Abstract
A method was developed to evaluate susceptibility of peach leaves to Plum pox virus (PPV) infection by aphids. We examined whether virus multiplication could be detected in aphid-inoculated detached leaves and if transmission efficiency of PPV by green peach aphids to detached leaves was comparable with that of peach seedlings. Results demonstrated that transmission efficiencies of viruliferous aphids transferred to detached peach leaves subsequently maintained on an agar layer for 3 weeks was not significantly different from that for intact seedlings. Overlaying infected PPV plum or peach leaf segments on the healthy peach leaves with subsequent application of aphids to the infected leaf pieces provided a comparable transmission efficiency. Reduced handling of the aphids using this method minimized the possibility of damaging the aphids and facilitated higher throughput testing. Comparable infection rates were obtained for detached leaves using either 50 or 25 viruliferous aphids per leaf. Residual PPV was not detected by direct quantitative reverse transcriptase polymerase chain reaction assay (DqRT-PCR) on non-host plants probed by viruliferous aphids. The effect of short-term storage temperatures pre- or post-inoculation did not significantly alter the susceptibility of peach leaves to PPV infection or the transmission rate. Application of the leaf overlay method to evaluate seasonal changes in susceptibility of peach leaves in the field is the subject of an ongoing study.
Résumé
Une méthode a été développée pour évaluer la sensibilité des feuilles de pêcher à l’infection par le virus de la sharka transmis par les pucerons. Nous avons vérifié si la multiplication du virus pouvait être détectée sur des feuilles détachées sur lesquelles se trouvaient des pucerons inoculés, et si l’efficacité de la transmission du virus de la sharka par le puceron vert du pêcher à des feuilles détachées était comparable à celle observée sur des semis de pêcher. Les résultats ont montré que l’efficacité de transmission de pucerons virulifères transférés sur des feuilles détachées de pêcher, gardées subséquemment sur une couche de gélose pendant trois semaines, n’était pas significativement différente de celle observée sur des semis intacts de pêcher. La superposition de morceaux de prunes ou de feuilles de pêcher infectés par le virus de la sharka à des feuilles saines de pêcher, suivie de l’application de pucerons sur les morceaux de feuilles infectées, a engendré une efficacité de transmission comparable. Cette méthode, qui tend à minimiser la manipulation des pucerons, a réduit le risque de mutiler ces derniers et a permis d’améliorer l’efficacité des essais. Des taux d’infection comparables ont été obtenus pour des feuilles détachées en utilisant 50 ou 25 pucerons virulifères par feuille. Aucun virus résiduel n’a été détecté par RT-PCR quantitative sur les plantes non hôtes piquées par les pucerons virulifères. Les températures d’entreposage à court terme avant ou après inoculation n’ont pas significativement influencé la sensibilité des feuilles de pêcher à l’infection par le virus de la sharka ou le taux de transmission. L’application de la méthode qui fait appel à la superposition de feuilles pour évaluer l’influence des variations saisonnières sur la sensibilité des feuilles de pêcher au champ fait l’objet d’une étude en cours.
Introduction
Plum pox virus (PPV), causal agent of plum pox or Sharka disease, is the most devastating viral disease of stone fruit (Prunus spp.) worldwide (Nemeth Citation1986). Different strains significantly limit stone fruit production in peaches, plums, apricots, nectarines, almonds and sweet and sour cherries in areas where they are established. In 1999, the Dideron strain of PPV (PPV-D) was first detected in North America in Pennsylvania in several peach and plum orchards (Levy et al. Citation2000; Damsteegt et al. Citation2001). The following year, PPV-D was subsequently detected in nectarine and peach in Ontario, Canada (Thompson et al. Citation2001) and an eradication programme was implemented by the Canadian Food Inspection Agency (CFIA).
Fig. 1 Addition of aphids to PPV-infected plum leaves positioned over detached peach leaves on a gel bed. Peach leaves were incubated 2 weeks in a containment room before being tested by DRT-PCR for PPV infection.
