Abstract
Clubroot caused by Plasmodiophora brassicae has been reported at sites across North America on brassica vegetables for more than 50 years. However, it had not been reported on canola (Brassica napus) on the Canadian prairies until the initial discovery of a cluster of 12 infested fields near Edmonton AB in 2003. The purpose of this review is to consolidate and summarize the data on the spread of P. brassicae on canola in Canada since 2003, to compare this pattern of distribution with observations from an infested site in Ontario, and to draw inferences about the relative importance of short- and long-distance transmission of the pathogen on clubroot distribution in the prairie region. Over the last decade, P. brassicae has spread across central Alberta, with the leading edge of the epidemic moving at about 20 km per year, resulting in more than 1850 fields confirmed infested. DNA of the pathogen has also been detected from soil collected at sites across Saskatchewan and Manitoba, and very slight clubroot symptoms have been observed at isolated sites across the prairies. Transport of resting spores in soil carried on farm equipment has been shown to be an important mechanism of short-distance dissemination in this region. Dispersal of resting spores with wind-borne soil may also have an important role in both short- and long-distance dissemination. Dispersal on seed does not appear to be an important factor in clubroot spread. In contrast to the rapid spread observed in Alberta, P. brassicae is spreading very slowly, if at all, at the site in Ontario. This likely reflects the relatively small size and strength of the inoculum source and the absence of susceptible hosts nearby at the site in Ontario, relative to the thousands of hectares of heavily infested fields that provide a large, strong inoculum source in central Alberta.
Résumé
Depuis plus de 50 ans et partout en Amérique du Nord, on rapporte que la hernie, causée par Plasmodiophora brassicae, s’attaque aux légumes de la famille des brassicacées. Toutefois, elle n’a pas été rapportée sur les Prairies canadiennes sur le canola (Brassica napus) avant sa découverte initiale, en 2003, dans une douzaine de champs près d’Edmonton en Alberta. Le but de cette revue est de consolider et de résumer les données relatives à la dissémination de P. brassicae sur le canola au Canada depuis 2003, d’en comparer la distribution avec d’autres sites infestés en Ontario et de tirer des conclusions quant à l’importance relative de la transmission sur courtes et longues distances de l’agent pathogène sur sa distribution dans les Prairies. Au cours de la dernière décennie, de P. brassicae s’est répandu dans le centre de l’Alberta, la pointe de l’épidémie gagnant environ 20 km par année, ce qui a engendré l’infestation confirmée de plus de 1850 champs. L’ADN de l’agent pathogène a également été détecté dans des échantillons de sol collectés en Saskatchewan et au Manitoba; des symptômes pour le moins bénins de la hernie ont été observés dans des sites isolés sur les Prairies. Il a déjà été démontré que, sur de courtes distances, le transport de spores de repos contenues dans le sol transporté par la machinerie agricole est un important mécanisme de dissémination dans cette région. Les particules de sol soufflées par le vent peuvent également jouer un rôle important dans la dispersion des spores de repos sur de courtes et de longues distances. Par ailleurs, leur dispersion sur les semences ne semble pas être un facteur aussi déterminant en ce qui a trait à la dissémination de la hernie. Comparativement à sa dissémination rapide sur les Prairies, en Ontario, P. brassicae se répand très lentement à partir du site infesté, voire du tout. De toute évidence, cela reflète le petit volume relatif et la faible force de la source d’inoculum ainsi que l’absence d’hôtes réceptifs à proximité du site ontarien, comparativement aux milliers d’hectares de champs lourdement infestés qui constituent une puissante et abondante source d’inoculum dans le centre de l’Alberta.
Introduction
Clubroot caused Plasmodiophora brassicae (Woronin) has been known to occur on brassica crops since Roman times. It is present worldwide and causes an estimated 10–15% annual yield loss (Dixon Citation2009). Plasmodiophora brassicae is an obligate pathogen with a remarkable life cycle that involves two motile zoospore stages, a mobile amoeboid phase that can slip from cell to cell within the host, and small (~3-µm dia.), sub-spherical to spherical resting spores (Kageyama & Asano Citation2009). In terms of pathogen dissemination, however, the only important stage of the life cycle is the resting spores. The resting spores are roughly the size of silt particles and bacteria, but have a tough, multi-layered cell wall that makes them highly resistant to both microbial breakdown in soil and standard sanitation treatments (Hwang et al. Citation2014). Once a field is infested by P. brassicae, resting spores can build up rapidly in the presence of susceptible crops (Hwang et al. Citation2013). The half-life of resting spores was estimated to be 3.6 in Sweden (Wallenhammar Citation1996) and 4.4 years in central Alberta (Hwang et al. Citation2013).
Canola is a quality designation for lines of oilseed rape (Brassica napus L.) that contain less than 2% erucic acid and lower than 30 µmol g−1 of glucosinolates in the seed. A few cultivars of B. rapa and B. juncea have also been developed that meet this quality standard. The oil is used primarily for human food, where it is recognized for its health and wellness benefits. In North America, canola is produced as a bulk commodity on large (e.g. 65 ha), often contiguous fields, using huge pieces of farm equipment on mineral soils. Most producers manage multiple fields, where canola is often grown in a short rotation. Canola has been grown across much of the prairie region of Canada for the last 30 years, but the intensity of production (frequency in cropping rotations) has been increasing steadily in recent years. The area seeded to canola on the Canadian prairies is almost 8 million ha per year, with a farm-gate value of about $9 billion (Rempel et al. Citation2014). In the USA, between 1 and 2 million ha of canola are produced annually, with almost all of the production located in North Dakota (USDA-NASS Citation2014a, Citation2014b).
