Abstract
The iconic tree species, Arbutus menziesii (Pacific madrona, madrone arbutus), has been in decline in the Pacific Northwest of North America for the past 40 years. It is thought that the fungal pathogen Neofusicoccum arbuti has contributed to the decline of this tree species. In recent years, there have been reports of declining arbutus in the coastal region of southern British Columbia, Canada. We conducted intensive sampling in a park with severely affected arbutus trees to determine the cause and prevalence of decline. The majority of arbutus trees sampled in this study had cankers associated with N. arbuti infection. We also sought to determine if N. arbuti has additional hosts that could act as a reservoir for this pathogen. Six new hosts of N. arbuti were identified, and a seventh was confirmed; these hosts spanned four taxonomic orders and included Amelanchier alnifolia, Cytisus scoparius (confirmed), Gaultheria shallon, Ilex aquifolium, Rosa sp., Sorbus sitchensis and Spiraea douglasii. Recovery of Neofusicoccum arbuti from both symptomatic and asymptomatic plant tissues of these hosts indicates that it potentially has a broad host range. It remains to be established if N. arbuti is also pathogenic to these hosts. These results highlight the importance of monitoring for this pathogen and assessing the extent of its geographic distribution and its ability to colonize various hosts.
Résumé
Depuis 40 ans, l’arbre emblématique Arbutus menziesii (arbousier d’Amérique, arbousier Madrono) est en proie au dépérissement sur la côte pacifique du Nord-Ouest américain. On croit que l’agent pathogène fongique Neofusicoccum arbuti en est le responsable. Récemment, on a rapporté des cas de dépérissement de l’arbousier dans la portion sud de la côte britanno-colombienne. Nous avons procédé à un échantillonnage intensif dans un parc où des arbousiers étaient gravement infectés afin de déterminer la cause et la prévalence du dépérissement. La majorité des arbousiers échantillonnés au cours de cette étude portaient des chancres associés à l’infection causée par N. arbuti. Nous avons également cherché à déterminer si d’autres hôtes pouvaient servir de réservoir à N. arbuti. Nous avons identifié six nouveaux hôtes de N. arbuti qui embrassent, et un septième a été confirmé; quatre ordres taxinomiques et incluent Amelanchier alnifolia, Cytisus scoparius (confirmé), Gaultheria shallon, Ilex aquifolium, Rosa sp., Sorbus sitchensis et Spiraea douglasii. La récupération de N. arbuti sur des tissus symptomatiques et asymptomatiques provenant de ces hôtes a montré qu’il peut posséder une vaste gamme d’hôtes. Il reste à établir si N. arbuti est également pathogène à l’égard de ces derniers. Ces résultats soulignent l’importance de mettre en place un suivi de cet agent pathogène et d’évaluer l’étendue de sa répartition géographique ainsi que sa capacité de coloniser divers hôtes.
Introduction
The iconic tree species Arbutus menziesii Pursh. (Pacific madrona, madrone or arbutus), in the family Ericaeae, which is distributed along the Pacific Coast of North America from southwest British Columbia to southern California (McDonald & Tappeiner Citation1990; Farr et al. Citation2005), has been experiencing a decline in parts of its range for the last 40 years (Davison Citation1972; Bressette Citation1995; Farr et al. Citation2005). Arbutus is a broad-leaved evergreen tree with white, urn-shaped flowers, orange-red berries and characteristic red-orange bark. The berries of arbutus are an important food source for wildlife and the trees are used by cavity-nesting bird species (Gurung et al. Citation1999). Arbutus is a shade-intolerant, drought-tolerant species, which in the Northwest part of its distribution, grows in the open on dry, rocky slopes, seldom far from the ocean shore (Alaback et al. Citation2004). It is an important component of several different forest types throughout its range, from the Coastal Douglas-fir biogeographic zone to the coast redwood-tanoak forests of California (McDonald & Tappeiner Citation1990).
Arbutus trees are increasingly being observed with dead or dying branches, crown dieback and cankers on the stem; in some cases, entire trees die (Hunt et al. Citation1992; Elliott Citation1999b; Elliott et al. Citation2002).
The decline of arbutus has been attributed to several causes: the removal of fire from the ecosystem, climate change, the Pacific Decadal Oscillation, habitat availability and pathogens (Bressette Citation1995; Elliott Citation1999b, Citation2005; Elliott et al. Citation2002). A range of fungal pathogens cause foliar blight, wood decay, root rots and cankers on arbutus (Hunt et al. Citation1992; Elliott Citation1999b). The three main diseases are: Arbutus canker, Phytophthora root rot (Phytophthora cactorum (Lebert & Cohn) J. Schröt) and Madrone canker (Fusicoccum aesculi Corda) (McDonald & Tappeiner Citation1990; Hunt et al. Citation1992; Elliott Citation1999a). Arbutus canker disease is the leading cause of cankers in the northwest portion of the arbutus tree range and is a result of infection by Neofusicoccum arbuti (D.F. Farr & M. Elliott) Crous, Slippers & A.J.L. Phillips (Davison Citation1972; Hunt et al. Citation1992; Bressette Citation1995; Elliott Citation1999b, Citation2005), a member of the Botryosphaeriaceae Theiss. & P. Syd. When an arbutus tree is stressed, infection by N. arbuti causes the development of cankers on the main stem. These cankers are usually sunken, with a sooty appearance, and often with a raised and convoluted margin (Davison Citation1972; Elliott Citation2005). If the tree is healthy enough, it may callus over the canker completely, but if the tree is stressed, many cankers can form, girdling small stems or branches and leading to water stress and dieback in the crown (Elliott Citation1999b, Citation2005). After 50% dieback in the crown, the trees enter a decline spiral, which can lead to mortality (Elliott et al. Citation2002). Canker development is greatly increased in drought-stressed trees (Elliott Citation1999b) and arbutus trees that have been subjected to heat damage or sudden exposure to sunlight (i.e. after logging) are more prone to infection by N. arbuti (Davison Citation1972; Bressette Citation1995).
