Abstract
Several bacterial diseases can significantly impact dry bean production in western Canada. The objectives of this study were to assess the incidence of multiple bacterial diseases on dry bean in southern Alberta and to determine the prevalent races causing halo blight (HB) in western Canadian dry bean fields. Field surveys combined with a pathogen isolation component were conducted in 2012–2014 to determine relative frequency of bacterial pathogens. Bacterial pathogens causing HB and brown spot were most commonly isolated from symptomatic tissues, whereas the pathogens causing common blight (CBB) and bacterial wilt were infrequently detected. In order to screen bean breeding lines for resistance to HB, the races of Pseudomonas syringae pv. phaseolicola (Pph) present in western Canada needed to be determined. A total of 114 Pph isolates were recovered from dry bean samples from Alberta, Saskatchewan and Manitoba in 2010–2013. Virulence testing of these isolates on the halo blight dry bean differential set indicated that only races 2 and 6 were present. These races were equally predominant in Manitoba, race 2 comprised 81% of Alberta isolates, and only race 2 occurred in Saskatchewan. Screening of select CBB-resistant breeding lines indicated that all were susceptible to a race 2 isolate of Pph. However, several commercial cultivars either currently grown, or recently registered for commercial production in Alberta, showed reduced susceptibility to HB. These results should be applicable to dry bean breeding programmes attempting to incorporate halo blight resistance into Canadian-adapted dry bean lines.
Résumé
Plusieurs maladies bactériennes peuvent compromettre la production de haricots secs dans l’Ouest canadien. Les objectifs de cette étude étaient d’évaluer l’incidence de plusieurs maladies bactériennes sur les haricots secs dans le sud de l’Alberta et d’identifier les races courantes causant la graisse du haricot (GH) dans les champs de l’Ouest canadien. Des études sur le terrain, combinées à l’isolement de l’agent pathogène, ont été menées de 2012 à 2014 pour déterminer la fréquence relative des agents pathogènes bactériens. Ceux causant la GB et la tache brune ont été le plus fréquemment isolés de tissus symptomatiques, tandis que les agents pathogènes causant la brûlure bactérienne (BB) et le flétrissement bactérien ont été rarement détectés. Afin de cribler des lignées de haricots relativement à une sélection ciblant la résistance à la GH, nous devions déterminer quelles étaient les races de Pseudomonas syringae pv. phaseolicola (Pph) que l’on trouvait dans l’Ouest canadien. En tout, de 2010 à 2013, 114 isolats de Pph ont été obtenus d’échantillons de haricots secs provenant de l’Alberta, de la Saskatchewan et du Manitoba. Des analyses de virulence menées sur ces isolats quant à la série différentielle de la graisse du haricot ont montré qu’il y avait seulement que les races 2 et 6. Celles-ci étaient aussi courantes l’une que l’autre au Manitoba, la race 2 comptait pour 81 % des isolats provenant de l’Alberta et, en Saskatchewan, il n’y avait que la race 2. Le criblage de lignées de sélection résistantes à la BB a montré que toutes étaient réceptives à l’égard d’un isolat de la race 2 de Pph. Toutefois, plusieurs cultivars commerciaux utilisés couramment ou récemment homologués en vue d’une production commerciale en Alberta ont affiché une certaine diminution de la sensibilité à la GH. Ces résultats devraient être appliqués aux programmes de sélection des haricots secs qui visent à incorporer la résistance à la graisse dans les lignées adaptées aux conditions canadiennes.
Introduction
Dry bean (Phaseolus vulgaris L.) is one of the most economically and nutritionally important grain legume crops worldwide, providing a primary source of protein and minerals for human diets (Broughton et al. Citation2003). It is an important crop in Canada, with a yearly production of over 100 000 ha and annual farm cash receipts in excess of $150 million CDN (Agriculture and Agri-Food Canada Citation2010). Approximately one-half of Canadian production is planted on the western prairies, mostly in the provinces of Alberta and Manitoba, with a smaller amount in Saskatchewan. Production in Alberta and Manitoba is centred on the small seeded (navy and black) and medium seeded (pinto, great northern, red and pink) bean market classes. Bean production in Alberta occurs only in the irrigated districts of southern Alberta, which is characterized by brown chernozemic soils, semiarid conditions and a short growing season (100–105 days to maturity) (Walburger et al. Citation2004). Virtually all of the dry bean production in Canada is for human consumption and is exported to other countries.
Bacterial diseases such as common blight, caused by Xanthomonas axonopodis pv. phaseoli (Smith 1897) Vauterin et al. 1995 (Xap) and X. fuscans subsp. fuscans (Burkholder) Starr & Burkholder 1942 (Xff), halo blight caused by Pseudomonas syringae pv. phaseolicola (Burkholder 1926) Gardan et al. 1992 (= P. savastanoi pv. phaseolicola, Pph), brown spot caused by P. s syringae pv. syringae van Hall 1902 (Pss) and bacterial wilt, caused by Curtobacterium flaccumfaciens pv. flaccumfaciens (Hedges 1922) Collins & Jones 1983 (Cff) are known to affect dry bean production worldwide. These pathogens have been detected in surveys of commercial dry bean crops in western Canada (Erickson & Balasubramanian Citation2008, Citation2010; Henriquez et al. Citation2012, Citation2014). Their relative importance varies annually and regionally, depending on climatic factors, management practices and the market class grown. Common bacterial blight (CBB) and halo blight (HB) are generally the most widespread bacterial diseases of dry bean in Canada (Bailey et al. Citation2003). Yield losses caused by these diseases are a result of leaf lesions that negatively impact photosynthesis and foliar growth during floral and pod development (Goodwin Citation1992; Rodríguez-Moreno et al. Citation2008). Despite numerous surveys of commercial dry bean fields on the western Canadian prairies, little information is known regarding the relative frequency of occurrence of these bacterial pathogens over the growing season.
CBB occurs under warm, humid conditions, and epidemics are exacerbated by damage to plants due to hail or wind, followed by irrigation (Bailey et al. Citation2003). Symptoms of CBB are necrotic brown lesions on the leaves that expand and coalesce to cover large areas of the leaf and stem. In advanced stages, the brown lesions become brittle, resulting in premature defoliation (by 7–9 days) before healthy plants (Bailey et al. Citation2003). Halo blight typically occurs under cool (20–25°C) moist conditions found early and later in the growing season (mid-July and early September). HB is characterized by the formation of large chlorotic zones which surround brown necrotic lesions on the leaves and result from production of phaseolotoxin by the pathogen, and are evident only at temperatures below 22°C (López-López et al. Citation2004). The symptoms of CBB and HB can be difficult to distinguish, especially later in the growing season when both pathogens may be present, and quantifying the impact of each pathogen on disease severity can be difficult (Bailey et al. Citation2003).
Both bacterial pathogens also infect the pods, resulting in direct yield loss and quality reduction due to discolouration and shrivelling of the seeds (Taylor & Dudley Citation1979). The pathogens can survive for extended periods on the seed, and planting of infected seed is the most common source of initial inoculum (Taylor & Dudley Citation1979; Weller & Saettler Citation1980).
