Abstract
A leaf spot disease caused by Phoma herbarum was observed in a plantation of cherry palm (Pseudophoenix sargentii) in Chiang Mai Province, Thailand. The fungus was isolated from lesions on leaves and pathogenicity tests showed that Ph. herbarum could infect P. sargentii, producing the same symptoms after inoculation as those observed naturally in the field. The fungus was identified based on morphological characteristics and confirmed using comparisons of DNA sequences of internal transcribed spacer (ITS)1, 2 and 5.8S rDNA. This is the first report of this disease on cherry palm.
Résumé
Une tache des feuilles, causée par Phoma herbarum, a été observée dans une plantation de palmier boucanier (Pseudophoenix sargentii) de la province de Chiang Mai, en Thaïlande. Le champignon a été isolé à partir de lésions trouvées sur des feuilles, et des tests de pathogénicité ont montré que P. herbarum pouvait infecter P. sargentii, produisant les mêmes symptômes après inoculation que ceux observés naturellement sur le terrain. Le champignon a été identifié sur la base des caractéristiques morphologiques et l’espèce a été confirmée par comparaison des régions 1 et 2 de l’espaceur transcrit interne (ITS) de l’ADN et de l’ADNr 5,8S. Il s’agit de la première mention de cette maladie sur le palmier boucanier.
Introduction
Cherry palm (Pseudophoenix sargentii H. Wendl. ex Sarg.) is a medium-sized palm which is native to Bahamas, Belize, Cuba, Dominica, Navassa Island, South Florida, USA and the Caribbean coast of Mexico (Nelson Citation1994; Henderson et al. Citation1995). Buccaneer palm, Florida cherry palm and Sargent’s cherry palm are other common names applied to this palm. It is highly tolerant to wind and salt, and grows well in warm temperate and tropical climates (Squire Citation2007). Nowadays, cherry palm is cultivated in the speciality horticulture trade as an ornamental palm and is an economically important plant (Henderson et al. Citation1995; Squire Citation2007). In Thailand, areas cultivated with cherry palm have increased, including private gardens, habitat gardens, and various municipal and commercial landscapes. However, as more plantations are established on less suitable sites, incidence and severity of diseases have increased.
Generally, diseases caused by phytoplasma species and Ganoderma butt rot disease caused by Ganoderma species were considered as the major diseases of ornamental palms. Leaf blight and leaf spot diseases caused by fungi are considered to be minor diseases; however, fungal infections can spread rapidly (Elliott Citation2005; Harrison et al. Citation2008; Downer et al. Citation2009; Hodel Citation2009; Vázquez-Euán et al. Citation2011) and these diseases affect both the seedling and mature stages of palms. Leaf blight and leaf spots in ornamental palm are caused by species of Alternaria, Annellophora, Bipolaris, Cercospora, Calonectria (Cylindrocladium), Exserohilum, Gliocladium, Pestalotiopsis, Phoma, Phyllachora, Pseudocercospora and Stigmina (Elliott et al. Citation2004; Elliott Citation2005; El-Deeb et al. Citation2007). There have been no prior reports on leaf spot of cherry palm in Thailand or elsewhere in the world. The aim of this study was to determine the aetiological agent of cherry palm leaf spot disease observed in northern Thailand.
Materials and methods
Sample collection and fungal isolation
Mature cherry palm leaves showing typical symptoms were collected from a palm plantation in Chiang Mai Province, Thailand (18°18ʹ16″N 98°21ʹ48′E, elevation 748 m) during July 2015. Samples were collected from two sites. Ten symptomatic leaves from 10 palms were kept in a plastic box with wet filter paper to induce sporulation. The single spore isolation method described by Choi et al. (Citation1999) was used. Conidial masses were suspended in 500 μL of sterile distilled water on sterile glass slides. The spore suspension was dropped onto 1.5% (w/v) water agar containing 0.5 mg L−1 of streptomycin sulphate. After 24 h incubation at 25°C, individual germinating conidia were selected and transferred directly to potato dextrose agar (PDA) and subcultured on fresh PDA. One fungal isolate (CMU-PP05) was selected and deposited in the Research Laboratory for Excellence in Sustainable Development of Biological Resources (SDBR), Faculty of Science, Chiang Mai University, Thailand.