Neofusicoccum arbuti has been reported on arbutus in the USA (Washington, Oregon and California) and Canada (British Columbia), and once in Cytisus scoparius (L.) Link (scotch broom), in an unreported location (Elliott Citation2005; Farr et al. Citation2005). Reports of N. arbuti on C. scoparius suggest that the host range of N. arbuti may be even broader than what is currently known. Complicating our understanding of the host and geographic range of N. arbuti is the recent description of N. nonquaesitum Inderb. Trouillas, Bostock & Michailides (Inderbitzin et al. Citation2010) and Neofusicoccum andinum (Mohali, Slippers & M.J. Wingf.) Mohali, Slippers & M.J. Wingf. (Crous et al. Citation2006; Mohali et al. Citation2006), both are closely related species to N. arbuti. It is possible that some reports of disease attributed to N. arbuti may instead be a result of infection by one of the sister species and therefore the documented host and geographic range of N. arbuti may not reflect the actual range. In recent years, declining arbutus trees with symptoms consistent with N. arbuti infection have been reported in Lighthouse Park, West Vancouver, Canada (Pynn Citation2006). The park is about 75 ha (185 acres) in size and is one of the few remaining examples of coastal Douglas-fir old growth forest in the Vancouver area (Coates & Mondor Citation1978).
The objectives of this study were to: (a) conduct a survey of Lighthouse Park to determine if the high incidence of tree cankers was linked with direct site-level human disturbance; (b) identify the pathogen(s) associated with the cankers; (c) assess the potential of other non-arbutus species to act as hosts of the pathogen; and (d) determine the genetic lineage of N. arbuti (if present) that occurs in Lighthouse Park.
Materials and methods
Incidence of cankers and level of disturbance
Survey
Over an 8-day period in late August and early September 2012, the extent of arbutus decline was assessed. Every accessible area of the park was examined, and the GPS coordinates of any location that contained four or more arbutus trees were recorded and designated as a site (). Non-arbutus woody plants, shrubs and trees in close proximity to the arbutus trees were identified. The percentage of arbutus trees affected by at least one canker was calculated. The site was assigned to a ‘disease class’ on a scale of 1 to 4, where 1 = 0–25% of trees with at least one canker, 2 = 26–50% of trees with at least one canker, 3 = 51–75% of trees with at least one canker, and 4 = 76–100% of trees with at least one canker.
Fig. 1 (Colour online) Arbutus sites identified in the survey of Lighthouse Park, prevalence of cankers (disease class), and human disturbance. Pie charts represent the prevalence of cankers (0–25%, 26–50%, 51–75% and 76–100%). Colours represent the level of human disturbance (green = none, yellow = some and red = common). Each chart corresponds to one site. Numbers denote sites picked for canker/vegetation sampling. Site 2 refers to two adjacent sites combined for sampling. Site 8 refers to two adjacent sites combined for sampling. *Indicates the two sites selected for broader sampling.
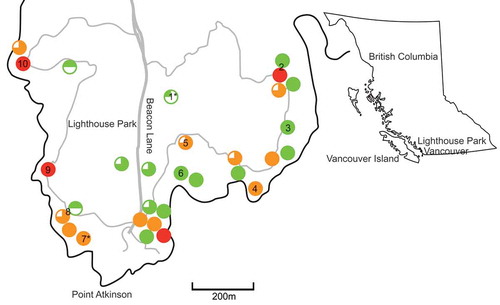
Direct site-level human-related disturbance such as tree carvings or trampled roots was also recorded at each site. The rating system for disturbance was qualitative and subjective i.e. none, some, common. Sites with ‘some’ human disturbance had one or two trees with trampled roots or carvings on their trunk, while sites with ‘common’ disturbance had several trees with moderate to heavy damage resulting from trampling or carving.
Statistics
To determine if the prevalence of cankers i.e. disease classes 1–4 differed significantly between sites with different levels of human disturbance, a chi-square analysis was performed. To establish if there was a correlation between canker presence and direct human disturbance, a Kendall rank-order correlation analysis (Kendall Citation1976) was implemented in R Core ver. 3.02 (Citation2013) using the Kendall package ver. 2.2 (McLeod Citation2011).
Fungi associated with arbutus and other hosts in lighthouse park
Sampling
Canker and non-canker tissue from both arbutus and non-arbutus vegetation were collected in late September and early October of 2012. In total, 10 sites were selected for sampling (in two cases, smaller adjacent sites were sampled together and counted as a single site as indicated in ), based on their distribution throughout the park, the inclusion of at least one ‘healthy’ site and one ‘very diseased’ site, and the number of trees. At each site, the frequency of cankers and the non-arbutus vegetation occurring in association with sampled arbutus was noted. At eight sites, three arbutus trees and three individual plants of non-arbutus species were sampled, and at the remaining two sites (one in disease class 1 and one in disease class 4), six arbutus trees and up to nine non-arbutus species were sampled (eight species at the site in disease class 1 and nine species at the site in disease class 4). At eight sites, stem or branch sections were taken from three species: Amelanchier alnifolia Nutt. (Saskatoon, service berry), Gaultheria shallon Pursh. (Salal), and another less frequent plant, either Cytisus scoparius (L.) Link (Scotch broom), Ilex aquifolium L. (Holly), Spiraea douglasii Hook. (Hardhack) or Vaccinium parvifolium Sm. (Red huckleberry). At the two sites with broad sampling, Paxistima myrsinites (Pursh) Raf. (Falsebox), Arctostaphylos uva-ursi (L.) Spreng. (Kinnikinnick, Bearberry), Rosa sp. (Rose), Prunus sp. (Cherry), Rubus discolor Weihe & Nees (Himalayan blackberry) and Sorbus sitchensis M. Roem (Sitka mountain-ash) were also collected. Non-arbutus plants were selected for sampling based on their proximity to the sampled arbutus. All of the non-arbutus plants sampled were within 30 m of a sampled arbutus tree. To keep the number of fungal cultures recovered at a manageable number, each non-arbutus species sampled at each site was represented by tissue samples from one plant.