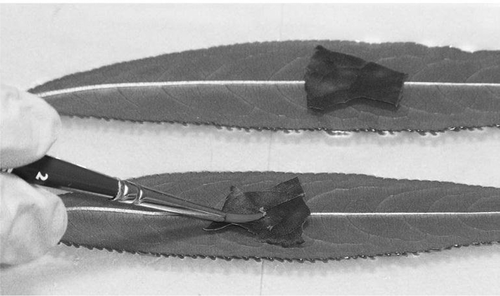
The efficiency of virus transmission is dependent on the frequency of occurrence of vectors and cultivar susceptibility to the pathogen. While numerous aphid species have been shown to transmit PPV-D in a non-persistent manner, the green peach aphid (Myzus persicae (Sulzer)), the spirea aphid (Aphis spiraecola (Patch)) and the soybean aphid (Aphis glycines (Matsumura)) represent the most prevalent and efficient vector species in Niagara orchards (Lowery et al. Citation2009). Incidence and populations of these species are variable over the growing season, linked with weather, presence of predator species and availability of feeding hosts. The application of oil sprays was recommended by the International PPV Expert Panel in 2009 as a protectant to reduce spread of PPV by aphid vectors in Prunus orchards over the entire growing season. Growers have resisted using oil sprays, however, due to concerns associated with cost and possible foliar phytotoxicity during the warmer summer months. Since 2007, ongoing studies in our laboratory have suggested that susceptibility of peach trees to aphid transmission of PPV may decrease over the summer. By studying susceptibility of peach trees to PPV over the growing season, it may be possible to reduce the number of oil sprays needed to provide protection and use oil only during periods of elevated susceptibility.
Under the eradication program, it is not possible to conduct field studies to evaluate the susceptibility of trees to aphid transmitted PPV. Although whole plants are necessary to examine host plant resistance in some systems (Klinger et al. Citation2005), other systems have shown that only parts of a plant, such as detached leaves, can be used for assessing resistance or virulence (Sams et al. Citation1975; Rufener et al. Citation1987; Sharma et al. Citation2005; Kalleshwaraswamy & Krishna Kumar Citation2008). This paper reports on the development and evaluation of a detached leaf method now in use in ongoing studies to evaluate foliar susceptibility to aphid transmitted PPV.
Materials and methods
Virus source
The Canadian isolate of the Dideron strain of PPV (PPV-D), characterized by Rochon et al. (Citation2003) that was used in this study was isolated from an infected peach tree in Niagara. Virus was maintained in plum (Prunus domestica L. ‘Stanley’) and peach (Prunus persica L. ‘Elberta’) seedlings for use in transmission trials. Seedlings were inoculated using the layered leaf method detailed below. Virus presence in the plants was confirmed by ELISA as described below after 3–4 weeks incubation.
Rearing of aphids
Green peach aphids (M. persicae Sulzer) were reared in ventilated Plexiglass cages on Bok Choy (Brassica rapa subspecies pekinensis ‘Heavy’ (422E), Stokes Seeds, St. Catharines, ON) and maintained under fluorescent lighting on a 16 h photoperiod. Ceramic plant watering spikes (Lee Valley Tools, Burlington, ON) were inserted in each pot to minimize exposure of the plants to outside aphid contamination through hand watering.
Manual serial aphid transfer inoculation method for seedlings
Third and fourth instar nymphs and apterous adult green peach aphids were transferred using a fine artist’s brush to 5 cm Petri dishes (VWR Scientific, Mississauga, ON) with tight-fitting lids and starved at ambient temperature (22°C) for a minimum of 2 h. Aphids were then transferred to leaf pieces from PPV-infected plum seedlings (P. domestica L. ‘Stanley’) in sealed Petri dishes for a 5 min acquisition access period (AAP) (). Following AAP, 50 aphids were transferred to the upper leaf surfaces of each of 5 or more peach seedlings (P. persica L. ‘Babygold’) in separate trials (). All seedlings were at the 5–6 leaf stage (c. 15 cm tall) and were pre-treated with the aphicide Pirimicarb (Pirimor® WG, Syngenta Inc., Guelph, ON) before the aphids were released. This minimized aphid escape and was found to result in complete aphid mortality within 48 h, as demonstrated in other systems with different hosts (Scott & Smilowitz Citation1980). Pirimicarb did not affect probing of apterous green peach aphids during the first day of transfer to leaves from treated potato (Lowery & Boiteau Citation1988) and had no effect on the rate of spread of Turnip mosaic virus under natural field conditions (Lowery et al. Citation1990). Following transfer of the viruliferous aphids, seedlings were placed in 20 lb polybags, sealed, and stored in the dark in plastic lidded Rubbermaid® tubs for 48 h after which time the bags were removed. The seedlings were then transferred to containment rooms and grown for an additional 3 weeks (22°C, 4100 lux halide lighting, 16 h photoperiod). Fully expanded apical leaves were macerated in ELISA extraction buffer (1:6, tissue:buffer) (Clark & Adams Citation1977). The suspension was further diluted using direct plant extraction buffer (DiPEB) and assayed by direct real-time reverse transcriptase Taqman probe based polymerase chain reaction assay (DqRT-PCR, Kim et al. Citation2008).