Fungal identification
Pure fungal isolate CMU-PP05 was grown on PDA at 25 ± 2°C in darkness for 3 weeks. Conventional morphological characters were used to tentatively identify the fungus isolate (Ellis Citation1971; Boerema et al. Citation2004). The morphological descriptions were based on at least 50 measurements of each structure under a light microscope (Olympus CX51, Japan). Molecular methods were used to confirm the identity of the isolate CMU-PP05. The fungus was grown on PDA at 25 ± 2°C in darkness for 1 week and genomic DNA was extracted using the SDS-CTAB method described by Kumla et al. (Citation2012). The internal transcribed spacer (ITS) region of ribosomal RNA gene was amplified by polymerase chain reaction (PCR) with ITS4 and ITS5 primers (White et al. Citation1990) under the following thermal conditions: 94°C for 2 min; 35 cycles of 95°C for 30 s, 50°C for 30 s, 72°C for 1 min; and 72°C for 10 min. PCR products were checked on 1% agarose gels stained with ethidium bromide under UV light and purified using NucleoSpin® Gel and PCR Clean-up Kit (Macherey-Nagel, Germany) following the manufacturer’s protocol. The purified PCR products were directly sequenced. Sanger sequencing was performed by 1ST Base Company (Kembangan, Malaysia) using the same PCR primers mentioned above. Sequences were manually trimmed and edited with Sequencher 4.0 (Gene Codes Corp., USA) and used to query via BLAST (http://blast.ddbj.nig.ac.jp/top-e.html).
Pathogenicity test
To confirm pathogenicity, the surfaces of 10 healthy P. sargentii leaves were disinfected with 70% ethanol (Sezer & Dolar Citation2012) and then placed in a plastic box with wet sterile filter paper. A conidial suspension of the fungus grown on PDA at 25 ± 2°C in darkness for 3 weeks was adjusted to 15 × 106 conidia mL−1 and 20 μL of the suspension were dropped onto the leaves. One drop was applied on each leaf and sterile distilled water was used as a control. The plastic boxes were stored in a growth chamber under 12 h light with fluorescent lamps at 25°C and 70% relative humidity for 3 weeks. The fungus was re-isolated from the disease tissues by the single spore isolation method (Choi et al. Citation1999) to confirm Koch’s postulates. The test was repeated twice.
Results and discussion
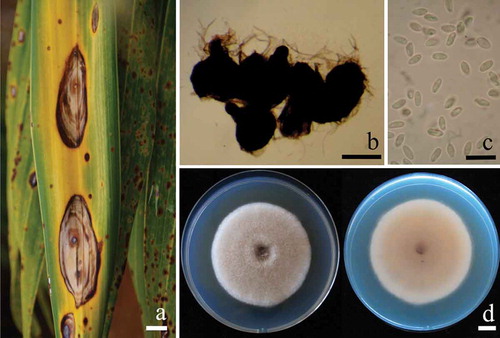
The initial symptoms of the disease appear as dark spots that gradually increased from 0.5 to 1.5 mm in diameter, changing from circular to elliptical lesions. Leaf spots were dark brown to black, surrounded with a yellow to rust brown zone. As lesions expanded, they became tan or grey in the centre, surrounded by a brownish zone (). In a humid environment, the infected area of leaf tissue contained blackish pycnidia from which masses of conidia were released. Pycnidia were subglobose, brown to dark brown, ostiolate and 100–250 μm in diameter (). The pycnidial wall was pseudoparenchymatous, composed of oblong to isodiametric cells, 3–4 layers, 5–9 μm in diameter. Conidiogenous cells were phialidic, hyaline, smooth, 3–4 × 2–3 μm. Conidia were hyaline, en masse hyaline to pinkish, oblong to ellipsoidal, unicellular, mostly eguttulate but sometimes with small inconspicuous polar guttules, 3–5 × 1–2 μm (). Fungal colonies on PDA grew to 50–60 mm in diameter within 1 week at room temperature (25 ± 2°C). The colonies were downy to woolly, with a regular margin, greyish surface initially and olive brown later, producing pale yellowish brown diffusible pigment. The reverse side was initially light brown (). Mycelia turned purplish-blue instantaneously after application of a drop 1N NaOH. Pycnidia and conidia were observed on PDA after 3 weeks of incubation at 25 ± 2°C in darkness. The fungus was initially identified as Phoma herbarum Westend. (Morgan-Jones Citation1998; Boerema et al. Citation2004) based on these morphological characteristics.
