Abstract
The root-knot nematode, Meloidogyne incognita, and the Phytophthora blight pathogen, Phytophthora capsici, cause root diseases in bell pepper under field conditions. However, limited information is available about the interactions between these two pathogens on different bell pepper cultivars. Greenhouse experiments were conducted on cultivars of bell pepper with different levels of resistance to P. capsici (‘Aristotle’, ‘Paladin’ and ‘Intruder’), by concomitant and sequential inoculation of both pathogens. The treatments included inoculating both pathogens simultaneously into the root zone, inoculating M. incognita 7 days before P. capsici, inoculating P. capsici 7 days before M. incognita, inoculating M. incognita or P. capsici alone, and an untreated control without any pathogens. In ‘Aristotle’ and ‘Intruder’, P. capsici inoculation with M. incognita simultaneously or 7 days before or after M. incognita reduced gall index significantly as compared with M. incognita alone. In ‘Paladin’, P. capsici inoculation 7 days before or after M. incognita lowered gall index compared with M. incognita – P. capsici simultaneous inoculation. Phytophthora blight severity was not influenced by M. incognita irrespective of inoculation methods used in the three genotypes. In ‘Aristotle’, M. incognita inoculation alone did not reduce fresh root weight, but P. capsici alone or combined with M. incognita reduced fresh root weight as compared with the untreated control. However, none of the treatments reduced fresh root weight in ‘Paladin’ or ‘Intruder’ as compared with the untreated control. The interaction between P. capsici and M. incognita seems to be one way in that P. capsici reduced galling of M. incognita, but the nematode had no effect on Phytophthora blight severity. The interaction was influenced by pepper cultivars, with a greater reduction in galling on ‘Aristotle’ and ‘Intruder’ than on ‘Paladin’.
Résumé
Le nématode à galles des racines, Meloidogyne incognita, et l’agent pathogène de l’infection à Phytophtora, Phytophthora capsici, causent, au champ, des maladies racinaires chez le poivron. Toutefois, l’information relative aux interactions entre ces deux agents pathogènes chez différents cultivars de poivrons est limitée. Des expériences ont été menées en serre sur des cultivars de poivrons affichant divers degrés de résistance à P. capsici (‘Aristotle’, ‘Paladin’ et ‘Intruder’), en leur inoculant de façon concomitante et séquentielle les deux agents pathogènes. Les traitements comprenaient l’inoculation simultanée des deux agents pathogènes dans la rhizosphère, l’inoculation de M. incognita sept jours avant l’inoculation de P. capsici, l’inoculation de P. capsici sept jours avant l’inoculation de M. incognita ainsi que l’inoculation de M. incognita seul ou de P. capsici seul. De plus, l’expérience comportait un groupe témoin qui n’a pas été inoculé. Chez les cultivars ‘Aristotle’ et ‘Intruder’, l’inoculation de P. capsici avec M. incognita simultanément ou sept jours avant ou après M. incognita a significativement réduit l’indice de la galle, comparativement à M. incognita seul. Chez le cultivar ‘Paladin’, l’inoculation de P. capsici sept jours avant ou après M. incognita a abaissé l’indice de la galle, comparativement à l’inoculation simultanée de P. capsici et M. incognita. La gravité de l’infection causée par Phytophtora n’a pas été influencée par M. incognita, et ce, indépendamment des techniques d’inoculation utilisées chez les trois génotypes. Chez ‘Aristotle’, l’inoculation de M. incognita seul n’a pas réduit le poids des racines fraîches, mais l’inoculation P. capsici seul ou combiné à M. incognita en a réduit le poids, comparativement aux plants témoins qui n’avaient pas été inoculés. Toutefois, aucun des traitements n’a réduit le poids des racines fraîches chez ‘Paladin’ ou ‘Intruder’, comparativement aux plants témoins qui n’avaient pas été inoculés. L’interaction entre P. capsici et M. incognita semble être une façon par laquelle P. capsici a réduit la formation de galles causées par M. incognita, mais le nématode n’a eu aucun effet sur la gravité de l’infection à Phytophtora. L’interaction a été influencée par les cultivars de poivron, ‘Aristotle’ et ‘Intruder’ affichant une plus forte réduction de la formation de galles que ‘Paladin’.