Table 1. Percentage infection of peach seedlings and detached peach leaves following manual serial aphid transmission of PPV from infected plum leaves.
Seedling and detached leaf manual serial aphid inoculation trials
In order to evaluate changes in susceptibility of field peach trees to PPV over the growing season, it is necessary to use detached leaves collected from the trees at various times during the summer. To examine whether virus multiplication could be detected in detached leaves, aphid inoculated detached seedling leaves were tested by PCR and simultaneously compared with the aphid inoculated seedlings as described above. Detached leaves were supported on a layer of 0.4% agar gel, midrib up, in a 24.5 × 24.5 × 2.5 cm (l × w × h) Nunclon TM polystyrene culture dish with lid (VWR Scientific, Mississauga, ON). Pirliss® 50DF (50% pirimicarb ai, Plant Products, Brampton, ON) was added to the agar (0.063% Pirliss, w/v) to minimize aphid escape and was found to result in complete aphid mortality within 48 h (Lowery & Boiteau Citation1988). Starved aphids were transferred onto PPV-infected peach (P. persica L. ‘Elberta’) leaf pieces for a 5 min AAP. Following acquisition, 50 viruliferous aphids were then transferred to each of the virus-free detached leaves in the agar plates that were then sealed with Parafilm® to minimize moisture loss. Plates were stored in the dark for 24 h and then incubated for 3 weeks in the containment room. Leaves were then assayed by DqRT-PCR as described. Fifty leaves each of apple and pear were also aphid-inoculated as described to determine whether assays detected any residual virus left in or on non-PPV hosts by probing aphids. Fifty peach leaves were used as controls.
Leaf overlay inoculation method
To reduce aphid handling, a layered leaf approach was also examined. A 1.0 × 1.0 cm piece of infected plum leaf was overlaid on the lower surface of each of six or more detached peach leaves supported on the agar gel layer in separate trials (). Twenty-five starved aphids were then transferred onto each of the PPV-infected plum/peach leaf pieces and the plates were sealed. Loss in turgor in the infected plum or peach leaf piece usually resulted in aphids moving onto the peach leaf within 6 h where they continued probing and feeding. Plates were stored in the dark for 24 h, incubated for 3 weeks in the containment room and leaves assayed for PPV as described. A comparison between 50 and 25 aphids per leaf using the detached leaf method was also made to compare transmission efficacies (). As a control, PPV-infected peach or plum leaves were layered over healthy peach leaves in the absence of aphids and did not transmit PPV to the healthy peach leaves. PPV is not known to be mechanically transmissible (OEPP/EPPO Citation1994).
Table 2. Comparison of PPV infection rates of detached leaves by the manual serial aphid transfer method and the leaf overlay method.
Table 3. Percentage infection of detached peach leaves influenced by the number of aphids applied to infected plum leaf segments overlaid on the peach leaves.
Pre- and post-inoculation temperature
The effect of short-term handling storage temperature of leaves pre- and post-inoculation with PPV was examined. A total of 62 peach leaves were collected from the field during the active growing season from 10-year-old peach (‘Babygold’) trees and randomly assigned to three temperature regimes of 4, 10, 20°C (± 1.0°C) for 48 h. Control treatments (ambient room temperature) were inoculated with PPV on the same day with 25 starved green peach aphids as described, while temperature-treated leaves were kept in the dark under the designated test temperatures prior to or after the 48 h of exposure to viruliferous aphids. Plates were then incubated for 3 weeks in the containment room and then assayed by DqRT-PCR as described. Experiments were repeated six times for each handling temperature.
Statistical analysis
SAS software was used (SAS Institute Citation1998). Treatment means were compared using the one-way ANOVA Welch’s test (Welch Citation1947). The chi-square test, Fisher’s exact test and contingency coefficients were used to analyse differences in transmission rates between the pre- and post-inoculation temperature treatments at the 95% confidence level.
