Abstract
Tomato marchitez virus (ToMarV) was observed infecting pepper as a natural new host in Sinaloa, Mexico during the annual growth season (September 2010 to May 2011). Symptoms typical of viral infection consisted of yellow mosaic, upward leaf curling, crinkling, and stunting. The presence of whiteflies was observed on the affected plants, indicating possible vectors of the virus. Samples from symptomatic plants tested negative for the presence of viruses in the genera Begomovirus and Crinivirus, both of which are whitefly-transmitted. The results from RT-PCR and sequencing analysis indicated that ToMarV (genus Torradovirus) was present in six out of 15 samples. Subsequently, sap from infected leaves was used to successfully transmit the virus by mechanical inoculation to three pepper cultivars. This is the first report of Tomato marchitez virus infection on pepper in Mexico.
Résumé
Durant la saison de croissance s’étendant de septembre 2010 à mai 2011, nous avons observé que le virus marchitez de la tomate (VMarTo) avait infecté un nouvel hôte naturel au Sinaloa, au Mexique, soit le piment. Les symptômes typiques de l’infection virale se traduisaient par de la mosaïque jaune, des feuilles recroquevillées vers le haut, des feuilles froissées et du rabougrissement. Nous avons également observé des aleurodes sur les plants infectés, vecteurs possibles du virus. Nous avons obtenu des résultats négatifs chez les échantillons de plants symptomatiques quant à la présence de virus des genres Begomovirus et Crinivirus, qui sont tous les deux transmis par des aleurodes. Les résultats obtenus par RT-PCR et analyse de séquences ont indiqué la présence du VMarTo (genre Torradovirus) dans 6 des 15 échantillons. Par la suite, la sève prélevée dans des feuilles infectées a été utilisée pour transmettre avec succès, par inoculation mécanique, le virus à trois cultivars de piment. Il s’agit du premier rapport d’une infection causée par le virus marchitez de la tomate chez le piment au Mexique.
Introduction
Pepper (Capsicum annuum L.) is an economically important crop in several countries including Mexico, which contributes to 20% of the total worldwide exports (FAOSTAT Citation2013). Pepper production in Mexico in 2012 was 2,379,736 tons with a value of US $ 30,936,568 (SIAP-SAGARPA Citation2012). Pepper cultivation and yield production can be affected by different viral diseases (Torres-Pacheco et al. Citation1996; Salati et al. Citation2010); among them are the whitefly-transmitted viruses belonging to the genera Begomovirus and Crinivirus, which have been previously reported to affect vegetable crops in Mexico (Garzón-Tiznado et al. Citation1993; Gámez-Jiménez et al. Citation2009; Méndez-Lozano et al. Citation2012). In 2007, a new virus transmitted by whiteflies was reported to infect tomato in Culiacán, Sinaloa, Mexico. Initially, it was named Tomato apex necrosis virus (ToANV) and later as Tomato marchitez virus (ToMarV) (Turina et al. Citation2007; Verbeek et al. Citation2008). Subsequently, the plant virus genus Torradovirus was created within the family Secoviridae to place two newly described virus species, namely Tomato torrado virus (ToTV) from Spain and Tomato marchitez virus (ToMarV) from Mexico (Sanfaçon et al. Citation2009). Lately, members of the new genus Torradovirus have been reported from Poland, France, Hungary, Panama, Guatemala, Australia, Italy, and Colombia (Pospieszny et al. Citation2007; Alfaro et al. Citation2009; Herrera-Vásquez et al. Citation2009; Verdin et al. Citation2009; Batuman et al. Citation2010; Davino et al. Citation2010; Gambley et al. Citation2010; Verbeek et al. Citation2010; Verbeek & Dullemans Citation2012). Members of this genus are transmitted by the whitefly species Trialeurodes vaporariorum (Pospieszny et al. Citation2010), Bemisia tabaci (Amari et al. Citation2008) and recently by Trialeurodes abutilonea (Verbeek et al. Citation2014). Under experimental conditions, ToTV could be transmitted to pepper, eggplant and potato as well as a range of indicator plants (Amari et al. Citation2008; Pospieszny et al. Citation2007; Verbeek et al. Citation2007, Citation2008). However, ToMarV could be transmitted only to a small number of experimental plants such as Nicotiana occidentalis, Chenopodium quinoa, and Physalis floridana (Verbeek et al. Citation2008).
