Abstract
Potato cyst nematodes are a major threat to potato production worldwide. In 2006, the isolation of the golden nematode, Globodera rostochiensis, in Quebec, Canada, led to the establishment of a quarantine area and the initiation of a research programme for the sustainable management of this regulated pest. In this study, the field efficacy of crop rotations and soil amendments was assessed in microplots for their potential to reduce populations of G. rostochiensis in the quarantine area. Crop rotation with a resistant potato cultivar was very effective in decreasing the population density of G. rostochiensis in soil. A single year with a cultivar carrying the H1 resistance gene reduced nematode populations by 62–95%. After 3 consecutive years of cropping to resistant potato, the number of viable eggs was zero in several microplots, and the overall population reduction was around 95%. Natural population decline with the cultivation of a non-host crop such as corn was around 30% per year. Trap cropping also showed reduced G. rostochiensis populations with results similar to the resistant cultivar. Sticky nightshade could not be established under Quebec’s climatic conditions and therefore was not a viable alternative for managing golden nematode in Quebec. Soil amendment with high rates of urea provided some reduction in populations but was not an economically viable option for managing potato cyst nematodes. Chicken manure and pig slurry did not provide a significant reduction of G. rostochiensis under Quebec field conditions.
Résumé
Les nématodes à kyste de la pomme de terre sont une menace majeure pour cette production à travers le monde. En 2006, la découverte du nématode doré, Globodera rostochiensis, au Québec (Canada), a mené à la mise en place d’une zone de quarantaine et au lancement d’un programme de recherche pour la gestion durable de ce ravageur réglementé. L’objectif de cette étude était d’évaluer l’efficacité de programmes de rotation des cultures et d’amendements de sol pour leur potentiel à réduire les populations de G. rostochiensis dans les conditions climatiques et les types de sol du Québec. L’utilisation d’un cultivar de pomme de terre résistant a été très efficace afin de réduire les densités de population de G. rostochiensis dans le sol. Une seule année de rotation avec un cultivar portant le gène de résistance H1 a permis une réduction allant de 62 à 95% des populations de nématodes. Après trois années consécutives de ce traitement, le nombre d’œufs viables était sous le niveau de détection dans plusieurs microparcelles avec une réduction globale d’environ 95%. Le déclin naturel des populations lors de la culture de plantes non-hôtes comme le maïs a été évalué à 30% par année et était linéaire lors de rotations pluriannuelles. Les cultures pièges ont également démontré un bon potentiel pour le contrôle des populations de G. rostochiensis avec des résultats similaires à la pomme de terre résistante. En revanche, la Morelle de Balbis n’est pas une alternative viable pour la gestion du nématode doré au Québec. Cette culture a fourni un niveau de contrôle, similaire à celui de la pomme de terre résistante mais n’a pas pu être établie par semis dans des conditions climatiques du Québec. Les amendements de sol avec de l’urée ont fourni un bon niveau de contrôle mais ne représentent pas une option économiquement viable. Les amendements avec du fumier de poulet ou du lisier de porc n’ont pas permis de réduire les populations de nématodes à kystes de la pomme de terre de façon satisfaisante.
Introduction
The potato cyst nematodes (PCN) – Globodera rostochiensis and G. pallida – remain the most important nematode threat to potato production worldwide (Turner & Evans Citation1998). In most countries, including Canada and the USA, PCN are quarantine organisms, and many efforts are being made by inspection agencies to contain them and to avoid any dispersal. In 2006, golden nematode, G. rostochiensis, was found in Quebec, Canada (Sun et al. Citation2007). In 2007 and 2009, new sampling in 11 representative infested fields confirmed that all the cysts were G. rostochiensis pathotype Ro1 (Mahran et al. Citation2010). For different reasons (shallow water table, proximity of residential homes, etc.) the regulatory agency did not try to eradicate this population with soil fumigation, as was done in British Columbia, Canada (Rott et al. Citation2010), and more recently in Idaho, USA (Animal and Plant Health Inspection Service Citation2009). Instead, it was decided to develop a more sustainable management programme using crop rotations and to test alternative practices to reduce the population densities.