Introduction
Phytophthora blight caused by the oomycete pathogen Phytophthora capsici Leon. is an important soilborne disease that was first reported in 1922 (Leonian Citation1922). This pathogen has a wide host range including a variety of solanaceous (peppers, eggplant, tomato) and cucurbitaceous (watermelon, squash, cucumber, pumpkin, zucchini) crops and beans (Erwin & Ribeiro Citation1996; Anderson & Garton Citation2000; Davidson et al. Citation2002; Gevens et al. Citation2008; Abbasi et al. Citation2011). Phytophthora capsici infects all plant parts of both seedlings and mature plants, including stems, roots, leaves and fruits. The pathogen causes root rot, crown rot, leaf blight, fruit rot and plant wilt. Phytophthora blight has become an important disease posing serious threat to pepper production worldwide (Hausbeck & Lamour Citation2004; Sholberg et al. Citation2007; Granke et al. Citation2012; Sanogo & Ji Citation2012, Citation2013; Cerkauskas et al. Citation2015).
Root-knot nematodes (Meloidogyne spp.) have a worldwide distribution and cause considerable damage to bell pepper production (Sikora & Fernández Citation2005; Thies et al. Citation2005). Four species of root-knot nematodes, M. incognita (Kofoid & White) Chitwood, M. arenaria (Neal) Chitwood, M. mayaguensis Rammah & Hirschmann and M. floridensis Handoo, parasitize pepper in the subtropics and tropics, with M. incognita being the dominant species (Sikora & Fernández Citation2005). This species not only infects peppers, but also other field crops such as cotton, corn and other vegetables, and has been shown to be extremely polyphagous (Perry et al. Citation2009). Due to withdrawal of several nematicides from the market, nematode populations are difficult to control in the field, especially nematodes such as M. incognita that could infect plants in several families and survive in the soil throughout the year.
Nematode and fungal interactions were shown as early as 1892 by Atkinson who observed increased Fusarium wilt disease in cotton when root-knot nematodes were present as compared with the fungal pathogen alone (Atkinson Citation1892). Many other studies reported synergistic nematode-fungal interactions in different crops, showing nematode infection predisposing the plant or breaking host resistance for fungal or oomycete pathogens and causing higher disease incidence and severity (Powell Citation1971; Golden & van Gundy Citation1975; Welty et al. Citation1980; Mai & Abawi Citation1987; McLean & Lawrence Citation1993; Prot Citation1993; Abdel-Momen & Starr Citation1998; Back et al. Citation2002). Root-knot nematodes or other nematodes may compromise host resistance and increase disease levels caused by pathogenic fungi or Phytophthora spp. For example, co-inoculating Fusarium oxysporum f. sp. ciceri Matuo & K. Sato (FOC) and Meloidogyne spp. led to a breakdown of resistance to certain races of FOC in chickpea (Maheswari et al. Citation1995; Castillo et al. Citation2003). In another study, M. incognita significantly increased disease caused by F. oxysporum f. sp. phaseoli Kendrick & Snyder on beans (France & Abawi Citation1994). Trujillo-Viramontes et al. (Citation2005) showed that the false root-knot nematode, Nacobbus aberrans (Thorne) Thorne & Allen, disrupted disease resistance in the breeding line of chili pepper, CM-334, which was highly resistant to P. capsici. Dong et al. (Citation2009) showed a significant interaction between the fungus Cylindrocladium parasiticum Crous, M.J. Wingfield & Alfenas and M. arenaria in runner peanuts, where the presence of M. arenaria and C. parasiticum increased root rot caused by C. parasiticum on some peanut cultivars compared with C. parasiticum alone. A synergistic interaction was also reported between M. incognita and Phytophthora palmivora Butler on betelvine, where vine mortality occurred when M. incognita was inoculated before P. palmivora (Jonathan et al. Citation1996). Alston et al. (Citation2003) reported that the reniform nematode, Rotylenchulus reniformis Linford & Oliveira, predisposed papaya and increased plant mortality due to P. palmivora after nematode attack.
