Abstract
China is one of the largest stripe rust epidemic areas in the world. Central Shaanxi, an important overwintering region, serves as a ‘bridge’ between the western over-summering and eastern epidemic regions. Understanding of resistance levels and Yr-gene distribution in regional wheat breeding lines may provide valuable recommendations for releasing resistant cultivars for managing the disease. A total of 183 wheat breeding lines developed for central Shaanxi were tested for seedling resistance to nine Chinese races of Puccinia striiformis f. sp. tritici (Pst) in the greenhouse. In field tests, entries were evaluated for stripe rust resistance in Yangling, Shaanxi, an overwintering region for the pathogen and artificially inoculated with selected races. In Tianshui, Gansu Province, an over-summering region, entries were evaluated under natural infection of Pst. Molecular markers for Yr5, Yr9, Yr10, Yr15, Yr17, Yr18, Yr26 and Yr61 were used to determine the presence and absence of the genes. Among the 183 entries, 4 (2.2%) entries had effective all-stage resistance; 15 (8.2%) entries had adult-plant resistance; and 164 (89.6%) entries were susceptible to one or more races, especially to the potentially important races V26/CM42 and V26/Gui22. Over 95% of entries showed seedling stage susceptibility in Tianshui across years. Resistance genes Yr9, Yr17 and Yr24/Yr26 were postulated in some of the breeding lines based on the seedling responses and molecular markers. Yr5, Yr10, Yr15, Yr18 and Yr61 were not present in any of the breeding lines. These results suggest that, when multiple races of Pst disperse into Shaanxi from southern Gansu Province, over 90% of wheat breeding lines are susceptible. Based on the results, recommendations are made for releasing or not releasing the individual lines, and a strategy of combining genes for effective all-stage and adult-plant resistance is proposed for developing wheat cultivars with high-level durable resistance to stripe rust.
Résumé
La Chine est une des plus grandes régions du monde qui est frappée par l’épidémie de rouille jaune. La région centrale du Shaanxi, une importante région quant à la survie hiémale de l’agent pathogène, sert de « pont » entre les régions de l’ouest et de l’est touchées en été par l’épidémie. La compréhension des degrés de résistance et de la distribution des gènes Yr dans les lignées généalogiques régionales peuvent aider à émettre de précieuses recommandations quant à la commercialisation de cultivars résistants afin de maîtriser la maladie. En tout, 183 lignées généalogiques de blé développées pour le Shaanxi ont été testées en serre en vue de la résistance des semis à 9 races chinoises de Puccinia striiformis f. sp. tritici (Pst). Dans les tests menés au champ, les entrées ont été évaluées en fonction de la résistance à la rouille jaune dans le district du Yangling, dans la province du Shaanxi, une région connue pour la survie hiémale de l’agent pathogène, et elles ont été inoculées artificiellement avec des races sélectionnées. À Tianshui, dans la province du Gansu, une région de survie estivale, les entrées ont été évaluées relativement à l’infection naturelle causée par Pst. Des marqueurs moléculaires pour les gènes Yr5, Yr9, Yr10, Yr15, Yr17, Yr18, Yr26 et Yr61 ont été utilisés pour détecter la présence ou l’absence de ces derniers. Parmi les 183 entrées, 4 entrées (2.2%) affichaient une résistance efficace à tous les stades; 15 entrées (8.2%) affichaient de la résistance aux stades adultes; et 164 entrées (89.6%) étaient réceptives à l’égard d’une ou de plusieurs races, particulièrement à l’égard des races V26/CM42 et V26/Gui22, potentiellement importantes. À Tianshui, au fil des années, plus de 95 % des entrées ont affiché de la réceptivité au stade de semis. Nous avons présumé de la présence des gènes de résistance Yr9, Yr17 et Yr24/Yr26 dans certaines des lignées généalogiques en fonction des réactions des semis et des marqueurs moléculaires utilisés. Par ailleurs, les gènes Yr5, Yr10, Yr15, Yr18 et Yr61 n’étaient présents dans aucune des lignées généalogiques. Ces résultats suggèrent que, lorsque plusieurs races de Pst se propagent dans le Shaanxi en provenance du sud de la province du Gansu, plus de 90% des lignées généalogiques de blé sont réceptives. En se basant sur ces résultats, des recommandations sont faites afin que les lignées à gène unique de résistance soient ou non commercialisées, et pour proposer une stratégie visant à combiner les gènes afin de développer des cultivars de blé qui afficheront un haut degré de résistance efficace et durable à la rouille jaune, et ce, à tous les stades de croissance de la plante.