During March 2011 (annual crop of 2010–2011), pepper plants in a commercial greenhouse located in Guasave Valley and an open field in La Cruz de Elota Valley from Sinaloa state were affected by an unusual disease. The symptoms and the presence of whiteflies (Bemisia tabaci) in the affected plants suggested a disease of viral aetiology. The objective of this study was to identify the causal agent inducing the virus symptoms in pepper crops in the state of Sinaloa, Mexico.
Materials and methods
Sampling
In March 2011, leaf samples from five symptomatic pepper plants each of cultivars ‘Mini Bell’ and ‘Anaheim’ were collected from a commercial greenhouse in Guasave Valley. Additionally, five samples of pepper cultivar ‘Bell’ were collected in an open field from La Cruz de Elota Valley; both places are located at Sinaloa State, Mexico. The disease incidence in the greenhouse and open field was estimated at around 15%.
Molecular identification
Total DNA and RNA extraction was performed from symptomatic pepper plants as described previously (Zhang et al. Citation1998; Singh et al. Citation2002). Initial attempts for viral detection were performed using a PCR test using degenerated primers for begomovirus (Ascencio-Ibáñez et al. Citation1999; Mauricio-Castillo et al. Citation2007) and RT-PCR with specific primers for two criniviruses: Tomato chlorosis virus and Tomato infectious chlorosis virus (Dovas et al. Citation2002). Furthermore, RT-PCR test was conducted for ToMarV using specific primers ToMarV-F (5´AGTGAGTTCCATTGAGATA3´) and ToMarV-R (5´TAAAGGAAATTATCGAGTTCC 3´), which amplify a ~ 517 bp fragment targeting the coat protein Vp24 and 3ʹUTR (Verbeek et al. Citation2008). PCR conditions were as follows: initial denaturation at 95°C for 3 min followed by 39 cycles at 95°C denaturation for 30 s, annealing at 48°C for 30 s, extension at 72°C for 36 s and a final extension at 72°C for 5 min. In order to increase the sensitivity of the detection, an internal set of primers pJER1123 (5´CAACCTCCTTCTGCCAACCAT3´) and pJER1124 (5´TCTAACCAAGCAGGGCACACG3´) were designed to amplify a 332 bp fragment for nested PCR. PCR conditions were as follows: initial denaturation at 95°C for 3 min followed by 34 cycles at 95°C denaturation for 30 s, annealing at 59.3°C for 30 s, extension at 72°C for 1 min and a final extension at 72°C for 3 min. An internal control that amplified a 188 bp fragment of the chloroplast ndhB gene (Thompson et al. Citation2003) was also included. The amplicons obtained from RT-PCR were cloned into pGEM-T easy vector system II (Promega Corporation, Madison, WI) and sequenced in both directions. The sequence of one amplicon was compared with sequences of other members of the genus Torradovirus reported in the NCBI/GenBank database, using the Clustal W alignment method of the sequence analysis software suite Lasergene (MegAlign, DNASTAR Inc., Madison, WI).
Infectivity test
Mechanical transmission was performed by leaf rub inoculation. Leaves from symptomatic plants were ground in 0.01 M-phosphate buffer, pH 7.4 (1:4 w/v) using a mortar and pestle. Eight plants at the four-leaf-stage of each of three pepper cultivars (‘Anaheim’, ‘M10' and ‘Orange’) were inoculated after carborundum abrasion (600 mesh) of the true leaves with extracted sap. All inoculated plants were placed in a growth chamber at 26/24°C (day/night) with a 12 h photoperiod. Plants were inspected for virus symptoms periodically for the next 4 weeks.
Results and discussion
Plant sampling and symptoms
A total of 15 leaf samples were collected from greenhouse-grown and open field pepper plants, representing a disease incidence of around 15% in both locations. The symptoms observed were yellow mosaic, upward leaf curling, crinkling and stunting (). In addition, plants in affected fields had presence of whiteflies on the leaves, suggesting that they could be a vector of viral pathogen. Previously, pepper crops have been reported to be affected by different whitefly-transmitted viruses, such as members of the genus Begomovirus: Pepper golden mosaic virus, Pepper huasteco yellow vain virus, and Tomato yellow leaf curl virus; and the genus Crinivirus: Tomato chlorosis virus (Garzón-Tiznado et al. Citation1993; Morilla et al. Citation2005; Fortes et al. Citation2012).