Results and discussion
Seedling and detached leaf manual serial aphid inoculation trials
Utilizing the manual serial transfer inoculation method, inoculation of intact peach seedlings with PPV using green peach aphids as the vector resulted in higher rates of infection compared with inoculation of detached leaves maintained on agar plates, but the difference was not significant (). To demonstrate that the positive PCR tests were the result of virus replication within the detached leaves and not the residue from the initial aphid inoculations, PPV was not detected from either apple or pear leaves probed by viruliferous aphids, while 16% of peach leaves exposed at the same time tested positive. Detached peach leaves were maintained for 3–4 weeks post-inoculation on the agar beds as described without any noticeable chlorosis or degradation, allowing ample time for virus multiplication. The addition of antimicrobial agents to the agar media would help prevent the growth of contaminant fungi and perhaps extend the viability of leaves for a longer length of time. For these experiments, we relied on fungicide sprays having been applied to peach trees in the field. Alternatively, the excised leaves could be treated with a fungicide prior to use. If required, the viability of leaves might be lengthened further with the addition of nutrients and growth regulators commonly used in plant tissue culture (e.g. Murashige & Skoog Citation1962).
Leaf overlay inoculation method
Serial transfer of aphids to infected leaf material and then to virus-free test plants following the initial starvation period is designed to reflect transmission of non-persistent viruses by transient alate aphids. This three-step procedure is laborious, however, and does not lend itself to studies where large numbers of aphids have to be physically transferred. The leaf overlay method was found to result in comparable transmission rates to the manual serial transfer method () and the reduced handling affords less opportunity to damage the aphid stylets or disrupt feeding. A large degree of variability can occur between experiments that is likely attributable to stages in aphid development, behavioural factors, plant leaf age and morphology, environmental factors (Smith et al. Citation1994) and the technical ability of personnel doing the aphid transfers. Several studies have used 100 or more aphids per leaf to ensure consistent transmission (Quiot et al. Citation1995; Damsteegt et al. Citation2001). Other studies, using the ‘free roaming method’, placed infected plants containing indeterminate numbers of aphids among healthy seedlings allowing aphids to move to the seedlings at their volition (Damsteegt et al. Citation2001, Citation2004). Generally, most researchers have found that 10–30 aphids per leaf or plant gave consistent virus transmission (Marénaud & Massonie Citation1977; Dosba et al. Citation1987; Labonne et al. Citation1994; Kamenova et al. Citation1998; Gildow et al. Citation2003). Our studies generally demonstrated acceptable transmission efficiencies with between 25 and 50 aphids (), although fewer than 25 aphids were not tested. Unless otherwise stated, all of our subsequent research has standardized on 25 aphids/leaf to reduce transfer times, allowing for more replications, while still maintaining sufficient inoculum pressure for consistent virus transmission. Virus levels in leaves inoculated with 50 or 25 aphids were moderately high, with PCR ct values averaging 25 compared with 18 in leaves from symptomatic seedlings grown in containment chambers that were used as controls. Although not permitted under containment guidelines at this facility, the most efficacious approach may be to rear aphids on infected plum seedlings and to apply excised leaf discs containing 25 or more aphids directly onto the target leaves. This would alleviate damage to the aphids resulting from the physical transfer process. The detached leaf method outlined here is flexible and allows for transfer of infected leaf pieces infested with aphids that would more closely simulate transmission of PPV between peach trees by colonizing aphids.
Pre- and post-inoculation temperature
Susceptibility of plants to virus infection is affected by environmental conditions such as temperature, relative humidity and light. Temperature has a significant effect on plant susceptibility to virus infection and virus multiplication rate as well as plant response to infection and disease symptoms (Kassanis Citation1957; Swenson Citation1963; Syller Citation1991). In the current study, peach leaves receiving different combinations of incubation temperatures for short storage times pre- and post-inoculation, as indicated in , did not show any significant differences in virus transmission rates. Similar observations have been reported from other studies; pre and post-inoculation treatments did not alter the susceptibility of host plants to Potato virus Y and Potato leafroll virus (Singh et al. Citation1988), Cucumber mosaic virus (Stimmann & Swenson Citation1967) or Bean yellow mosaic virus (BYMV) (Swenson Citation1968). According to Szittya et al. (Citation2003), under cold conditions, plants tend to become more susceptible to virus infection. Susceptibility of bean and pea plants to BYMV inoculated by aphids has been reported to increase when plants were kept pre-inoculation at 18 and 15°C, respectively, while plants kept post-inoculation at 30°C resulted in more infected plants (Swenson & Sohi Citation1961). Our pre- and post-conditioning studies did not show any effect of temperature on susceptibility of peach leaves to PPV over the short period of 48 h, suggesting that leaves can be harvested from the field and kept chilled prior to use.