Introduction
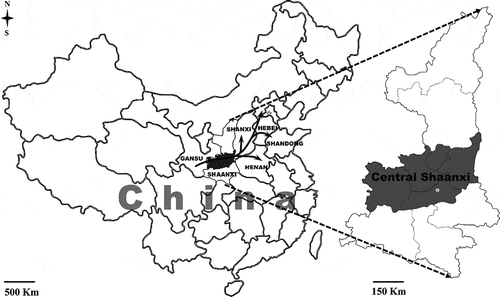
Stripe (yellow) rust, caused by Puccinia striiformis Westend. f. sp. tritici Erikss. (Pst), is one of the most damaging diseases in almost all wheat growing areas in the world (Stubbs Citation1985; Chen Citation2005; Hovmøller et al. Citation2010; Wellings Citation2011). Severe epidemics have occurred in China many times, causing yield losses of several million tonnes (Wan et al. Citation2007; Chen et al. Citation2009, Citation2014; Kang et al. Citation2010) and China is considered to be one of the largest stripe rust epidemic regions in the world (Stubbs Citation1985). Based on historical epidemiological data of stripe rust, the wheat-growing regions in China can be divided into the western over-summering region, the overwintering region and the eastern epidemic region (Li & Zeng Citation2002; Zeng & Luo Citation2006; Wan et al. Citation2007). Previous studies demonstrated that urediniospores from the over-summering region, particularly southern and eastern Gansu Province, spread eastward to eastern China (Brown & Hovmøller Citation2002; Li & Zeng Citation2002). Central Shaanxi is the most significant overwintering region where autumn-sown winter wheat can be infected in late autumn and early winter. The pathogen survives mainly as latent mycelia in infected wheat leaves until temperatures increase in the following spring. Urediniospores produced in the overwintering region can cause multiple infections within the region, and also can be disseminated by wind to major winter wheat regions to the east (Li & Zeng Citation2002; Kang et al. Citation2010; Chen et al. Citation2014). Thus, central Shaanxi serves as a ‘bridge’ for the pathogen to migrate from the western over-summering region to the eastern epidemic region ().
Fig. 1 Location of central Shaanxi (shadowed) in China and dispersal of Puccinia striiformis f. sp. tritici in China. The main area of winter wheat cultivation includes Shaanxi, Shanxi, Henan, Hebei and Shandong provinces. Solid black arrows indicate dispersal of urediniospores from southern Gansu to the main wheat-growing regions in autumn.
Stripe rust severity is affected by host resistance levels, the time of initial infection in relation to the phenology of the host, and weather conditions (Rapilly Citation1979; Chen Citation2013). Growing resistant cultivars is the most effective control strategy, as it has no additional cost to farmers and is environmentally desirable (Li & Zeng Citation2002; Wellings Citation2011; Chen Citation2013; Ellis et al. Citation2014). Long-term control of the disease depends on characterization and deployment of genetically diverse sources of resistance (Chen Citation2005; Wellings Citation2011; Chen et al. Citation2014). Considerable work has been done in different epidemic regions of China. The major cultivars and elite breeding lines from Shannxi, Gansu and Sichuan Provinces have been studied for stripe rust resistance genes (Wang et al. Citation1994, Citation2007; Zeng et al. Citation2014). These studies indicate that the resistance of wheat cultivars in central Shaanxi is mainly controlled by only a few genes, such as Yr9, and only a few cultivars have adult-plant resistance, which is largely inadequate for managing the disease. In order to substantially reduce pathogen spread from southern Gansu Province to eastern regions, it is extremely important to clearly characterize and deploy resistance genes in wheat breeding lines in central Shaanxi as breeding lines may become leading cultivars in the future. However, no information is available regarding stripe rust resistance genes in wheat breeding lines in this region. Furthermore, it is not clear if the lines developed in this region are resistant in southern Gansu Province. Therefore, information on the reaction of wheat breeding lines in central Shaanxi to different Pst races could be used to make recommendations for whether to release these wheat breeding lines and to manage the disease. The objectives of this study were to: (1) determine the reaction and type of resistance in breeding lines; and (2) postulate the resistance gene(s) in the breeding lines, developed for central Shaanxi.
