of the results (). Knowledge on the diversity of both the pathogen and the host is a pre-requisite to understanding the mechanisms that govern any host-pathogen interaction. In the case of V. dahliae, such diversity has been studied in pathogen populations from different geographic and host origins, but such knowledge has not been fully exploited when investigating the concomitant host-pathogen interactions.
Fig. 2 (Colour online) Diagram showing a system aimed at the dissection of host-pathogen interactions in terms of reactions that are specific to highly versus weakly aggressive isolates when inoculated to susceptible versus resistant host lines, in different combinations. Comparative studies using this system may encompass pathogenicity, proteomics, gene expression, metabolomics and other tests such as microarray and RNAseq analyses where materials from treatments 1 to 6 are compared to each other and with the different controls. cDNA from the different treatments shown here can be further used with subtractive methods: i.e.: SH/cDNA-AFLP, which may lead to the identification of new potential plant susceptibility and/or resistance genes, as well as those from the pathogen potentially controlling defence suppressors or elicitors. For example subtractive hybridizations involving ‘3 minus 2’ and ‘6 minus 5’, compared with ‘HA’ and ‘WA’, helped identify potential pathogenicity factors of P. infestans and V. dahliae. More concrete examples using this system are shown in Henriquez & Daayf (Citation2010) and El-Bebany et al. (Citation2011). H2O: sterilized water; WA: weakly aggressive isolate; HA: highly aggressive isolate; S: susceptible line; R: resistant line. Numbers 1 to 6 indicate interaction treatments.
Structure of V. dahliae populations and impact on the pathogen interactions with host plants
A major challenge in dealing with fungal pathogens is their ability to adapt to new environments and chemicals. Successful pathogens continually undergo changes in the structure of their populations, and V. dahliae is no exception. It is well known for the plasticity of its genome, which allows it to be highly variable (Klosterman et al. Citation2009). While sexual reproduction is considered to be the main driver for the evolution of virulence in other plant pathogens, asexual pathogens such as V. dahliae (Fradin & Thomma Citation2006), which mainly have mutations to adapt to their environment and hosts, fare not only as one of the most destructive plant pathogens, but also a highly variable one. Such variability poses challenges in making plant selections for breeders using specific strains in their testing, or for agrochemical companies testing new active ingredients against existing strains. The task becomes even more complex when such variability is combined with the pathogen’s strong responsiveness to environmental clues, resulting in an even more diverse population of the pathogen. The diversity of V. dahliae has been extensively studied, using strains from different crops and origins, and covering everything from morphology to several ‘omics’ in describing V. dahliae strains (Klosterman et al. Citation2009; El-Bebany et al. Citation2010, Citation2011). Most studies made an effort to link genetic diversity markers with phenotypes such as pathogenicity, virulence and vegetative compatibility (Daayf et al. Citation1995; Alkher et al. Citation2009a, Citation2009b; El-Bebany et al. Citation2013b). Some progress has been made in the latter area as a good marker that relates well to pathogenicity. Studies using isolates from Africa, Asia, Europe and North America, showed correlations between V. dahliae’s VCGs and their pathotype and race status on cotton and tomato (Daayf et al. Citation1995), potato (Joaquim & Rowe Citation1991) and other plant species (Collado-Romero et al. Citation2006). More recently, molecular markers such as SNPs, RFLP, RAPD, RAMS and others using PCR-based techniques have been explored to explain the diversity and variability of V. dahliae (Nazar et al. Citation1991; Pantou & Typas Citation2005; Collado-Romero et al. Citation2006; El-Bebany et al. Citation2013a; Gharbi et al. Citation2014a, Citation2014b), while other studies used proteomics (El-Bebany et al. Citation2010), transcriptomics (El-Bebany et al. Citation2011), and DNA sequencing (Pantou & Typas Citation2006).
Host species diversity and impact on verticillium wilt
The host range of V. dahliae is wide, with verticillium wilt affecting many economically important crops, including plant species once considered as non-hosts (Subbarao et al. Citation1997). Nevertheless, some isolates can exhibit a certain level of specificity to preferential hosts. For example, Verticillium isolates from tobacco (Taylor Citation1969), peppermint (Horner Citation1954) and cocoa (Resende et al. Citation1994) were reported to cause disease in a restricted number of host species. Another study by Bhat & Subbarao (Citation1999) showed that V. dahliae isolates from artichoke, lettuce, potato, strawberry, tomato and watermelon had no host range specificity, whereas those from bell pepper, cabbage, cauliflower, cotton, eggplant and mint were pathogenic to a restricted group of host species. Some studies may be carried out using a limited number of isolates, especially if multiple host species are assessed at the same time. Therefore, extrapolating the results to all isolates originating from a certain host may not always be justified. One must keep in mind that when an isolate is collected from a given host plant, there is no certainty of how many generations it has been on that same crop (Alkher et al. Citation2009b). Alkher et al. (Citation2009a) investigated cross-pathogenicity of V. dahliae between potato and sunflower using several isolates from each of the two crops, and showed pathogenic diversity within isolates from each crop. Those from potato were highly aggressive on both potato and sunflower, whereas those from sunflower did not cause much damage on potato (Alkher et al. Citation2009a).
Variability of V. dahliae and its pathogenic abilities offer numerous possibilities for studying the mechanisms by which pathogens are able to adapt to new hosts under different conditions. Understanding the components and mechanisms of such pathogenic abilities will be useful in searching for innovative alternatives to manage verticillium wilt and other soilborne diseases in economically important crops. For this purpose, taking V. dahliae’s specific traits into consideration when dissecting host responses to this pathogen will help generate more efficient disease management approaches.
Components of plant defences against verticillium wilts
Defence and counter-defence mechanisms have been investigated in different host-pathogen systems during the last 20 years (Göhre & Robatzek Citation2008; El Hadrami et al. Citation2009; Niks & Marcel Citation2009; El-Oirdi et al. Citation2011). More recently, there has been mention of ‘extreme resistance’ as a host counter-counter defence strategy (Sansregret et al. Citation2013). Several of these aspects involving both fungal and oomycete pathogens have been reviewed recently (Alba et al. Citation2011; El Hadrami et al. Citation2012), but it is noticeable that similar studies involving soilborne pathogens are lacking (Ramos et al. Citation1997; Daayf et al. Citation2003).
Considering the current advances in molecular plant pathology, relatively little is known about plant defences that are specific to vascular and/or root pathogens. Many years ago, authors used phytochemistry, histology, histochemistry and electron microscopy to document changes in plant tissues infected by these pathogens (Goodman & Plurad Citation1971; Hückelhoven Citation2007), revealing the implication of structural, i.e. tyloses (Dixon & Pegg Citation1969), as well as metabolic responses, i.e. phytoalexins (Hammerschmidt Citation1999) and signalling molecules (Derksen et al. Citation2013b), in plant resistance to pathogens. Molecular techniques have been deployed to understand defence mechanisms such as the induction of PR-proteins (Van Loon et al. Citation2006), the expression of known defence genes and the identification and cloning of R and avr genes (Gebhardt & Valkonen Citation2001; Kawchuk et al. Citation2001; Tyler Citation2002). Unfortunately, there seems to be a gap between some of the early studies, involving structural or biochemical descriptions, and current investigations, which exclusively use molecular techniques to understand these host-pathogen interactions. For example, many recent papers assess plant defence genes involved in pathways that are important for resistance, without analysing the end product of such pathways, i.e. phytoalexins. In cotton, one of the most studied hosts of V. dahliae, phytoalexins were originally considered as key players in plant resistance to this pathogen on the basis of biochemical and histochemical investigations by the teams of Bell, Beckman, Stipanovic and Mace (Bell Citation1969; Mace et al. Citation1985). Nowadays, several teams are investigating the molecular aspects of this interaction, but little is done to connect the most current data with the earliest findings.