Over the past decades, numerous cultural methods have been investigated for managing PCN, such as rotation with non-host plants, use of resistant varieties, cover crops, green manure, keeping fields fallow, and the application of various soil amendments (Brodie Citation1982; Barker & Koenning Citation1998). Past experiments have shown that a single year of rotation with a non-host crop or fallow soil was sufficient to reduce the number of viable eggs under field conditions by 15–35% (Rawsthorne & Brodie Citation1986). Trap cropping or early harvesting can also be used to reduce the PCN population by simply destroying it before the nematodes have reached maturity (LaMondia & Brodie Citation1986; Whitehead & Turner Citation1998; Scholte & Vos Citation2000). However, this method requires very good knowledge of the PCN life cycle and the variations that can occur each year, so that nematode reproduction and population increases can be avoided (Ebrahimi et al. Citation2014). In Quebec, it was demonstrated that the proper timing for destroying potato trap crops was between 28 and 42 days after planting, depending on heat accumulation, at the appearance of white females on roots (Mimee et al. Citation2015).
In the Netherlands, sticky nightshade (Solanum sisymbriifolium) has been used as an immune host plant to induce hatching of Globodera spp. without any reproduction (Timmermans et al. Citation2006). Green manures of Brassicaceae plants, such as rapeseed and brown mustard, have received much attention as biofumigants. The decomposition of these plants after ploughing produces precursor compounds of allyl isothiocyanates, which are reported to have some in vitro toxicity against G. pallida (Avato et al. Citation2013; Brolsma et al. Citation2014). However, obtaining effective concentrations of these compounds in the field could be more complicated (Brolsma et al. Citation2014). Soil amendments with N sources (urea, chicken manure) have also been reported to have deleterious effects on plant-parasitic nematodes (Mian & Rodríguez-Kábana Citation1982). However, the incorporation of this matter into the soil can cause new problems and has to be monitored closely to avoid phytotoxicity (Sasanelli et al. Citation2006; Renčo et al. Citation2011).
The objective of this study was to assess the field efficacy of crop rotations and soil amendments for their potential to reduce populations of G. rostochiensis in the climatic conditions and soil types found in the PCN quarantine areas in Quebec, Canada.
Materials and methods
Sites and cropping systems
All the experiments were carried out in microplots (1 × 2 m) established in two quarantine areas of the province of Quebec, Canada, from 2009 to 2012. Two sites that had different soil textures and were naturally infested with high populations of G. rostochiensis were used. The first site, located in St-Amable, had a loamy sand texture, and the second site, located in St-Dominique, had a muck soil. The initial numbers of viable eggs per gram of soil for each site and year are presented in . Unless otherwise indicated, all the trials were repeated on both sites over 4 years with the same design, which comprised eight microplots (replicates) per treatment. The cultivars used were ‘Snowden’ for susceptible potato, ‘Andover’ for resistant potato (carrying the H1 gene, resistance level 9 according to EPPO Citation2006), genotype ‘4004ʹ for sticky nightshade, Pioneer ‘38M58 RR’ for corn, AERC Inc. ‘CFPM-101ʹ for forage pearl millet, and ‘Caliente’ for brown mustard. The crops were fertilized according to the recommendations in the reference fertilization guide (Centre de référence en agriculture et agroalimentaire du Québec Citation2011). The soil was tilled before planting and spring sampling. Weed control was performed manually three or four times during the growing season. An insecticide treatment (Admire® from Bayer Crop Science and Coragen™ from DuPont Canada) were applied following label recommendations once to manage Colorado potato beetle when actively feeding larvae appeared.
Table 1. Average number of initial viable eggs per gram of soil (range) in sandy soil (St-Amable) and muck soil (St-Dominique).