In general, control of bacterial pathogens of dry bean is primarily based on the use of pathogen-free seed, but this method is not completely effective because pathogen inoculum may be harboured in non-host and asymptomatic tissues (Cafati & Saettler Citation1980; Gent et al. Citation2005). Chemical sprays using foliar-applied copper products is also used, but the strategy is only moderately effective (Taylor et al. Citation1996a). The method requires multiple applications, making dry bean production more costly and less desirable for producers, and increases the risk of developing resistance in pathogens (Garrett & Schwartz Citation1998; Vanneste et al. Citation2003). Seed treatment with streptomycin has been used to control seed-borne bacterial pathogens (Vidaver Citation2002; Stockwell & Duffy Citation2012), but concerns about antibiotic resistance have resulted in tighter restrictions in Canada, and the permit to import streptomycin-treated bean seed from the USA expires in December 2016 (Bett & Banniza Citation2014).
Development of resistant cultivars against bacterial diseases is a promising and cost-effective alternative. Although most commercial bean cultivars currently grown in western Canada are susceptible to common and halo blights, molecular markers for common blight resistance have been identified (Yu et al. Citation2004; Duncan et al. Citation2012) and high-yielding cultivars with improved resistance to common blight are available, or under development, in several market classes (Michaels et al. Citation2006; Smith et al. Citation2012; Navabi et al. Citation2013; Boersma et al. Citation2014, Citation2015; Balasubramanian et al. Citation2015). Halo blight resistance is governed by gene-for-gene interactions (Arnold et al. Citation2011), but quantitative resistance has also been described (Taylor et al. Citation1996b). An understanding of the race structure of pathogenic organisms is required for resistance breeding and deployment of resistance genes to that pathogen (Taylor et al. Citation1996a). Nine races of HB were detected based on interactions with eight differential bean cultivars and five pairs of avirulence and resistance genes (Taylor et al. Citation1996a, Citation1996b). In a large-scale study of Pph isolates in 1996, races 1, 2, 5, 6 and 7 were reported to occur worldwide, with race 6 being predominant (Taylor et al. Citation1996a). However, only one isolate from Canada was included in this study, and was identified as belonging to race 5. To our knowledge, no other studies describing races of Pph present in Canada have been published.
The objectives of this study were to: (1) determine the relative frequency and distribution of bacterial pathogens of bean in western Canada; (2) determine the race structure of halo blight infecting bean in western Canada; (3) determine if any of the halo blight isolates collected are resistant to commonly used copper compounds; and (4) test selected bean lines from Agriculture and Agri-Food Canada dry bean breeding programmes for resistance to the predominant race of halo blight.
Materials and methods
Foliar disease survey and identification of pathogens
Foliar disease surveys were conducted bi-weekly in the dry bean growing region of southern Alberta from late June until mid-August during the years 2012, 2013 and 2014. Each field was selected arbitrarily, and sampled in a U-shaped pattern by selecting 10 sites approximately 20 m apart, with each site consisting of a 3 m long section of row (Howard & Huang Citation1983). The incidences of bacterial diseases in each crop were calculated as the percentage of infected plants, averaged over the 10 sites. Disease severity was estimated at each site according to the following scale: (1) none (0% of leaf area infected), (2) trace (<1%), (3) mild (1–10%), (4) moderate (11–25%), (5) severe (26–50%), (6) very severe (>50%) (Erickson & Chatterton Citation2012). Bean leaves with symptoms of bacterial diseases were collected from new growth during the vegetative growth stage, and symptomatic bean pods were collected during the reproductive stage.
Diseased tissues were returned to the laboratory for isolation and identification of pathogens. For each sample, one gram of symptomatic plant tissue was crushed in sterile distilled water and the supernatant was collected using a pipette. A loopful (10 μL) of the extract was streaked onto Milk-Tween agar (Goszczynska & Serfontein Citation1998) and the resulting growth was sub-cultured to obtain single colony isolates. Single colonies were assigned a presumptive identification according to their reaction on the medium, and compared with growth of previously identified isolates of the five bacterial pathogens (Pph, Pss, Xap, Xff and Cff) obtained from the Lethbridge Research and Development Centre culture collection. The isolates were also streaked onto King’s B medium (King et al. Citation1954), and those isolates that displayed UV fluorescence were tested using the Pph ELISA kit for halo blight (Agdia, Elkhart, IN) according to the manufacturer’s instructions.
To confirm presumptive morphological identifications, three single colonies of each isolate were tested in one of two multiplex PCR reactions designed to amplify either the group of Xanthomonas or Pseudomonas spp. The Xanthomonas assay used published, species-specific primers for Xap, Xff and Xanthomonas spp. () (Audy et al. Citation1996; Toth et al. Citation1998; Parkinson et al. Citation2007). The Pseudomonas assay used published species-specific primers for Pph, Pss and one genus-specific Pseudomonas primer set () (Audy et al. Citation1996; Sorensen et al. Citation1998; Widmer et al. Citation1998; Rico et al. Citation2003). Bacteria were suspended in sterile distilled water to achieve a final OD600 of 0.01 using a NanoVue Plus spectrophotometer (GE Healthcare, Pittsburgh, NJ). To compare identification methods, the crude plant extract, obtained as described above, was also used in a multiplex PCR assay containing primers for all five bacterial pathogens () (Audy et al. Citation1996; Sorensen et al. Citation1998; Stevens et al. Citation1998; Toth et al. Citation1998; Tegli et al. Citation2002).
Table 1. Primer sets, sequences and amplicon size used to identify bacterial pathogens using single-colony PCR or multiplex PCR using crude plant extracts as the template.
PCR amplification assays were performed in a 25-μL reaction mixture containing 2.5 μL of bacteria suspension or crude plant extract, master mix according to manufacturer’s instructions, and 0.2 μM of each primer using the Qiagen Multiplex PCR Kit (Qiagen, Germantown, MD). Reactions were performed in a MasterCycler Pro thermocycler (Eppendorf, Mississauga, ON) with an initial denaturation step at 95°C for 15 min, followed by 25–30 cycles of denaturation (30 s at 94°C), primer annealing (90 s at 60°C) and primer extension (90 s at 72°C), and a final extension step at 72°C for 10 min. DNA extracted from standard isolates of each of the pathogenic bacteria in the Lethbridge Research and Development Centre culture collection, or crude bacterial suspension containing a mix of the five species, were used as positive controls.
Due to inconsistent identification of Xanthomonas spp. using PCR and plate culturing techniques, several Xanthomonas-like isolates were also tested for their pathogenicity on the susceptible dry bean cultivar ‘CDC Sol’. The stems of 10-day-old bean seedlings, grown in Cornell Mix in 4 cm diameter plastic pots, were wounded between the first and second node with a sterile needle dipped into a fresh (24–48 h) bacterial culture grown on nutrient agar (NA, Difco; Mississauga, ON) (International Seed Testing Association, Citation2007). LRC isolates Xap 916 and Xff 917 served as positive controls, while control plants were wounded with a sterile needle dipped in NA only. Plants were grown in a greenhouse (conditions described below), and the length of stem lesions (cm) was measured with a ruler 14 days after inoculations. Five seedlings per isolate were inoculated, treatments were arranged in a randomized complete block design, and the experiment was carried out twice. Identification of these unknown isolates was attempted by sequencing of the 16S ribosomal DNA using universal primers and standard PCR protocol (Jensen et al. Citation1993). The resulting PCR products were sequenced using Genome Quebec sequencing services, and sequences compared with the 16S ribosomal project database to determine most likely identity (Wang et al. Citation2007).