Fig. 1 (Colour online) Symptoms associated with Tomato marchitez virus (ToMarV) in pepper plants. a, Plants from an open field in La Cruz de Elota exhibiting yellow mosaic, upward leaf curling and crinkling; b, Plants of cultivar ‘Orange’ 30 days post-inoculation – (left) mock inoculated plant and (right) infected plant exhibiting leaf yellowing and stunting; c, Leaf pepper plants of cultivar ‘Orange’ 15 dpi showing vein necrosis (white arrow).

Molecular detection and identification
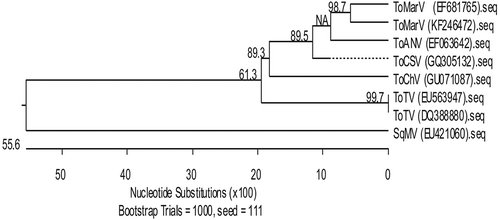
Initial attempts to detect Begomovirus and Crinivirus (ToCV and TICV) in the collected samples by PCR and RT-PCR were negative (data not shown). A viral disease caused by ToMarV was reported previously affecting tomato in Sinaloa (Verbeek et al. Citation2008). In addition, under experimental conditions, ToTV has been shown to be transmitted to pepper plants (Amari et al. Citation2008). Therefore, leaf samples were analysed for ToMarV infection by RT-PCR using specific primers (ToMarV-F/ToMarV-R), but the samples resulted in no amplification of the expected size amplicon (). However, when using nested-RT PCR for ToMarV detection in pepper by using primer set pJER1123 and pJER1124, six out of 15 leaf samples provided PCR products of the expected size of 332 bp (). Dovas et al. (Citation2002) have reported a multiplex nested PCR assay that can reliably detect and discriminate two criniviruses in tomato crops, such as TICV and ToCV, with the detection sensitivity improved. It is important to mention that pepper samples were negative for crinivirus using the method of Dovas et al. (Citation2002) (data not shown). RT-PCR assay of the internal control (ndhB gene) resulted in the amplification of the expected size of 188 bp (). The PCR fragment from three representative samples amplified with ToMarV primers was cloned and sequenced in both directions ( lanes 1, 2 and 13). These sequences showed 100% nucleotide sequence identity with each other and one was deposited in GenBank (Accession number KF246472). In the phylogenetic analysis, the isolate showed 98.7% nucleotide sequence identity to ToMarV reported in Culiacán, Mexico (GenBank Accession number EF681765) ().
Fig. 2 Molecular detection by RT PCR of Tomato marchitez virus (ToMarV) in pepper plants collected in Guasave and La Cruz de Elota Valleys. a, Electrophoretic gel after PCR using primers ToMarV-F and ToMarV-R. No bands were detected except in the positive control (+). b, Electrophoretic gel after nested PCR using primers pJER1123 and pJER1124. The expected band size of 332 bp was detected in six samples. c, Electrophoretic gel after PCR of ndhB gene, used as internal control. Lane (-) negative control, lane 1 kb, molecular marker (1 Kb); lane (+) positive control; lanes 1–5, pepper samples from ‘Mini Bell’ from Guasave; lanes 6–10, pepper samples from ‘Bell’ from La Cruz de Elota; lanes 11–15, pepper samples from ‘Anaheim’ from Guasave.
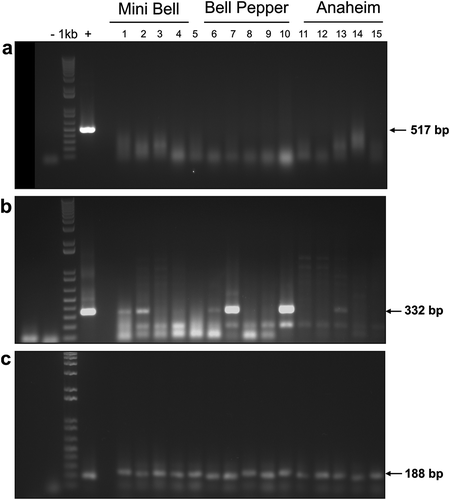
Fig. 3 Similarity dendrogram derived from sequences of the partial Vp24 gene and 3ʹUTR region of selected Torradovirus. The isolate from this study is shown as GenBank Accession number KF246472. Distances were determined by clustal W alignment method with weighed residue weight table in the program MegAlign of the sequence analysis software suite Lasergene (DNASTAR Inc.). Bootstrap confidence values are shown on the branches.