Little is known about the interactions between M. incognita and P. capsici in cultivars of bell pepper or other vegetable crops. There are a few commercial bell pepper cultivars, such as ‘Aristotle’, ‘Paladin’ and ‘Intruder’, which have different levels of resistance to P. capsici. It is not known whether co-infection of M. incognita and P. capsici compromises this resistance. The objective of this research was to determine the effects of co-inoculation of M. incognita and P. capsici on commercial cultivars of bell pepper with resistance to P. capsici. Understanding interactions between M. incognita and P. capsici may contribute to development of more effective management strategies for both M. incognita and P. capsici in pepper production.
Materials and methods
Plant growth and inoculum production
Three bell pepper cultivars, ‘Aristotle’, ‘Paladin’ and ‘Intruder’ were seeded separately into Styrofoam trays with 288 cells containing a commercial potting mix (Miracle-Gro LLC, Marysville, OH) and maintained in a greenhouse. All three cultivars are susceptible to M. incognita, but have varying degrees of resistance to P. capsici: ‘Aristotle’ (Seminis, Oxnard, CA, USA) has a low level of resistance, ‘Paladin’ (Rogers, Holland, MI, USA) has an intermediate level, and the new cultivar ‘Intruder’ (Syngenta Seeds Inc., Naples, FL, USA) has a high level of resistance (Ji et al., unpublished; Yin et al. Citation2012). Five weeks after seeding, plants were transplanted individually into 10-cm pots containing the potting mix. The plants were maintained on a greenhouse bench for 2 more weeks before inoculation with pathogens.
Cultures of M. incognita race 3 were maintained in eggplant (‘Black Beauty’). The galled roots, obtained from 3-month-old greenhouse cultures, were placed in a mist chamber for 2–7 days for the eggs to hatch and second-stage juveniles (J2) were collected on a 25 μm pore sieve every 2–3 days and stored at 4°C for no more than 7 days before use as inoculum. A P. capsici strain PC121 (A2) isolated from bell pepper in Tifton, GA, was grown on V8 juice agar dishes wrapped with Parafilm at 25°C for 5 days. The dishes, with Parafilm removed, were then placed under fluorescent lights at room temperature (23 ± 2°C) to induce sporangial production. Two days after incubation under light, the dishes were flooded with sterile distilled water and kept at 4°C for 30 min and then at room temperature for 0.5 to 1 h to stimulate zoospore release. Zoospores were harvested into 50 mL sterile polypropylene tubes (Fisher Scientific, Pittsburgh, PA, USA), and the concentration was adjusted to 2000 zoospores mL−1 with sterile distilled water using a hemocytometer.
Treatments and disease assessment
The following six treatments were applied to the root zone of each of the three pepper cultivars. (1) P. capsici only, (2) M. incognita only, (3) M. incognita + P. capsici simultaneous inoculation at 0 day, (4) M. incognita inoculation 7 days prior to P. capsici inoculation, (5) M. incognita inoculation 7 days after P. capsici inoculation and (6) untreated control without M. incognita or P. capsici inoculation. The plants were inoculated with P. capsici on the same day and M. incognita was used for inoculation on different days as described above. For inoculation with nematodes, each pot received 1000 J2s around the base of the stem and covered with potting soil. To inoculate with P. capsici, each plant received 5 mL of zoospore suspension (2000 zoospores mL−1) to the soil surface around the plant (Yin et al. Citation2012). The experiment was arranged in randomized complete block design with 10 replications for each treatment and was repeated once under the same conditions. The plants were maintained in the greenhouse at 20ºC (night) to 28ºC (day) with approximately 12 h photoperiod and watered once or twice a day and fertilized with 24-8-16 N-P-K every 10 days.