In accordance with the canker sampling methods in Elliott (Citation1999b), a hammer and chisel were used to take chips from the canker margin i.e. the raised edge of the canker where the healthy and diseased tissues merge. Between each canker sampling, the chisel was sterilized with ethanol. To sample non-arbutus vegetation, stem or branch cuttings were taken using ethanol-sterilized pruners. If cankers were seen on the stems or branches, those stems/branches were collected.
Fungal isolation
Samples were surface-sterilized in bleach and ethanol, and rinsed several times in autoclaved, deionized water as described by Taylor et al. (Citation2009). Each sample was cut into three or four pieces and plated on 2% malt extract agar (MEA) as outlined in Farr et al. (Citation2005). Cultures were allowed to grow for c. 1 week and were sub-cultured until a pure culture was obtained. Cultures were grouped based on morphology and growth characteristics into Botryosphaeriaceae-like (with fluffy white to grey-green cultures) and non-Botryosphaeriaceae (Slippers & Wingfield Citation2007; Phillips et al. Citation2013). Pure cultures were obtained by repeated sub-culturing. To collect mycelium for DNA extraction, mycelium from cultures were scraped using a scalpel and placed in 1.5 mL Eppendorf Flex-Tubes® and stored at −80°C.
Sample preparation and PCR
All cultures originating from arbutus and all Botryosphaeriaceae-like cultures from non-arbutus vegetation were prepared for PCR using a modified method from Moreth and Schmidt (Citation2000). While still frozen, small pieces of mycelium were placed into 1.5 mL tubes containing two tungsten beads. Samples were then blended in a Retsch Mixer Mill MM 300 at maximum speed for 3 min. Tungsten beads were removed and 1 mL of sterile, deionized water was added to each tube. The solution was then vortexed and placed in a water bath set at 96°C for 5 min. Reusable locks were placed on the tubes to prevent them from popping open in the water bath. The boiled mycelium solution was used directly for PCR.
Because the identities of the fungi were unknown and variable, a ‘touchdown’ PCR (Don et al. Citation1991) was used to amplify the ribosomal internal transcribed spacer (ITS) gene using the standard ITS1F (5ʹ- CTT GGT CAT TTA GAG GAA GTA A-3ʹ) (Gardes & Bruns Citation1993) and ITS4R (5ʹ-TCC TCC GCT TAT TGA TAT GC-3ʹ) primers (White et al. Citation1990). A touchdown PCR cycles through multiple annealing temperatures. In this case, annealing temperatures ranged from 52°C to 57°C. PCR was carried out in a BIORAD T100 Thermal cycler and an Applied Biosystems 2720 Thermal cycler using the following protocol: denaturation at 95°C for 3 min, followed by a second denaturation step (95°C for 30 s), a variable annealing step that started at 57°C for 30 s and decreased by 1°C at each cycle, and an elongation step (72°C for 1 min). Steps 2 through 4 were repeated five times. This was followed by 34 cycles of denaturation (95°C for 30 s), annealing (55°C for 30 s) and elongation (72°C for 1 min). The protocol finished with a 10-min elongation at 72°C.
PCR products were purified and sequenced by the CHUL Research Centre (Laval University, http://www.sequences.crchul.ulaval.ca/eng/index.html) using an ABI 3730xl Data Analyser (Applied Biosystems). Sequences were aligned using default parameters, and manually edited by trimming of ends using Geneious Pro version 6.0.5 created by Biomatters (available from http://www.geneious.com). A BLAST (Basic Local Alignment Search Tool) search in the NCBI (National Center for Biotechnology Information) database was used to identify species, and the species with the highest pairwise identity match was noted.
Molecular identification
To ensure that all available N. arbuti genetic diversity was included in the analysis, GenBank BLAST searches were conducted where the first 100 sequences were downloaded and inspected for similarity with the N. arbuti type (CBS 116131; GenBank accession no. AY819720) and a GenBank word search with the binomial ‘Neofusicoccum arbuti’ was also performed. To ensure that N. arbuti was correctly characterized, sequences were visualized and aligned as previously mentioned and compared with N. arbuti, N. nonquaesitum (CBS 126655; GenBank accession no. GU251163) and N. andinum (CBS 117453; GenBank accession no. AY693976).
To identify non-Botryosphaeriaceae cultures, GenBank BLAST searches were conducted with nucleotide searches limited to sequences from type material.
To establish which lineage (Farr et al. Citation2005) of N. arbuti was present in Lighthouse Park, ribosomal Internal Transcribed Spacer (ITS) sequences were compared between 55 sequenced isolates from Lighthouse Park and six isolates that had been previously collected from British Columbia (GenBank no. AY819725), central California (GenBank no. AY819724), northern California (GenBank no. AY819721, AY819722 and AY819723) and Washington (GenBank no. AY819720).
Results
Incidence of cankers
Thirty sites in the park contained arbutus trees, and cankers () were visible on arbutus trees at all sites (). Whilst there was some variability in the prevalence of cankers from one site to another, the majority of the sites were classified as disease class 4 as they contained at least one canker on over 75% of the arbutus trees (). In total, 19 sites belonged to disease class 4, eight sites were in class 3, two sites were in class 2, and one site was in class 1.