Rotations and trap cropping
The potential of rotation with resistant potato or non-host plants and of trap cropping for reducing population densities of G. rostochiensis was evaluated. Six rotation plants (susceptible potato, resistant potato, corn, nightshade, pearl millet and mustard) were compared in 1-year programmes (repeated three times from 2010 to 2012 in different microplots) or programmes lasting 3 consecutive years (repeated twice from 2009 to 2011 and 2010 to 2012 in different microplots). Brown mustard was cut and buried at full bloom. Sticky nightshade was sown in a greenhouse in trays in April of each year and 2-month-old plants were transplanted in early June or at the start of the frost-free period into three staggered rows (33 cm between rows) at a density of 18 plants per microplot (90 000 plants ha−1). For trap cropping, susceptible potato was planted at the standard density of six plants per microplot and pulled out after 5, 6, 7 or 8 weeks. Rye was grown for the remainder of the season. In addition, two or three successive plantings of susceptible potato pulled out after 5 weeks or of susceptible potato planted at a high density of 50 plants per microplot, pulled out after 5 weeks, and followed by rye were also compared with resistant potato (planted at standard or high density).
Soil amendments
Different soil amendments were tested for their ability to reduce G. rostochiensis populations (in loamy sand soil only). All the amendments were applied to corn. Urea (46-0-0) was applied at 2 or 4 t ha−1, fresh chicken manure was applied at 15 or 30 t ha−1, and pig slurry (solid fraction) was applied at 5 or 10 t ha−1. These products were distributed uniformly on the soil surface and mixed to a depth of 10 cm with a rototiller. For urea, each microplot was sprinkled with 45 L of water (1.3 mm). To avoid phytotoxicity, planting was delayed for 4 weeks after urea treatments and 2 weeks after chicken manure and pig slurry treatments. No further fertilization was applied to the microplots that received these latter treatments. The corn treated with urea was fertilized with phosphorus and potassium only.
Soil sampling, extraction method and analysis
Populations of G. rostochiensis were assessed in both the spring and autumn of each year. Before seeding and after harvesting, 16 sub-samples per microplot were collected to a depth of 0–20 cm using a trowel. After drying, 300 cm3 of soil was measured for each composite sample and used for cyst extraction using the Fenwick can procedure (Fenwick Citation1940). The number of cysts, egg viability rate (%) and reproduction rate (Pf/Pi) were calculated. Egg viability was assessed on recovered cysts (max. 50 cysts) deposited in a 1.5-mL microtube containing 1 mL of tap water. Using a pellet pestle, the cysts were ruptured, and the entire content was deposited in a counting dish for observation under an inverted microscope. Egg viability was determined visually based on the internal morphology of both living and dead egg and juveniles (EPPO Citation2013). The reproduction rate was determined by calculating the change in the number of viable eggs per gram of dry soil during a growing season or a rotation programme (3 years) by dividing the final number (Pf) by the initial number (Pi). Statistical analyses were performed in the SAS software program by a two-way analysis of variance using the General Linear Model procedure (SAS Institute Inc., Cary, NC). Means were separated using Duncan’s Multiple Range test at P ≤ 0.05.
Results
Crop rotations
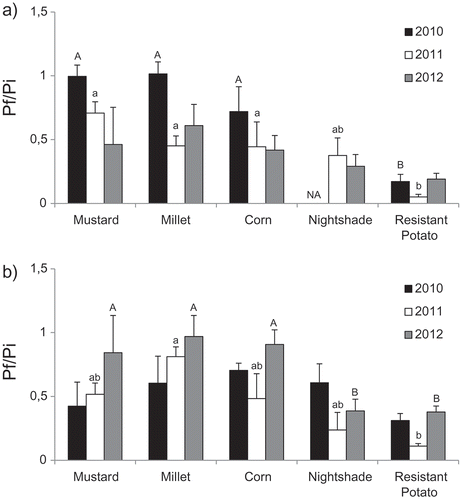
Crop rotations with resistant potato or non-host plants had a significant impact on the G. rostochiensis reproduction rate. After 1 year of cropping, resistant potato generated the lowest Pf/Pi value, followed by nightshade and non-host crops (mustard, millet and corn) (). For resistant potato and nightshade, the Pf/Pi values were slightly higher in muck soil than in loamy sand soil, although the soil type did not cause any significant difference between the treatments. Overall, a 1-year rotation with resistant potato reduced nematode populations ((1−pf/pi) × 100%) by 86.3% (81–95%) in loamy sand soil and 73.3% (62–89%) in muck soil (). Nightshade also resulted in reductions of G. rostochiensis populations, by 66.7% (62–71%) in loamy sand soil and 59.0% (40–76%) in muck soil . Non-host crops had a less pronounced effect than resistant potato and nightshade; the natural population decline was estimated at 35.3% (0–58%) in loamy sand soil and 28.7% (3–58%) in muck soil, over a single season. For a given year*site, non-host crops were not significantly different between each other. On the other hand, resistant potato was significantly more effective in reducing G. rostochiensis populations than non-host crops four year*site out of six.