Halo blight race determination
Experiments were conducted to determine the race identity of halo blight isolates from the disease surveys described above. Additional isolates obtained from dry bean fields, which were not part of the systematic surveys, in Alberta from 2010 to 2014, in Saskatchewan from 2011 to 2013, and in Manitoba from 2005 to 2014, were also screened for race identification. The isolates were categorized into race groupings using PCR primers specific to known avirulence genes (Stevens et al. Citation1998; Tsiamis et al. Citation2000) and placed in a race-grouping based on presence of DNA bands indicating presence of hopX1 (AvrPphE, all races), hopAR1 (AvrPphF, races 1, 5, 7 and 9), or hopF1 (AvrPphB, races 3 and 4) (). Presence of hopX1, and absence of hopAR1 and hopF1, indicated the isolate belonged to either race 2 or 6.
The halo blight virulence pattern of each isolate was confirmed on the set of eight differential bean lines (Taylor et al. Citation1996a) using a detached leaf assay. Bean plants were grown in Cornell Peat-Lite Mix (Boodley & Sheldrake Citation1977) in 15 cm diameter plastic pots in a growth chamber at 20 ± 2ºC with a mixture of fluorescent and incandescent light and a 16 h photoperiod. Bacterial inoculum was prepared by growing each isolate on nutrient agar (NA) at 20 ± 2ºC for 48 h, suspending the resulting growth in sterile distilled water, and adjusting the concentration of the suspension to 105 cfu μL−1 based on OD600 absorbance readings using a NanoVue Plus spectrophotometer. Fresh, fully expanded leaflets from trifoliate leaves were excised, washed in distilled water, and placed on wet paper towel in plastic trays with the petiole embedded in the towel. Each leaflet was injured twice (once on each side of the midrib) with an orienteering punch (Suunto; Vantaa, Finland) and sprayed with the bacterial inoculum suspension. Trays containing the inoculated leaflets were placed in transparent plastic bags, incubated in the growth chamber for 7 days, and scored for presence or absence of symptoms around the wound sites. Each isolate was tested twice, with 3 replicates (trays) in each test. Observed disease reaction data from each halo blight isolate were compared with the expected result for each race by calculating the per cent similarity using the formula S = [1−(nmis/ntot)]×100, where S = per cent similarity of the observed data to the expected data; nmis = the number of mismatched pairs of data; and ntot = the total number of pairs of data. The tested isolate was then deemed to belong to the race with the highest per cent similarity.
Copper sensitivity testing of Pph isolates
The halo blight isolates used in the race determination study were also assessed for their sensitivity to copper sulphate (CuSO4 · 5H2O; Fisher; Ottawa, ON) and copper (II) hydroxide (Cu(OH)2 from Parasol) (Nufarm; Calgary, AB). Stock solutions of the compounds (100 mM) were prepared using sterile distilled water and were sterilized by passing through a 200 µm filter. Copper-amended media were prepared by aseptically adding the stock solution to tryptone-yeast agar (tryptone 1 g L−1; yeast extract 2 g L−1; glycerol 2 mL L−1; agar 15 g L−1) at 60–80ºC, to achieve final concentrations of 0 (control), 0.25, 0.5, 1.0 or 1.25 mM of copper sulphate or copper hydroxide, and then pouring the amended media into 90-mm Petri dishes using 20 mL per dish. Single colonies of each isolate were transferred onto the amended media, incubated at room temperature for 48 h, and scored for presence or absence of growth by comparing with the control treatment. There were three replicates (plates) of each treatment per isolate, and the experiment was conducted twice.
Testing Canadian dry bean lines for halo blight resistance
Two groups of bean cultivars were assessed for resistance to halo blight in greenhouse tests. The first group consisted of 15 dry bean breeding lines developed at AAFC in Morden, MB, some with known resistance to common blight (Boersma et al. Citation2014). The second group was composed primarily of 26 dry bean cultivars that are currently grown in western Canada, recently registered for commercial production or breeding lines of interest. Older cultivars that are not currently in production were also included in the test to compare responses within market classes. The susceptible cultivar ‘Canadian Wonder’ (Taylor et al. Citation1996a) was included in all tests as a susceptible control.
Bean plants were grown in Cornell Peat-Lite Mix in 15 cm diameter plastic pots in a greenhouse with a 16 h photoperiod and temperature of 22 ± 2ºC during the day and 18 ± 2ºC at night. Bacterial inoculum was prepared by growing P. syringae pv. phaseolicola isolate AB2012.002.1 (race 2) on nutrient agar at 20 ± 2ºC for 48 h, suspending the resulting growth in sterile distilled water, and adjusting the concentration of the suspension to 105 cfu mL−1. Fresh, fully expanded leaflets from trifoliate leaves were inoculated in situ by dipping an orienteering punch into the suspension and punching each leaflet twice (once on either side of the midrib). The inoculated plants were grown in the same greenhouse conditions described above, but were kept in moist conditions (>95% relative humidity) for 7 days, and then each inoculation site was rated for severity of infection using the scale of Innes et al. (Citation1984), where 1 = necrosis only in area of inoculation (highly resistant); 2 = trace of water soaking around the inoculation site (resistant); 3 = more extensive water soaking around the inoculation site (slightly susceptible); 4 = small lesions distributed over leaf (susceptible); and 5 = larger lesions distributed over leaf (fully susceptible). A total of 40 sites (two inoculation sites per leaflet, two leaflets per plant, and 10 plants) were inoculated for each cultivar in each test, and plants were arranged in a randomized complete block design. The test was performed twice.
Analysis of variance (ANOVA) at the P = 0.05 level was used to determine significant differences between cultivars for the halo blight severity data. Duncan’s multiple range test was used to compare the cultivar means. The data for each test were analysed separately, and then pooled to perform a combined analysis, using SAS/STAT™ computer software, version 9.1.3 (SAS Institute Inc Citation2004). Pooling of multiple trials was deemed appropriate based on similar scores for the check cultivar ‘Canadian Wonder’, which was included in every test.
Results
Weather conditions
In 2012, frequent rainfalls occurred in June, while little rainfall occurred in July and August, as is typical for southern Alberta. Average night-time and day-time temperatures in these months ranged from 9–12°C and 22–28°C, respectively, providing ideal conditions for halo blight development by Pph. In 2013, rainfall amounts were lower in June and higher in July than observed in 2012, while night-time and day-time temperatures were similar to 2012. In 2014, there were frequent rainfalls in June and August, but there was little precipitation in July. Night-time and day-time temperatures ranged from 8–11°C and 20–28°C, respectively, although August was cooler than normal with daily average temperature of 25°C.