Severity of Phytophthora blight was assessed 4 weeks after inoculation with P. capsici using a 0–5 scale modified from Ristaino (Citation1990) where 0 = no disease; 1 = stem necrosis without girdling; 2 = stem necrosis with girdling; 3 = stem necrosis with <50% defoliation; 4 = stem necrosis with >50% defoliation; and 5 = dead. Gall indices for M. incognita were determined after washing the roots of adhering soil particles. The index was on a 0–10 scale based on the percentage of root system with galls (0 = no galls; 1 = 1–10%; … 10 = 91–100%) as described previously (Aryal et al. Citation2011). Fresh root weight was measured after blot drying the roots on paper towels.
Analysis of variance (Proc. GLM; SAS Institute, Cary, NC) was used to determine whether pepper cultivar, pathogen combination, or their interaction influenced M. incognita gall index, Phytophthora blight severity, and fresh root weight. Because there was no interaction (P > 0.05) between experimental trials, the data were combined across trials in the figures. Treatment means were separated by Fisher’s protected least significant difference (LSD) test at P ≤ 0.05.
Results
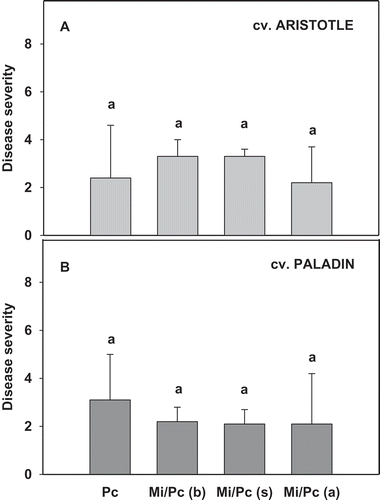
Inoculation of M. incognita did not affect blight severity in any of the three pepper cultivars and there was no interaction between cultivar and M. incognita inoculation on the disease severity (). The cultivar ‘Paladin’ had mean Phytophthora blight severity between 2.1 to 3.1 on the 0–5 scale and ‘Aristotle’ between 2.2 to 3.3. The cultivar ‘Intruder’ did not show any disease when inoculated with P. capsici alone or with M. incognita.
Fig. 1 Effects of Phytophthora capsici and Meloidogyne incognita co-inoculation as compared with P. capsici alone on Phytophthora blight severity in two bell pepper cultivars: (a) ‘Aristotle’ and (b) ‘Paladin’. Mean values presented were combined results from two experiments (experiment × treatment, P = 0.38; N = 20). Pc = P. capsici and Mi = M. incognita. Mi/Pc (b), Mi/Pc (s), and Mi/Pc (a) indicate M. incognita was inoculated before, at the same time as, and after P. capsici, respectively. Treatments within each cultivar with different letters are significantly different according to the Fisher’s protected least significant difference (P ≤ 0.05) test. Error bars are standard errors of the means of two experiments.