Fig. 1 Effect of 1-year cropping with different plants on population densities of Globodera rostochiensis expressed as Pf/Pi, where Pf is the final population and Pi is the initial population, with both populations expressed as the number of viable eggs per gram of soil. Results are shown for (a) St-Amable (loamy sand) and (b) St-Dominique (muck soil). Treatments with different letters in a single year were significantly different (Duncan; P ≤ 0.05).
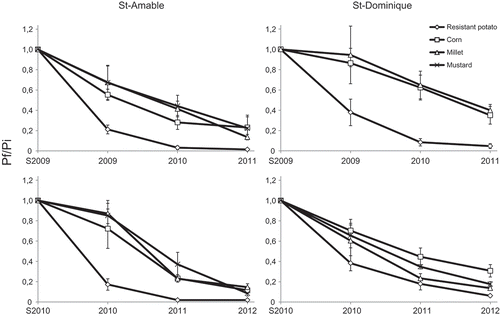
The changes in the populations of G. rostochiensis in microplots with continuous cultivation of susceptible potato, resistant potato or non-host plants were monitored over 3 years. Resistant potato had a significant effect on the reproduction rate and population decline was very fast. On the other hand, non-host plants generated a steady but slower reduction of populations (). Nightshade, however, could not be included because of establishment problems. After 3 consecutive years of the same rotation crop, the number of viable eggs was very low in several microplots. Populations were reduced by more than 97% after 3 years of resistant potato in loamy sand soil (). All the treatments, when used continuously over that period, caused reductions in the number of viable eggs of G. rostochiensis. Mustard, for example, reduced populations by more than 80% in both soil types (). Overall, the effect was less pronounced in muck soil.
Fig. 2 Effect of 3 years of cropping with different plants on population densities of Globodera rostochiensis expressed as Pf/Pi, where Pf is the final population and Pi is the initial population (Spring of first year), with both populations expressed as the number of viable eggs per gram of soil. Results are shown for St-Amable (loamy sand) and St-Dominique (muck soil) for two periods of 3 years (2009–2011 and 2010–2012). Data for ‘Mustard’ from 2009–2011 in St-Dominique are not available.
Trap cropping
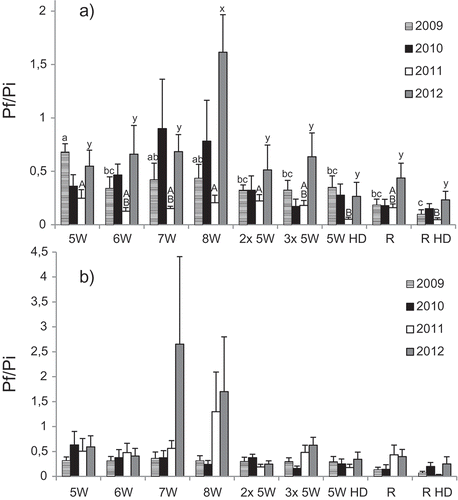
Trap cropping had a negative effect on nematode reproduction rates. Overall, the method generated results similar to the utilization of resistant potatoes (). No significant differences were observed in St-Dominique (muck soil) between the treatments while minor differences in some site*year were observed in St-Amable (loamy sand). The methods involving two or three successive plantings of susceptible potato pulled out after 5 weeks were not more effective than a single trapping with the plants destroyed after 5 weeks. Also, destroying the plants 7 or 8 weeks after planting was less effective than pulling them out after 5 or 6 weeks on both experimental sites and showed high reproduction rates in some microplots in different site*year.