Materials and methods
Wheat plant materials
A total of 183 advanced wheat breeding lines developed by different programmes from 2009 to 2011 for central Shaanxi were used in this study. Seeds of these lines were kindly provided by Prof. Baotong Wang (College of Plant Protection, Northwest A&F University). The wheat cultivars (or lines) ‘Avocet S’, ‘Mingxian 169’ and ‘Xiaoyan 22’ were used as the susceptible controls, ‘92R137’ (Yr26) and ’Pindong34 ’ (Yr61) were included in comparative response tests in the stripe rust assessment. A set of Yr single-gene lines provided by Prof. Xianming Chen (USDA-ARS, Pullman, WA, USA) was included in the study as references for individual stripe rust resistance genes ().
Table 1. The Yr single-gene lines used as references for individual Yr genes for resistance to stripe rust caused by Puccinia striiformis f. sp. tritici.
Pst races
Nine races (CYR29, CYR31, CYR32, CYR33, Sull-4, Sull-5, Sull-7, V26/CH42, V26/Gui22), which represent different race groups, were used to test for race-specific resistance at the seedling stage under greenhouse conditions. Two races (CYR32 and CYR33) that are predominant in China (Chen et al. Citation2009) were used to inoculate the field nursery at Yangling, Shaanxi Province. All races were obtained from the Institute of Plant Pathology, Northwest A&F University. The virulence/avirulence patterns of the races were confirmed by testing on ‘Avocet’ near-isogenic wheat lines used to differentiate Pst races ().
Table 2. Virulence (V) and avirulence (A) of Puccinia striiformis f. sp. tritici races used in this study and their virulence spectra on Yr single-gene lines and Yr26 donor line at seedling stage.
Seedling tests
Seedling tests for the 183 entries were conducted in a greenhouse in Yangling. For each entry, 8–10 plants were grown in a 9 × 9 × 9 cm pot filled with standard peat soil. Seedlings at the two-leaf stage (14 days after planting) were separately inoculated with urediniospores mixed with talc at a ratio of approximately 1:20. Inoculated plants were incubated at 10°C in a dew chamber in the dark for 24 h, and then transferred to a greenhouse at 17 ± 2°C with 14 h light (22 000 lx) photoperiod. When stripe rust was fully developed on susceptible checks ‘Mingxian 169’ and ‘Avoset S’ (about 18–20 days after inoculation), the infection type (IT) was recorded using a 0–9 scale (Line & Qayoum Citation1992). Plants with ITs 0 to 6 were considered resistant and the inoculated race avirulent, and plants with ITs 7-9 were considered susceptible and the race virulent. In order to confirm and clarify ITs of the entries, all seedling tests were conducted three times at different times (November 2012–March 2013).
Field tests in the artificially inoculated nursery at Yangling
The 183 entries and susceptible check ‘Xiaoyan 22’ were planted in early October at the Northwest A&F University Research Farms in Yangling during the 2012, 2013 and 2014 wheat-growing seasons. Yangling is located in central Shaanxi, an overwintering region. Entries in each season were arranged in a randomized complete block design with two replications, and inoculated by spraying a mineral oil suspension of mixed urediniospores of predominant races CYR32 (50%) and CYR33 (50%) at a ratio of approximately 1:300 when flag leaves emerged. Infection types and disease severity (DS) were recorded at least twice starting when the susceptible check had at least 30% severity using the Peterson et al. (Citation1948) scale. Disease severity was assessed visually using the percentage of diseased leaf area covered by stripe rust. Both IT and DS in the last assessment, which had the highest disease severity, were used to determine the reaction of the entries.
Field tests in Tianshui under natural infection
During the 2012–13 and 2013–14 wheat growing seasons, all entries were planted in a field in the Tianshui region located in eastern Gansu Province. The nursery was managed using the common practices in the region and had natural stripe rust infection. Seeds were sown in mid-September. Infection types of seedlings were recorded in late November and early December when the plants were at the tillering stage. Both IT and DS were recorded in late May and early June when the crops were at boot and milk stages, respectively, and DS on the susceptible check reached 40-80 %.