Infectivity test
Mechanical transmission was conducted by leaf rub inoculation onto three pepper cultivars (‘Anaheim’, ‘M10' and 'Orange') using sap from leaves of symptomatic plants ( lane 2) The inoculated leaves developed symptoms of yellow mosaic and systemic yellowing, crinkling and stunting were observed in at least two plants from each cultivar (). Similar symptoms of ToTV infection in pepper plants inoculated experimentally were described previously (Amari et al. Citation2008). In addition, vein necrosis was also observed at 15 days post inoculation (dpi) in one plant of cultivar 'Orange' (), similar to that described previously for ToTV (Verdin et al. Citation2009). Molecular detection of ToMarV in inoculated leaves of the following pepper cultivars confirmed the presence of the virus in nine out of 24 plants at 15 dpi – the virus was present in two of eight plants of 'Anaheim', five of eight plants of 'M10', and two of eight plants of ‘Orange’. In summary, these results indicate that ToMarV causes a pepper disease, indicating that this virus has begun to infect new crops of agricultural importance. However, the economic impact in the region is unknown. To our knowledge, this is the first report of natural infection of ToMarV affecting pepper in Mexico.
Acknowledgements
We thank Instituto Politécnico Nacional (SIP 20131613) for financial support of this research project. RAC was supported by fellowships from the Consejo Nacional de Ciencia y Tecnología (CONACyT, Mexico) and from PIFI program of IPN. We wish to thank Prof. Andreas Voloudakis for critically reviewing this manuscript.
References
- Alfaro A, Bese G, Córdoba C, Cebrián MC, Herrera JA, Forray A, Jordá C. 2009. First report of tomato torrado virus infecting tomato in Hungary. Plant Dis. 93:554.
- Amari K, Gonzalez-Ibeas D, Gómez P, Sempere RN, Sanchez-Pina MA, Aranda MA, Diaz-Pendon JA, Navas-Castillo J, Moriones E, Blanca J, et al. 2008. Tomato torrado virus is transmitted by Bemisia tabaci and infects pepper and eggplant in addition to tomato. Plant Dis. 92:1139.
- Ascencio-Ibáñez JT, Diaz-Plaza R, Méndez-Lozano J, Monsalve- Fonnegra ZI, Argüello-Astorga GR, Rivera-Bustamante RF. 1999. First report of tomato yellow leaf curl geminivirus in Yucatán, México. Plant Dis. 83:1178.
- Batuman O, Kuo YW, Palmeri M, Rojas MR, Gilbertson RL. 2010. Tomato chocolate spot virus, a member of a new torradovirus species that causes a necrosis-associated disease of tomato in Guatemala. Arch Virol. 155:857–869.
- Davino S, Bivona L, Iacono G, Davino M. 2010. First report of tomato torrado virus infecting tomato in Italy. Plant Dis. 94:1172.
- Dovas CI, Katis NI, Avgelis AD. 2002. Multiplex detection of criniviruses associated with epidemics of a yellowing disease of tomato in Greece. Plant Dis. 86:1345–1349.
- Food and Agriculture Organization of the United Nations. 2013. FAOSTAT, Available from: http://faostat.fao.org
- Fortes M, Moriones E, Navas J. 2012. Tomato chlorosis virus in pepper: prevalence in commercial crops in southeastern Spain and symptomatology under experimental conditions. Plant Pathol. 61:994–1001.
- Gambley CF, Thomas JE, Persley DM, Hall BH. 2010. First report of tomato torrado virus on tomato from Australia. Plant Dis. 94:486.
- Gámez-Jiménez C, Romero-Romero JL, Santos-Cervantes ME, Leyva-López NE, Méndez-Lozano J. 2009. Tomatillo (Physalis ixocarpa) as a natural new host for tomato yellow leaf curl virus in Sinaloa, Mexico. Plant Dis. 93:545.
- Garzón-Tiznado JA, Torres-Pacheco I, Ascencio-Ibañez JT, Herrera-Estrella L, Rivera-Bustamante RF. 1993. Inoculation of peppers with infectious clones of a new geminivirus by a biobalistic procedure. Phytopathology. 83:514–521.
- Herrera-Vásquez JA, Alfaro A, Córdoba MC, Cebrián MC, Font MI. 2009. First report of tomato torrado virus infecting tomato in single and mixed infections with cucumber mosaic virus in Panama. Plant Dis. 93:198.
- Mauricio-Castillo JA, Argüello-Astorga GR, Ambriz-Granado S, Alpuche-Solís AG. 2007. First report of tomato golden mottle virus on Lycopersicon esculentum and Solanum rostratum in Mexico. Plant Dis. 91:1513.