Prior to 2003, the canola crop on the Canadian prairies was one of the few substantial areas of brassica crop production in the world that was not affected by clubroot. The pathogen had been reported from many other parts of the USA, including California, Ohio, Wisconsin, and even Hawaii (Williams Citation1966; Rowe Citation1980). Similarly, clubroot had been confirmed on brassica vegetables in many areas of Canada, and there were anecdotal reports of a few infested garden sites in Alberta (Howard et al. Citation2010). Also, it was known that clubroot could infect and cause damage on canola in other regions of Canada (Morasse et al. Citation1997; Pageau et al. Citation2006). However, the clubroot-free status of the canola crop in the Canadian prairies changed in 2003 when severe clubroot was first identified in several fields near Edmonton, Alberta (Tewari et al. Citation2005). The initial report of clubroot on canola generated considerable concern in the region, given knowledge of the difficulties experienced with managing clubroot in other regions and on other crops. Nonetheless, as a soil-borne pathogen, spread of P. brassicae was expected to occur slowly (Gossen et al. Citation2013b).
The mechanism(s) of transmission of P. brassicae inoculum in canola on the Canadian prairies has been reviewed recently (Gossen et al. Citation2014; Strelkov & Hwang Citation2014). Transmission of the pathogen from field to field in infested soil on heavy equipment appears to be an important method of short-distance dissemination (Cao et al. Citation2009) and may also be a mechanism for long-distance dissemination. The spore concentrations observed in many infested fields is often ≥107 spores g−1, and hundreds of kilograms of infested soil can be carried from field to field on heavy equipment such as tractors and cultivators (Hwang et al. Citation2014; ). As a result, more than enough resting spores can be transported on equipment that is moved from an infested field to a clean field to initiate a new infestation. Also, recent studies on the importance of wind erosion for the dispersal of P. brassicae demonstrated that substantial amounts of inoculum are present in wind-blown dust from infested fields in Alberta (Rennie et al. Citation2015). This indicates that dispersal of inoculum by wind may be an important mechanism for both short and long-distance transmission of P. brassicae.
Fig. 1 (Colour online) Factors that may be important in introducing the resting spores to clubroot-free sites: a, Electron micrograph of resting spores of Plasmodiophora brassicae (photo courtesy of A. Deora, University of Guelph, Guelph, ON); b, clouds of dust produced by wind erosion (photo courtesy of H. de Gooier, University of Saskatchewan, Saskatoon, SK); c, infested soil on farm equipment (photo courtesy of R.J. Howard, Alberta Agriculture and Food, Brooks, AB); and d, gullies formed by water erosion (photo courtesy of J. Schoenau, University of Saskatchewan, Saskatoon, SK).

Dispersal of resting spores on seed produced in infested fields is a potential mechanism of long-distance transmission that has not been studied in detail. A low but repeatable incidence of seed-to-seedling transmission was demonstrated under optimal conditions in a greenhouse study (Rennie et al. Citation2011). Seed cleaning or seed treatment with a fungicide reduced this transmission to trace levels. The risk of transmission directly on canola seed, which is routinely cleaned and treated prior to planting, was therefore considered small (Rennie et al. Citation2011). Seed of other crops, such as field pea and potato tubers, could also carry P. brassicae-infested soil as an external contaminant, and might thereby also serve to disperse the pathogen. However, the risk of planting infested canola seed could not be assessed in the field at sites where the pathogen was already present because of high levels of background infection.
The objectives of this review are to: (i) summarize the results of more than a decade of surveys that document the rapid spread of P. brassicae across the canola production area on the Canadian prairies; (ii) contrast this rapid spread with the slow dispersal observed at a site in southern Ontario; (iii) evaluate the importance of seed-to-seedling transmission in long-distance dispersal of P. brassicae; and (iv) assess how the information from surveys and studies on the mechanisms of dispersal are changing the understanding of the relative importance of factors involved in dispersal of clubroot.
Surveys of clubroot on canola in Alberta
In 2003, clubroot on canola was reported for the first time on the northern Great Plains of North America in 12 commercial fields near Edmonton (Tewari et al. Citation2005). A survey for clubroot was initiated by a team of researchers at the University of Alberta in 2004 and has been continued each year to the present. The initial surveys were relatively small, involving 41 fields in 2004, 112 fields in 2005, and 250 fields in 2006. In recent years, clubroot incidence and severity have been examined in more than 400 commercial canola crops in Alberta each year. Over time, the focus of the survey has expanded from the area around Edmonton, where many fields were known to be infested, to regions on the periphery of the infestation, to alert producers to new infested sites in their area. Most of the surveyed crops were located on fields not previously surveyed for clubroot, or previously surveyed and found to be negative for the disease. Surveys were conducted mainly in September, shortly after swathing, to provide ease of access to mature crops before the clubbed roots started to break down. At least 50 roots were sampled in a 20 to 30 m2 area near the entrance to the field. If no symptoms of clubroot were found, no additional sampling was performed. This approach was taken because many clubroot infestations were observed to be most severe at the field entrance (Cao et al. Citation2009). If clubroot was found, then the roots of all plants within a 1 m2 area at each of 10 locations along the arms of a ‘W’ sampling pattern were assessed (generally > 100 plants). The severity of symptoms on each plant was assessed on a 0–3 scale, where 0 = no clubbing, 1 = a few small clubs, 2 = moderate clubbing and 3 = severe clubbing (adapted from Kuginuki et al. Citation1999). The individual ratings were then used to calculate a disease severity index (DSI) for each field (Horiuchi & Hori Citation1980, modified by Strelkov et al. Citation2006b). In 2013 and 2014, a total of 53 crops surveyed were clubroot-resistant canola hybrids, with the remainder being susceptible hybrids or of unknown resistance.
In addition to the main clubroot survey each year, trained inspectors for local governments conducted surveys and submitted samples for identification to certified testing facilities, starting in 2008. In most instances, the locations of fields with confirmed clubroot infestations were included in the survey tallies.