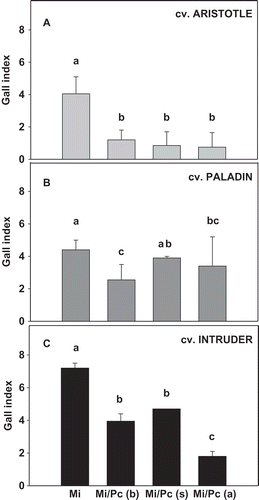
The bell pepper cultivar ‘Intruder’ had higher (P ≤ 0.05) gall indices than the cultivars ‘Paladin’ and ‘Aristotle’, with a gall rating of 7.3, 4.4 and 4.1 for ‘Intruder’, ‘Paladin’ and ‘Aristotle’, respectively, when inoculated with M. incognita alone. Across cultivars, plants inoculated with M. incognita 7 days after P. capsici had the lowest (P ≤ 0.05) gall indices as compared with M. incognita inoculation alone. The effect of P. capsici on root galling by M. incognita was not consistent among cultivars (cultivar × P. capsici interaction, P < 0.0001). The per cent reduction in gall index due to inoculation of M. incognita 7 days after P. capsici was 82.9% in ‘Aristotle’, 75.2% in ‘Intruder’ and 22.7% in ‘Paladin’ compared with the gall index in M. incognita alone (). Inoculation of M. incognita 7 days before P. capsici inoculation also resulted in less (P ≤ 0.05) galling in all three cultivars as compared with M. incognita alone with the highest reduction in ‘Aristotle’ at 70.7%, followed by 45.5% and 40.9% reduction in ‘Intruder’ and in ‘Paladin’, respectively. Simultaneous inoculation with P. capsici reduced gall index (P ≤ 0.05) compared with M. incognita alone by 35.9% in ‘Intruder’ and 80.5% in ‘Aristotle’. In ‘Paladin’, simultaneous inoculation of M. incognita with P. capsici did not reduce gall indices.
Fig. 2 Effects of Phytophthora capsici and Meloidogyne incognita co-inoculation as compared with M. incognita alone on gall index in three bell pepper cultivars: (a) ‘Aristotle’, (b) ‘Paladin’ and (c) ‘Intruder’. Mean values are combined results from two trials (trial × treatment, P = 0.53; N = 20). Pc = P. capsici and Mi = M. incognita. Mi/Pc (b), Mi/Pc (s) and Mi/Pc (a) indicate M. incognita was inoculated before, at the same time as, and after P. capsici, respectively. Treatments within each cultivar with different letters are significantly different according to Fisher’s protected least significant difference test (P ≤ 0.05). Error bars are standard errors of the means of two experiments.
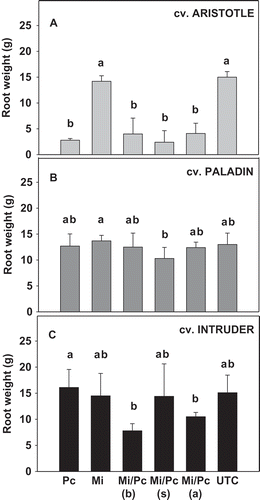
There was an interaction (P < 0.0001) between cultivar and pathogen inoculation on fresh root weight. When the cultivars were analysed separately, inoculation with P. capsici alone or combined with M. incognita reduced (P ≤ 0.05) fresh root weight in ‘Aristotle’ by 71.8–85.3% compared with the untreated control (). In contrast, neither P. capsici alone or in combination with M. incognita reduced fresh root weight compared with the untreated control in ‘Paladin’ () or ‘Intruder’ (). Inoculation of M. incognita alone did not reduce fresh root weight compared with the untreated control in any of the cultivars.
Fig. 3 Effects of Phytophthora capsici and Meloidogyne incognita co-inoculation on fresh root weight of three bell pepper cultivars: (a) ‘Aristotle’, (b) ‘Paladin’ and (c) ‘Intruder’. Mean values are combined results from two trial (trial × treatment, P = 0.93; N = 20). Pc = P. capsici, Mi = M. incognita and UTC = untreated control. Mi/Pc (b), Mi/Pc (s), and Mi/Pc (a) indicate M. incognita was inoculated before, at the same time as, and after P. capsici, respectively. Treatments within each cultivar with different letters are significantly different according to Fisher’s protected least significant difference test (P ≤ 0.05). Error bars are standard errors of the means of two experiments.