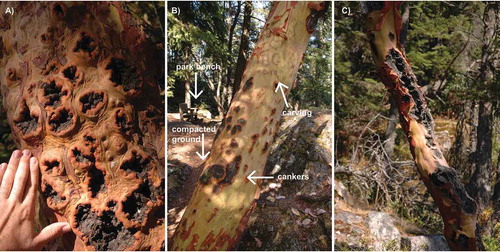
Fig. 2 (Colour online) Diseased arbutus showing symptoms consistent with infection by Neofusicoccum arbuti. (A) Close up of trunk showing numerous sunken blackened cankers with a raised, uneven margin; (B) trunk of arbutus showing evidence of canker infection and human carving at a site that has been modified for human activities; (C) severe canker infection, canker extending beyond the cambium deeper into the tree.
Association of direct site-level human disturbance with canker prevalence
There was no significant difference between the prevalence of cankers (disease class 1–4) at sites that exhibited different levels of site-level human disturbance (none, some, common); Χ2 = 7.041, P = 0.341, df = 6). There was no significant correlation between the level of human disturbance on the site and the prevalence of canker severity (T = 0.199, P = 0.256). However, the arbutus on sites that were impacted by levels of human disturbance ranging from ‘some’ to ‘common’ also belonged to disease class 3 or 4 (> 51% trees on the site contained at least one canker) (). In addition, arbutus trees at all sites that had a ‘common’ level of human disturbance had more than 75% canker prevalence (disease class 3) and only the arbutus trees at sites with less than 25% canker prevalence (disease class 1) exhibited no human disturbance.
Fig. 3 Plot demonstrating the prevalence of cankers at each site (% of trees that contain cankers: one or more cankers present in <25% of trees at a site [disease class 1], 26–50% of trees [disease class 2], 51–75% [disease class 3], or >76% of trees at a site [disease class 4]) and the level of direct human disturbance (none, some or common) on arbutus in Lighthouse Park. Numbers in the circles represent the number of sites that fall into these categories.
![Fig. 3 Plot demonstrating the prevalence of cankers at each site (% of trees that contain cankers: one or more cankers present in <25% of trees at a site [disease class 1], 26–50% of trees [disease class 2], 51–75% [disease class 3], or >76% of trees at a site [disease class 4]) and the level of direct human disturbance (none, some or common) on arbutus in Lighthouse Park. Numbers in the circles represent the number of sites that fall into these categories.](/cms/asset/e56dcd4d-fbf7-43b5-9bd9-89eaf89768ba/tcjp_a_1135476_f0003_b.gif)
Fungi associated with arbutus in lighthouse park
Neofusicoccum arbuti was the dominant fungal species isolated from cankers on arbutus (). This species was isolated from arbutus at every site sampled and was found in the majority (87%) of cankers sampled. One isolate was recovered from the smooth un-cankered stem of an apparently healthy arbutus at the ‘healthy’ site with the lowest canker prevalence (disease class 1). Non-Botryosphaeriaceae cultures were obtained from seven canker samples; from three of these, cultures were identified as N. arbuti. In addition to N. arbuti, seven species of fungi were isolated from arbutus (). Of these, two fungal species, Cytospora sp. Ehrenb. (KP026326) and Absidia sp. Tiegh. (KP026328), were isolated from an old, cracked lesion with a burnt appearance. A Diaporthe sp. Nitschke (KP026322) was isolated from a raised bump, or ‘blister’, that was different from the typical canker associated with N. arbuti infection. Trichoderma viride Pers. (KP026329) and a Candida sp. Berkhout (KP026318) were isolated from typical cankers (N. arbuti was also isolated from the same canker that T. viride was isolated from). Three species were isolated from asymptomatic stems of arbutus: Cytospora sp. (KR362301), Unknown 1 (KP026325) and Unknown 2 (KP026320).
Table 1. Disease class and the frequency and distribution of fungi isolated from Arbutus menziesii from 10 sites () in Lighthouse Park, Vancouver, BC, Canada. Unless otherwise indicated, all samples were collected from cankers (c) typical of Neofusicoccum arbutus infection. ITSrDNA sequences were deposited in GenBank. Best match accession numbers are indicated in brackets followed by identity (%) in NCBI BLAST searches.
Fungi associated with other hosts in lighthouse park
All Botryosphaeriaceae-like cultures isolated from asymptomatic non-arbutus hosts were identified as N. arbuti except for one, a culture identified as Diplodia mutila (Fr.) Mont. (KJ361837, 99% pairwise identity match to sequence from type species – CBS 136014 in a BLAST search in NCBI GenBank) and was isolated from Amelanchier alnifolia. In total, N. arbuti was isolated from seven of the 12 non-arbutus hosts sampled (). New hosts identified from isolations of asymptomatic woody tissues include Amelanchier alnifolia, Gaultheria shallon, Ilex aquifolium, Rosa sp., Sorbus sitchensis and Spiraea douglasii. Cytisus scoparius was also confirmed as a host (as reported by Elliott Citation2005). Neofusicoccum arbuti was also isolated from cankers on dead shoots of Gaultheria shallon.
Table 2. Occurrence of Botryosphaeriaceae species on plants in close proximity to Arbutus menziesii in 10 sites () in Lighthouse Park, Vancouver, BC, Canada. ITSrDNA sequences were deposited in GenBank. Associated accession numbers are indicated in brackets.
Lineage of neofusicoccum arbuti
All isolates of N. arbuti collected in this study had identical sequences in the ITS region. Sequences of N. arbuti collected from each of the different hosts from Lighthouse Park were deposited in GenBank (accession numbers: KP026316–KP026323). The Lighthouse Park isolates shared a single nucleotide polymorphism (A) with other isolates from British Columbia, central California, Sierra Nevada mountains (no publicly available sequence data, SNP site described in Elliott Citation2005) and Washington, whilst isolates from Oregon (no publicly available sequence data, SNP site described in Elliott Citation2005) and northern California contained the alternate allele (G). Analyses of GenBank sequences that showed a high similarity to N. arbuti or were named ‘Neofusicoccum arbuti’ revealed some incorrect species name assignments ().