Identification of Yr genes using molecular markers
Genomic DNA was extracted from leaves with sodium dodecyl sulfonate (SDS) (Song et al. Citation1994). Primers of markers for identifying specific Yr genes were synthesized by Sangon Biotech Co, Ltd (Shanghai, China). Molecular markers for eight stripe rust resistance genes were used. Yr9 and Yr17 were intensively used in wheat breeding programmes from the 1970s to 1990s in China and many other countries (Francis et al. Citation1995; Zhou et al. Citation2004). Yr10 and Yr24/Yr26 have recently become susceptible because of the emergence of new races in China (Liu et al. Citation2010; Han et al. Citation2015). Yr5, Yr15 and Yr61 are race-specific resistance genes effective against all Chinese Pst races. Yr18, an adult-plant resistance (APR) gene, is effective but provides partial resistance against the Pst population in China (Sharma-Poudyal et al. Citation2013; Zhou et al. Citation2014). Yr5, Yr9, Yr10, Yr15, Yr17, Yr18 and Yr26 were identified according to the methods described by Zeng et al. (Citation2014), and Yr61 was detected according to the method described by Zhou et al. (Citation2014). Wheat lines with corresponding Yr genes and ‘Avocet S’ were used as the positive and negative control, respectively.
Results
Stripe rust resistance
The 183 entries in the seedling stage grown in the greenhouse were inoculated separately with races CYR29, CYR31, CYR32, CYR33, Su11-4, Su11-5, Su11-7, V26/CH42 and V26/Gui22. Supplemental shows the reactions of entries to the nine races at the seedling stage in the greenhouse tests, at the seedling stage in field tests at Tianshui, and at the adult-plant stage in field tests at both Yangling and Tianshui in 2012–2014. Based on the seedling and field reactions, 183 entries could be classified into six groups based on the characteristics of resistance or susceptibility (). In group 1, four (2.2%) entries (‘Shaan 319’, ‘Shaannong 389ʹ, ‘Shaan 019’ and ‘Xiaoyan 461’) were resistant in all tests, except ‘Xiaoyan 461’ was susceptible in Tianshui in 2014, indicating that these entries have effective all-stage resistance. Group 2 consisted of 13 entries (7.1%) that were resistant in all field tests, but had susceptible reactions to some races in the greenhouse seedling tests, suggesting that these entries have both all-stage resistance and adult-plant resistance. Two entries (‘Heiyou’ and ‘Xinong 822’; 1.1%) in group 3 were susceptible in all seedling tests, but resistant in all field tests, indicating only adult-plant resistance. Group 4 consisted of 71 (38.8%) entries that were resistant to one or more races in the seedling tests and in the Yangling field, but susceptible in the Tianshui fields. Group 5 had 78 (42.6%) entries that were resistant to one or more races in the seedling tests, but susceptible in the fields of both Yangling and Tianshui. Fifteen (8.2%) entries in group 6 were susceptible in all of the greenhouse and field tests. Thus, the 164 (89.6%) entries in groups 4, 5 and 6 have no effective resistance against the Pst populations in the fields, and should be considered susceptible.
Table 3. Groups of wheat breeding lines based on resistant or susceptible reactions to Puccinia striiformis f. sp. tritici at seedling stage in greenhouse and field tests and adult-plant stage in the field tests.
Yr genes
The presence of molecular markers for Yr5, Yr9, Yr10, Yr15, Yr17, Yr18, Yr26 and Yr61 in the tested wheat entries are shown in Supplemental . Two markers were chosen to detect each gene, and the Yr gene(s) was considered to be present in the entry when both markers were detected. All entries were tested for the markers of Yr9 and Yr17. Based on the marker tests and resistance phenotypes, 90 (49.2%) entries had Yr9 and 19 (10.4%) entries had Yr17. Ninety entries that were resistant to races CYR32 and CYR33 but susceptible to races V26/CH42 and V26/Gui22 were tested with markers for Yr10 and Yr24/Yr26. No lines were positive for the Yr10 markers, but three (1.6%) entries (‘Shannmai 54’, ‘Xinong 389’ and ‘Wunong 882’) were positive for Yr24/Yr26. Nineteen entries that were resistant at the adult-plant stage were tested with molecular markers for Yr5, Yr15, Yr18 and Yr61. None of the resistant entries had Yr5, Yr15, Yr18 or Yr61, indicating that these entries may carry a novel resistance gene or an effective combination of resistance genes. However, some entries showed differential reactions, presumably due to other Yr gene(s) which were not detected in this study.