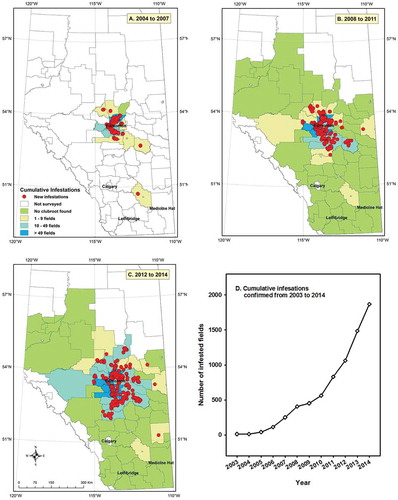
In 2004, a small clubroot survey focused primarily on fields close to the original discovery failed to identify any additional infested fields (Strelkov et al. Citation2005). However, the survey area and the number of infested fields subsequently expanded rapidly. In the first few years of the survey, there was an intense focus on the area around Edmonton, where the initial infested fields had been found (). By 2008, fields in almost all of the agricultural counties in Alberta were being assessed (). From 2011 to 2014, the focus shifted away from counties that were known to be heavily infested and towards the periphery of the infestation (), to assist producers by identifying fields at high risk of clubroot infestation. To highlight the location of newly infested fields in each time interval, they are indicated in with red dots. By 2007, more than 250 infested fields had been identified and confirmed, more than 800 infested fields were confirmed by 2011, and 1868 fields confirmed by 2014 (). Summaries of each annual survey are available in the Canadian Plant Disease Survey (Strelkov et al. Citation2005–2015).
Rapid spread of clubroot in Alberta
In each of the clubroot-infested fields identified in 2003, there were large patches where the incidence of symptomatic plants was high and symptoms were severe (Tewari et al. Citation2005). Based on our subsequent experience with the rate and pattern of development of clubroot epidemics in canola fields, we conclude that the pathogen must have been present in these fields for several plantings of susceptible crops to allow build up to such high levels. Similarly, clubroot severity was high at the infested site identified for the first time in North Dakota in 2013 (Chittem et al. Citation2014), which indicates that the pathogen had been present but undetected in the area for some time.
In the early years of the clubroot surveys in Alberta, it was difficult to determine if the pathogen was moving rapidly from field to field, if rapid expansion of the area included in the survey led to identification of fields where the pathogen had been established but not reported previously, or if both factors were contributing to the rapid increase in the number of fields confirmed as infested. It was not until 2008 that the area surveyed extended substantially beyond the leading edge of the infestation (). Clearly, increases in the area covered by the survey contributed to the initial rapid increase in the number of infested fields from 2004 to 2007. However, the number of confirmed infested fields has continued to increase exponentially since 2008 (). During that period, infested fields continued to be identified ever further away from the epicentre of the epidemic. The result was a steady increase in the number of clubroot-infested fields across central Alberta.
Based on the results presented in , the leading edge of the clubroot infestation in central Alberta progressed at about 20 km per year. This rate, although slow relative to the progress of pathogens like the cereal rusts, was unexpectedly rapid (Gossen et al. Citation2013b) given that the pathogen does not produce air-borne spores. Initially, dispersal of clubroot appeared to be occurring most quickly towards the south-east, in the direction of the prevailing wind (a, b). However, more rapid dispersal to the north and south has been observed since 2010 ().
Surveys and spread of clubroot in Saskatchewan and Manitoba
An annual survey for clubroot has been conducted by trained professional staff on >100 canola fields across the production area in both Saskatchewan and Manitoba for the past 6 years, using the method described previously (e.g. Dokken-Bouchard et al. Citation2010, Citation2012; Kubinec et al. Citation2014) Also, soil samples from more than 600 random canola fields from each of Saskatchewan and Manitoba were assessed between 2010 and 2014 for the presence of P. brassicae using molecular markers (Strelkov, unpublished). During soil sampling, about 1 kg of top soil was collected at 10 random locations in each field, near the field entrance. Samples were air dried overnight, thoroughly mixed, a subsample was collected and ground to a fine powder, and each soil sample was tested using conventional PCR analysis for DNA of P. brassicae (Cao et al. Citation2009).
In Saskatchewan, mild symptoms of clubroot (small areas of swollen or thickened roots, which often indicate low levels of initial inoculum) have been confirmed as clubroot from two isolated canola breeding nurseries. Also, the presence of low levels of resting spores of P. brassicae has been confirmed in soil samples from a commercial field, based on a bioassay of field soil following a positive PCR test (Dokken-Bouchard et al. Citation2010, Citation2012). However, surveys of more than 100 random canola fields per year across the production area have failed to identify clubroot symptoms in any crop. In Manitoba, mild clubroot symptoms were first identified at an experimental site in 2005 (Cao et al. Citation2009). Since then, surveys of more than 100 random canola fields per year across the production area have identified clubroot symptoms in only one crop and a second field was identified from a sample sent in to a diagnostic laboratory (Kubinec et al. Citation2014). However, there have been positive PCR tests for DNA of P. brassicae from soil collected from commercial canola fields across Manitoba (Kubinec et al. Citation2014; Strelkov et al. Citation2014b) and Saskatchewan (unpublished). Finally, clubroot was reported for the first time on a canola crop in North Dakota in 2013 (Chittem et al. Citation2014).
Mechanism(s) of dispersal of clubroot
There were several instances where an infested field identified in the clubroot survey was separated from the area of infestation by one or more counties that were free of clubroot (), and these fields often exhibited large patches of severe clubroot. This indicated that these fields had been infested for several cropping cycles before the infestation was detected. The mechanism of spread to these fields has not been identified, but may involve long-distance transmission.