Table 3. Reevaluation of ITSrDNA sequences erroneously associated with Neofusicoccum arbuti in GenBank.
Discussion
The results from this study show that N. arbuti is the primary fungal species associated with cankers on Arbutus menziesii at Lighthouse Park. They also indicate that N. arbuti has a much broader host range than previously reported and that the geographic range of this pathogen encompasses western North America and Canada.
Neofusicoccum arbuti was confirmed as the dominant species associated with cankers on arbutus in Lighthouse Park. Approximately 75% of the arbutus trees that were examined exhibited symptomatic cankers consistent with infection by N. arbuti (Elliott Citation1999b; Farr et al. Citation2005). Additionally, this pathogen was isolated from 87% of cankers that were sampled. This is consistent with the results of previous canker surveys on arbutus elsewhere (Elliott Citation1999b; Elliott et al. Citation2002).
Cankered arbutus trees were found at all 30 sites sampled. The healthiest site (disease class 1: 0–25% cankers) did not appear to be visibly impacted by human disturbance (disturbance level ‘none’). All 15 of the sites that experienced ‘some’ or ‘common’ disturbance levels were also classified as disease class 3 or 4 (> 51% trees on the site contained at least one canker). However, our results did not show any statistical correlation between direct site-level stress and canker severity. This suggests a system-wide stress, or alternative factors, as the main drivers behind the decline of arbutus.
Decline of native tree species such as arbutus may not have a single primary cause; instead, a combination of factors such as climate change, modified land management (vegetation clearing for agriculture, fire management, logging, urban development), pests and pathogens can contribute to tree decline. Arbutus in Lighthouse Park are subjected to both indirect and direct stresses facilitated by human influences. This encompasses indirect broad-scale climate change-related stress as well as stress related to the park itself: management of fire regimes, proximity to paths and carvings on trees. Fires have been entirely removed from Lighthouse Park (Catherine Berris Associates Inc. Citation2004). In the absence of fires, arbutus, which is an early successional and shade-intolerant species, is shaded out by taller tree species such as conifers (Bressette Citation1995; Elliott Citation2005). Fire also plays a role in reducing inoculum by destroying diseased plant material (Elliott et al. Citation2002). Site-level disturbance, such as the peeling off of the bark, carving of trunks and trampling of the roots, can increase potential inoculation points and the level of stress arbutus trees are subjected to (Davison Citation1972; Bressette Citation1995; Elliott Citation1999b). A similar decline of Corymbia calophylla (Lindl.) K.D.Hill & L.A.S.Johnson has been reported in the south-west of Western Australia (Paap et al. Citation2008).
Climate change predictions for the Pacific Northwest region (including Washington, Oregon, Idaho, western Montana and southern British Columbia) vary from model to model, although on average, a warming trend is predicted with a change of +1.1°C in annual temperature by the 2020s, +1.8°C by the 2040s, and +3°C by the 2080s (Mote & Salathé Citation2010). Predicted changes in precipitation are less pronounced, with an average of +1 to +2% increase (Mote & Salathé Citation2010). The modest predicted change in precipitation may be misleading, as some models predict that seasons will become more pronounced, i.e. wetter autumns and winters and drier summers are to be expected (Mote & Salathé Citation2010). A change in climate conditions may result in increased plant stress, especially in cases such as arbutus in Lighthouse Park, which tends to occupy marginal sites (exposed granite outcrops). This additional stress may shift the balance between arbutus and N. arbuti, by increasing the susceptibility of the host or increasing the frequency of the canker-causing life stage of the latent pathogen. It is also possible that the geographic range of N. arbuti will expand with the changing climate.
The host range of N. arbuti is much wider than previously reported. The pathogen was isolated from asymptomatic tissues of seven non-arbutus hosts (and in one case from canker tissues on dead shoots of Gaultheria shallon), both native and introduced, from four different taxonomic orders: Rosales Bercht. & J.Presl, Ericales Bercht. & J.Presl, Fabales Bromhead and Aquifoliales Senft. This increases the known host range of N. arbuti from two (Elliott Citation2005; Farr et al. Citation2005) to eight, and includes some abundant native hosts, such as G. shallon, as well as invasive plants such as Cytisus scoparius. It remains to be determined if infection by N. arbuti can also be manifested as disease symptoms in these new hosts.
Given that N. arbuti is found in a broad range of plant species (Elliott Citation2005; Farr et al. Citation2005; this study), it appears the fungus is not ‘host’ limited; rather, external factors such as climate may limit the continued establishment of the fungus and manifestation of the pathogenic life stage. This climate-limiting trend on the spread and establishment of Botryosphaeriaceae species has been observed in other instances and is thought to be just as important as, if not more, than host range in determining their distribution (Slippers & Wingfield Citation2007; Sakalidis et al. Citation2011a).
Since N. arbuti appears to asymptomatically colonize a broad range of host species, including native plant species such as Salal and Saskatoon berry (also used commercially in floral arrangements) and exotic plant species such as English holly and Scotch broom, the anthropogenic spread of this pathogen into new environments is a possibility. Future work could focus on pathogenicity trials on non-arbutus hosts to determine if these hosts can act as an inoculum source and if N. arbuti can cause disease symptoms in these hosts.
A report of N. arbuti infecting several cultivars of commercial blueberry (Vaccinium corymbosum L.) in Río Negro, Chile in 2009 (Espinoza et al. Citation2009) turned out to be N. nonquaesitum, which was formally described the following year from California (Inderbitzin et al. Citation2010). McDonald and Eskalen (Citation2011) collected several strains of ‘a Neofusicoccum sp. closely related to N. nonquaesitum’ from avocado (Persea americana Mill.) in California, which were designated ‘N. arbuti’ in GenBank. A re-evaluation of these latter sequences indicates one is a ‘Neofusicoccum sp.’ (HQ529769); whilst the remaining two strains (HQ529770 and HQ529771) could be designated N. nonquaesitum. One of these strains (HQ529771) contained two SNPs and two single base-pair insertions, suggesting either diversity in the ITS sequence of N. nonquaesitum, or the presence of a cryptic species closely related to N. nonquaesitum. A report of N. arbuti on Dendrobium sp. Sw in China (unpublished, GenBank accession no. GU441599) should be described as a Neofusicoccum sp. This re-assessment of the strains previously described as N. arbuti indicates this species actually appears to be restricted to western North America and Canada, specifically California, Oregon, Washington and British Columbia.