Discussion
Much of the research in race identification and virulence evolution of the stripe rust pathogen during the past 30 years has shown that changes in races and their frequencies have corresponded to stripe rust epidemics, which in turn led to changes in wheat cultivars in China (Wan et al. Citation2004, Citation2007; Liu et al. Citation2012; Chen et al. Citation2009, Citation2014; Duan et al. Citation2010; Jia et al. Citation2012; Wang et al. Citation2014). Race CYR29, first detected in 1985, caused epidemics on cultivars carrying Yr9 in the 1990s, and races CYR31 and CYR32, virulent to wheat cultivars with Yr3b and Yr4b (such as ‘Hybrid 46’, ‘Fan 6’, and its derivatives), resulted in yield losses of 1.3 million tonnes in 2002 (Wan et al. Citation2007). Race CYR33, with virulence on ‘Suwon 11’, was first detected in 1997 and formally named in 2008 and has been one of the most predominant races (Chen et al. Citation2009; Kang et al. Citation2010). Races Sull-4, Sull-5 and Sull-7 are also virulent on ‘Suwon 11’, and their frequencies ranged between 3.0% and 10.0% from 2003 to 2013 (Chen et al. Citation2009; Kang et al. Citation2010; Li et al. Citation2012; Han et al. Citation2015). Race V26/CH42, discovered from the wheat cultivar ‘Chuanmai 42’ (YrCH42 = Yr26) in 2008, was virulent on cultivars with Yr26 (Liu et al. Citation2010). Several Yr26-virulent races with broader virulence spectra have been identified recently (Wang et al. Citation2014). An even more serious problem is that Yr26-virulent races are becoming prevalent as their frequency has increased to 10.3% in 2013 (Han et al. Citation2015), and the total frequency of CYR32, CYR33 and V26 has exceeded 70% (Zeng et al. Citation2015). Therefore, it is important to develop breeding lines with effective resistance to the currently prevalent and potentially threatening races.
In order to characterize stripe rust resistance in wheat cultivars accurately and objectively, different races and environmental conditions were used in screening for disease resistance (Walker Citation1965). In the present study, 183 entries were tested for resistance against nine races in seedling tests, and to races CYR32 and CYR33 under artificial inoculation conditions at the adult plant stage in Yangling. The entries were also planted in Tianshui for natural infection because Tianshui is considered a hot-spot region for stripe rust in China, the number of races is large and the Pst population structure is complex (Li & Zeng Citation2002; Duan et al. Citation2010; Chen et al. Citation2014). Four entries in group 1 were resistant to all nine races in the greenhouse tests and three of them were also resistant at the seedling stage in the Tianshui field nursery. These results indicated that one of the four entries with all-stage resistance were no longer resistant to the natural population in Tianhui. Fifteen entries in group 2 and group 3 were susceptible to at least one race in seedling tests, but resistant in all field tests. Entries of this type may have great effectiveness to control stripe rust for their adult-plant resistance. These 15 breeding lines could be released and used in wheat production programmes. Seventy-one entries in group 4 displayed moderate (30R) to high levels (5R) of responses to stripe rust at Yangling, but were susceptible (40S–100S) in Tianshui. This may indicate race-specific adult-plant resistance that might not be effective against races in Tianshui, or a low level of high-temperature adult-plant (HTAP) resistance, which might not fully express under the relatively low temperatures. The resistance in this group is not adequate to control stripe rust; therefore, these lines need additional adult-plant resistance genes and/or effective all-stage resistance genes. It is worth noting that the entries in this group were resistant to predominant races (CYR32 and/or CYR33) at the seedling stage, but were susceptible to race V26 or Su11 race series. These entries should not be used in wheat production because growing these cultivars could produce a large quantity of Pst inoculum that might spread to the downwind endemic regions. Additionally, the entries in groups 5 and 6 should not be released since they were susceptible in all field tests and do not have any effective resistance genes.