- Méndez-Lozano J, Magallanes-Tapia MA, Romero-Romero JL, Camacho-Beltrán E, Orduño-Vega WL, Leyva-López NE, Santos-Cervantes ME, Félix-Gastélum R. 2012. Tomato infectious chlorosis virus associated with tomato diseases in Baja California, Mexico. Plant Dis. 96:1229.
- Morilla G, Janssen D, García S, Moriones E, Cuadrado IM, Bejarano ER. 2005. Pepper (Capsicum annuum) is a dead-end host for tomato yellow leaf curl virus. Phytopathology. 95:1089–1097.
- Pospieszny H, Borodynko N, Obrępalska-Stęplowska A, Hasiow B. 2007. The first report of tomato torrado virus in Poland. Plant Dis. 91:1364.
- Pospieszny H, Budziszewska M, Hasiów-Jaroszewska B, Obrepalska-Steplowska A, Borodynko N. 2010. Biological and molecular characterization of Polish isolates of tomato torrado virus. J. Phytopathol. 158:56–62.
- Salati R, Shorey M, Briggs A, Calderon J, Rojas MR, Chen LF, Gilbertson RL, Palmieri M. 2010. First report of tomato yellow leaf curl virus infecting tomato, tomatillo, and pepper in Guatemala. Plant Dis. 94:482.
- Sanfaçon H, Wellink J, Le Gall O, Karasev A, Van der Vlugt R, Wetzel T. 2009. Secoviridae: a proposed family of plant viruses within the order Picornavirales that combines the families Sequiviridae and Comoviridae, the unassigned genera Cheravirus and Sadwavirus, and the proposed genus Torradovirus. Arch Virol. 154:899–907.
- SIAP-SAGARPA. 2012. Servicio de Información Agroalimentaria y Pesquera de la Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación. http://www.siap.sagarpa.gob.mx/
- Singh RP, Nie X, Singh M, Coffin R, Duplessis P. 2002. Sodium sulphite inhibition of potato and cherry polyphenolics in nucleic acid extraction for virus detection by RT-PCR. J Virol Methods. 99:123–131.
- Thompson JR, Wetzel S, Klerks MM. 2003. Multiplex RT- PCR detection of four aphid-borne strawberry viruses in Fragaria spp. in combination with a plant mRNA specific internal control. J Virol Methods. 111:85–93.
- Torres-Pacheco I, Garzón-Tiznado JA, Brown JK, Becerra-Flora A, Rivera-Bustamante RF. 1996. Detection and distribution of geminiviruses in Mexico and the southern United States. Phytopathology. 86:1186–1192.
- Turina M, Ricker MD, Lenzi R, Masenga V, Ciuffo M. 2007. A severe disease of tomato in the Culiacan area (Sinaloa, Mexico) is caused by a new picorna-like viral species. Plant Dis. 91:932–941.
- Verbeek M, Dullemans A, Van den Heuvel H, Maris P, Van der Vlugt R. 2010. Tomato chocolate virus: A new plant virus infecting tomato and a proposed member of the genus Torradovirus. Arch Virol. 155:751–755.
- Verbeek M, Dullemans AM. 2012. First report of tomato torrado virus infecting tomato in Colombia. Plant Dis. 96:592.
- Verbeek M, Dullemans AM, Van den Heuvel JFJ, Maris PC, Van der Vlugt RAA. 2007. Identification and characterization of tomato torrado virus, a new plant picorna-like virus from tomato. Arch Virol. 152:881–890.
- Verbeek M, Dullemans AM, Van den Heuvel JFJ, Maris PC, Van der Vlugt RAA. 2008. Tomato marchitez virus, a new plant picorna-like virus from tomato related to tomato torrado virus. Arch Virol. 153:127–134.
- Verbeek M, Van Bekkum P, Dullemans Annette M, Van der Vlugt René AA. 2014. Torradoviruses are transmitted in a semi-persistent and stylet-borne manner by three whitefly vectors. Virus Res. 186:55–60.
- Verdin E, Gognalons P, Wipf C, Bornard I, Ridray G, Schoen L, Lecoq H. 2009. First report of tomato torrado virus in tomato crops in France. Plant Dis. 93:1352.
- Zhang Y-P, Uyemoto JK, Kirkpatrick BC. 1998. A small-scale procedure for extracting nucleic acids from woody plants infected with various phytopathogens for PCR assay. J Virol Methods. 71:45–50.