Foliar disease survey and identification of pathogens
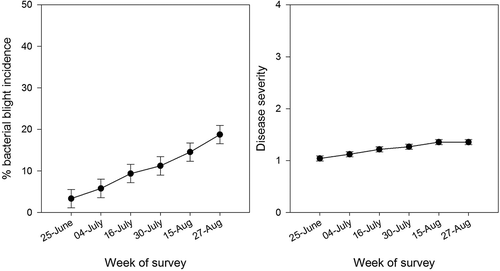
Symptoms of bacterial blights were observed in commercial bean fields in southern Alberta during 2012, 2013 and 2014, although the number of fields with symptoms was low in 2013 compared with other years (, ). The frequency of fields with moderate, severe and very severe leaf symptoms in 2012 was 30%, 18% and 9%, respectively. Disease incidence and severity increased steadily throughout the survey period in 2012 (), but disease levels were too low to show the same trend in 2013 and 2014. Obvious symptoms of bacterial wilt were not observed in the fields during the 3 years of the survey.
Fig. 1 (Colour online) Symptoms of bacterial diseases observed during field surveys conducted in Alberta in 2012, with corresponding pathogens isolated from leaves in the lab. (a) Large yellow halos surrounding small necrotic lesions typical of halo blight; Pph isolated; (b) Brown spot symptoms showing spotty brown necrotic lesions with slight yellow margins; Pss isolated. (c) Symptoms typical of brown spot, but both Pph and Pss were isolated; (d) Early symptoms of common blight with brown necrotic area and yellow margins; both Xff and Pph isolated; (e) Foliar symptoms of bacterial wilt with spreading brown, senescent lesions and slight yellow margins; both Cff and Pph were isolated; and (f) symptoms typical of abiotic injury yielding fluorescent Pseudomonads only.
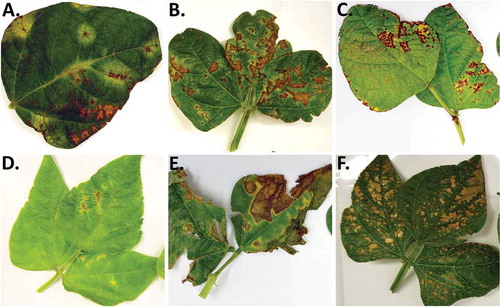
Table 2. Occurrence and severity of bacterial disease symptoms observed during surveys of commercial dry bean fields in southern Alberta.
Isolations onto MT agar from plant materials collected during the survey detected the presence of Pph, Pss, and Xap or Xff in commercial bean fields (), although symptoms were not always indicative of the pathogen isolated (). In 2012, a total of 55 samples were collected from surveyed fields, whereas in 2014 only 13 samples in total were collected from the fields. No samples were collected from fields surveyed in 2013. Based on single-colony PCR from bacterial cultures isolated on milk-tween agar, the most frequently isolated pathogen in 2012 was Pph (40%), followed by Pss (29%). In 2014, Pss (38%) was most commonly isolated, followed by Pph (31%) (). Multiple bacterial infections occurred in 15% of leaf samples in 2012 but only one sample had multiple infections in 2014 (data not shown). Detection frequencies of Pss and Xap/Xff using multiplex PCR directly from plant extracts were in agreement with results from single colony PCR from isolated bacteria for these species (). However, frequency of detection of Pph from plant extracts was always lower when compared with single colony PCR. Cff was only detected in multiplex PCR assays with plant extracts, as isolations onto Cff-selective media were not performed due to difficulty in making the media and since bacterial wilt symptoms were not obvious in the field.
Table 3. Frequency of isolation (%) of bacterial pathogens from bean tissue samples collected in Alberta during 2012 and 2014 using three different identification methods: colony morphology on milk-tween agar, single colony PCR and PCR from plant extract.
Bacteria that appeared to be Xap or Xff based on morphology on milk-tween agar were recovered from 38% and 15% of samples in 2012 and 2014, respectively (). However, the characteristic development of brown pigmentation on this medium was not always observed. When these isolates were identified using single colony PCR with Xap and Xff specific primers, only 5% of samples yielded positive results for Xff in 2012 and 0 isolates tested positive for Xap (). These Xanthomonas-like bacteria () were isolated from leaves with bacterial blight symptoms and produced characteristic yellow colonies with slight to large halos on milk-tween agar. Sequencing of the 16S ribosomal DNA from select isolates from this group identified these isolates as either Xanthomonas campestris or Pseudomonas sp. Pathogenicity tests of selected isolates from this group demonstrated reduced or no virulence compared with positive Xap and Xff controls ().
Table 4. Identification of Xanthomonas-like bacteria isolated from bean leaf samples using 16S ribosomal sequences and length of lesions on bean stems ‘CDC Sol’ following injection of bacterial suspensions.
Halo blight race determination
A total of 114 Pph isolates were assayed for halo blight race determination. PCR assays indicated the presence of hopX1, and absence of hopF1 and HopAR1, based on expected DNA band sizes, for all of the isolates analysed, suggesting that they all belonged to either race 2 or 6. Detached leaf assays confirmed this grouping, and further indicated the presence of both races in western Canada (). Race 2 was the most common, comprising 75% of the isolates, with the remaining 25% being race 6. The percentage similarity ranged from 100 to 83% for isolates deemed to be race 2, and from 98 to 81% for isolates deemed to be race 6. Both races occurred in Alberta and Manitoba, but only race 2 was detected in Saskatchewan. In Alberta, 81% of isolates belonged to race 2, making it the predominant race in this province. In Manitoba, races 2 and 6 were present in equal proportions overall, but differences in race frequency were apparent from year to year. Both races were found in all 3 years of the study from isolates collected during the survey (2012 and 2014) or from dry bean breeding plots and other fields that were not included in the survey (2012–2014), as well as in samples obtained from culture collections isolated in 2011 and earlier.
Table 5. Race characterization of Pseudomonas syringae pv. phaseolicola (Pph) isolates collected from commercial dry bean fields in western Canada in 2011–2014, as determined by reaction on the set of eight differential bean lines.
Copper sensitivity of Pph
Results of the copper sensitivity studies showed that the majority of halo blight isolates were sensitive to both copper sulphate and copper hydroxide. Of the 114 isolates tested, two were able to grow on medium amended with copper compounds. The isolates AB2012.004.1 and AB2012.004.12 both originated from the same commercial field in Alberta in 2012, and were tolerant to copper sulphate at concentrations up to 0.5 mM and to copper hydroxide at concentrations up to 1.0 mM (data not shown). Both isolates belonged to race 2. All other isolates did not grow at the lowest concentration (0.25 mM) tested.
Testing Canadian dry bean lines for halo blight resistance
Fifteen dry bean lines from the black, navy and pinto market classes with known resistance to common blight were screened for their response to halo blight race 2. All of the cultivars developed lesions on inoculated leaves, with characteristic yellow halos developing around each lesion. Halo blight severity indices ranged from 4.1 (susceptible) for the dark red kidney bean ‘Canadian Wonder’ to 3.5 (susceptible) for the navy bean ‘NA6-27-2ʹ (). There was no difference in the halo blight reactions among lines that are resistant or susceptible to CBB, and statistical analysis revealed no significant (P > 0.05) differences among the cultivars with respect to halo blight severity index.