Similarly, the frequent identification of P. brassicae in soil based on PCR assessments, and isolated instances of clubroot symptoms reported from widely dispersed locations in Saskatchewan, Manitoba and North Dakota indicate that one or more mechanisms of long-distance transmission of the pathogen are operating in the region. In Saskatchewan and Manitoba, symptom severity (when present) has consistently been trace to slight, and patches of infected plants are limited to small areas of affected fields. Also, bioassays of soil collected from fields identified as infested based on PCR analyses, which are conducted under conditions highly conducive for infection, have only rarely resulted in any clubroot symptoms (unpublished). This indicated that the concentration of resting spores at each of these sites was too low to produce consistent infection under most circumstances. However, localized concentration of spores can increase quickly if infection does occur (e.g. Hwang et al. Citation2013), so even low levels of resting spores have the potential to expand into an outbreak when conditions are conducive for infection.
Despite this indication that long-distance transmission of the pathogen can be important, the general pattern of dispersal observed in Alberta was that the county nearest to the leading edge of the infestation developed a few infested fields, the number of infested fields increased over the course of several years, and then the next county developed a few infested fields. Another line of evidence that clubroot was spreading rapidly was that the disease was confirmed in a number of fields that had been rated as clubroot-free in previous assessments (Strelkov et al. Citation2014b). Finally, clubroot levels in most newly identified fields were low to moderate, and most infestations occurred near the field entrance (unpublished). This indicated that the infestations were recent, associated with introduction of infested soil on field equipment (Cao et al. Citation2009) from nearby fields. We conclude that the rapid increase in the number of infested fields confirmed in Alberta from 2008 to 2014 was due primarily to continued spread of the pathogen over this period.
Indications of rapid short-distance spread of clubroot in the direction of the prevailing wind from 2007–2011 were supported by a recent report that concentrations of up to 2.2 × 105 resting spores mL−1 of dust were collected in dust samplers adjacent to four infested fields in Alberta (Rennie et al. Citation2015). Wind erosion of infested soil may also have a role in long-distance transmission (). Once up into the air column, spores can be moved hundreds of kilometres before being re-deposited. It is extremely likely that resting spores are resilient to exposure to factors such as UV light, based on their pigmentation and the lack of efficacy of standard sanitation treatments (Hwang et al. Citation2014), and so are likely to survive the rigours of this long-distance movement. Movement of resting spores with dust could account for low concentrations of spores in soil at widely dispersed locations, and occasional, widely scattered foci of new infections. Additional studies on the relative importance of wind-borne resting spores on clubroot transmission are required.
In the 2013 survey, movement of inoculum through water was implicated for the first time in transmission of P. brassicae in Alberta; two infested fields were identified downstream from a lone infested field on the edge of a small stream (Strelkov et al. Citation2014a). This supports previous studies in Australia and China (Donald et al. Citation2006; Yu et al. Citation2013), which indicated that water-borne inoculum can have an important role in clubroot dispersal. Dispersal of resting spores by water in the northern Great Plains is most likely to occur over relatively short distances, associated with localized flooding and overland run-off (). However, there may be an important exception to this general pattern. The Red River, which runs through the canola production areas of North Dakota and Manitoba, routinely floods in early spring, affecting thousands of hectares of crop land. Run-off from infested fields in North Dakota may eventually transport large quantities of resting spores through the extensive floodplain of the Red River.
One important factor that influences the pattern of clubroot dispersal is the intensity of canola production, with a high frequency of susceptible canola in cropping rotations being conducive for development of clubroot. The intensity of canola production is very high in a band that extends from Edmonton east across Saskatchewan and into Manitoba, but generally declines to the south (associated with progressively lower average rainfall) and to the north (fewer frost-free days). Movement of clubroot westward has occurred relatively slowly because agricultural production to the west quickly gives way to forests and mountains, which represent a natural limitation for westward spread.
It should be noted that a field with a moderate level of clubroot was identified far to the south of the initial focus of infection in the early years of the clubroot survey in Alberta (). The pathotype of P. brassicae that predominates at this site (pathotype 5, based on the differentials of Williams Citation1966) is different from the one that predominates near Edmonton (pathotype 3; Cao et al. Citation2009). It is likely that the presence of P. brassicae in southern Alberta represents a separate introduction from outside the prairie region, but additional work is required to provide definitive information on the original source of the clubroot infestation on canola in Alberta and North Dakota.
Slow spread of clubroot in Ontario
Vegetable research has been conducted at the Muck Crops Research Station (MCRS) of the University of Guelph, located in the Holland Marsh in southern Ontario, since 1947. Research trials on clubroot management were conducted at the MCRS each year from 1989–1992, 1999–2002 and 2008–2014, and numerous other research trials involving brassica vegetables have also been conducted every year throughout this period. One portion of the site is heavily infested with resting spores of P. brassicae, but susceptible hosts grown in other areas of this small (<5 ha) research station develop little or no clubroot (e.g. Gludovacz et al. Citation2014). Compared with the area around Edmonton, where clubroot is severe and spreading rapidly, this site has a longer growing period each season when temperatures are above the minimum for clubroot development (Sharma et al. Citation2011a, Citation2011b), has higher and more consistent rainfall, and a slightly acidic pH of about 6.2. This combination of factors indicates that conditions are highly conducive for clubroot development at this site (Gossen et al. Citation2013a).
In 2014, replicated field experiments of clubroot-susceptible brassica vegetables conducted at three sites at the MCRS were rated for clubroot incidence and severity. These sites were selected to assess the distribution of clubroot at sites that are accessed routinely by vehicles, equipment and workers from the MCRS, with no sanitation measures in place to reduce potential transfer of resting spores. One field trial was located just 20 m east of the area heavily infested with clubroot at the MCRS. The clubroot-susceptible broccoli ‘Diplomat’ was transplanted on 10 June, and the plants were assessed for clubroot on 14 August. A second trial was located 1 km from the MCRS; seedlings of the clubroot-susceptible cauliflower ‘Snow Queen’ were transplanted on 25 June and the plants were assessed for clubroot on 15 September. Research based out of the MCRS has been conducted on this site for 6 years. At the third site, about 4 km from the MCRS on a mineral soil adjacent to the Holland Marsh (pH 7.1, 2% organic matter), the clubroot susceptible cabbage ‘Adaptor’ was transplanted on 15 June and assessed on 30 September. This site had been a research site for the MCRS for 14 years. Approximately 930 broccoli plants, 300 cabbage plants and 900 cauliflower plants were assessed.