All isolates of N. arbuti collected at Lighthouse Park were identical in the ITS region and matched other isolates found in Washington, Southern California and the Sierra Nevada mountains. These isolates exhibited a single polymorphism difference in the ITS region when compared with N. arbuti found in Oregon and northern California (Elliott Citation2005). The single SNP in the ITS region that divides currently known N. arbuti may suggest the possible existence of lineages or cryptic species. Cryptic species have been identified in the Neofusicoccum parvum–N. ribis species complex by sequencing additional regions other than ITS, such as EF1-α, β-tubulin and RPB2 (Sakalidis et al. Citation2011a). In a study by Inderbitzin et al. (Citation2010), in which six gene regions (ITS, β-tubulin, EF1-a, glyceraldehyde-3-phosphate dehydrogenase, histone H3 and heat shock protein) of six isolates of N. arbuti were sequenced, no SNPs (except the previously mentioned SNP in the ITS region) were found. The presence of ITS lineage variation could have an impact on the epidemiology of pathogen races causing diseases in trees, as observed in the North American (Scleroderris canker) and European (Brunchorstia disease) races of Gremmeniella abietina (Lagerb.) Morelet, which differ at only two sites in the ITS sequence, yet only the European race can attack and kill large pine trees (Hamelin & Rail Citation1997). To explain the current distribution of N. arbuti, determine the natural range of the pathogen and assist in assigning new detections to source populations, widespread sampling of isolates and amplification of microsatellites or SNP sites for a population study would be required.
In addition to N. arbuti, seven other fungal species were isolated from arbutus. Over half of the non-Botryosphaeriaceae fungi were isolated from trees that appeared healthy or had non-typical cankers (raised bumps or blisters that may have been cankers that were healed over by the tree or had not yet broken the bark surface). Some of the cultures did not show sequence similarity with any known species available in GenBank, highlighting the diverse and undescribed biodiversity that is often found in native ecosystems (Buée et al. Citation2009; Sakalidis et al. Citation2011b).
Cytospora spp. Ehrenb. are latent endophytes known to cause cankers and dieback and which are secondary invaders found in association with cankers caused by other pathogens, such as Botryosphaeria dothidea (Moug.) Ces. & De Not. (Bettucci et al. Citation1999; Adams et al. Citation2005; Sinclair & Lyon Citation2005). Cytospora spp. are found in over 85 host species, primarily woody trees and shrubs, and are capable of causing serious damage in fruit crops and orchards (Adams et al. Citation2005; Sinclair & Lyon Citation2005). In this study, it is possible that Cytospora spp. were acting as endophytes in the healthy trees they were isolated from or as a secondary pathogen in the older cankers. Pathogenicity studies are required to confirm the role of Cytospora on arbutus.
A Diaporthe sp. Nitschke was also isolated from arbutus. The genus Diaporthe, which has anamorphs belonging to Phomopsis (Sacc.) Bubák, is comprised of hundreds of species that range in pathogenicity from highly aggressive to opportunistic saprophytes found exclusively in necrotic plant tissues (Sinclair & Lyon Citation2005). The Diaporthe sp. in this study was isolated from a raised bump, a ‘blister’, not from a typical canker. It is not known whether it caused the formation of the bump or if it was living as saprophyte in a canker underneath that had healed over.
Trichoderma spp. Pers. have been isolated from a number of arbutus cankers by Elliott (Citation1999b) and were more frequent in older cankers (Elliott Citation1999b; Elliott et al. Citation2002). Seedlings inoculated solely with Trichoderma did not express any symptoms of disease (Elliott Citation1999b; Elliott et al. Citation2002). We recovered Trichoderma viride Pers. from arbutus cankers in the present study. Trichoderma viride (teleomorph = Hypocrea rufa (Pers.) Fr.) is one of the most widely distributed species of fungi and is commonly used as a biocontrol agent (Hermosa et al. Citation2000). Since Jaklitsch et al. (Citation2006) showed that the majority of species in the Trichoderma genus are indistinguishable in the ITS 1 and ITS 2 region, species identification in this genus may not be possible using only the ITS region. As a result, unless verified by other gene regions that provide species level resolution, BLAST search matches of the ITS sequence data to T. viride may also match other Trichoderma species. Since we only examined the ITS region in our study, it is possible that the species isolated from arbutus was a Trichoderma sp. different from T. viride.
A Candida sp. Berkhout was isolated from arbutus cankers in this study. Candida is a group of yeast fungi commonly associated with humans and other animals, causing disease (candidiasis) in primarily immunodeficient hosts (Coutinho Citation2009). However, Candida is also found in forest soils (Hui et al. Citation2012) and rotting wood (Barbosa et al. Citation2009). The isolate of Candida sp. from arbutus may have been a saprophyte. Similarly, species in the genus Absidia Tiegh. are ubiquitous in soil, and are common airborne contaminants (Cvetnić & Pepeljnjak Citation1997; Donnison Citation2000). In immune-compromised humans, Absidia spp. have been known to cause lung disease and other health issues (Ribes et al. Citation2000). Therefore, the occurrence of non-pathogenic fungi in arbutus cankers is not uncommon as shown in this study.