Marker-assisted selection is conducted to identify the presence of resistance genes and speed up the selection process if useful markers are available (Chen Citation2013). Yr5, Yr15, Yr18 and Yr61 were found effective against stripe rust in China. Yr10 and Yr26 were effective against predominant races CYR32 and CYR33, but susceptible to the V26 new race series. When the molecular markers were used to detect Yr5, Yr10, Yr15, Yr18 and Yr61, the targeted bands were only observed in the positive controls, indicating none of the entries have these genes. Thus, the four resistant lines in group 1 carry either uncharacterized resistance genes or effective combinations of genes. Further genetic studies are needed to characterize the resistance genes in these lines to assess their value as sources of stripe rust resistance.
Zhuang (Citation2003) reported that during the history of wheat breeding in China, there was no evidence of using Yr10 and Yr15. No lines were positive for the Yr10 and Yr15 markers in Chinese wheat cultivars (Zeng et al. Citation2014). Gene Yr61 is rarely used in breeding, probably due to its recent identification or other agronomic reasons (Zhou et al. Citation2014). Yr26 was found in several Triticum aestivum–Haynaldia villosa 6VS/6AL translocation lines (‘92R89’, ‘92R90’, ‘92R137’, ‘92R149’, ‘92R178’) developed by the Cytogenetics Institute, Nanjing Agricultural University (Wang et al. Citation2008; Zhang et al. Citation2013). The marker data for the presence of Yr26 in the identified entries ‘Shaanmai 54’, ‘Shaanong 389’ and ‘Wunong 882’ were consistent with their pedigrees. When tested with races V26/CH42 and V26/Gui22 with virulence to Yr26, these entries were susceptible suggesting that Yr26 should not be used singly. The complex of Yr17, Lr37 and Sr38 rust resistance genes, which confers resistance to stripe rust, leaf rust and stem rust, respectively, have been used in breeding programmes in different parts of the world (Helguera et al. Citation2003). Based on the presence of these markers, Yr17 was identified in 19 of 183 (10.3%) entries. Gene Yr9 was widely used in breeding programmes from the 1970s to 1980s in China.
In the present study, 90 out of the 183 (49.2%) entries were found to have Yr9, indicating that Yr9 exists extensively in the cultivars and genetic stocks used in breeding programmes of central Shaanxi. These results are consistent with previous reports (Wang et al. Citation1994; Zeng et al. Citation2014). The above results show that the previously resistant materials are still used in wheat breeding, possibly because of their other desirable traits. Therefore, effective all-stage resistance genes (such as Yr5, Yr15 and Yr61) and HTAP resistance genes (such as Yr18, Yr29, Yr36, Yr39, Yr52, Yr59 and Yr62) (Chen Citation2013) should be used in combinations in breeding programmes to develop wheat cultivars with high-level and durable resistance. Moreover, there is an ongoing need to identify new resistance genes and develop new resistant germplasm.
As suggested by He et al. (Citation2011), growing cultivars with effective race-specific resistance and durable adult-plant resistance is a good strategy for control of stripe rust in the area where the disease occurs frequently. Han et al. (Citation2010) also suggested that expanding the growth area of cultivars with combination of effective all-stage resistance and adult-plant resistance may be an appropriate approach for management of the disease in the central Shaanxi region. In this study, we evaluated wheat breeding lines for reactions to stripe rust in central Shaanxi and the results show that most of the breeding lines are susceptible or have low levels of resistance. This study suggests that more effort should be taken in developing stripe rust resistant cultivars.
Supplement Table 1
Download MS Excel (39.8 KB)Supplemental material
Supplemental data for this article can be accessed online here: http://dx.doi.org/10.1080/07060661.2016.1206039
Additional information
Funding
References
- Brown JK, Hovmøller MS. 2002. Aerial dispersal of pathogens on the global and continental scales and its impact on plant disease. Science. 297:537–541.
- Chen WQ, Wellings C, Chen XM, Kang ZS, Liu TG. 2014. Wheat stripe (yellow) rust caused by Puccinia striiformis f. sp. tritici. Mol Plant Pathol. 15:433–446.
- Chen WQ, Wu LR, Liu TG, Xu SC, Jin SL, Peng YL, Wang BT. 2009. Race dynamics, diversity, and virulence evolution in Puccinia striiformis f. sp. tritici, the causal agent of wheat stripe rust in China from 2003 to 2007. Plant Dis. 93:1093–1101.
- Chen XM. 2005. Epidemiology and control of stripe rust [Puccinia striiformis f. sp tritici] on wheat. Can J Plant Pathol. 27:314–337.