Plant tissue responses to V. dahliae belong to a wide range of structural and/or biochemical categories, and can be either constitutive, i.e. part of the host plant before infection (Mueller & Morgham Citation1993), or induced in response to infection (Daayf et al. Citation1997). An important aspect of defence responses in any host-pathogen interaction is the manner in which they are coordinated against the pathogen. Harrison and Beckman (Citation1982) suggested a two-step system to explain the sequence of events leading to cotton plant resistance against Verticillium, with an initial ultrastructural reorganization of vessel contact cells immediately above the penetration site (Harrison & Beckman Citation1982), followed by release of antifungal substances that coat the pathogen (Mueller & Morgham Citation1993). Another known structural response to vascular pathogens is the formation of tyloses (Dixon & Pegg Citation1969), which occlude stem vessels of plants infected by V. dahliae, thereby restricting the spread of secondary conidia throughout the plant (Mace Citation1978; Beckman Citation1987). Using both cotton species Gossypium hirsutum and G. barbadense, considered as susceptible and tolerant to verticillium wilt, respectively, Daayf et al. (Citation1997) reported structural responses to inoculation with a very aggressive isolate of V. dahliae that included the accumulation of paramural deposits and cell wall coatings, but no tyloses were formed. Such reactions were more pronounced in the tolerant than the susceptible cultivar. Further histochemical analysis in the same study showed the importance of coordinated responses of cotton to the same isolate (Daayf et al. Citation1997). Many papers described the next step as being the release of secondary metabolites into the vessels’ lumens to restrict the pathogen’s progress, including sesquiterpenes followed by phenolics (Harrison & Beckman Citation1982; Daayf et al. Citation1997). The sesquiterpenes involved in response to V. dahliae in cotton were at much higher levels in tolerant than susceptible lines (Mace et al. Citation1974, Citation1976). On the other hand, phenolic compounds were considered to be less important in the initial defence of cotton cells (Bell Citation1969) until Daayf et al. (Citation1997) assessed their involvement, along with sesquiterpenes, in this interaction, and showed that several of them were directly involved in cotton responses to V. dahliae. These studies tackled earlier defence events as they were completed in the roots (Daayf et al. Citation1997), as opposed to all prior studies, which used stem tissues to investigate this interaction. Using transmission electron microscopy, immunocytochemistry and phytochemistry, Daayf et al. (Citation1997) described the coordinated accumulation of callose in parenchyma cells and release of phenolic and sesquiterpene phytoalexins in the vessels colonized by V. dahliae. Cotton plants infected by V. dahliae accumulated four sesquiterpene phytoalexins – hemigossypol, methoxyhemigossypol, desoxyhemigossypol and desoxymethoxyhemigossypol. Desoxyhemigossypol is the most toxic to this pathogen and the most soluble at the known pH of infected xylem of cotton stems, probably making it the most important sesquiterpene phytoalexin in this interaction (Mace et al. Citation1985). The phenolic compounds detected in this system included flavans, flavonols and coumarins (Daayf et al. Citation1997).
In potato, V. dahliae is the main component of the complex causing early dying syndrome (Rowe & Powelson Citation2002), one of the most important diseases in this crop. In order to assess the differential expression of defence genes in this interaction, Derksen et al. (Citation2013a) used a quadratic system including two potato varieties with contrasting levels of susceptibility to V. dahliae and inoculated them with two isolates possessing different levels of aggressiveness. The study first focused on genes previously shown to be related to the SA signalling pathway, namely PAL1, PAL2, PR-1, PR-2 and PR-5, and assessed their expression in both roots and leaves. In the roots, expression of PAL1, PR-1 and PR-2 was higher in the less susceptible cultivar 7 d after inoculation, whereas PAL-2 transcripts only increased after 21 d. Interestingly, in the leaves, only PAL1, PAL2 had higher expression in the moderately resistant line. These and previous data (Derksen Citation2011) suggested that not only the jasmonic acid (Thaler et al. Citation2004) and ethylene (Mansoori & Smith Citation2005) pathways, but also the salicylic acid pathway can play an important signalling role in potato defence against V. dahliae. At the secondary metabolites level, the flavonoid rutin was recently identified as a component of the potato response to infection by both Phytophthora infestans (Henriquez et al. Citation2012a) and V. dahliae (El Hadrami et al. Citation2011; El-Bebany et al. Citation2013a), in line with the differential PAL responses reported by Derksen et al. (Citation2013a).
Other reactions more recently reported include miRNA and the hypersensitive response. For example, MiRNA from eggplant (Solanum melongena L.) were detected in response to inoculation with V. dahliae (Yang et al. Citation2013a). Liebrand et al. (Citation2013) showed that the tomato orthologue of the Arabidopsis thaliana SOBIR1/EVR interacts with V. dahliae’s Ve1 and was required for the Ve1-mediated hypersensitive response. However, work by the group of Thomma (Zhang et al. Citation2013c) suggested that a hypersensitive response is not absolutely required for plant resistance to Verticillium, although it may occur in some species as a consequence of Ve1/Ave1-induced defence signalling. In pathosystems involving vascular/root pathogens, however, what is considered a hypersensitive response is debatable, especially when compared with foliar diseases where leaf necrosis is readily visible as a response of a gene-for-gene interaction. Several markers such as ROS production in response to foliar pathogens can be used to describe the events leading to or accompanying HR, but do not replace the actual tissue changes that would be visible and directly affect the pathogen.
Several studies have attempted to induce resistance to soilborne plant diseases using biological inducers such as chitosan (El Hassni et al. Citation2004a), PGPRs (El Hassni et al. Citation2007; Dihazi et al. Citation2012), or hypoaggressive isolates of the pathogen (El Hassni et al. Citation2004b), while other studies transformed plants with foreign genes in an effort to establish disease resistance (Yang et al. Citation2013b). After transformation into potato, the ribosomal protein L13a from the wild eggplant Solanum torvum boosted resistance to V. dahliae (Yang et al. Citation2013b). Similarly, overexpressing a serine/threonine protein kinase from cotton (GbSTK) in Arabidopsis enhanced resistance to V. dahliae (Zhang et al. Citation2013b), and induced the expression of PR proteins PR-4, PR-5 and EREBP. As a result, transformed plants were better able to mitigate the negative effects from the reactive oxygen species resulting from the interaction (Zhang et al. Citation2013b). In a study by the same group, depicting the transcriptome profile of cotton species G. barbadense inoculated with V. dahliae, 3027 genes had homology with genes known to have a role in plant defence in other species (Zhang et al. Citation2013a), illustrating the transcriptional complexity of defence responses to V. dahliae in cotton, and probably in other species both in quality and quantity.
Pathogenicity factors of V. dahliae
Microsclerotia, the resting structures of V. dahliae, are the main inoculum source for this pathogen. They are usually dormant in the soil, but when a host is available and conditions are conducive for disease, they germinate, generally in response to the host root exudates (Mol & Van Riessen Citation1995), and proceed to infection. There are conflicting reports about the effect of inoculum pressure on successful infections in different crops and geographic origins, and more studies are needed to determine the factors limiting successful infections and how inoculum pressure modulates such interactions.