Discussion
Inoculation with M. incognita did not significantly enhance susceptibility of the bell pepper cultivars to P. capsici in this study. It appeared that there was only a one-way antagonistic interaction between P. capsici and M. incognita, where inoculation with P. capsici decreased gall development of root-knot nematode in the three cultivars of bell pepper evaluated. This is in contrast with a number of studies that showed the interactions between nematodes and other fungal or oomycete pathogens were synergistic, where nematodes increased severity of the diseases caused by other pathogens. In a study with seven rootstocks of pepper, co-inoculation with M. incognita and P. capsici did not increase the percentage of plants affected by P. capsici (Ros et al. Citation2014). In another study, Thies et al. (Citation2011) reported that M. incognita when co-inoculated with P. capsici had no effect on Phytophthora blight severity in bell pepper. However, the authors did not report if P. capsici had any influence on M. incognita infection. Our study is the first report demonstrating an antagonistic interaction, where inoculation with P. capsici decreased root galling caused by M. incognita in bell pepper cultivars with different levels of resistance to P. capsici.
The reduction of M. incognita gall index when P. capsici was co-inoculated to the three bell pepper cultivars was consistent irrespective of host resistance of the cultivars to P. capsici. The mechanisms behind the reduction in root galling by M. incognita in the presence of P. capsici are unknown. Based on the life cycles of these pathogens, both M. incognita and P. capsici initially infect the fibrous root cortex region. It has been shown that within a few hours of inoculation, P. capsici zoospores were encysted and germinated irrespective of whether the cultivar was sensitive or resistant to the pathogen (Palloix et al. Citation1988). Therefore, infection by P. capsici may be faster than M. incognita in the root zone and competition for entry or other physiological factors may be involved, resulting in reduction of M. incognita infection when P. capsici is present.
The severity of black shank of tobacco caused by Phytophthora nicotianae Breda de Haan increased when plant-parasitic nematodes were present, especially root-knot nematodes (Meloidogyne spp.) (Powell & Nusbaum Citation1960). During these nematode–Phytophthora interactions, black shank resistant tobacco cultivars were affected by severe disease losses. Histopathological studies showed that P. nicotianae successfully colonized wherever nematodes caused damage on the roots due to feeding. This colonization by P. nicotianae resulted in the disruption of gall tissue in and around Meloidogyne spp. feeding sites (Powell & Nusbaum Citation1960). Similar effects were observed with the root-knot nematode species M. javanica and P. nicotianae in tobacco that showed roots of the resistant variety NC95 infested with the nematode were more extensively colonized by P. nicotianae than non-galled root tissue (Miller Citation1968). In the present study, M. incognita did not increase or decrease P. capsici infection but P. capsici decreased root galling by M. incognita. There is a likelihood of P. capsici damaging the gall tissue and thereby halting further giant cell development (Powell & Nusbaum Citation1960).
Antagonistic interactions between plant-parasitic nematodes and other nematodes and/or pathogens also exist, although less information is available. For example, El-Borai et al. (Citation2002) showed that citrus nematode (Tylenchulus semipenetrans Cobb) reduced root infection by P. nicotianae in citrus; however, the mechanism of reduction was not determined. Another study showed that the root-knot nematode, M. incognita, reduced reniform nematode, Rotylenchulus reniformis, in cotton during concomitant inoculation by inducing systemic acquired resistance in the plant (Aryal et al. Citation2011). It is believed that most of these interactions involve either direct or indirect complex physiological, molecular or biochemical associations (El-Borai et al. Citation2002).
In addition to synergistic and antagonistic interactions between nematodes and other pathogens, a few reports also showed that nematode and fungal co-infection did not alter disease development by either pathogen. In some circumstances, disease development varied by genotypes. Positive or negative interactions between M. artiellia Franklin and F. oxysporum f. sp. ciceris occurred on some genotypes of chickpea, while in other genotypes there was no interaction between the pathogens (Navas-Cortés et al. Citation2008). All combined, there are several possible outcomes of nematode–fungus co-infection, including nematode reducing fungal disease development, fungus reducing nematode development, both nematode and fungus decreasing each other’s development, and no interaction between the two pathogens.