- Chen XM. 2013. High-temperature adult-plant resistance, key for sustainable control of stripe rust. Amer J Plant Sci. 04:608–627.
- Duan XY, Tellier A, Wan AM, Leconte M, De Vallavieille-Pope C, Enjalbert J. 2010. Puccinia striiformis f. sp. tritici presents high diversity and recombination in the over-summering zone of Gansu, China. Mycologia. 102:44–53.
- Ellis JG, Lagudah ES, Spielmeyer W, Dodds PN. 2014. The past, present and future of breeding rust resistant wheat. Front Plant Sci. 5:1–13.
- Francis HA, Leitch AR, Koebner RM. 1995. Conversion of a RAPD-generated PCR product, containing a novel dispersed repetitive element, into a fast and robust assay for the presence of rye chromatin in wheat. Theor Appl Genet. 90:636–642.
- Han DJ, Wang QL, Chen XM, Zeng QD, Wu JH, Xue WB, Zhan GM, Huang LL, Kang ZS. 2015. Emerging Yr26-virulent races of Puccinia striiformis f. sp. tritici are threatening wheat production in the Sichuan Basin, China. Plant Dis. 99:754–760.
- Han DJ, Wang QL, Zhang L, Wei GR, Zeng QD, Zhao J, Wang XJ, Huang LL, Kang ZS. 2010. Evaluation of resistance of current wheat cultivars to stripe rust in Northwest China, North China and the Middle and Lower Reaches of Changjiang River epidemic area. Sci Agric Sin. 43:2889–2896.
- He ZH, Lan CX, Chen XM, Zou YC, Zhuang QS, Xia XC. 2011. Progress and perspective in research of adult-plant resistance to stripe rust and powdery mildew in wheat. Sci Agric Sin. 44:2193–2215.
- Helguera M, Khan IA, Kolmer J, Lijavetzky D, Li ZQ, Dubcovsky J. 2003. PCR assays for the Lr37-Yr17-Sr38 cluster of rust resistance genes and their use to develop isogenic hard red spring wheat lines. Crop Sci. 43:1839–1847.
- Hovmøller MS, Walter S, Justesen AF. 2010. Escalating threat of wheat rusts. Science. 329:369–369.
- Jia QZ, Huang J, Cao SQ, Zhang B, Wang XM, Jin SL. 2012. Discovery of new stripe rust strain infecting Chinese major wheat resistant material Guinong 22 and its preliminary pathogenicity analysis. Gansu Agr Sci Technol. 32:3–5.
- Kang ZS, Zhao J, Han DJ, Zhang HC, Wang XJ, Wang CF, Han QM, Guo J, Huang LL. 2010. Status of wheat rust research and control in China. In: BGRI 2010 technical workshop oral presentations. p. 50–69 Available from http://www.globalrust.org/all-bgri-abstracts &combine=&field_abstract_tags_tid=All&page=6
- Li Q, Li GB, Wang BT, Wang F, Kang ZS. 2012. Pathogenic changes of the stripe rust fungus of wheat in Shaanxi Province during 2006-2010. Acta Phytopathol Sin. 38:133–136.
- Li ZQ, Zeng SM. 2002. Wheat rust in China. Beijing: China Agriculture Press.
- Line RF, Qayoum A. 1992. Virulence, aggressiveness, evolution, and distribution of races of Puccinia striiformis (the cause of stripe rust of wheat) in North America 1968-1987. US Dep. Agric. Agric. Res. Serv. Tech. Bull. 1788.
- Liu TG, Peng YL, Chen WQ, Zhang ZY. 2010. First detection of virulence in Puccinia striiformis f. sp. tritici in China to resistance genes Yr24 (= Yr26) present in wheat cultivar Chuanmai 42. Plant Dis. 94:1163.
- Liu TG, Wang BT, Jia QZ, Zhang ZY, Li Q, Cao SQ, Peng YL, Jin SL, Li MJ, Liu B, et al. 2012. Physiologic specialization of Puccinia striiformis f. sp. tritici in China during 2010-2011. J Triticeae Crops. 3:574–578.
- Peterson RF, Campell AB, Hannah A. 1948. A diagrammatic scale for estimating rust intensity on leaves and stems of cereals. Can J Res. 26:496–500.
- Rapilly F. 1979. Yellow rust epidemiology. Annu Rev Phytopathol. 17:59–73.