Clubroot severity on broccoli at the site adjacent to the main infested area at the MCRS was less than 50%. Clubroot severity on cauliflower at the research site 1 km from the MCRS was about 20%, with the most severe clubroot symptoms observed near the edges of the plot. No clubroot was observed on cabbage at the mineral soil site.
Rapid spread of P. brassicae within and among brassica vegetable production areas has also been reported in Australia (Donald & Porter Citation2014) and China (Chai et al. Citation2014), and P. brassicae is ubiquitous on oilseed rape in many parts of Europe (Wallenhammar et al. Citation2014). In contrast, the pathogen appears to have spread only slowly, if at all, in the Holland Marsh in Ontario. Clubroot was first reported in Ontario in 1923 on rutabaga, and was observed again on several fields of vegetables in 1930 (Reyes et al. Citation1974). The first report of clubroot in the Holland Marsh was of a single field in 1953. The pathogen was subsequently spread throughout the Holland Marsh by flooding during Hurricane Hazel in 1954. In 1955, clubroot was present in most fields of cabbage and cauliflower, including fields 8 km from the original infested site (Canada Department of Agriculture Citation1956), and was moderate to severe at the Muck Crops Research Station (MCRS) (Canadian Department of Agriculture Citation1958). In recent years, however, clubroot is no longer widespread on the Holland Marsh. Although clubroot severity was often near 100% in the main infested block at the MCRS (Saude et al. Citation2012; Gludovacz et al. Citation2014; Cranmer et al. Citation2015), mean severity was less than 50% on susceptible cabbage just 100 m from the infested block in 1989 (McDonald et al. Citation1990), 42% within 100 m of the infested block from 1999–2001 (McDonald et al. Citation2004) and 0–30% just 50 m from the infested block in 2011–2012 (Sharma et al. Citation2013).
Assessments of clubroot severity in 2014 as part of the current examination supported the general observation that clubroot occurred at low levels, if at all, just a short distance from the main infested area at the MCRS. Levels of infestation were low to moderate just a few metres from the infested block, were low at an off-station research site just 1 km from the MCRS, and there was no clubroot detected at another off-station research site 4 km from the MCRS. This indicated that little or no effective dispersal of P. brassicae has occurred from this heavily infested site over the last 15 years, even though no sanitation protocols have been in place and machinery used to establish, maintain and harvest plots in the clubroot block was also used routinely at the other sites. The high organic matter (muck) soil at this site does not cling to the tyres and chassis of farm equipment as tenaciously as a typical mineral soil. As a result, it is possible that this soil type is less likely to be moved in substantial amounts on equipment from field to field, or even within a field, than a mineral soil (McDonald et al. Citation2015), which would reduce or eliminate an important mechanism of short-distance transmission of the pathogen. Comparison with movement of P. brassicae on canola in northern Quebec (Pageau et al. Citation2006) is not possible because no surveys of distribution in that area have been published.
Assessment of genotypic diversity of P. brassicae on oilseed rape crops from across Germany demonstrated that there was limited genetic diversity within the three main regions where oilseed rape is produced, but substantial diversity among regions (Strehlow et al. Citation2014). This indicated that there was little movement of inoculum of P. brassicae from region to region, and points to the importance of short-distance pathogen dispersal, e.g. on farm equipment (), rather than long-distance movement of inoculum, e.g. wind dispersal, as the main mechanism of pathogen dispersal to new fields in Germany.
Potential for seed transmission of clubroot
To examine the risk associated with pathogen spread through infested seed, a study was conducted near the MCRS at a mineral-soil site adjacent to the Holland Marsh (pH 8.0, 4.5% organic matter). The site was selected because it was managed by the MCRS, so the cropping rotation of the site was known with certainty. Although P. brassicae is endemic in the region, no brassica crops had been planted at the site for decades, so the site was presumed to be free of the pathogen. In the first trial, seed of the clubroot-susceptible canola ‘45H26’ was inoculated with resting spores of P. brassicae pathotype 3 (Williams differential system) to result in 0, 103, 104, 105 or 106 resting spores per seed. The trial was laid out in a randomized complete block design with four replicates. Each experimental unit consisted of four 5-m-long rows, 40 cm apart. Each plot was direct seeded on 15 July, 2009 at about 40 seeds per m of row. On 15 September, 50 plants per replicate were uprooted and examined for clubroot.
The trial was repeated in 2010 on an adjacent portion of the site with high organic matter and lower pH (pH 7.7, 42% organic matter), seeded on 15 June and assessed for clubroot on 4 October. To assess the possibility that some of the resting spores had survived in the soil and would cause infection in a subsequent crop, the entire plot area was seeded to the moderately susceptible canola ‘Invigor 5030’ on 16 June 2011. Sulphur fertilizer was applied to the site to lower the pH at 2 weeks prior to seeding. All of the plants were uprooted and roots were assessed for clubbing symptoms on 2 September. In 2012, the entire site was seeded to Shanghai pak choy (B. rapa subsp. chinensis (Rupr.) var. communis Tsen and Lee), which is highly susceptible to P. brassicae (Sharma et al. Citation2013). At 6 weeks after seeding, all of the plants (about 2000) were uprooted and assessed for clubroot symptoms.
No plants with clubroot symptoms were detected in the initial trial. However, it should be noted that the soil pH at this site was high enough to partially suppress infection, even under otherwise conducive conditions of moisture and temperature (Gossen et al. Citation2013a). Therefore, the absence of clubroot infection only indicates that high levels of inoculum on seed may not result in infection when conditions are not conducive for infection.