Fig. 3 Effect of trap cropping on population densities of Globodera rostochiensis expressed as Pf/Pi, where Pf is the final population and Pi is the initial population, with both populations expressed as the number of viable eggs per gram of soil. Results are shown for (a) St-Amable (loamy sand) and (b) St-Dominique (muck soil). 5 W, 6 W, 7 W and 8 W = susceptible potato pulled out after 5, 6, 7 and 8 weeks, respectively, and followed by rye for the remainder of the season; 2× 5 W and 3× 5 W = 2 and 3 successive plantings of susceptible potato pulled out after 5 weeks, respectively; 5 W HD = susceptible potato planted at high density (50 plants per microplot) and pulled out after 5 weeks; R = resistant potato; R HD = resistant potato planted at high density (50 plants per microplot). Treatments with different letters in a single year were significantly different (Duncan; P ≤ 0.05).
Soil amendments
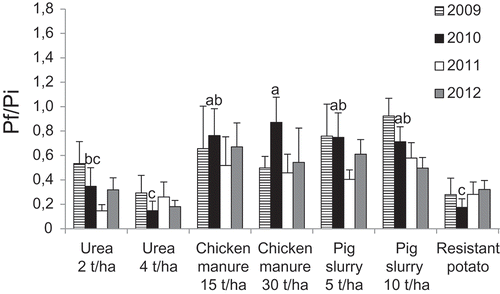
Urea provided the most potent reduction in G. rostochiensis populations, with reproduction rate values (Pf/Pi) similar to resistant potato for applications of 2 and 4 t ha−1 (). Chicken manure and pig slurry were more variable in their efficacy and did not result in significant population decreases.
Fig. 4 Effect of soil amendments on population densities of Globodera rostochiensis in St-Amable (loamy sand) expressed as Pf/Pi, where Pf is the final population and Pi is the initial population, with both populations expressed as the number of viable eggs per gram of soil. Treatments with different letters in a single year were significantly different (Duncan; P ≤ 0.05).
Discussion
The discovery of golden nematode in Quebec has had a huge impact on potato production. The establishment of a quarantine area, although challenging for growers, was required to limit the spread of this serious pest. However, the willingness of governments to continue the production of potatoes in these areas and to not attempt chemical eradication was a relief. Chemical eradication could have endangered aquifers and was a concern because of the proximity of residential areas. The decision to avoid chemical treatment meant that a sustainable and effective nematode management programme had to be established. The use of resistant cultivars proved very effective in decreasing the number of viable eggs per gram of soil in this study. This finding also confirms that G. rostochiensis in Quebec was a susceptible pathotype. Mahran et al. (Citation2010) showed that the population in St-Amable was pathotype Ro1, but nothing was known about the population in St-Dominique. In the long term, the repeated use of resistant cultivars might exert a selection pressure and favour the development of rare virulent genotypes. It has already been shown that this phenomenon led to the establishment of G. pallida in the UK (Trudgill et al. Citation2003) and G. rostochiensis pathotype Ro2 in the state of New York, USA (Brodie Citation1995).
Rotations with non-host crops such as corn also helped, to a lesser extent, to reduce nematode populations. However, alternative crops such as pearl millet and mustard were not a better choice in reducing the number of viable eggs. Mustard was reported to have bio-fumigant properties (Avato et al. Citation2013); however, our results indicate that the reduction in population density was equivalent to natural population decline. Consequently, there is no advantage to using pearl millet and mustard instead of a profitable crop such as corn, unless they fill a particular need, including serving as green manure to increase organic matter (mustard) or to control other nematodes such as Pratylenchus, a role for which millet proved effective in a previous study (Bélair et al. Citation2005).