Table 4. Effect of pre- and post-incubation temperatures on PPV multiplication in peach leaves collected from the field during the growing season.
The detached leaf assay procedure outlined in this study produced PPV infection rates on leaves inoculated by viruliferous M. persicae that were equivalent to those using intact peach seedlings. Utilization of this technique will allow for a rapid evaluation of changes in host suitability in trees growing under field conditions and could be used in other studies that were previously difficult to perform with whole trees. Previous research we conducted on the host range of PPV, for example, required culture of inoculated woody plants for many months, often with an intervening period of cold, before the plants tested positive using DqRT-PCR (data not shown). Based on our results with leaves collected from peach trees in the field, the detached leaf technique could provide reliable results over a period of 2–3 weeks using a minimal amount of space.
Acknowledgements
Funding for this project was provided from the National Plum Pox Virus Research Program, Agriculture and Agri-Food Canada. The authors thank Rebecca Matthews, Darlene Nesbitt, Michael Szczepanek and Neil Bodimeade for their technical assistance in aphid transmission trials and DqRT-PCR assays.
References
- Clark MF, Adams AN. 1977. Characteristics of the microplate method of enzyme-linked immunosorbent assay for the detection of plant viruses. J Gen Virol. 34:475–483.
- Damsteegt V, Stone AL, Luster DG, Gildow FE, Levy L, Welliver R. 2001. Preliminary characterization of a North American isolate of Plum pox virus from naturally infected peach and plum orchards in Pennsylvannia, USA. Acta Hortic. 550:145–152.
- Damsteegt VD, Stone AL, Schneider WL, Luster DG, Gildow FE. 2004. Potential Prunus host range of PPV-PENN isolates by aphid transmission. Proceedings of the XIXth International Symposium on Fruit Tree Virus Diseases. Acta Hortic. 657: 201–205.
- Dosba F, Maison P, Lansac M, Massonie G. 1987. Experimental transmission of Plum pox virus (PPV) to Prunus mahaleb and Prunus avium. J Phytopathol. 120:199–204.
- Gildow FE, Damsteegt VD, Stone AL, Schneider W, Luster DG, Levy L. 2003. Transmission of North American isolates of Plum pox virus: Identification of aphid vectors and species-specific transmission from infected stone fruits. Proceedings of the 19th International Symposium on Virus and Virus-Like Diseases of Temperate Fruit Crops, Valencia, Spain, pp. 47.
- Kalleshwaraswamy CM, Krishna Kumar NK. 2008. Transmission efficiency of Papaya ringspot virus by three aphid species. Phytopathology. 98:541–546.
- Kamenova I, Lohuis D, Peters D. 1998. Comparison of aphid transmission of Plum pox virus isolates and purification of their helper components. Bulg J Agric Sci. 4:9–16.
- Kassanis B. 1957. Effects of changing temperature on plant virus diseases. Adv Virus Res. 4:212–241.
- Kim W-S, Stobbs LW, Lehman SM, James D, Svircev AM. 2008. Direct real-time PCR detection of Plum pox virus in field surveys in Ontario. Can J Plant Pathol. 30:308–317.
- Klinger J, Creasy R, Gao L, Nair RM, Calix AS, Jacob HS, Edwards OR, Singh KB. 2005. Aphid resistance in Medicago truncatula involves antixenosis and phloem-specific, inducible antibiosis, and maps to a single locus flanked by NBS-LRR resistance gene analogs. Plant Physiol. 137:1445–1455.
- Labonne G, Lauriaut F, Won M, Quiot JB. 1994. Dissémination du plum pox potyvirus par les pucerons: Analyse des vecteurs potentiels du virus dans un verger d’abricotiers. OEPP/EPPO Bulletin. 24:681–690.
- Levy L, Damsteegt V, Welliver R. 2000. First report of Plum pox virus (Sharka disease) in Prunus persica in the United States. Plant Dis. 84:202.