In the second trial, no plants with clubroot symptoms were found in the first year (2010). In 2011, when the plot area was planted to a susceptible canola cultivar, one plant was observed with root thickening that may have been mild symptoms of clubroot. Unfortunately, the presence of P. brassicae could not be confirmed based on inoculation of susceptible seedlings, and no sample remained for subsequent testing. No symptoms of clubroot were observed on the Shanghai pak choy seeded in 2012. It is important to note, however, that soil pH at this site was higher than optimum for the pathogen infection and symptom development.
In the current assessment, there was no confirmed evidence of any seed-to-seedling transmission of clubroot from spores inoculated on seed. These data support previous conclusions (Gossen et al. Citation2014; Strelkov & Hwang Citation2014) that infested seed represents a low risk of transmission of P. brassicae, and that cleaned, treated seed represents almost no risk. Therefore, we conclude that it is unlikely that the introduction of P. brassicae in central Alberta, southern Alberta and North Dakota occurred as a result of pathogen transmission on infested seed.
Conclusions
The system of canola production on the Canadian prairies has proven to be highly conducive for dispersal of P. brassicae (Gossen et al. Citation2013b; Strelkov & Hwang Citation2014), with the leading edge of the original clubroot infestation in Alberta moving outwards at about 20 km per year. If one visualizes the heavily infested and contiguous large fields in central Alberta as a focal source of inoculum surrounded by susceptible hosts, compared with the single heavily infested block at the MCRS as a miniscule point source with few or no susceptible hosts nearby, it should be no surprise that clubroot has moved rapidly across Alberta, but has not shown the same capacity to spread from site to site at the Holland Marsh in Ontario.
There is circumstantial evidence that there have been at least two separate introductions of P. brassicae inoculum pathogenic on canola to the prairie region, with one introduction near Edmonton and a second one in southern Alberta. The likelihood of multiple introductions has been overlooked but requires consideration because it has implications for the genetic and pathogenic variability of the pathogen population. High variability may in turn affect the rate that host resistance can be overcome. There is compelling evidence that occasional long-distance dispersal of the pathogen is occurring in the prairie region, with the most likely candidates being movement of soil on equipment and air-borne dispersal of spores with infested soil. Short distance movement of spores via wind has recently been confirmed in Alberta, and we believe that it is simply a matter of time before long-distance dispersal of air-borne resting spores is also demonstrated. Seed transmission does not appear to be an important mechanism for transmission of the pathogen. Similarly, movement of spores in water is likely not a major mechanism of pathogen dispersal in the prairie region, but that could change if clubroot were to become established in the Red River Valley, where large-scale flooding is a frequent occurrence.
At present, genetic resistance represents the key strategy for managing clubroot of canola on the Canadian prairies (Hwang et al. Citation2014). New cultivars with additional sources of resistance are urgently required, because the resistance of the initial cohort of clubroot-resistance canola cultivars has already been eroded at several sites in Alberta (Canola Council of Canada Citation2014; Strelkov, unpublished). However, the existing resistant cultivars can still be utilized effectively by producers near or in advance of the leading edge of the infestation. Use of resistant cultivars in these areas could reduce the rate of clubroot spread by reducing or eliminating almost all crop infection from low concentrations of resting spores, e.g. wind-borne spores.
Use of resistant cultivars alone is unlikely to halt the advance of P. brassicae, but could reduce the rate and impact of clubroot spread when used together with sanitation of equipment brought from infested fields, early seeding (Hwang et al. Citation2014) and a longer (>2 years) break from canola (Peng et al. Citation2013, Citation2015). Effective use of existing resistant cultivars and longer crop rotations are especially important to provide a much-needed reprieve to allow development of canola cultivars with improved resistance and development of alternative clubroot management strategies.
Acknowledgements
We thank all of the agricultural fieldmen, producers and summer students who have contributed to the clubroot surveys, and L. Riches and the crew at the Muck Crops Research Station for technical assistance.
Additional information
Funding
References
- Canada Department of Agriculture. 1956. 36th annual report of the Canadian plant disease survey. Can Plant Dis Surv. 36:52–53.
- Canadian Department of Agriculture. 1958. Can Plant Dis Surv. 36:52.
- Canola Council of Canada. 2014. Equipment sanitation is first line of defense to help prevent spread of a different clubroot pathotype. [cited 2014 Nov 11]. Available from: http://www.canolacouncil.org/news/equipment-sanitation-is-first-line-of-defense-to-help-prevent-spread-of-different-clubroot-pathotype/
- Cao T, Manolii VP, Hwang S, Howard RJ, Strelkov SE. 2009. Virulence and spread of Plasmodiophora brassicae [clubroot] in Alberta, Canada. Can J Plant Pathol. 31:321–329.
- Chai AL, Xie XW, Shi YX, Li BJ. 2014. Research status of clubroot (Plasmodiophora brassicae) on cruciferous crops in China. Can J Plant Pathol. 36:142–153.
- Chittem K, Mansouripour S, Rio Mendoza LE. 2014. First report of clubroot on canola caused by Plasmodiophora brassicae in North Dakota. Plant Dis. 98:1438.
- Cranmer TJ, Gossen BD, McDonald MR. 2015. Environmental parameters influencing clubroot incidence and severity on canola. Can J Plant Pathol. 37:254 (abstr.).
- Dixon GR. 2009. The occurrence and economic impact of Plasmodiophora brassicae and clubroot disease. J Plant Growth Regul. 28:194‒202.
- Dokken-Bouchard FL, Anderson K, Bassendowski KA, Bouchard A, Brown B, Cranston R, Cowell LE, Cruise D, Gugel RK, Hicks L, et al. 2012. Survey of canola diseases in Saskatchewan, 2011. Can Plant Dis Surv. 92:125–129.