Many studies have reported on V. dahliae gene transcripts, peptides, and other molecules that are produced either constitutively, i.e. in culture, or during pathogen interactions with a host. However, few studies investigated the functional involvement of such genes or proteins in pathogenicity on their host species (Dobinson et al. Citation2004; Liu et al. Citation2013; Santhanam & Thomma Citation2013), and fewer have considered the diversity of this pathogen in such analyses. To establish disease on a host plant, Verticillium species deliver molecules outside and into the host cells, including enzymes, other proteins and an array of secondary metabolites (Klosterman et al. Citation2011). In addition, there are reports indicating that this pathogen can also inactivate host defence responses at the site of infection (Gold & Robb Citation1995) or during colonization (El Hadrami et al. Citation2011). However, very few studies confirmed defence suppression by V. dahliae (El Hadrami et al. Citation2011), and the mechanisms by which the pathogen defeats and/or escapes host defences are not fully understood. In other pathosystems, such mechanisms range from early suppression of defence gene transcription (Wang et al. Citation2004b, Citation2008) to later degradation of plant bioactive defence molecules such as phytoalexins (Stassen & Van Den Ackerveken Citation2011; Henriquez et al. Citation2012a, Citation2012b; Doehlemann & Hemetsberger Citation2013). Such counter-defence mechanisms may also involve the circumventing of defence responses indirectly through interference with the host’s own signalling cross-talk (El Oirdi et al. Citation2011). Therefore, many questions remain unanswered about the components and the mechanisms underlying V. dahliae’s strategies to successfully circumvent the defence responses of its hosts. For example, very few studies made the link between molecules produced by the pathogen and their potential effect on a specific defence reaction, i.e. V. dahliae’s quercetinase was shown to be involved in the degradation of flavonoid phytoalexins in potato after infection (El Hadrami et al. Citation2011).
Effector genes and proteins
During the process of infection, in addition to cell-wall degrading enzymes, Verticillium species produce and release other molecules implicated in necrosis and wilt symptoms (Pegg & Brady Citation2002). Such molecules were sometimes called elicitors, pathogenicity factors or virulence factors, among others. More recently, the term 'effectors’ has also been used to describe such factors. Several of these molecules have been identified and characterized in Verticillum species, i.e. Sge1 that differentially regulates expression of effector genes (Santhanam & Thomma Citation2013) and a necrosis- and ethylene-inducing protein (VdNEP) both identified in V. dahliae (Wang et al. Citation2004a; Yao et al. Citation2011). The latter protein contains 233 amino acids with a high sequence similarity to NEP proteins in other pathogenic fungi (Wang et al. Citation2004a). Infiltration of cotton cotyledons and leaves with VdNEP caused dehydration and wilting symptoms, suggesting that VdNEP is a pathogenicity factor in V. dahliae on cotton (Wang et al. Citation2004a). However, although similar symptoms, including chlorosis, necrosis and vascular discolouration, were observed on sunflower cotyledons and leaves artificially infiltrated with VdNEP protein synthesized in vitro (Yao et al. Citation2011), defence responses were also induced under such conditions. This suggested that VdNEP protein served not only as a host non-specific pathogenicity factor from V. dahliae, but also as a defence inducer (Yao et al. Citation2011). This raises evolutionary questions on this pathogen, in relation to other pathogens’ virulence factors that became avirulence factors after the host developed new mechanisms to recognize them.
VMK1, a gene encoding a mitogen-activated protein, was isolated from V. dahliae, and its role in pathogenicity was demonstrated via transposon mutagenesis and subsequent Agrobacterium tumefaciens-mediated transformation of a mutant allele into V. dahliae (Dobinson et al. Citation2004). Mutant strains (vmk1) had significantly reduced virulence on several host species belonging to different families, including cotton and tomato, indicating that this gene is a pathogenicity determinant in V. dahliae. These mutant strains also showed a reduction in conidia and microsclerotia formation, suggesting a crucial role of this gene in multiple cellular functions (Rauyaree et al. Citation2005). Mitogen-activated protein kinases (MAPKs) are highly conserved proteins within fungi. Mutagenesis-based functional analyses of these proteins in several pathogenic fungi have shed light on the key roles of some MAPKs in fungal pathogenicity in different host-pathogen interactions (Xu Citation2000).
VDH1, a small, secreted, moderately hydrophobic protein homologous to hydrophobins was also isolated from a cDNA library developed from microsclerotia of V. dahliae. Hydrophobins are key factors in different developmental and pathogenic processes, including the formation of both hyphae and spores (Talbot Citation2001; Wösten Citation2001). Mutant vdh1 developed via targeted mutation of the gene exhibited drastic reduction in microsclerotia formation, suggesting that VDH1 is another key gene in the development and production of microsclerotia (Klimes & Dobinson Citation2006). Microsclerotia represent the initial stage of the disease cycle, and are important components in the infection process by providing germinating mycelia that invade host cells. From that view, VDH1 is an important contributor to the disease cycle.
The cAMP-dependent protein kinase A (PKA) is another factor that has been well investigated in different pathogens including Verticillium species. The cyclic AMP (cAMP)-dependent signalling pathway controls the adaptation of pathogenic fungi to nutritional deficiency and stress as well as fungal development, sexual reproduction and virulence (Kronstad et al. Citation1998; Yamauchi et al. Citation2004). In silico-search identified two PKA catalytic subunits in the V. dahliae genome, namely VdPKAC1 and VdPKAC2. Despite their ability to infect the roots of tomato and eggplant, vdpkac1 mutants caused substantially lower disease severity (Tzima et al. Citation2010), thus confirming their contribution to the pathogenicity of V. dahliae. In tests on culture media, these mutants produced lower amounts of ethylene, a molecule that is essential for symptom induction in many wilt diseases. They also produced many less conidia than wild type strains, indicating that VdPKAC1 plays critical and multiple roles in V. dahliae, including virulence, conidia formation and ethylene biosynthesis (Tzima et al. Citation2010).
A glutamic acid-rich protein (GARP1) has been cloned from a V. dahliae strain isolated from cotton and named VdGRAP1 (Gao et al. Citation2010). In silico analysis detected no significant sequence similarity to any known annotated genes, suggesting that this gene might be unique to V. dahliae (Gao et al. Citation2010). Studies using Agrobacterium tumefaciens-mediated insertional mutagenesis suggested that this gene might play a role in V. dahliae`s pathogenicity and development. The vdgrap1 mutants were defective in microsclerotia production as well as in their ability to infect cotton plants (Gao et al. Citation2010).
Another gene, Vlaro2, this time cloned from a V. longisporum isolated from Brassica napus, plays a role in the pathogen’s life cycle (Singh et al. Citation2010). This gene encodes chorismate synthase in V. longisporum, a protein essential for the production of three aromatic amino acids (Singh et al. Citation2010). The role of Vlaro2 in the pathogenicity of V. longisporum was elucidated using RNA-based gene silencing technology (Singh et al. Citation2010), which showed a reduced ability to infect B. napus and A. thaliana by the Vlaro2 mutants as compared with the wild type. It is an important gene for the fungus adaptation to imbalanced amino acid levels in the surrounding environment. When certain amino acids are available in the environment, they are absorbed by an uptake system and their biosynthesis is minimized, whereas if they are present in an insufficient amount, Vlaro2 activates amino acid biosynthesis (Singh et al. Citation2010).
A recently identified effector in V. dahliae is the Sucrose Non-Fermenting Protein Kinase 1 (VdSNF1), which plays a role in virulence and in regulating several cell-wall degrading enzymes (Tzima et al. Citation2011). Under glucose starvation, SNF1 reactivates catabolite-repressed genes (Hardie et al. Citation1998). In yeast, it mediates glucose repression of several genes and plays a role in invasive and filamentous growth (Palecek et al. Citation2002). In plant pathogenic fungi, disruption of SNF1 lowered the expression of several cell-wall degrading enzyme (CWDE) genes, and mutant strains were unable to initiate disease on their hosts (Tonukari et al. Citation2000; Ospina-Giraldo et al. Citation2003). For instance, in Fusarium oxysporum, disruption of FoSNF1, in addition to reducing the expression of CWDE genes, precluded the F. oxysporum from severely infecting Arabidopsis and cabbage plants (Ospina-Giraldo et al. Citation2003). In V. dahliae, vdsnf1 mutants grew very slowly on media containing pectin or galactose as carbon sources. Also, the mutant strains were unable to cause severe infection on tomato or eggplant, due to a weak ability to colonize the host roots (Tzima et al. Citation2011).