Recently, the use of sticky nightshade has been attracting considerable attention as an alternative method of controlling PCN (Dandurand & Knudsen CitationForthcoming). Sticky nightshade stimulates the hatching of cysts without allowing the nematodes to become established and complete their life cycle. This mechanism is different from what occurs with the use of resistant potato, because the latter allows some new cysts to be produced on the roots, although in amounts less than 1% of that produced on a susceptible cultivar. With this information in mind, our initial hypothesis was that sticky nightshade would provide a higher level of control in comparison to resistant potato. However, given the results obtained over several years of research, we conclude that sticky nightshade is not a viable alternative for managing golden nematode in Quebec. While providing a level of control that was more or less equal to that of resistant potato varieties, sticky nightshade does not establish well in Quebec’s cool and wet spring soils. Multiple attempts to direct-seed this plant failed on both experimental sites, because its long germination time allowed annual grasses to choke out emerging seedlings (G. Bélair, unpublished data). In our field trials, we used transplants that had been started in a greenhouse. Apart from being laborious to transplant, the use of grown plants could have lowered the concentration of hatching agent produced by the roots, thus not affecting hatching and population decline as much as seedlings. For these reasons, we conclude that sticky nightshade is not an effective or economically viable management alternative for golden nematode in Quebec.
In temperate regions, the decline of PCN populations is estimated at around 30% per year in the absence of a suitable host (Evans & Stone Citation1977). In Europe, recent models of population dynamics of PCN were developed and integrated into the NemaDecide software program for decision making in the management of PCN (Been et al. Citation2005). NemaDecide predicts an annual decline of 50% in the first year and 33% in subsequent years in the number of viable eggs of G. rostochiensis with a non-host crop. If we calculate the average of all declines recorded on both of our experimental sites during the first year of a rotation with a non-host crop, we obtain an average decline of 33% for G. rostochiensis populations in Quebec. For resistant varieties, the average decline for the first year of the 3-year rotations was 70%. According to NemaDecide, annual reductions of 56% in the first year and 72% in subsequent years in the number of viable eggs of G. rostochiensis are predicted for resistant cultivars. Thus, the field data obtained during this study appear to be consistent with the models included in NemaDecide. The software is therefore a good tool for decision making in this region.
With the trap cropping technique, PCN populations are reduced by triggering egg hatching with root exudates and by destroying the plants before any nematode reproduction occurs. The most rapid decline in population density occurs near the potato plants, where the exudate concentration is higher, and decreases as the distance from the plants increases (LaMondia & Brodie Citation1986; Rawsthorne & Brodie Citation1986). Therefore, if we significantly increase the density of potato plants to cover the entire cultivated land, hatching should be maximized and trapping improved. Our results seem to agree with this approach, with greater reduction observed using resistant potatoes at high density, although not significantly different from standard density. If the main objective of regulatory authorities is to reduce the density of this quarantine organism below damage level as soon as possible, high-density cropping with resistant potato is certainly the most cost-efficient way of achieving this. Based on our results, 2 or 3 years of cropping with resistant potato would be needed to decrease populations below this level, depending on the initial densities. Because a 5-week period after planting was needed to stimulate hatching and provide a significant level of reduction, it is theoretically possible to proceed with a 5-week trapping period early in the spring (April–early May), destroy the crop, and grow a non-host crop such as corn to provide the grower with income that year.
Soil amendment with urea appeared to provide a good reduction in the number of viable eggs, with results similar to those obtained with resistant potato. The efficacy of urea against several nematode species has been shown at rates above 300 kg N ha−1 (Rodríguez-Kábana Citation1986). In Quebec, the recommended rate of N for potato varies with the soil type and also the requirements of the cultivar, but for comparison purposes, we could apply 150–200 kg N ha−1. Our soil amendments with urea at 2 or 4 t ha−1 were much greater than the recommendation for growing potato in a sandy soil. At these rates, soil runoff of N leachates into surrounding freshwater bodies will occur. These nitrogenous compounds were demonstrated to cause harmful environmental and human health effects (Bobbink et al. Citation1998; Chiu et al. Citation2007; Dubrovsky et al. Citation2010). Also, because the cost of such high rates of urea would surpass the costs related to soil fumigation with a standard nematicide, it would be more appropriate to reconsider the option of performing hot-spot soil fumigation to reduce the probability that this quarantine organism would spread in the area. Chicken manure and pig slurry failed to have a significant effect on G. rostochiensis populations and should not be retained as viable options for suppressing PCN under Quebec field conditions.