- Lowery DT, Boiteau G. 1988. Effects of five insecticides on the probing, walking, and settling behavior of the green peach aphid and the buckthorn aphid (Homoptera: Aphididae) on potato. J Econ Entomol. 81:208–214.
- Lowery DT, Sears MK, Harmer CS. 1990. Control of turnip mosaic virus of rutabaga with applications of oil, whitewash, and insecticides. J Econ Entomol. 83:2352–2356.
- Lowery T, Vickers T, Bittner L. 2009. Research update: aphid transmission, host range and management of PPV. Report to the PPV International Expert Panel 2009 Annual Meeting, St. Catharines, ON, Canada.
- Marénaud C, Massonie G. 1977. Variability of Plum pox virus isolates. Ann Phytopathol. 9:107–121.
- Murashige T, Skoog F. 1962. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 15:473–497.
- Nemeth M. 1986. Virus, Mycoplasma and Rickettsia diseases of fruit trees. Akademia Kiado. Forestry series. Vol. 10, Budapest: Springer; 750 pp.
- OEPP/EPPO. 1994. Plum pox potyvirus. Bulletin. 24:721–730
- Quiot JB, Boeglin M, Adamolle C, Candresse T, Labonne G, Renaud LY. 1995. Behaviour of two isolates of Plum pox virus inoculated on peach and apricot trees: first results. Acta Hortic. 386:290–297.
- Rochon D, Theilmann J, James D, Reade R, Yang L, Upton C. 2003. Partial molecular characterization of Plum pox virus isolates occurring in Canada. Can J Plant Pathol. 25:198–208.
- Rufener GK, Hammond RB, Cooper RL, St. Martin SK. 1987. Larval antibiosis screening technique for Mexican bean beetle resistance in soybean. Crop Sci. 27:598–600.
- Sams DW, Lauer FI, Radcliffe EB. 1975. Excised leaflet test for evaluating resistance to green peach aphid in tuber-bearing Solanum germplasm. J Econ Entomol. 68:607–609.
- SAS Institute. 1998. SAS users guide: statistics. Version 2. Cary, NC: SAS Institute.
- Scott L, Smilowitz Z. 1980. Selective Toxicity of Pirimicarb, Carbaryl and Methamidophos to Green Peach Aphid, (Myzus persicae) (Sulzer), Coleomegilla maculata lengi (Timberlake) and Chrysopa oculata Say. Environ Entomol. 9:752–755
- Sharma HC, Pampapathy G, Dhillon MK, Ridsdill-Smith JT. 2005. Detached leaf assay to screen for host plant resistance to Helicoverpa armigera. J Econ Entomol. 98:568–576.
- Singh MN, Khurana SM, Nagaich BB, Agrawal HO. 1988. Environmental factors influencing aphid transmission of potato virus Y and potato leafroll virus. Potato Res. 31:501–509.
- Smith CM, Khan ZR, Pathak MD. 1994. Techniques for evaluating insect resistance in host crops. Boca Raton, FL, USA: CRC Press.
- Stimmann MW, Swenson KG. 1967. Effects of temperature and light on plant susceptibility to Cucumber mosaic virus by aphid transmission. Phytopathology. 57:1072–1073.
- Swenson KG, Sohi SS. 1961. Factors determining the rate of bean yellow mosaic virus transmission by the aphid Myzus persicae (Abstract). Phytopathology. 51:67.
- Swenson KG. 1963. Effects of insect and virus host plants on transmission of viruses by insects. Ann N Y Acad Sci. 105:730–740.
- Swenson KG. 1968. Relation of environment and nutrition to plant susceptibility to bean yellow mosaic virus by aphid transmission. Tech Bull Oreg Aes. 106:23.
- Syller J. 1991. The effects of temperature on the susceptibility of potato plants to infection and accumulation of Potato leafroll virus. J Phytopathol. 133:216–224.
- Szittya G, Silhavy D, Molnar A, Havelda Z, Lovas A, Lakatos L, Banfalvi Z, Burgyan J. 2003. Low temperature inhibits RNA silencing-mediated defence by the control of siRNA generation. EMBO J. 22:633–640.
- Thompson D, McCann M, MacLeod M, Lye D, Green M, James D. 2001. First report of Plum pox potyvirus in Ontario, Canada. Plant Dis. 85:97.
- Welch BL. 1947. The generalization of “Student’s” problem when several different population variances are involved. Biometrika. 34:28–35.