G proteins are other effectors found to control the level of virulence and the development of pathogenic fungi including V. dahliae (Doehlemann et al. Citation2006; Charoensopharat et al. Citation2008; Tzima et al. Citation2011). Their involvement in growth, sporulation, mating and virulence has been investigated in several fungal pathogens using mutagenesis (Charoensopharat et al. Citation2008). In F. oxysporum, Gα subunit genes FGA1, FGA2, in addition to FGB1 gene, were shown to be crucial for virulence (Jain et al. Citation2002). Mutants fga1 and fgb1 had abnormal colony morphology, reduced conidia formation on culture media and lower disease severity on the host, while fga2 mutants were unable to initiate infection or cause disease. These observations suggested that the three G protein subunit genes are involved in the signal transduction pathway of F. oxysporum (Jain et al. Citation2002, Citation2005). The role of G proteins in V. dahliae pathogenicity has also been studied (Tzima et al. Citation2012). Similarly to the F. oxysporum FGA1 subunit, disruption of V. dahliae Gβ subunit (VGB) gene reduced virulence on tomato, increased the production of microsclerotia and conidia and reduced ethylene production as compared with wild type strains (Tzima et al. Citation2012).
Most recently, a specific secreted protein (VdSSP1) has been isolated from a highly aggressive strain of V. dahliae, and seemed to be specific to this fungal pathogen because no homologous genes were identified in the non-redundant protein sequence database (Liu et al. Citation2013). The role of this gene in the pathogen life cycle has been functionally analysed using mutants. A mutant (vdssp1) showed a noticeable decrease in virulence on cotton seedlings and growth on media containing pectin and starch as carbon sources. These findings pointed out that this VdSSP1 protein is critical for the pathogenicity of V. dahliae (Liu et al. Citation2013).
El-Bebany et al. (Citation2010) conducted a proteomic analysis to compare proteomes of highly and weakly aggressive isolates of V. dahliae, and identified peptides that were produced in vitro by only one or the other isolate. Peptides that were exclusive to the highly aggressive isolate included genes involved in stress tolerance, sporulation and microsclerotia formation, as well as suppression of plant defence responses. Interestingly, except for a few peptides related to antibiotic resistance, most of the differential peptides in the weakly aggressive isolate were involved in basic metabolic functions, confirming the assumption that more pathogenicity factors would be expressed in the highly, not the weakly aggressive isolate (El-Bebany et al. Citation2010). Adapting a suppressive hybridization technique in combination with cDNA AFLP (Henriquez & Daayf Citation2010), a follow-up transcriptomics study showed that both isolates had more differentially expressed genes induced in response to root extracts from a susceptible potato cultivar than from a moderately resistant one (El-Bebany et al. Citation2011). Many of these were associated with V. dahliae pathogenicity based on their similarity with the transcript sequences isolated during the pathogenic growth of this and other fungi (Neumann & Dobinson Citation2003). Functional analysis of many of these genes is currently being conducted in several labs.
In addition to the aforementioned pathogenicity effectors, there are other genes that have been identified in Verticillium species, including glyoxalase 1 (VdGLO1) and trypsin protease 1 (VTP1). However, their role in pathogenicity needs to be determined (Dobinson et al. Citation2004; Klimes & Dobinson Citation2006; El-Bebany et al. Citation2010, Citation2011). Furthermore, genome-wide searches of Verticillium species database may identify several potential effectors, i.e. G-protein-coupled receptors (GPCRs), which are thought to play important roles in the pathogen life cycle including morphogenesis, mating, and virulence (Zheng et al. Citation2010). Nonetheless, functional analyses of these genes are needed to confirm their involvement in pathogenesis.
Enzymes
The plant cell wall is one of the first barriers for plant pathogens attempting to infect their hosts. To break down this barrier, Verticillium species release cell-wall degrading enzymes upon infection, allowing them to penetrate and move more efficiently within the plant’s vascular system (Bidochka et al. Citation1999; Fradin & Thomma Citation2006). The reduction in host colonization and wilt symptoms of tomato plants inoculated with V. albo-atrum pectinase mutants as compared with the wild type strain suggested that this pathogen uses a mixture of cell-wall degrading enzymes to colonize the host (Durrands & Cooper Citation1988). A well-studied group of these enzymes is the pectinolytic enzyme group which targets and breaches pectin-rich membranes between vessel elements (Bishop & Cooper Citation1983). Even in vitro, several polysaccharide-degrading enzymes were observed in V. albo-atrum cultures grown on tomato cell wall-containing media (Cooper & Wood Citation1975). In particular, the production of endo-polygalacturonases (endo-PG), exo-arabinases and endopolygalacturonate trans-eliminases (endo-PGTE) increased after 2 days in culture, while that of endo-xylanases and cellulases did after 6 days (Cooper & Wood Citation1975). The application of such enzymes in vitro caused necrosis and wilt symptoms, such as endo-pectin lyase on tomato plants (Cooper & Wood Citation1980).
Secondary metabolites
The production of different forms of metabolites, which are toxic to plants and therefore called toxins, is well documented in the literature. They include different biochemical structures such as polypeptides, glycoproteins, amino acid derivatives, terpenoids, sterols and quinones (Pegg Citation1965; Kono et al. Citation1981; Stoessl Citation1981). Their role was demonstrated in pathogenicity of fungal pathogens such as Alternaria spp. and Helminthosporium spp. (Arntzen et al. Citation1973). In vascular wilt pathogens including Verticillium, the role of toxins in symptoms severity is important (Daly & Deverall Citation1983), although their production is not always a prerequisite for infection. The literature on toxins from Verticillium species is very limited, and the work described so far is at its initial stages. Verticillium toxins include high molecular weight protein-lipopolysaccharaide (PLP) complex and glycoproteins. Glycopeptide toxins isolated from a V. dahliae isolate on potato were associated with the development of wilt symptoms on a susceptible potato cultivar (Buchner et al. Citation1982). Recently, a new low molecular weight toxin, cinnamyl acetate, was isolated from V. dahliae causing disease on olive trees (Laouane et al. Citation2011). Similar observations were made on other V. dahliae hosts including cotton, tomato, potato and alfalfa (Ireland & Leath Citation1987; Nachmias et al. Citation1987; Clovis et al. Citation2006). However, the consistency of toxin production is inconsistent in different hosts. The difficulty with V. dahliae toxin analysis is the inability to isolate such toxins in highly pure forms for further testing. Much can be achieved if further studies were allocated to toxin identification and analysis in soilborne fungal pathogens such as V. dahliae.
Conclusions
Dealing with a global pathogen such as V. dahliae requires more funding from both the public and private sectors to support research on this pathogen, and more collaborative initiatives among researchers. It also requires joint efforts involving multiple complementary disciplines. Furthermore, developing more sustainable management solutions for verticillium and other wilts requires more awareness that managing the deleterious impacts of soilborne pathogens requires specific approaches, not necessarily similar to those used for foliar pathogens. A better understanding of the conditions that make such pathogens even more damaging and more global in occurrence should be achieved, including more specific knowledge on how crops are impacted by climate change and intensification of monocultures, in addition to the plasticity of pathogens’ genomes. With the advent of new technologies, new methods have been developed to take advantage of the data available on genomes, including those of fungal and oomycete pathogens (Kamoun Citation2006; Klosterman et al. Citation2011). A tremendous amount of information can be extracted simply by comparative genomics methods, but in most cases, functional analyses to study the role of specific genes, and their interactions with other genes or pathways, are becoming necessary to identify their functions (Fradin et al. Citation2014). Such studies can be carried out at different ‘omic’ levels but will usually need validation in vivo either with the pathogen alone, or while interacting with its host plant. Regarding Verticillium species and the losses they cause, the outcomes of disease management efforts would be much more positive if most or all of the factors mentioned above were integrated into the verticillium wilt equation. This means many more resources and efforts into collaborative studies, and better integration of environmental and sustainability considerations into the future.