Table 6. Response of common blight resistant dry bean breeding lines from the AAFC-Morden bean breeding programme to P. syringae pv. phaseolicola isolate AB2012.002 (race 2) collected in Alberta, as determined in greenhouse trials.
A second group of 26 dry bean genotypes was screened for their reaction to the same halo blight isolate. The genotypes were from the black, cranberry, great northern, dark red kidney, light red kidney, navy, pink, pinto, red and yellow bean market classes, and included cultivars that are currently in, or have recently been registered for, commercial production in Canada. Two dry bean germplasm lines 92BG-7 and I9365-31 (Miklas et al. Citation1998) were also included in the above list. Although none of the cultivars were fully resistant, a wide range of reactions to inoculation was observed. The halo blight severity indices ranged from 5.0 (highly susceptible) for the pinto bean cultivar ‘Othello’, to 1.8 (resistant) for the black bean cultivar ‘AAC Black Diamond 2ʹ (). Analysis of variance revealed significant (P < 0.0001) differences among cultivars with respect to halo blight severity, and 18 of the cultivars were less susceptible than the control cultivar ‘Canadian Wonder’. The cultivars with reduced susceptibility were from a variety of market classes.
Table 7. Halo blight disease reaction of selected bean genotypes from commonly grown commercial market classes to P. syringae pv. phaseolicola isolate AB2012.002 (race 2) collected in Alberta, as determined in greenhouse trials.
Discussion
Throughout the 3 years of surveying dry bean fields in western Canada, incidence and severity of bacterial blights were low, with highest ratings occurring in 2012, and low levels in 2013 and 2014. Halo blight and brown spot were the most prevalent bacterial blight pathogens isolated from symptomatic leaf and pod tissues. All bacterial blights are favoured by high humidity, provided by either rainstorms or irrigation, and by wounding of the foliage that occurs during hail or windstorms (Serfontein Citation1994). All dry bean fields in Alberta are irrigated frequently, thus providing ideal conditions for development of bacterial blights. Therefore, preference for different temperatures may be a primary factor distinguishing pathogen infection and disease development; Pph prefers temperatures lower than 25°C, Pss prefers warm temperatures lower than 30°C, while Xap and Xff thrive at temperatures greater than 30°C (Schwartz et al. Citation2005). Cff has the highest temperature preference, as it is most damaging at temperatures greater than 32°C (Schwartz et al. Citation2005). The diagnostic halo symptom resulting from the production of phaseolotoxin by Pph is only displayed at temperatures below 22°C (López-López et al. Citation2004). Therefore, average temperatures in 2012–2014 probably favoured halo blight and brown spot pathogens, as mean temperatures rarely exceeded 30°C in July and August in dry bean growing regions of southern Alberta. Despite favourable temperatures occurring for halo blight and brown spot development in July and August of 2013, very low levels of bacterial blights were observed in the surveyed fields.
It has been reported that common bacterial blight is the most prevalent bacterial disease in southern Alberta in surveys conducted prior to 2012 (Erickson & Balasubramanian Citation2008, Citation2010). However, Xap and Xff, the pathogens causing common bacterial blight, were rarely isolated from leaves that displayed characteristic symptoms of bacterial blights. Use of milk-tween agar to identify Xap and Xff does not allow differentiation between pathogenic and non-pathogenic Xanthomonas spp. (Goszczynska & Serfontein Citation1998). PCR identification of presumptive Xanthomonas-like isolates, based on morphology on MT agar, indicated that most were non-pathogenic Xanthomonas and Pseudomonas spp. which are common epiphytic bacteria (Gilbertson et al. Citation1990; Garrett & Schwartz Citation1998; Hirano & Upper Citation2000). These results were confirmed by pathogenicity testing. Similar findings were reported by Bett and Banniza (Citation2014), where a significant proportion (45%) of Xanthomonas spp. isolated from dry bean plants from across Canada were also non-pathogenic, and all five isolates collected from Alberta were non-pathogenic. They also reported that pathogenic Xap and Xff strains could not be differentiated from non-pathogenic strains on milk-tween agar, but that in most cases, X4c/X4e and Xf1/Xf2 markers were successful in identifying pathogenic Xap and Xff isolates, respectively. In our study, only five, or 9% of the 53 presumptive Xanthomonas isolates on milk-tween agar were positive for the Xff marker. This indicates that further research into Xanthomonas populations on dry bean in Alberta is needed to determine temporal dynamics and genetic variation of isolates causing CBB.
There are several reasons why bacterial blights remained low in the 3 years that bean crops were surveyed. Southern Alberta is typically a semi-arid environment. Therefore, relative humidity, when crops are not being irrigated, is low, thus limiting the spread of bacterial pathogens which are dependent on high humidity (Schwartz et al. Citation2005). Secondly, the majority of producers in southern Alberta plant certified seed produced in Idaho and Wyoming, USA, where the arid growing conditions reduce bacterial blights and seed-borne contamination (Duncan et al. Citation2014). Most fields contracted for dry bean production were also planted with seeds treated with agricultural streptomycin to reduce risk of spread of pathogens from seed to seedlings (Blair Roth, Viterra Bean Division, pers. communication). This is in contrast to other dry bean production areas in Canada (i.e. Ontario, Manitoba and Saskatchewan) where warmer temperatures and higher humidity are normal, and seed, which is not treated with streptomycin, is used. These conditions can result in much higher levels of common bacterial blight (Henriquez et al. Citation2012, Citation2014) than was observed in southern Alberta. Furthermore, many producers in southern Alberta use preventative copper sprays immediately after hail damage occurs, and during the flowering period in mid-July when environmental conditions are most favourable for bacterial blights (Jim Rex, Viterra Bean Division, pers. communication). Although producer spray records were not obtained in the surveys, copper residue on leaves was observed on many crops, indicating that spraying is a routine practice in southern Alberta.
When comparing methodologies to differentiate the five bacterial pathogens, isolation onto semi-selective milk-tween agar followed by PCR confirmation of bacteria cultures with species-specific primers was the most successful confirmation for Pss and Pph. Multiplex PCR amplification of target species directly from crude plant extracts often failed to detect Pph, or results were not always reproducible. However, this method was reliable for detection of Pss when compared with detection frequencies resulting from single colony PCR. Use of DNA extracted from symptomatic plant tissues in the multiplex PCR assay did not significantly improve detection results for Pph, nor did the use of a single primer-set for Pph in the PCR assay, rather than the multiplex reaction (data not shown). Isolations on agar media are time-consuming, and require several different semi-selective media to differentiate between the five bacterial pathogens of dry bean. For this reason, Cff was only detected in PCR performed with extracts from plant tissues, as reagents used in the selective media to identify Cff are highly specific (EPPO Citation2011). Therefore, the method of detection using multiplex PCR directly with plant extracts may be useful for the rapid detection of bacterial pathogens of dry bean, but this methodology requires further refinement in order to improve Pph, Xap and Xff detection.