We have shown in this study that the reduction of cyst populations was following general assumptions for H1-resistant potato cultivars. We are therefore able to conclude with good confidence that the utilization of these plants is an effective tool to reduce G. rostochiensis in the climatic conditions of Quebec, Canada. Currently, the growers are allowed to grow a H1-resistant potato crop once every 3 years with non-host crops in the remaining years. This is sufficient to maintain the nematode populations at a very low level. Although some cysts may survive for extended periods, there is no short-term plan to deregulate those fields. Rott et al. (Citation2010) showed that fields with no known infractions were free of viable cysts after 30 years of quarantine in British Columbia. Thus, if we exclude the possibility of a new introduction, avoid any quarantine infractions, and follow the correct and recommended rotation programme, golden nematode could be completely eliminated from these fields in a few decades.
Acknowledgements
The authors thank Yvon Fournier and Catalin Cebuc for their technical assistance. We also wish to thank George Laplante for his expertise and guidance throughout the golden nematode project. This work was supported by the Developing Innovative Agri-Products programme of Agriculture and Agri-Food Canada, the Research Partnership Strategy programme of the Canadian Food Inspection Agency, and the Les Buissons Research Center.
Additional information
Funding
References
- Animal and Plant Health Inspection Service. 2009. Idaho pale potato cyst nematode cooperative program: PCN eradication program enters third year. Idaho Falls, ID: USDA/APHIS Plant Protection & Quarantine, Idaho State Department of Agriculture. (USDA publication; 4 (1)).
- Avato P, D’Addabbo T, Leonetti P, Argentieri MP. 2013. Nematicidal potential of Brassicaceae. Phytochem Rev. 12:791–802.
- Barker KR, Koenning SR. 1998. Developing sustainable systems for nematode management. Annu Rev Phytopathol. 36:165–205.
- Been TH, Schomaker CH, Molendijk LPG. 2005. NemaDecide: A decision support system for the management of potato cyst nematodes. In: Haverkort AJ, Struik PC, editors. Potato in progress: Science meets practice. Wageningen: Wageningen Academic Publishers; p. 143–156.
- Bélair G, Dauphinais N, Fournier Y, Dangi OP, Clément MF. 2005. Effect of forage and grain pearl millet on Pratylenchus penetrans and potato yields in Quebec. J Nematol. 37:78–82.
- Bobbink R, Hornung M, Roelofs JGM. 1998. The effects of air-borne nitrogen pollutants on species diversity and semi-natural European vegetation. J Ecol. 86:717–738.
- Brodie BB. 1982. Possible use of potato as a trap crop for controlling Globodera rostochiensis populations [abstract]. J Nematol. 14:432.
- Brodie BB. 1995. The occurrence of a second pathotype of potato cyst nematode in New York. J Nematol. 27:493–494.
- Brolsma KM, van der Salm RJ, Hoffland E, de Goede RGM. 2014. Hatching of Globodera pallida is inhibited by 2-propenyl isothiocyanate in vitro but not by incorporation of Brassica juncea tissue in soil. Appl Soil Ecol. 84:6–11.
- Centre de référence en agriculture et agroalimentaire du Québec. 2011. Guide de référence en fertilisation. 2nd ed. Quebec City: CRAAQ.
- Chiu H-F, Tsai S-S, Yang C-Y. 2007. Nitrate in drinking water and risk of death from bladder cancer: An ecological case–control study in Taiwan. J Toxicol Environ Health Part. 70:1000–1004.
- Dandurand LM, Knudsen G. Forthcoming. Effect of the trap crop Solanum sisymbriifolium and two biocontrol fungi on reproduction of the potato cyst nematode, Globodera pallida. Ann Appl Biol. doi:10.1111/aab.12295
- Dubrovsky NM, Burow KR, Clark GM, Gronberg JAM, Hamilton PA, Hitt KJ, Mueller DK, Munn MD, Nolan BT, Puckett LJ, et al. 2010. The quality of our nation’s waters: Nutrients in the nation’s streams and groundwater, 1992–2004. Reston, VA: U.S. Geological Survey (USGS Circular 1350).
- Ebrahimi N, Viaene N, Demeulemeester K, Moens M. 2014. Observations on the life cycle of potato cyst nematodes, Globodera rostochiensis and G. pallida, on early potato cultivars. Nematology. 16:937–952.