Acknowledgements
Thanks to Dr A. Dakouri for gathering some of the literature and other material used to prepare this review and to Drs Chris Rampitsch and Jim Menzies for reviewing the initial version of this manuscript. Dr Daayf’s work on Verticillium has been supported by grants from NSERC, MRAC (PIN) Manitoba, ARDI Manitoba, and industry partners, including McCain Foods, Peak of the Market and KPPA.
References
- Alba JM, Glas JJ, Schimmel BCJ, Kant MR. 2011. Avoidance and suppression of plant defenses by herbivores and pathogens. J Plant Interac. 6:221–227.
- Alkher H, El Hadrami A, Rashid KY, Adam LR, Daayf F. 2009a. Cross-pathogenicity of Verticillium dahliae between potato and sunflower. Eur J Plant Pathol. 124:505–519.
- Alkher H, El Hadrami A, Rashid KY, Adam LR, Daayf F. 2009b. Pathogenic variation of Verticillium dahliae after serial passages through potato and sunflower. Can J Plant Pathol. 31:427–438.
- Arntzen CJ, Koeppe DE, Miller RJ, Peverly JH. 1973. The effect of pathotoxin from Helminthosporium maydis (race T) on energy-linked processes of corn seedlings. Physiol Plant Pathol. 3:79–89.
- Barbara DJ, Clewes E. 2003. Plant pathogenic Verticillium species: how many of them are there? Mol Plant Pathol. 4:297–305.
- Beckman CH. 1987. The nature of wilt diseases of plants. St Paul (MN): APS Press.
- Bell AA. 1969. Phytoalexin production and Verticillium wilt resistance in cotton. Phytopathology. 59:1119–1127.
- Bhat RG, Subbarao KV. 1999. Host range specificity in Verticillium dahliae. Phytopathology. 89:1218–1225.
- Bidochka MJ, St Leger RJ, Stuart A, Gowanlock K. 1999. Nuclear rDNA phylogeny in the fungal genus Verticillium and its relationship to insect and plant virulence, extracellular proteases and carbohydrases. Microbiol.-UK. 145:955–963.
- Bishop CD, Cooper RM. 1983. An ultrastructural study of vascular colonization in three vascular wilt diseases I. Colonization of susceptible cultivars. Physiol Plant Pathol. 23:323–343.
- Buchner V, Nachmias A, Burstein Y. 1982. Isolation and partial characterization of a phytotoxic glycopeptide from a protein- lipopolysaccharide complex produced by a potato isolate of Verticillium dahliae. FEBS Lett. 138:261–264.
- Charoensopharat K, Thummabenjapone P, Sirithorn P, Thammasirirak S. 2008. Antibacterial substance produced by Streptomyces sp. No. 87. Afr J Biotech. 7:1362–1368.
- Clovis SP, Jennifer AS, Bruce RL. 2006. Phytotoxicty on cotton explants of an 18.5 KDa protein from culture filtrates of Verticillium dahliae. Physiol Mol Plant Pathol. 67:308–318.
- Collado-Romero M, Mercado-Blanco J, Olivares-García C, Valverde-Corredor A, Jiménez-Díaz RM. 2006. Molecular variability within and among Verticillium dahliae vegetative compatibility groups determined by fluorescent amplified fragment length polymorphism and polymerase chain reaction markers. Phytopathology. 96:485–495.
- Cooper R, Wood RKS. 1975. Regulation of synthesis of cell wall degrading enzymes by Veticillium albo-atrum and Fusarium oxysporum f. sp. lycopersici. Physiol Plant Pathol. 5:135–156.
- Cooper R, Wood RKS. 1980. Cell wall degrading enzymes of vascular wilt fungi. III. Possible involvement of endo-pectin lyase in Verticillium wilt of tomato. Physiol Plant Pathol. 16:285–300.
- Daayf F, El Bellaj M, El Hassni M, J’Aiti F, El Hadrami I. 2003. Elicitation of soluble phenolics in date palm (Phoenix dactylifera L.) callus by Fusarium oxysporum f. sp. albedinis culture medium. Environ Exp Bot. 49:41–47.
- Daayf F, Nicole M, Bélanger RR, Geiger J-P. 1998. Hyaline mutants from Verticillium dahliae: an example of selection and characterization of strains aimed for host-parasite interaction studies. Plant Pathol. 47:523–529.
- Daayf F, Nicole M, Boher B, Pando-Bahuon A, Geiger JP. 1997. Early defense reactions of cotton (Gossypium sp.) to Verticillium dahliae Kleb. Eur J Plant Pathol. 103:125–136.
- Daayf F, Nicole M, Geiger J-P. 1995. Differentiation of Verticillium dahliae strains on the basis of vegetative compatibility and pathogenicity on susceptible and tolerant cotton plants. Eur J Plant Pathol. 101:69–79.
- Daly JM, Deverall BJ. 1983. Toxins and Plant Pathogenesis. New York (NY): Academic Press.
- Derksen H 2011. Expression of defense signaling genes in the potato-Verticillium dahliae interaction [dissertation], Winnipeg (MB): University of Manitoba, 131 pp.
- Derksen H, Badawi M, Henriquez MA, Yao Z, El-Bebany AF, Daayf F. 2013a. Differential expression of potato defence genes associated with the salicylic acid defence signalling pathway in response to weakly and highly aggressive isolates of Verticillium dahliae. J Phytopathol. 161:142–153.
- Derksen H, Rampitsch C, Daayf F. 2013b. Signaling cross-talk in plant disease resistance. Plant Sci. 207:79–87.
- Dihazi A, Jaiti F, Taktak W, Kilani Feki O, Jaoua S, Driouich A, Baaziz M, Daayf F, Serghini MA. 2012. Use of two bacteria for biological control of bayoud disease caused by Fusarium oxysporum in date palm (Phoenix dactylifera L.) seedlings. Plant Physiol Biochem. 55:7–15.
- Dixon GR, Pegg GF. 1969. Hyphal lysis and tylose formation in tomato cultivars infected by Verticillium albo-atrum. Trans Br Mycol Soc. 53:109–118.
- Dobinson KF, Grant SJ, Kang S. 2004. Cloning and targeted disruption, via Agrobacterium tumefaciens-mediated transformation, of a trypsin protease gene from the vascular wilt fungus Verticillium dahliae. Curr Genet. 45:104–110.
- Doehlemann G, Berndt P, Hahn M. 2006. Different signalling pathways involving a Galpha protein, cAMP and a MAP kinase control germination of Botrytis cinerea conidia. Mol Microbiol. 59:821–835.
- Doehlemann G, Hemetsberger C. 2013. Apoplastic immunity and its suppression by filamentous plant pathogens. New Phytol. 198:1001–1016.
- Durrands PK, Cooper RM. 1988. The role of pectinases in vascular wilt disease as determined by defined mutants of Verticillium albo-atrum. Physiol Mol Plant Pathol. 32:363–371.
- El Hadrami A, Adam LR, Daayf F. 2011. Biocontrol treatments confer protection against Verticillium dahliae infection of potato by inducing anti-microbial metabolites. Mol Plant-Microbe Inter. 24:328–335.