In addition to determining predominant bacterial pathogens present in southern Alberta, the other primary objective of this study was to determine the prevalent races of Pph present in dry bean fields across the Prairie provinces. Few studies on the race structure of Pph in dry bean growing regions worldwide have been conducted since 1996, but a study conducted in Spain in 2003 reported that six races were present, with race 7 being the most prevalent, followed by races 6 and 1 (Rico et al. Citation2003). In contrast, only two races, 2 and 6, were detected from western Canada in this study. This is similar to the results from sampling studies of dry bean fields in North Dakota conducted during 1995–2002, where only races 2 and 6 were detected, but race 6 was predominant (Lamppa et al. Citation2002). Development of a detached leaf assay to rapidly screen a large number of isolates on the differential cultivar set provided very clear-cut and reproducible results. A similar method has been used previously to successfully screen dry bean cultivars for resistance to Sclerotinia sclerotiorum (Lib.) deBary and Botrytis cinerea Pers. (Huang et al. Citation2002). In order to screen a large number of isolates, Pph isolates were not only collected from surveyed bean fields in southern Alberta, but also from dry bean fields in Manitoba and Saskatchewan, as well as dry bean breeding and research plots in Alberta. Isolates collected prior to 2011, and present in culture or research collections in Alberta and Manitoba, were also included to determine if the race distribution had changed over time. Of the 114 isolates tested, all belonged to either race 2 or 6, with race 2 being predominant at 75% of total. This pattern remained consistent over time and location, suggesting that race structure has likely remained stable for the past several years. However, continual monitoring of race structure of Pph isolates from dry bean fields will be necessary to confirm this.
Pph isolates were also screened for their response to copper compounds, as copper bactericides are used extensively in dry bean production fields. Resistance to copper bactericides in P. syringae has been documented previously (Garrett & Schwartz Citation1998; Gutiérrez-Barranquero et al. Citation2013). Of the 114 isolates screened at varying copper concentrations, a very low percentage (1.7% or two isolates) was able to grow on copper-amended medium. Both isolates were collected from the same field on 15 and 27 August 2012, and were both race 2, indicating that resistance had developed in a population from only one field. This is a very low percentage of isolates in comparison to development of copper resistance in P. syringae from other cropping systems, particularly fruit tree production. Among Pss isolates from mango (Mangifera indica L.) plantations in Spain and Portugal, 59% were resistant to copper sulphate (Cazorla et al. Citation2002), while 40% of P. syringae isolates from almond (Prunus dulcis (Mill.) Webb) in California were resistant to copper (Andersen et al. Citation1991). However, a previous study on dry bean in 1998, showed that Pph developed resistance to copper compounds infrequently, probably due to the fact that only 2–4 applications of copper-based bactericides were made on dry bean in a season, compared with more than 6 applications on other horticultural crops (Garrett & Schwartz Citation1998). Therefore, selection pressure for development of copper resistance in dry bean fields is low. Nonetheless, a 1.7% occurrence does indicate that the potential for copper resistance in Pph populations on dry bean in southern Alberta exists, and that continuing resistance management by minimizing applications of this bactericide group is essential.
Dry bean lines that were bred for resistance to CBB were screened for their reactions to Pph race 2. It is anticipated that as development and release of cultivars with resistance to CBB increases, routine copper sprays will no longer be employed, potentially allowing halo blight to emerge as a more important disease. Although host resistance to multiple unrelated bacterial diseases is not expected, it is nonetheless important to screen cultivars with CBB resistance against Pph to determine the risk of halo blight epidemics. Results from this study indicated that almost all bean lines that have CBB resistance were susceptible to halo blight. Only the CBB-resistant cultivar ‘AAC Black Diamond 2ʹ showed resistance to Pph race 2, which was similar to its CBB-susceptible parent ‘AC Black Diamond’. Evaluation of cultivars recently grown in southern Alberta indicated that most cultivars are categorized as slightly susceptible to resistant to race 2 of halo blight, with the exception of ‘Winchester’ and ‘Myasi’, which were susceptible. The two most common cultivars, ‘AC Resolute’ and ‘AC Island’, comprising approximately 70% of the acreage in 2014 (Agriculture Financial Services Corporation Citation2015), both had slightly susceptible reactions. This reaction is typified by plants becoming infected, but disease not spreading throughout the plant (Innes et al. Citation1984), which could explain why halo blight disease severity levels remained low in surveyed fields while incidence slightly increased.
Bean lines and cultivars used in this study were only tested for their reaction to race 2, and not race 6, partly because race 2 was the predominant race in Alberta. Resistance reactions of P. vulgaris to Pph are either under qualitative or quantitative genetic control (Taylor et al. Citation1996b; Miklas et al. Citation2011; Duncan et al. Citation2014; Trabanco et al. Citation2014). For example, race-specific resistance to race 2 is conferred by the single dominant gene Pse-2 (Miklas et al. Citation2011). In contrast, race 6 is pathogenic on all of the differential genotypes, as well as other accessions and cultivars screened to date (Taylor et al. Citation1996b; Bozkurt & Soylu Citation2011); therefore, opportunities for breeding for qualitative resistance to race 6 are less likely than breeding for this type of resistance to race 2. However, resistance to race 6, controlled by two independently inherited recessive genes, was recently identified in the breeding line US14HBR6 (Duncan et al. Citation2014). As both races 2 and 6 are common in western Canada, any attempts to breed for resistance to Pph in Canadian-adapted lines will need to combine resistance to both races. Quantitative resistance to race 6 was demonstrated by Trabanco et al. (Citation2014) and Taylor et al. (Citation1996b), who showed that 21 out of 1048 bean accessions had a quantitative response to Pph infection, regardless of race-type. Several cultivars bred for adaptation in southern Alberta (e.g. ‘AAC Black Diamond 2ʹ, ‘AAC Burdett’, ‘Medicine Hat’ and ‘AC Island’) showed slightly susceptible to resistant responses to Pph, indicating that resistance is likely a quantitative trait, and not due to presence of Pse-2 gene, which confers resistance to race 2. Future studies will need to examine the reactions of the lines and cultivars tested in this study to race 6 to determine whether observed resistance levels to race 2 were due to a race-specific reaction, or to quantitative resistance, and if the latter, whether this quantitative resistance is also effective against race 6.
Acknowledgements
Additional bacterial isolates were kindly provided by Dr R.J. Howard, Alberta Agriculture and Rural Development. The technical support of C. Mueller and W.C. Penner is gratefully acknowledged.
Additional information
Funding
References
- Agriculture and Agri-Food Canada. 2010. Overview of the Canadian Pulse Industry 2009. Publication No. 10578E. 31 pp. Market Analysis and Information Section, Agriculture and Agri-Food Canada, Ottawa Ontario.
- Agriculture Financial Services Corporation. 2015. Yield Alberta 2015. Supplement to the Alberta Farmer Express, March 2, 2015. Farm Business Communications, Winnipeg Manitoba.