- Sharma-Poudyal D, Chen XM, Wan AM, Zhan GM, Kang ZS, Cao SQ, Jin SL, Morgounov A, Akin B, Mert Z, et al. 2013. Virulence characterization of international collections of the wheat stripe rust pathogen, Puccinia striiformis f. sp. tritici. Plant Dis. 97:379–386.
- Song WN, Ko L, Henry RJ. 1994. Polymorphisms in the α-amy1 gene of wild and cultivated barley revealed by the polymerase chain reaction. Theor Appl Genet. 89:509–513.
- Stubbs RW. 1985. Stripe rust. In: Roelfs AP, Bushnell WR, editors. The cereal rusts. Vol. II. New York (NY): Academic Press; p. 61–101.
- Walker JC. 1965. Use of environmental factors in screening for disease resistance. Annu Rev Phytopathol. 3:197–208.
- Wan AM, Chen XM, He ZH. 2007. Wheat stripe rust in China. Aust J Agr Res. 58:605–619.
- Wan AM, Zhao ZH, Chen XM, He ZH, Jin SL, Jia QZ, Yao G, Yang JX, Wang BT, Li GB, et al. 2004. Wheat stripe rust epidemic and virulence of Puccinia striiformis f. sp tritici in China in 2002. Plant Dis. 88:896–904.
- Wang BT, Li GB, Li Q, Wang F, Kang ZS. 2007. Cluster analysis of major and candidate cultivars of wheat for dominant populations of stripe rust in seedling stage from Shaanxi, Gansu, Sichuan and Henan provinces. Acta Phytopathol Sin. 34:500–506.
- Wang CM, Zhang YP, Han DJ, Kang Z, Li GP, Cao AZ, Chen PD. 2008. SSR and STS markers for wheat stripe rust resistance gene Yr26. Euphytica. 159:359–366.
- Wang FL, Wu LR, Wan AM. 1994. Postulated genes for resistance to stripe rust in seedlings of wheat cultivars from Shaanxi, Gansu and Sichuan Provinces. Acta Agron Sin. 20:589–594.
- Wang FP, Zhan GM, Wei GR, Huang LL, Kang ZS, Han QM. 2014. Analysis of Puccinia striiformis f. sp. tritici population virulence on two wheat cultivars from different over-summering zones in Longnan. J Triticeae Crops. 8:1146–1152.
- Wellings CR. 2011. Global status of stripe rust: a review of historical and current threats. Euphytica. 179:129–141.
- Zeng Q-D, Han D-J, Wang Q-L, Yuan F-P, Wu J-H, Zhang L, Wang X-J, Huang -L-L, Chen X-M, Kang Z-S. 2014. Stripe rust resistance and genes in Chinese wheat cultivars and breeding lines. Euphytica. 196:271–284.
- Zeng QD, Shen C, Yuan FP, Wang QL, Wu JH, Xue WB, Zhan GM, Yao S, Chen W, Huang LL, et al. 2015. The resistance evaluation of the Yr genes to the main prevalent pathotypes of Puccinia striiformis f. sp. tritici in China. Acta Phytopathol Sin. 45:641–650.
- Zeng S, Luo Y. 2006. Long-distance spread and interregional epidemics of wheat stripe rust in China. Plant Dis. 90:980–988.
- Zhang XJ, Han DJ, Zeng QD, Duan YH, Yuan FP, Shi JD, Wang QL, Wu JH, Huang LL, Kang ZS. 2013. Fine mapping of wheat stripe rust resistance gene Yr26 based on collinearity of wheat with Brachypodium distachyon and rice. PloS ONE. 8:e57885.
- Zhou XL, Han DJ, Chen XM, Gou HL, Guo SJ, Rong L, Wang QL, Huang LL, Kang ZS. 2014. Characterization and molecular mapping of stripe rust resistance gene Yr61 in winter wheat cultivar Pindong 34. Theor Appl Genet. 127:2349–2358.
- Zhou Y, He ZH, Zhang GS, Xia LQ, Chen XM, Gao YC, Jing ZB, Yu GJ. 2004. Utilization of 1BL/1RS translocation in wheat breeding in China. Acta Agron Sin. 30:531–535.
- Zhuang QS. 2003. Wheat improvement and pedigree analysis in China. Beijing: China Agriculture Press.