Diseases symptoms due to infection by N. arbuti and other latent pathogens in the Botryosphaeriaceae are often only expressed when the host is weakened by suboptimal growing conditions. Thus, increased drought frequency and intense weather events due to climate change may increase the potential for wide-scale decline of arbutus and lead to a potential increase of the disease in non-arbutus hosts. Management strategies designed to improve tree health may involve both the reduction of deleterious human activities and the planting of arbutus in areas located within the optimal ranges of the species.
For individual trees, which may be valuable or culturally important, certain fungicide regimes may be effective in reducing canker size and preventing new infections (Elliott & Edmonds Citation2008). However, the best treatments are arboricultural methods, such as reduction or removal of sources of inoculum (i.e. heavily diseased trees), proper pruning methods that promote healing, and management of competing vegetation (Elliott Citation2005). Unfortunately, since the host range of N. arbuti extends to native and ubiquitous species other than arbutus, efforts to reduce inoculum levels in a natural forest context may be difficult.
Acknowledgements
The authors would like to thank the anonymous reviewers for their comments and suggestions. We would also like to extend their sincere gratitude to Dr Allan Carroll, Dr Sarah Gergel, Avneet Brar, Padmini Herath, Stéphanie Beauseigle, Angie Dale and the rest of Richard Hamelin’s team for their assistance in the laboratory and encouragement. Thanks to Dan Henegar, Manager of Parks Arboriculture and Horticulture for the District of West Vancouver, for permission to sample in Lighthouse Park.
Additional information
Funding
References
- Adams GC, Wingfield MJ, Common R, Roux J. 2005. Phylogenetic relationships and morphology of Cytospora species and related teleomorphs (Ascomycota, Diaporthales, Valsaceae) from Eucalyptus. Stud Mycol. 52:1–144.
- Alaback P, Antos J, Goward T, Lertzman K, MacKinnon A, Pojar J, Pojar R, Reed A, Turner N, Vitt D. 2004. Plants of the Pacific Northwest coast: Washington, Oregon, British Columbia & Alaska.. Pojar J and MacKinnon A editors. Vancouver (BC): Lone Pine Publishing; p. 49, 53, 71, 72, 74, 81, 83.
- Barbosa AC, Cadete RM, Gomes FCO, Lachance M-A, Rosa CA. 2009. Candida materiae sp. nov., a yeast species isolated from rotting wood in the Atlantic rain forest. Int J Syst Evol Microbiol. 59:2104–2106.
- Bettucci L, Alonso R, Tiscornia S. 1999. Endophytic mycobiota of healthy twigs and the assemblage of species associated with twig lesions of Eucalyptus globulus and E. grandis in Uruguay. Mycol Res. 103:468–472.
- Bressette DK 1995. Determining causes of decline of Pacific madrone in urban landscapes of the Pacific Northwest [MSc dissertation]. Seattle (WA): College of Forest Resources, University of Washington.
- Buée M, Reich M, Murat C, Morin E, Nilsson RH, Uroz S, Martin F. 2009. Pyrosequencing analyses of forest soils reveal an unexpectedly high fungal diversity. New Phytol. 184:449–456.
- Catherine Berris Associates Inc. 2004. Lighthouse Park management plan. [Online]. West Vancouver (BC): City of West Vancouver. Available from: http://westvancouver.ca/uploadedFiles/Parks_and_Environment/Parks/LHP_MANAGEMENT_PLAN_FINAL.pdf
- Coates B, Mondor C. 1978. An assessment of the national significance of Lighthouse Park. Victoria (BC): National Parks Branch, Parks System Planning, Parks Canada, Parks & Outdoor Recreation Division, Government of British Columbia.
- Coutinho HDM. 2009. Factors influencing the virulence of Candida spp. West Indian Med J. 58:160–163.
- Crous PW, Slippers B, Wingfield MJ, Rheeder J, Marasas WFO, Philips AJL, Alves A, Burgess T, Barber P, Groenewalk JZE, Groenewald JZ. 2006. Phylogenetic lineages in the Botryosphaeriaceae. Stud Mycol. 55:235–253.
- Cvetnić Z, Pepeljnjak S. 1997. Distribution and mycotoxin-producing ability of some fungal isolates from the air. Atmos Environ. 31:491–495.
- Davison AD. 1972. Factors affecting development of madrone canker. Plant Dis Rept. 56:50–52.
- Don RH, Cox PT, Wainwright BJ, Baker K, Mattick JS. 1991. “Touchdown” PCR to circumvent spurious priming during gene amplification. Nucleic Acids Res. 19:4008.
- Donnison L. 2000. Management influences on soil microbial communities and their function in botanically diverse haymeadows of northern England and Wales. Soil Biol Biochem. 32:253–263.
- Elliott M. 1999a. Diseases of Pacific Madrone. In: Adams AB, Hamilton CW, editors. The decline of Pacific madrone (Arbutus menziesii Pursh): Current theory and research directions, Proceedings of the April 28, 1995 Symposium. Seattle (WA): Center for Urban Horticulture, University of Washington; p. 48–60.
- Elliott M 1999b. The decline of Pacific madrone (Arbutus menziesii Pursh) in urban and natural environments: its causes and management [MSc dissertation]. Seattle (WA): College of Forest Resources, University of Washington.
- Elliott M 2005. A canker disease of Pacific madrone (Arbutus menziesii) caused by the fungal pathogen Fusicoccum arbuti (Botryosphaeriaceae) [PhD dissertation]. Seattle (WA): College of Forest Resources, University of Washington.
- Elliott M, Edmonds RL. 2008. Injected treatments for management of madrone canker. Arboric Urban For. 34:110–115.
- Elliott M, Edmonds RL, Mayer S. 2002. Role of fungal diseases in decline of Pacific madrone. Northwest Sci. 76:293–303.
- Espinoza JG, Briceño EX, Chávez ER, Úrbez-Torres JR, Latorre BA. 2009. Neofusicoccum spp. associated with stem canker and dieback of blueberry in Chile. Plant Dis. 93:1187–1194.