- Dokken-Bouchard FL, Bouchard AJ, Ippolito J, Peng G, Strelkov SE, Kirkham C, Kutcher HR. 2010. Detection of Plasmodiophora brassicae in Saskatchewan. Can Plant Dis Surv. 90:126.
- Donald EC, Porter IJ. 2014. Clubroot in Australia: the history and impact of Plasmodiophora brassicae in Brassica crops and research efforts directed towards its control. Can J Plant Pathol. 36:66–84.
- Donald EC, Porter IJ, Faggian R, Lancaster RA. 2006. An integrated approach to the control of clubroot in vegetable brassica crops. Acta Hort. 706:283–300.
- Gludovacz TV, Deora A, McDonald MR, Gossen BD. 2014. Cortical colonization by Plasmodiophora brassicae in susceptible and resistant cabbage cultivars. Eur J Plant Pathol. 140:859–862.
- Gossen BD, Deora A, Peng G, Hwang SF, McDonald MR. 2014. Effect of environmental parameters on clubroot development and the risk of pathogen spread. Can J Plant Pathol. 36:37–48.
- Gossen BD, Kasinathan H, Cao T, Manolii VP, Strelkov SE, Hwang SF, McDonald MR. 2013a. Influence of pH and temperature on infection and symptom development of clubroot in canola. Can J Plant Pathol. 35:294−303.
- Gossen BD, McDonald MR, Hwang SF, Strelkov SE, Peng G. 2013b. Comparison of clubroot (Plasmodiophora brassicae) development and management on canola and Brassica vegetables. Can J Plant Pathol. 35:175−191.
- Horiuchi S, Hori M. 1980. A simple greenhouse technique for obtaining high levels of clubroot incidence. Bull Chugoku Natl Agric Exp Stn E (Environ Div). 17:33‒55.
- Howard RJ, Strelkov SE, Harding MW. 2010. Clubroot of cruciferous crops -new perspectives on an old disease. Can J Plant Pathol. 32:43–57.
- Hwang SF, Ahmed HU, Zhou Q, Rashid A, Strelkov SE, Gossen BD, Peng G, Turnbull GD. 2013. Effect of susceptible and resistant canola plants on Plasmodiophora brassicae resting spore populations in the soil. Plant Pathol. 62:404–412.
- Hwang SF, Howard RJ, Strelkov SE, Gossen BD, Peng G. 2014. Management of clubroot (Plasmodiophora brassicae) on canola (Brassica napus) in western Canada. Can J Plant Pathol. 36:49–65.
- Kageyama K, Asano T. 2009. Life cycle of Plasmodiophora brassicae. J Plant Growth Regul. 28:203‒211.
- Kubinec AM, Derksen H, Desjardins M, McLaren DL. 2014. Monitoring and occurrence of clubroot in Manitoba in 2013. Can Plant Dis Surv. 94:184–185. Available from: www.phytopath.ca/cpds.shtml
- Kuginuki Y, Hiroaki Y, Hirai M. 1999. Variation in virulence of Plasmodiophora brassicae in Japan tested with clubroot-resistant cultivars of Chinese cabbage (Brassica rapa L. spp. pekinensis). Eur J Plant Pathol. 105:327‒332.
- McDonald MR, Deora A, Al-Daoud F, Gossen BD. 2015. Why is clubroot a problem on canola in Alberta but not in Ontario, Canada? In: Proceedings of the International Rapeseed Congress; 2015 Jul 6–9. Saskatoon, SK.
- McDonald MR, Hovius S, Janse S. 1990. Muck vegetable cultivar trials and research report. no.40. 78 pp. Available from: http://www.uoguelph.ca/muckcrop/pdfs/mcdonaldm1990-muckvegculttrialscmp.ocr.pdf
- McDonald MR, Kornatowska B, McKeown AW. 2004. Management of clubroot of Asian Brassica crops grown on organic soils. Acta Hort. 635:25–30.
- Morasse I, Pageau D, Lafond J. 1997. Attention à la herniedes crucifères dans le canola. Grandes Cultures. 7:22–23.
- Pageau D, Lajeunesse J, Lafond J. 2006. Impact of clubroot (Plasmodiophora brassicae) on the yield and quality of canola. Can J Plant Pathol. 28:137–143.
- Peng G, Pageau D, Strelkov SE, Gossen BD, Hwang SE, Lahlali R. 2015. A > 2-year rotation reduces Plasmodiophora brassicae resting spores in soil and the impact of clubroot on canola. Eur J Agron. 70:78–84.
- Peng G, Pageau D, Strelkov SE, Lahlali R, Hwang SF, Hynes RK, Anderson K, McDonald MR, Gossen BD, Turkington KT, et al. 2013. Assessment of crop rotation, cultivar resistance and Bacillus subtilis biofungicide for control of clubroot on canola. Acta Hort. 1005:591–598.
- Rempel CB, Hutton SN, Jurke CJ. 2014. Clubroot and the importance of canola in Canada. Can J Plant Pathol. 36:19–26.
- Rennie DC, Holtz MD, Turkington TK, Leboldus JM, Hwang SF, Howard RJ, Strelkov SE. 2015. Movement of Plasmodiophora brassicae resting spores in windblown dust. Can J Plant Pathol. 37:188–196.
- Rennie DC, Manoli VP, Cao T, Hwang SF, Howard RJ, Strelkov SE. 2011. Direct evidence of surface infestation of seeds and tubers by Plasmodiophora brassicae and quantification of spore loads. Plant Pathol. 60:811‒819.
- Reyes AA, Davidson RT, Marks CF. 1974. Races, pathogenicity and chemical control of Plasmodiophora brassicae in Ontario. Phytopathology. 64:173–177.
- Rowe RC. 1980. Evaluation of radish cultivars for resistance to clubroot (Plasmodiophora brassicae) race 6 for midwestern United States. Plant Dis. 64:462–464.