- El Hadrami A, El Hadrami I, Daayf F. 2009. Suppression of induced plant defence responses by Fungal and Oomycete Pathogens. In: Bouarab K, Brisson N, Daayf F, editors. Molecular plant-microbe interactions. Wallingford: CAB International; p. 231–268.
- El Hadrami A, El-Bebany AF, Yao Z, Wang X, Adam LR, El Hadrami I, Daayf F. 2012. Plants versus fungi and oomycetes: pathogenesis, defense and counter-defense in the proteomics era. Int J Mol Sci. 13:7237–7259.
- El Hassni M, El Hadrami A, Daayf F, Ait Barka E, El Hadrami I. 2004a. Chitosan, antifungal product against Fusarium oxysporum f. sp. albedinis and elicitor of defence reactions in date palm roots. Phytopathol Mediter. 43:195–204.
- El Hassni M, El Hadrami A, Daayf F, Chérif M, Ait Barka E, El Hadrami I. 2007. Biological control of Bayoud disease in date palm: selection of microorganisms inhibiting the causal agent and inducing defense reactions. Environ Exp Bot. 59:224–234.
- El Hassni M, J’Aiti F, Dihazi A, Ait Barka E, Daayf F, El Hadrami I. 2004b. Enhancement of defence responses against Bayoud disease by treatment of date palm seedlings with an hypoaggressive Fusarium oxysporum Isolate. J Phytopathol. 152:182–189.
- El Oirdi M, Abd El Rahman T, Rigano L, El Hadrami A, Rodriguez MC, Daayf F, Vojnov A, Bouarab K. 2011. Botrytis cinerea manipulates the antagonistic effects between immune pathways to promote disease development in tomato. Plant Cell. 23:2405–2421.
- El-Bebany AF, Adam LR, Daayf F. 2013a. Differential accumulation of phenolic compounds in potato in response to weakly and highly aggressive isolates of Verticillium dahliae. Can J Plant Pathol. 35:232–240.
- El-Bebany AF, Alkher H, Adam LR, Daayf F. 2013b. Vegetative compatibility of Verticillium dahliae isolates from potato and sunflower using nitrate-nonutilizing (nit) mutants and PCR-based approaches. Can J Plant Pathol. 35:1–9.
- El-Bebany AF, Henriquez MA, Badawi M, Adam LR, El Hadrami A, Daayf F. 2011. Induction of putative pathogenicity-related genes in Verticillium dahliae in response to elicitation with potato root extracts. Environ Exp Bot. 72:251–257.
- El-Bebany AF, Rampitsch C, Daayf F. 2010. Proteomic analysis of the phytopathogenic soilborne fungus Verticillium dahliae reveals differential protein expression in isolates that differ in aggressiveness. Proteomics. 10:289–303.
- Fradin EF, Thomma BPHJ. 2006. Physiology and molecular aspects of Verticillium wilt diseases caused by V. dahliae and V. albo-atrum. Mol Plant Pathol. 7:71–86.
- Fradin EF, Zhang Z, Royenich H, Song Y, Liebrand TWH, Masini L, Van Den Berg GC, Joosten MHAJ, Thomma BPHJ. 2014. Functional analysis of the tomato immune receptor Ve1 through domain swaps with its non-functional homolog Ve2. PLoS One. doi:10.1371/journal.pone.0088208
- Gao F, Zhou BJ, Li GY, Jia PS, Li H, Zhao YL, Zhao P, Xia GX, Guo HS. 2010. A glutamic acid-rich protein identified in Verticillium dahliae from an insertional mutagenesis affects microsclerotial formation and pathogenicity. PLoS One. 5:e15319.
- Gebhardt C, Valkonen JPT. 2001. Organization of genes controlling disease resistance in the potato genome. Annu Rev Phytopathol. 39:79–102.
- Gharbi Y, Triki MA, Jolodara A, Trabelsi R, Gdoura R, Daayf F. 2014a. Genetic diversity of Verticillium dahliae from olive trees in Tunisia based on RAMS and IGS-RFLP analyses. Can J Plant Pathol. 36:491–500. doi:10.1080/07060661.2014.964776
- Gharbi Y, Triki MA, Trabelsi R, Fendri I, Daayf F, Gdoura R. 2014b. Genetic structure of Verticillium dahliae isolates infecting olive tree in Tunisia using AFLP, Pathogenicity and PCR Markers. Plant Pathol. doi:10.1111/ppa.12323.
- Göhre V, Robatzek S. 2008. Breaking the barriers: microbial effector molecules subvert plant immunity. Annu Rev Phytopathol. 46:189–215.
- Gold J, Robb J. 1995. The role of the coating response in Craigella tomatoes infected with Verticillium dahliae, races 1 and 2. Physiol Mol Plant Pathol. 47:141–157.
- Goodman RN, Plurad SB. 1971. Ultrastructural changes in tobacco undergoing the hypersensitive reaction caused by plant pathogenic bacteria. Physiol Plant Pathol. 1:11–15.
- Hammerschmidt R. 1999. Phytoalexins: what have we learned after 60 years? Annu Rev Phytopathol. 37:285–306.
- Hardie DG, Carling D, Carlson M. 1998. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu Rev Biochem. 67:821–855.
- Harrison NA, Beckman CH. 1982. Time/space relationships of colonization and host response in wilt-resistant and wilt-susceptible cotton (Gossypium) cultivars inoculated with Verticillium dahliae and Fusarium oxysporum f. sp. vasinfectum. Physiol Plant Pathol. 21:193–207.
- Henriquez MA, Adam LR, Daayf F. 2012a. Alteration of secondary metabolites’ profiles in potato leaves in response to weakly and highly aggressive isolates of Phytophthora infestans. Plant Physiol Biochem. 57:8–14.
- Henriquez MA, Daayf F. 2010. Identification and cloning of differentially expressed genes involved in the interaction between potato and Phytophthora infestans using a subtractive hybridization and cDNA-AFLP combinational approach. J Integr Plant Biol. 52:453–467.
- Henriquez MA, Wolski EA, Molina OI, Adam LR, Andreu AB, Daayf F. 2012b. Effects of glucans and eicosapentaenoic acid on differential regulation of phenylpropanoid and mevalonic pathways during potato response to Phytophthora infestans. Plant Physiol Biochem. 60:119–128.
- Horner CE. 1954. Pathogenicity of Verticillium isolates to peppermint. Phytopathology. 44:239–242.
- Hückelhoven R. 2007. Cell wall-associated mechanisms of disease resistance and susceptibility. Ann Rev Phytopathol. 45:101–127.
- Ireland KF, Leath KT. 1987. Potential of using culture filtrates from Verticillium albo-atrum to evaluate alfalfa germ plasm for resistance to Verticillium Wilt. Plant Dis. 71:900–903.
- Jain S, Akiyama K, Mae K, Ohguchi T, Takata R. 2002. Targeted disruption of a G protein α subunit gene results in reduced pathogenicity in Fusarium oxysporum. Curr Genet. 41:407–413.
- Jain S, Akiyama K, Takata R, Ohguchi T. 2005. Signaling via the G protein subunit FGA2 is necessary for pathogenesis in Fusarium oxysporum. FEMS Microbiol Lett. 243:165–172.
- Joaquim TR, Rowe RC. 1991. Vegetative compatibility and virulence of strains of Verticillium dahliae from soil and potato plants. Phytopathology. 81:552–558.
- Johnson DA, Dung JKS. 2010. Verticillium wilt of potato - the pathogen, disease and management. Can J Plant Pathol. 32:58–67.
- Kamoun S. 2006. A catalogue of the effector secretome of plant pathogenic oomycetes. Annu Rev Phytopathol. 44:41–60.