Fig. 1a, (Colour online) Natural symptoms of Pseudophoenix sargentii leaf spot caused by Phoma herbarum. b, pycnidia of Ph. herbarum taken from P. sargentii leaf. c, conidia of Ph. herbarum. d, 1-week old colonies of Ph. herbarum CMU-PP05 on PDA viewed from both the top side (left) and bottom side (right). Scale bars: a = 10 mm; b = 100 μm; c = 10 μm; d = 10 mm.
The partial ITS1, ITS2 and 5.8S rDNA sequence of this fungus, containing 510 bp, was deposited in GenBank as KU058400. BLAST searches of the database showed that this sequence had 99% similarity with Ph. herbarum strain BLE15 (FN868459) and strain Js 6551 (KT004577). Therefore, the fungus that caused leaf spot disease on cherry palm was identified as Ph. herbarum, based on both morphological and molecular characteristics.
For the pathogenicity test, the symptoms seen in the plantation were observed on inoculated P. sargentii leaves following 3 weeks of incubation. The same fungus was re-isolated from the disease lesions and cultured on PDA to complete Koch’s postulates. Prior to this research, no leaf spot disease has been reported on cherry palm. Therefore, we propose to add Phoma leaf spot disease caused by Ph. herbarum as a new disease on cherry palm.
Acknowledgements
This work was supported by grants from the Thailand Research Fund for Research Team Association Grant RTA5880006, and Chiang Mai University, Thailand. We are grateful to Mr Santhiti Vadthanarat for taking the field photographs and Mr Keegan Kennedy for improving the English text.
References
- Boerema GH, de Gruyter J, Noordeloos ME, Hamers MEC. 2004. Phoma identification manual. Differentiation of specific and infra-specific taxa in culture. Oxfordshire: CABI Publishing. CAB International.
- Choi YW, Hyde KD, Ho WWH. 1999. Single spore isolation of fungi. Fungal Divers. 3:29–38.
- Downer AJ, Uchida JY, Hodel DR, Elliott ML. 2009. Lethal palm diseases common in the United States. HortTechnol. 19:710–716.
- El-Deeb HM, Lashin SM, Arab YA. 2007. Distribution and pathogenesis of date palm fungi in Egypt. Acta Hortic. 736:421–429.
- Elliott ML. 2005. Leaf spots and leaf blights of palm. Gainesville: Plant Pathology Department, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences. University of Florida.
- Elliott ML, Broschat TK, Uchida JY, Simone GW. 2004. Compendium of ornamental palm disease and disorders. Minnesota: APS Press.
- Ellis MB. 1971. Dematiaceous hyphomycetes. London: Commonwealth Mycological Institute.
- Harrison NA, Helmick EE, Elliott ML. 2008. Lethal yellowing-type diseases of palms associated with phytoplasmas newly identified in Florida, USA. Ann Appl Biol. 153:85–94.
- Henderson A, Galeano G, Bernal R. 1995. Field guide to the palms of the Americas. New Jersey: Princeton University Press.
- Hodel DR. 2009. Palms in the landscape. Diseases Part I. Western Arborist. 35:12–20.
- Kumla J, Suwannarach N, Bussaban B, Lumyong S, Danell E. 2012. Basidiome formation of an edible wild, putatively ectomycorrhizal fungus, Phlebopus portentosus without host plant. Mycologia. 104:597–603.
- Morgan-Jones G. 1998. Studies in the genus Phoma. XIV. Concerning Phoma herbarum, the type species, a widespread saprophyte. Mycotaxon. 33:81–90.
- Nelson G. 1994. The trees of Florida. Florida: Pineapple Press.
- Sezer A, Dolar FS. 2012. Colletotrichum acutatum, a new pathogen of hazelnut. J Phytopathol. 160:428–430.
- Squire D. 2007. Palms and cycads: a complete guide to selecting, growing and propagating. Chicago: Chicago Review Press.
- Vázquez-Euán R, Harrison N, Narvaez M, Oropeza C. 2011. Occurrence of a 16SrIV group phytoplasma not previously associated with palm species in Yucatan, Mexico. Plant Dis. 95:256–262.
- White TJ, Bruns TD, Lee S, Taylor JW. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: A guide to methods and applications. San Diego: Academic; p. 315–322.