- Saude C, McKeown A, Gossen BD, McDonald MR. 2012. Effect of host resistance and fungicide application on clubroot pathotype 6 in green cabbage and napa cabbage. Hort Technol. 22:311–319.
- Sharma K, Gossen BD, Howard RJ, Gludovacz TV, McDonald MR. 2013. Reaction of selected Brassica vegetable crops to Canadian pathotypes of Plasmodiophora brassicae. Can J Plant Pathol. 35:371–383.
- Sharma K, Gossen BD, McDonald MR. 2011a. Effect of temperature on primary infection by Plasmodiophora brassicae and initiation of clubroot symptoms. Plant Pathol. 60:830–838.
- Sharma K, Gossen BD, McDonald MR. 2011b. Effect of temperature on cortical infection by Plasmodiophora brassicae and clubroot severity. Phytopathology. 101:1424–1432.
- Strehlow B, De Mol F, Struck C. 2014. History of oilseed rape cropping and geographic origin affect the genetic structure of Plasmodiophora brassicae populations. Phytopathology. 104:532–538.
- Strelkov SE, Cao T, Manolii VP, Lange RM, Smith-Degenhardt E, Orchard D, Tewari JP. 2006a. Incidence of clubroot of canola in Alberta in 2005. Can Plant Dis Surv. 86:91–93.
- Strelkov SE, Hwang SF. 2014. Clubroot in the Canadian canola crop: 10 years into the outbreak. Can J Plant Pathol. 36:27–36.
- Strelkov SE, Manolii VP, Cao T, Hwang SF, Orchard D. 2007. Incidence of clubroot on canola in Alberta in 2006. Can Plant Dis Surv. 87:109‒111.
- Strelkov SE, Manolii VP, Harding MW, Hwang SF, Fei W, Rong S, Burke DA, Pugh CA, Nielsen JM, Barnes A, et al. 2015. The spread of clubroot on canola in Alberta in 2014. Can Plant Dis Surv. 95:155–159.
- Strelkov SE, Manolii VP, Harding MW, Hwang SF, Poscente N, Lisowski SLI, Pugh CA, Burke DA. 2014a. The occurrence of clubroot on canola in Alberta in 2013. Can Plant Dis Surv. 94:158–161.
- Strelkov SE, Manolii VP, Howard RJ, Rennie DC, Hwang SF, Manolii AV, Liu J, Cao T, Xiao Q. 2009. Incidence of clubroot on canola in central Alberta in 2008. Can Plant Dis Surv. 89:110‒112.
- Strelkov SE, Manolii VP, Hwang SF. 2014b. Continued dissemination of Plasmodiophora brassicae (clubroot) on Canadian canola. Can J Plant Pathol. 36:130 (abstr.).
- Strelkov SE, Manolii VP, Hwang SF, Howard RJ, Manolii AV, Zhou Q, Holtz M, Yang Y. 2008. Incidence of clubroot on canola in Alberta in 2007. Can Plant Dis Surv. 88:101‒102.
- Strelkov SE, Manolii VP, Liu J, Jurke C, Rennie DC, Orchard D, Hwang SF, Laflamme P. 2012. The occurrence of clubroot on canola in Alberta in 2011. Can Plant Dis Surv. 92:122‒124.
- Strelkov SE, Manolii VP, Márquez-Zequera I, Manolii E, Hwang SF. 2010. Incidence of clubroot on canola in Alberta in 2009. Can Plant Dis Surv. 90:123‒125.
- Strelkov SE, Manolii VP, Rennie DC, Manolii EV, Fu H, Strelkov IS, Hwang SF, Howard RJ, Harding MW. 2013. The occurrence of clubroot on canola in Alberta in 2012. Can Plant Dis Surv. 93:145‒148.
- Strelkov SE, Manolii VP, Rennie DC, Xiao Q, Cui D, Hwang SF. 2011. The occurrence of clubroot on canola in Alberta in 2010. Can Plant Di Surv. 91:109‒111.
- Strelkov SE, Tewari JP, Hartman M, Orchard D. 2005. Clubroot on canola in Alberta in 2003 and 2004. Can Plant Dis Surv. 85:72‒73.
- Strelkov SE, Tewari JP, Smith-Degenhardt E. 2006b. Characterization of Plasmodiophora brassicae populations from Alberta, Canada. Can J Plant Pathol. 28:467‒474.
- Tewari JP, Strelkov SE, Orchard D, Hartman M, Lange RM, Turkington TK. 2005. Identification of clubroot of crucifers on canola (Brassica napus) in Alberta. Plant Dis. 67:758‒762.
- USDA-NASS. 2014a Apr. Crop production: historical track record. [ cited 2014 Nov 11]. Available from: http://usda.mannlib.cornell.edu/MannUsda/viewDocumentInfo.do?documentID=1593
- USDA-NASS. 2014b Nov. Field Crops: Final Estimates 2007–2012. Stat Bull No 1044. [ cited 2014 Nov 11]. Available from: http://usda.mannlib.cornell.edu/MannUsda/viewDocumentInfo.do?documentID=1529
- Wallenhammar AC. 1996. Prevalence of Plasmodiophora brassicae in a spring oilseed rape growing area in central Sweden and factors influencing soil infestation levels. Plant Pathol. 45:710–719.
- Wallenhammar AC, Almquist C, Schwelm A, Roos J, Marzec-Schmidt K, Jonsson A, Dixelius C. 2014. Clubroot, a persistent threat to Swedish oilseed rape production. Can J Plant Pathol. 36:135–141.
- Williams PH. 1966. A system for the determination of races of Plasmodiophora brassicae that infect cabbage and rutabaga. Phytopathology. 56:624–626.
- Yu X-K, Wu Y-X, Mao Z-C, He Y-Q. 2013. Water-mediated dissemination and chemical control of cabbage clubroot disease. J Huazhong Agric Univ. 1:48–53.