- Kawchuk LM, Hachey J, Lynch DR, Kulcsar F, Van Rooijen G, Waterer DR, Robertson A, Kokko E, Byers R, Howard RJ, et al. 2001. Tomato Ve disease resistance genes encode cell surface-like receptors. Proc Natl Acad Sci USA. 98:6511–6515.
- Klimes A, Dobinson KF. 2006. A hydrophobin gene, VDH1, is involved in microsclerotial development and spore viability in the plant pathogen Verticillium dahliae. Fungal Genet Biol. 43:283–294.
- Klosterman SJ, Atallah ZK, Vallad GE, Subbarao KV. 2009. Diversity, pathogenicity, and management of Verticillium species. Annu Rev Phytopathol. 47:39–62.
- Klosterman SJ, Subbarao KV, Kang SC, Veronese P, Gold SE, Thomma BPHJ, Chen ZH, Henrissat B. et al. 2011. Comparative genomics yields insights into niche adaptation of plant vascular wilt pathogens. PLoS Pathogens. 7:e1002137. doi:10.1371/journal.ppat.1002137
- Kono Y, Knoche HW, Daly JM. 1981. Structure of host–specific toxin. In: Durbin RD, editor. Toxins in Plant Diseases. Academic Press; p. 221–257.
- Krikun J, Bernier CC. 1987. Infection of several crop species by two isolates of Verticillium dahliae. Can J Plant Pathol. 9:241–245.
- Kronstad J, De Maria A, Funnell D, Laidlaw RD, Lee N, De Sá MM, Ramesh M. 1998. Signaling via cAMP in fungi: Interconnections with mitogen-activated protein kinase pathways. Arch Microbiol. 170:395–404.
- Laouane H, Lazrek HB, Sedra MH. 2011. Synthesis and toxicity evaluation of cinnamyl acetate: a new phytotoxin produced by a strain of Verticillium dahliae pathogenic on olive tree. Inter J Agr Biol. 13:444–446.
- Liebrand TWH, van den Berg GCM, Zhang Z, Smit P, Cordewener JHG, America AHP, Sklenar J, Jones AME, Tameling WIL, Robatzek S, et al. 2013. Receptor-like kinase SOBIR1/EVR interacts with receptor-like proteins in plant immunity against fungal infection. Proc Natl Acad Sci USA. 110:10010–10015.
- Liu S-Y, Chen J-Y, Wang J-L, Li L, Xiao H-L, Adam SM, Dai X-F. 2013. Molecular characterization and functional analysis of a specific secreted protein from highly virulent defoliating Verticillium dahliae. Gene. 529:307–316.
- Mace ME. 1978. Contributions of tyloses and terpenoid aldehyde phytoalexins to Verticillium wilt resistance in cotton. Physiol Plant Pathol. 12:1–11.
- Mace ME, Bell AA, Beckman CH. 1976. Histochemistry and identification of disease-induced terpenoid aldehydes in Verticillium-wilt resistant and-susceptible cottons. Can J Bot. 54:2095–2099.
- Mace ME, Bell AA, Stipanovic RD. 1974. Histochemistry and isolation of gossypol and related terpenoids in roots of cotton seedlings. Phytopathology. 64:1297–1302.
- Mace ME, Stipanovic RD, Bell AA. 1985. Toxicity and role of terpenoid phytoalexins in verticillium wilt resistance in cotton. Physiol Plant Pathol. 26:209–218.
- Mansoori B, Smith CJ. 2005. Elicitation of ethylene by Verticillium albo-atrum phytotoxins in potato. J Phytopathol. 153:143–149.
- Mol L, Van Riessen HW. 1995. Effect of plant roots on the germination of microsclerotia of Verticillum dahliae. Eur J Plant Pathol. 101:673–678.
- Molina OI, Tenuta M, El Hadrami A, Buckley K, Cavers C, Daayf F. 2014. Potato early dying and yield responses to compost, green manures, seed meal and chemical treatments. Am J Potato Res. 91:414–428.
- Mueller WC, Morgham AT. 1993. Ultrastructure of the vascular responses of cotton to Verticillium dahliae. Can J Bot. 71:32–36.
- Nachmias A, Buchner V, Tsror V, Burtein Y, Keen N. 1987. Differential phytotoxicity of peptides from culture fluids of Verticillium dahliae race 1 and 2 and their relationship to pathogenicity of the fungi on tomato. Phytopathology. 77:506–510.
- Nazar RN, Hu X, Schmidt J, Culham D, Robb J. 1991. Potential use of PCR-amplified ribosomal intergenic sequences in the detection and differentiation of verticillium wilt pathogens. Physiol Mol Plant Pathol. 39:1–11.
- Neumann MJ, Dobinson KF. 2003. Sequence tag analysis of gene expression during pathogenic growth and microsclerotia development in the vascular wilt pathogen Verticillium dahliae. Fungal Genet Biol. 38:54–62.
- Niks RE, Marcel TC. 2009. Nonhost and basal resistance: How to explain specificity? New Phytol. 182:817–828.
- Ospina-Giraldo M, Mullins E, Kang S. 2003. Loss of function of the Fusarium oxysporum SNF1 gene reduces virulence on cabbage and Arabidopsis. Curr Genet. 44:49–57.
- Palecek SP, Parikh AS, Huh JH, Kron SJ. 2002. Depression of Saccharomyces cerevisiae invasive growth on non-glucose carbon sources requires the Snf1 kinase. Mol Microbiol. 45:453–469.
- Pantou MP, Kouvelis VN, Typas MA. 2006. The complete mitochondrial genome of the vascular wilt fungus Verticillium dahliae: A novel gene order for Verticillium and a diagnostic tool for species identification. Curr Genet. 50:125–136.
- Pantou MP, Typas MA. 2005. Electrophoretic karyotype and gene mapping of the vascular wilt fungus Verticillium dahliae. FEMS Microbiol Lett. 245:213–220.
- Pegg GF. 1965. Phytotoxin production by Verticillium albo-atrum Reinke et Berthold. Nature. 208:1228–1229.
- Pegg GF, Brady BL. 2002. Verticillium wilts. Wallingford (UK): CAB International.
- Peters RD, Sturz AV, Carter MR, Sanderson JB. 2003. Developing disease-suppressive soils through crop rotation and tillage management practices. Soil Till Res. 72:181–192.
- Powelson ML, Rowe RC. 1993. Biology and management of early dying of potatoes. Annu Rev Phytopathol. 31:111–126.
- Ramos T, El Bellaj M, El Idrissi-Tourane A, Daayf F, El Hadrami I. 1997. Phenolamides of palm Rachis, components of defense reaction of date palm against Fusarium oxysporum f. sp albedinis, the causal Agent of Bayoud. J Phytopathol. 145:487–493.
- Rauyaree P, Ospina-Giraldo MD, Kang S, Bhat RG, Subbarao KV, Grant SJ, Dobinson KF. 2005. Mutations in VMK1, a mitogen-activated protein kinase gene, affect microsclerotia formation and pathogenicity in Verticillium dahliae. Curr Genet. 48:109–116.
- Resende MLV, Flood J, Cooper RM. 1994. Host specialization of Verticillium dahliae, with emphasis on isolates from cocoa (Theobroma cacao). Plant Pathol. 43:104–111.
- Robb J. 2007. Verticillium tolerance: resistance, susceptibility, or mutualism? Can J Bot. 85:903–910.
- Rowe RC, Powelson ML. 2002. Potato early dying: management challenges in a changing production environment. Plant Dis. 86:1184–1193.
- Sansregret R, Dufour V, Langlois M, Daayf F, Dunoyer P, Voinnet O, Bouarab K. 2013. Extreme resistance as a host counter-counter defense against viral suppression of RNA silencing. PLoS Pathogens. 9:e1003435.
- Santhanam P, Thomma BPHJ. 2013. Verticillium dahliae Sge1 differentially regulates expression of candidate effector genes. Mol Plant-Microbe Inter. 26:249–256.