- Andersen G, Menkissoglou O, Lindow S. 1991. Occurence and properties of copper-tolerant strains of Pseudomonas syringae isolated from fruit tress in California. Phytopathology. 81:648–656.
- Arnold DL, Lovell HC, Jackson RW, Mansfield JW. 2011. Pseudomonas syringae pv. phaseolicola: from ‘has bean’ to supermodel. Mol Plant Pathol. 12:617–627.
- Audy P, Braat CE, Saindon G, Huang HC, Laroche A. 1996. A rapid and sensitive PCR-based assay for concurrent detection of bacteria causing common and halo blights in bean seed. Phytopathology. 86:361–366.
- Bailey KL, Gossen BD, Gugel RK, Morrall RAA. 2003. Diseases of field crops in Canada. Saskatoon, SK.: University Extension Press, University of Saskatchewan.
- Balasubramanian PM, Mündel HH, Conner RL, Chatterton S, Hou A. 2015. AAC black diamond 2 dry bean. Can J Plant Sci. 95:437–440.
- Bett KE, Banniza S. 2014. Population study of Xanthomonas spp. from bean growing regions of Canada and response of bean cultivars to pathogen inoculation. Can J Plant Pathol. 36:341–353.
- Boersma J, Hou A, Gillard C, McRae KB, Conner RL. 2015. Impact of common bacterial blight on the yield, seed weight and seed discoloration of different classes of dry beans (Phaseolus vulgaris L.). Can J Plant Sci. 95:703–710.
- Boersma JG, Conner RL, Balasubramanian PM, Navabi A, Yu K, Hou A. 2014. Combining resistance to common bacterial blight, anthracnose, and bean common mosaic virus into Manitoba-adapted dry bean (Phaseolus vulgaris L.) cultivars. Can J Plant Sci. 94:405–415.
- Boodley JW, Sheldrake R Jr. 1977. Cornell peat-lite mixes for commercial plant growing. New York State College of Agriculture and Life Sciences Information Bulletin 43. 8 pp. Cornell University, Ithaca, New York.
- Bozkurt IA, Soylu S. 2011. Determination of responses of different bean cultivars against races of Pseudomonas syringae pv. phaseolicola, causal agent of halo blight of bean. Euphytica. 179:417–425.
- Broughton WJ, Hernández G, Blair M, Beebe S, Gepts P, Vanderleyden J. 2003. Beans (Phaseolus spp.) - model food legumes. Plant Soil. 252:55–128.
- Cafati CR, Saettler AW. 1980. Role of nonhost species as alternate inoculum sources of Xanthomonas phaseoli. Plant Dis. 64:194–196.
- Cazorla FM, Arrebola E, Sesma A, Pérez-García A, Codina JC, Murillo J, De Vicente A. 2002. Copper resistance in Pseudomonas syringae strains isolated from mango is encoded mainly by plasmids. Phytopathology. 92:909–916.
- Duncan RW, Gilbertson RL, Lema M, Singh SP. 2014. Inheritance of resistance to the widely distributed race 6 of Pseudomonas syringae pv. phaseolicola in common bean pinto US14HBR6. Can J Plant Sci. 94:923–928.
- Duncan RW, Gilbertson RL, Singh SP. 2012. Direct and marker-assisted selection for resistance to common bacterial blight in common bean. Crop Sci. 52:1511–1521.
- EPPO. 2011. Curtobacterium flaccumfaciens pv. flaccumfaciens. EPPO Bulletin. 41:320–328.
- Erickson RS, Balasubramanian P. 2010. Survey of diseases of dry bean in southern Alberta in 2009. Can Plant Dis Surv. 90:121–122.
- Erickson RS, Balasubramanian PM. 2008. Survey of diseases of dry bean in southern Alberta in 2007. Can Plant Dis Surv. 88:99.
- Erickson RS, Chatterton S. 2012. Survey of diseases of dry bean in southern Alberta in 2011. Can Plant Dis Surv. 92:118–119.
- Garrett KA, Schwartz HF. 1998. Epiphytic Pseudomonas syringae on dry beans treated with copper-based bactericides. Plant Dis. 82:30–35.
- Gent DH, Lang JM, Schwartz HF. 2005. Epiphytic survival of Xanthomonas axonopodis pv. allii and X. axonopodis pv. phaseoli on leguminous hosts and onion. Plant Dis. 89:558–564.
- Gilbertson RL, Rand RE, Hagedorn DJ. 1990. Survival of Xanthomonas campestris pv. phaseoli and pectolytic strains of X. campestris in bean debris. Plant Dis. 74:322–327.
- Goodwin PH. 1992. Effect of common bacterial blight on leaf photosynthesis of bean. Can J Plant Pathol. 14:203–206.
- Goszczynska T, Serfontein JJ. 1998. Milk-Tween agar, a semiselective medium for isolation and differentiation of Pseudomonas syringae pv. syringae, Pseudomonas syringae pv. phaseolicola and Xanthomonas axonopodis pv. phaseoli. J Microbiol Meth. 32:65–72.
- Gutiérrez-Barranquero JA, de Vicente A, Carrión VJ, Sundin GW, Cazorla FM. 2013. Recruitment and rearrangement of three different genetic determinants into a conjugative plasmid increase copper resistance in Pseudomonas syringae. Appl Environ Microbiol. 79:1028–1033.
- Henriquez MA, McLaren DL, Conner RL, Penner WC, Kerley T. 2012. Diseases of field bean in Manitoba in 2011. Can Plant Dis Surv. 92:120–121.
- Henriquez MA, McLaren DL, Conner RL, Penner WC, Kerley TJ. 2014. Diseases of field bean in Manitoba in 2013. Can Plant Dis Surv. 94:154–155.
- Hirano SS, Upper CD. 2000. Bacteria in the leaf ecosystem with emphasis on Pseudomonas syringae—a pathogen, ice nucleus, and epiphyte. Microbiol Mol Biol Rev. 64:624–653.
- Howard RJ, Huang HC. 1983. Studies of pulse crop diseases in southern Alberta in 1982: survey of commercial fields of dry beans for white mold disease. Brooks, AB: Alta Hort Res Cent., Alta Agric; p. 20.
- Huang HC, Muendel -H-H, Saindon G, Erickson RS, Chang M-H. 2002. Physiological resistance of dry bean (Phaseolus vulgaris L.) to Sclerotinia sclerotiorum (Lib.) de Bary and Botrytis cinerea Pers.:Fr. Rev Mex Fitopatol. 20:182–186.
- Innes NL, Conway J, Taylor JD. 1984. Resistance to halo-blight in the Cambridge accessions V4604 and V4058 of Phaseolus beans. Ann Appl Biol. 104:307–314.
- International Seed Testing Association. 2007. 7-021: Detection of Xanthomonas axonopodis pv. phaseoli and Xanthomonas axonopodis pv. phaseoli var. fuscans on Phaseolus vulgaris. Bassersdorf, Switzerland: ISTA.
- Jensen MA, Webster JA, Straus N. 1993. Rapid identification of bacteria on the basis of polymerase chain reaction-amplified ribosomal DNA spacer polymorphisms. Appl Environ Microbiol. 59:945–952.