- Farr DF, Elliott M, Rossman AY, Edmonds RL. 2005. Fusicoccum arbuti sp. nov. causing cankers on Pacific madrone in western North America with notes on Fusicoccum dimidiatum, the correct name for Scytalidium dimidiatum and Nattrassia mangiferae. Mycologia. 97:730–741.
- Gardes M, Bruns TD. 1993. ITS primers with enhanced specificity for basidiomycetes - application to the identification of mycorrhizae and rusts. Mol Ecol. 2:113–118.
- Gurung J, Adams AB, Raphael MG. 1999. A review of the use of Pacific madrone by nesting, pollinating and frugivorous birds. In: Adams AB, Hamilton CW, editors. The decline of Pacific madrone (Arbutus menziesii Pursh): Current theory and research directions, Proceedings of the April 28, 1995 Symposium. Seattle (WA): Center for Urban Horticulture, University of Washington; p. 27–34.
- Hamelin RC, Rail J. 1997. Phylogeny of Gremmeniella spp. based on sequences of the 5.8S rDNA and internal transcribed spacer region. Can J Bot. 75:693–698.
- Hermosa MR, Grondona I, Iturriaga EA, Diaz-Minguez JM, Castro C, Monte E, Garcia-Acha I. 2000. Molecular characterization and identification of biocontrol isolates of Trichoderma spp. Appl Environ Microbiol. 66:1890–1898.
- Hui F-L, Ke T, Niu Q-H, Du P-C. 2012. Candida baotianensis sp. nov., an ascomycetous yeast species from forest soil in China. J Gen Appl Microbiol. 58:59–63.
- Hunt RS, Callan B, Funk A. 1992. Common pests of arbutus in British Columbia. Forest Pest Leaflet; no. 63. Victoria (BC): Canadian Forest Service.
- Inderbitzin P, Bostock RM, Trouillas FP, Michailides TJ. 2010. A six locus phylogeny reveals high species diversity in Botryosphaeriaceae from California almond. Mycologia. 102:1350–1368.
- Jaklitsch WM, Samuels GJ, Dodd SL, Lu B-S, Druzhinina IS. 2006. Hypocrea rufa/Trichoderma viride: A reassessment, and description of five closely related species with and without warted conidia. Stud Mycol. 56:135–177.
- Kendall MG. 1976. Rank Correlation Methods. 4th ed. London: Griffin.
- McDonald PM, Tappeiner JC. 1990. Arbutus menziesii Pursh Pacific madrone. In: Burns RM, Honkala BH, editors. Silvics of North America. Vol. 2. Washington (DC): USDA Forest Service; pp. 124–132.
- McDonald V, Eskalen A. 2011. Botryosphaeriaceae species associated with avocado branch cankers in California. Plant Dis. 95:1465–1473.
- McLeod AI 2011. Kendall: Kendall rank correlation and Mann-Kendall trend test. R package version 2.2. Available from: http://CRAN.R-project.org/package=Kendall
- Mohali S, Slippers B, Wingfield MJ. 2006. Two new Fusicoccum species from Acacia and Eucalyptus in Venezuela, based on morphology and DNA sequence data. Mycol Res. 110:405–413.
- Moreth U, Schmidt O. 2000. Identification of indoor rot fungi by taxon-specific priming polymerase chain reaction. Holzforschung. 54:1–8.
- Mote PW, Salathé EP. 2010. Future climate in the Pacific Northwest. Clim Change. 102:29–50.
- Paap T, Burgess TI, McComb JA, Shearer BL, Hardy G. 2008. Quambalaria species, including Q. coyrecup sp. nov., implicated in canker and shoot blight diseases causing decline of Corymbia species in the southwest of Western Australia. Mycol Res. 112:57–69.
- Phillips AJL, Alves A, Abdollahzadeh J, Slippers B, Wingfield MJ, Groenewald JZ, Crous PW. 2013. The Botryosphaeriaceae: Genera and species known from culture. Stud Mycol. 76:51–167.
- Pynn L 2006. Arbutus trees dying at an alarming rate in West Van. [Internet]. Vancouver (BC): Vancouver Sun. [ cited 2015 January 16]. Available from: http://www.canada.com/story.html?id=70f97756-5a14-4467-98f5-eb00cc1c7470
- R Core Team. 2013. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna (Austria). Available from: http://www.R-project.org/.
- Ribes JA, Vanover-Sams CL, Baker DJ. 2000. Zygomycetes in human disease. Clin Microbiol Rev. 13:236–301.
- Sakalidis ML, Hardy G, Burgess TI. 2011a. Use of the Genealogical Sorting Index (GSI) to delineate species boundaries in the Neofusicoccum parvum-Neofusicoccum ribis species complex. Mol Phylogenet Evol. 60:333–344.
- Sakalidis ML, Hardy G, Burgess TI. 2011b. Endophytes as potential pathogens of the baobab species Adansonia gregorii: A focus on the Botryosphaeriaceae. Fungal Ecol. 4:1–14.
- Sinclair WA, Lyon HH. 2005. Diseases of trees and shrubs. 2nd ed. Ithaca (NY): Comstock Publishing Associates, Cornell University Press.
- Slippers B, Wingfield MJ. 2007. Botryosphaeriaceae as endophytes and latent pathogens of woody plants: Diversity, ecology and impact. Fungal Biol Rev. 21:90–106.
- Taylor K, Barber PA, GEStJ H, Burgess TI. 2009. Botryosphaeriaceae from tuart (Eucalyptus gomphocephala) woodland, including descriptions of four new species. Mycol Res. 113:337–353.
- White T, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innes MA, Gelfand DH, Sninsky JJ, White TJ, editors. Protocols: A guide to methods and applications. San Diego (CA): Academic Press; p. 315–322.