- EPPO. 2006. PM 3/68 (1) Testing of potato varieties to assess resistance to Globodera rostochiensis and Globodera pallida. Bull OEPP/EPPO Bull. 36:419–420.
- EPPO. 2013. PM 7/40 (3) Globodera rostochiensis and Globodera pallida. Bull OEPP/EPPO Bull. 43:119–138.
- Evans K, Stone AR. 1977. A review of the distribution and biology of the potato cyst-nematodes Globodera rostochiensis and G. pallida. Int J Pest Manage. 23:178–189.
- Fenwick DW. 1940. Methods for the recovery and counting of cysts of Heterodera schachtii from soil. J Helminthol. 18:155–172.
- LaMondia JA, Brodie BB. 1986. The effect of potato trap crops and fallow on decline of Globodera rostochiensis. Ann Appl Biol. 108:347–352.
- Mahran A, Turner S, Martin T, Yu Q, Miller S, Sun F. 2010. The golden potato cyst nematode Globodera rostochiensis pathotype Ro1 in the Saint-Amable regulated area in Quebec, Canada. Plant Dis. 94:1510.
- Mian IH, Rodríguez-Kábana R. 1982. Organic amendments with high tannin and phenolic contents for control of Meloidogyne arenaria in infested soil. Nematropica. 12:221–234.
- Mimee B, Dauphinais N, Bélair G. 2015. Life cycle of the golden cyst nematode, Globodera rostochiensis, in Quebec, Canada. J Nematol. 47:290–295.
- Rawsthorne D, Brodie BB. 1986. Root growth of susceptible and resistant potato cultivars and population dynamics of Globodera rostochiensis in the field. J Nematol. 18:501–504.
- Renčo M, Sasanelli N, Kováčik P. 2011. The effect of soil compost treatments on potato cyst nematodes Globodera rostochiensis and Globodera pallida. Helminthologia. 48:184–194.
- Rodríguez-Kábana R. 1986. Organic and inorganic nitrogen amendments to soil as nematode suppressants. J Nematol. 18:129–135.
- Rott M, Lawrence T, Belton M, Sun F, Kyle D. 2010. Occurrence and detection of Globodera rostochiensis on Vancouver Island, British Columbia: An update. Plant Dis. 94:1367–1371.
- Sasanelli N, D’Addabbo T, Papajova I, Greco P. 2006. Suppressive effect of composted olive mill wastes soil amendments on root-knot nematodes on tobacco. In: Brunelli A, Canova A, Collina M, Editors. Atti, Giornate Fitopatologiche. Riccione, Italy: Università di Bologna; p. 249–254.
- Scholte K, Vos J. 2000. Effects of potential trap crops and planting date on soil infestation with potato cyst nematodes and root-knot nematodes. Ann Appl Biol. 137:153–164.
- Sun F, Miller S, Wood S, Côté M-J. 2007. Occurrence of potato cyst nematode, Globodera rostochiensis, on potato in the Saint-Amable region, Quebec, Canada. Plant Dis. 91:908.
- Timmermans BGH, Vos J, Stomph TJ, Van Nieuwburg J, Van der Putten PEL. 2006. Growth duration and root length density of Solanum sisymbriifolium (Lam.) as determinants of hatching of Globodera pallida (Stone). Ann Appl Biol. 148:213–222.
- Trudgill DL, Elliott MJ, Evans K, Phillips MS. 2003. The white potato cyst nematode (Globodera pallida) – A critical analysis of the threat in Britain. Ann Appl Biol. 143:73–80.
- Turner SJ, Evans K. 1998. The origins, global distribution and biology of potato cyst nematodes (Globodera rostochiensis (Woll.) and Globodera pallida Stone). In: Marks RJ, Brodie BB, editors. Potato cyst nematodes: Biology, distribution and control. Cambridge, UK: Cambridge University Press; p. 7–26.
- Whitehead AG, Turner SJ. 1998. Management and regulatory control strategies for potato cyst nematodes (Globodera rostochiensis and Globodera pallida). In: Marks RJ, Brodie BB, editors. Potato cyst nematodes: Biology, distribution and control. Cambridge, UK: Cambridge University Press; p. 135–152.