- King EO, Ward MK, Raney DE. 1954. Two simple media for the demonstration of pyocyanin and fluorescein. J Lab Clin Med. 44:301–307.
- Lamppa RS, Gross PL, del Río LE. 2002. Identification of races of Pseudomonas syringae pv. phaseolicola present in North Dakota. Phytopathology. 92:S139 (abstr.).
- López-López K, Hernández-Flores JL, Cruz-Aguilar M, Alvarez-Morales A. 2004. In Pseudomonas syringae pv. phaseolicola, expression of the argK gene, encoding the phaseolotoxin-resistant ornithine carbamoyltransferase, is regulated indirectly by temperature and directly by a precursor resembling carbamoylphosphate. J Bacteriol. 186:146–153.
- Michaels TE, Smith TH, Larson J, Beattie AD, Pauls KP. 2006. OAC Rex common bean. Can J Plant Sci. 86:733–736.
- Miklas PN, Fourie D, Trapp J, Larsen RC, Chavarro C, Blair MW, Gepts P. 2011. Genetic characterization and molecular mapping Pse-2 gene for resistance to halo blight in common bean. Crop Sci. 51:2439–2448.
- Miklas PN, Grafton KF, Kelly JD, Steadman JR, Silbernagel MJ. 1998. Registration of four white mold resistant dry bean germplasm lines: I9365-3, I9365-5, I9365-31 and 92BG-7. Crop Sci. 38:1728.
- Navabi A, Rupert T, Park SJ, Yu K, Smith TH, Pauls KP. 2013. Apex common bean. Can J Plant Sci. 93:131–135.
- Parkinson N, Aritua V, Heeney J, Cowie C, Bew J, Stead D. 2007. Phylogenetic analysis of Xanthomonas species by comparison of partial gyrase B gene sequences. Int J Syst Evol Microbiol. 57:2881–2887.
- Rico A, López R, Asensio C, Aizpún MT, Asensio -S, Manzanera MC, Murillo J. 2003. Nontoxigenic strains of Pseudomonas syringae pv. phaseolicola are a main cause of halo blight of beans in Spain and escape current detection methods. Phytopathology. 93:1553–1559.
- Rodríguez-Moreno L, Pineda M, Soukupová J, Macho AP, Beuzón CR, Barón M, Ramos C. 2008. Early detection of bean infection by Pseudomonas syringae in asymptomatic leaf areas using chlorophyll fluorescence imaging. Photosynthesis Res. 96:27–35.
- SAS Institute Inc. 2004. Statistical Analysis Software, version 9.1.3. Cary, North Carolina.
- Schwartz HF, Steadman JR, Hall R, Forster RL. 2005. Compendium of bean diseases. 2nd ed. APS Press, The American Phytopathological Society. St. Paul, Minnesota.
- Serfontein JJ. 1994. Occurrence of bacterial brown spot of dry beans in the Transvaal province of South Africa. Plant Pathol. 43:597–599.
- Smith TH, Michaels TE, Navabi A, Pauls KP. 2012. Rexeter common bean. Can J Plant Sci. 92:351–353.
- Sorensen KN, Kim K-H, Takemoto JY. 1998. PCR detection of cyclic lipodepsinonapeptide-producing Pseudomonas syringae pv. syringae and similarity of strains. Appl Environ Microbiol. 64:226–230.
- Stevens C, Bennett MA, Athanassopoulos E, Tsiamis G, Taylor JD, Mansfield JW. 1998. Sequence variations in alleles of the avirulence gene avrPphE.R2 from Pseudomonas syringae pv. phaseolicola lead to loss of recognition of the AvrPphE protein within bean cells and a gain in cultivar-specific virulence. Mol Microbiol. 29:165–177.
- Stockwell V, Duffy B. 2012. Use of antibiotics in plant agriculture. Rev Sci Tech. 31:199–210.
- Taylor JD, Dudley CL. 1979. Studies of halo blight seed infection and disease transmission in dwarf beans. Ann Appl Biol. 93:267–277.
- Taylor JD, Teverson DM, Allen DJ, Pastor-Corrales MA. 1996a. Identification and origin of races of Pseudomonas syringae pv. phaseolicola from Africa and other bean growing areas. Plant Pathol. 45:469–478.
- Taylor JD, Teverson DM, Davis JHC. 1996b. Sources of resistance to Pseudomonas syringae pv. phaseolicola races in Phaseolus vulgaris. Plant Pathol. 45:479–485.
- Tegli S, Sereni A, Surico G. 2002. PCR-based assay for the detection of Curtobacterium flaccumfaciens pv. flaccumfaciens in bean seeds. Lett Appl Microbiol. 35:331–337.
- Toth IK, Hyman LJ, Taylor R, Birch PRJ. 1998. PCR-based detection of Xanthomonas campestris pv. phaseoli var. fuscans in plant material and its differentiation from X. c. pv. phaseoli. J Appl Microbiol. 85:327–336.
- Trabanco N, Asensio-Manzanera MC, Pérez-Vega E, Ibeas A, Campa A, Ferreira JJ. 2014. Identification of quantitative trait loci involved in the response of common bean to Pseudomonas syringae pv. phaseolicola. Mol Breed. 33:577–588.
- Tsiamis G, Mansfield JW, Hockenhull R, Jackson RW, Sesma A, Athanassopoulos E, Bennett MA, Stevens C, Vivian A, Taylor JD, Murillo J. 2000. Cultivar-specific avirulence and virulence functions assigned to avrPphF in Pseudomonas syringae pv. phaseolicola, the cause of bean halo-blight disease. EMBO J. 19:3204–3214.
- Vanneste J, Voyle M, Zydenbos S. 2003. Genetic basis of copper resistance in New Zealand strains of Pseudomonas syringae. N Z Plant Prot. 56:109–112.
- Vidaver AK. 2002. Uses of antimicrobials in plant agriculture. Clin Infect Dis. 34:S107–S110.
- Walburger AM, McKenzie RH, Seward K, Ulrich K. 2004. The economics of sustainable agriculture alternatives on the semiarid Canadian prairies: the case for legumes and organic amendments. J Sustainable Agric. 24:51–69.
- Wang Q, Garrity GM, Tiedje JM, Cole JR. 2007. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 73:5261–5267.
- Weller DM, Saettler AW. 1980. Evaluation of seedborne Xanthomonas phaseoli and Xanthomonas phaseoli var. fuscans as primary inocula in bean blights. Phytopathology. 70:148–152.
- Widmer F, Seidler RJ, Gillevet PM, Watrud LS, Di Giovanni GD. 1998. A highly selective PCR protocol for detecting 16S rRNA genes of the genus Pseudomonas (sensu stricto) in environmental samples. Appl Environ Microbiol. 64:2545–2553.
- Yu K, Park S, Zhang B, Haffner M, Poysa V. 2004. An SSR marker in the nitrate reductase gene of common bean is tightly linked to a major gene conferring resistance to common bacterial blight. Euphytica. 138:89–95.