- Singh S, Braus-Stromeyer SA, Timpner C, Von Tran T, Lohaus G, Reusche M, Knüfer J, Teichmann T, Von Tiedemann A, Braus GH. 2010. Silencing of Vlaro2 for chorismate synthase revealed that the phytopathogen Verticillium longisporum induces the cross-pathway control in the xylem. Appl Microbiol Biotechnol. 85:1961–1976.
- Stassen JHM, Van Den Ackerveken G. 2011. How do oomycete effectors interfere with plant life? Curr Opin Plant Biol. 14:407–414.
- Stoessl A. 1981. Structure and biogenetic relations: fungal nonhost-specific. In: Durbin RD, editor. Toxins in Plant Diseases. Academic Press; p. 109–219.
- Subbarao KV, Hubbard JC, Greathead AS, Spencer GA. 1997. Verticillium wilt. In: Davis RM, Subbarao KV, Raid RN, Kurtz KA, editors. Compendium of Lettuce Diseases. St. Paul (MN): The American Phytopathological Society; p. 26–27.
- Talbot NJ. 2001. Fungal hydrophobins. In: Howard RJ, Gow NAR, editors. The Mycota VIII Biology of the Fungal Cell. New York (NY): Springer; p. 145–156.
- Taylor JB. 1969. Host specificity of Verticillium dahliae to tobacco. NZ J Sci. 12:709–712.
- Thaler JS, Owen B, Higgins VJ. 2004. The role of the jasmonate response in plant susceptibility to diverse pathogens with a range of lifestyles. Plant Physiol. 135:530–538.
- Tjamos EC, Paplomatas EJ. 1988. Long-term effect of soil solarization in controlling verticillium wilt of globe artichokes in Greece. Plant Pathol. 37:507–515.
- Tonukari NJ, Scott-Craig JS, Walton JD. 2000. TheCochliobolus carbonum SNF1 gene is required for cell wall-degrading enzyme expression and virulence on maize. Plant Cell. 12:237–248.
- Tyler BM. 2002. Molecular basis of recognition between Phytophthora pathogens and their hosts. Annu Rev Phytopathol. 40:137–167.
- Tzima A, Paplomatas EJ, Rauyaree P, Kang S. 2010. Roles of the catalytic subunit of cAMP-dependent protein kinase A in virulence and development of the soil borne plant pathogen Verticillium dahliae. Fungal Genet Biol. 47:406–415.
- Tzima AK, Paplomatas EJ, Rauyaree P, Ospina-Giraldo MD, Kang S. 2011. VdSNF1, the sucrose nonfermenting protein kinase gene of Verticillium dahliae, is required for virulence and expression of genes involved in cell-wall degradation. Mol Plant-Microbe Inter. 24:129–142.
- Tzima AK, Paplomatas EJ, Tsitsigiannis DI, Kang S. 2012. The G protein beta subunit controls virulence and multiple growth- and development-related traits in Verticillium dahliae. Fungal Genet Biol. 49:271–283.
- Uppal AK, El Hadrami A, Adam LR, Tenuta M, Daayf F. 2007. Pathogenic variability of Verticillium dahliae Kleb. isolates from potato fields in Manitoba and screening of bacteria for their biocontrol. Can J Plant Pathol. 29:141–152.
- Uppal AK, El Hadrami A, Adam LR, Tenuta M, Daayf F. 2008. Biological control of potato Verticillium wilt under controlled and field conditions using selected bacterial antagonists and plant extracts. Biol Control. 44:90–100.
- Van Loon LC, Rep M, Pieterse CMJ. 2006. Significance of inducible defense-related proteins in infected plants. Annu Rev Phytopathol. 44:135–162.
- Wang J-Y, Cai Y, Gou J-Y, Mao Y-B, Xu Y-H, Jiang W-H, Chen X-Y. 2004a. VdNEP, an elicitor from Verticillium dahliae, induces cotton plant wilting. Appl Environ Microbiol. 70:4989–4995.
- Wang X, El Hadrami A, Adam L, Daayf F. 2004b. US-1 and US-8 genotypes of Phytophthora infestans differentially affect local, proximal and distal gene expression of phenylalanine ammonia-lyase and 3-hydroxy, 3-methylglutaryl CoA reductase in potato leaves. Physiol Mol Plant Pathol. 65:157–167.
- Wang X, El Hadrami A, Adam LR, Daayf F. 2008. Differential activation and suppression of potato defence responses by Phytophthora infestans isolates representing US-1 and US-8 genotypes. Plant Pathol. 57:1026–1037.
- Wilhelm S. 1955. Longevity of Verticillium wilt fungus in the laboratory and field. Phytopathology. 45:180–181.
- Woolliams GE. 1966. Host range and symptomatology of Verticillium dahliae in economic, weed, and native plants in interior British Columbia. Can J Plant Sci. 46:661–669.
- Wösten HAB. 2001. Hydrophobins: multipurpose proteins. Annu Rev Microbiol. 55:625–646.
- Xiao CL, Subbarao KV, Schulbach KF, Koike ST. 1998. Effects of crop rotation and irrigation on Verticillium dahliae microsclerotia in soil and wilt in cauliflower. Phytopathology. 88:1046–1055.
- Xu J-R. 2000. MAP kinases in fungal pathogens. Fungal Genet Biol. 31:137–152.
- Yamauchi J, Takayanagi N, Komeda K, Takano Y, Okuno T. 2004. cAMP-PKA signaling regulates multiple steps of fungal infection cooperatively with Cmk1 MAP kinase in Colletotrichum lagenarium. Mol Plant-Microbe Inter. 17:1355–1365.
- Yang L, Jue DW, Li W, Zhang RJ, Chen M, Yang Q. 2013a. Identification of MiRNA from eggplant (Solanum melongena L.) by small RNA deep sequencing and their response to Verticillium dahliae infection. PLoS One. 8:e72840. doi:10.1371/journal.pone.0072840
- Yang L, Xie C, Li W, Zhang RJ, Jue DW, Yang Q. 2013b. Expression of a wild eggplant ribosomal protein L13a in potato enhances resistance to Verticillium dahliae. Plant Cell Tissue Org Cult. 115:329–340.
- Yao Z, Rashid KY, Adam LR, Daayf F. 2011. Verticillium dahliae’s VdNEP acts both as a plant defense elicitor and a pathogenicity factor in the interaction with Helianthus annuus. Can J Plant Pathol. 33:375–388.
- Zhang Y, Wang XF, Ding ZG, Ma Q, Zhang GR, Zhang SL, Li ZK, Wu LQ, Zhang GY, Ma ZY. 2013a. Transcriptome profiling of Gossypium barbadense inoculated with Verticillium dahliae provides a resource for cotton improvement. BMC Genomics. 14. doi:10.1186/1471-2164-14-637
- Zhang Y, Wang XF, Li YY, Wu LZ, Zhou HM, Zhang GY, Ma ZY. 2013b. Ectopic expression of a novel Ser/Thr protein kinase from cotton (Gossypium barbadense), enhances resistance to Verticillium dahliae infection and oxidative stress in Arabidopsis. Plant Cell Rep. 32:1703–1713.
- Zhang Z, Van Esse HP, Van Damme M, Fradin EF, Liu C-M, Thomma BPHJ. 2013c. Ve1-mediated resistance against Verticillium does not involve a hypersensitive response in Arabidopsis. Mol Plant Pathol. 14:719–727.
- Zheng H, Zhou L, Dou T, Han X, Cai Y, Zhan X, Tang C, Huang J, Wu Q. 2010. Genome-wide prediction of G protein-coupled receptors in Verticillium spp. Fungal Biol. 114:359–368.