In conclusion, this study demonstrated that there was a one-way interaction between M. incognita and P. capsici when co-inoculated on bell pepper. The nematode did not increase or decrease disease caused by P. capsici, but P. capsici decreased root galling by M. incognita. Further research is needed to determine if the phenomenon occurs under field conditions and if the different treatments affect pepper yield. Additional studies are also needed to elucidate the mechanisms involved in reduction of root galling due to P. capsici infection. These studies will provide a more comprehensive understanding of the interactions between these two important soilborne pathogens.
Acknowledgements
The authors thank Michael Purvis and David Clements for providing technical assistance. This work was supported by a USDA-NIFA grant for integrated management of Phytophthora blight on vegetables.
References
- Abbasi PA, Lazarovits G, Weselowski B. 2011. Effectiveness of AG3 phosphonate formulation in suppressing phytophthora blight in cucumber and bell pepper plants under growth room conditions. Can J Plant Pathol. 33:150–158.
- Abdel-Momen SM, Starr JL. 1998. Meloidogyne javanica-Rhizoctonia solani disease complex of peanut. Fundam Appl Nematol. 21:611–616.
- Alston DG, Sipes BS, Uchida J, Schmitt DP, Chia CL. 2003. Interactive effects of Rotylenchulus reniformis and Phytophthora palmivora on papaya (Carica papaya L.) survival and growth in greenhouse pots. Nematropica. 33:73–85.
- Anderson TR, Garton R. 2000. First report of blight of field peppers caused by Phytophthora capsici in Ontario. Plant Dis. 84:705.
- Aryal SK, Davis RF, Stevenson KL, Timper P, Ji P. 2011. Induction of systemic acquired resistance by Rotylenchulus reniformis and Meloidogyne incognita in cotton following separate and concomitant inoculations. J Nematol. 43:160–165.
- Atkinson GF. 1892. Some diseases of cotton. Bull Ala Agric Exp Stn. 41:61–65.
- Back MA, Haydock PPJ, Jenkinson P. 2002. Disease complexes involving plant parasitic nematodes and soil borne pathogens. Plant Pathol. 51:683–697.
- Castillo P, Navas-Cortes JA, Gomar-Tinoco D, Di Vito M, Jimenez-Diaz RM. 2003. Interactions between Meloidogyne artiellia, the cereal and legume root-knot nematode, and Fusarium oxysporum f. sp. ciceris race 5 in chickpea. Phytopathology. 93:1513–1523.
- Cerkauskas RF, Ferguson G, MacNair C. 2015. Management of Phytophthora blight (Phytophthora capsici) on vegetables in Ontario: some greenhouse and field aspects. Can J Plant Pathol. 37:285–304.
- Davidson CR, Carroll RB, Evans TA, Mulrooney RP, Kim SH. 2002. First report of Phytophthora capsici infecting lima bean (Phaseolus lunatus) in the Mid-Atlantic region. Plant Dis. 86:1049–1052.
- Dong WB, Brenneman TB, Holbrook CC, Timper P, Culbreath AK. 2009. The interaction between Meloidogyne arenaria and Cylindrocladium parasiticum in runner peanut. Plant Pathol. 58:71–79.
- El-Borai FE, Duncan LW, Graham JH. 2002. Infection of citrus roots reduces root infection by Phytophthora nicotianae. J Nematol. 34:384–389.
- Erwin DC, Ribeiro OK. 1996. Phytophthora diseases worldwide. St. Paul (MN): The American Phytopathological Society.
- France RA, Abawi GS. 1994. Interaction between Meloidogyne incognita and Fusarium oxysporum f.sp. phaseoli on selected bean genotypes. J Nematol. 26:467–474.
- Gevens AJ, Donahoo RS, Lamour KH, Hausbeck MK. 2008. Characterization of Phytophthora capsici causing foliar and pod blight of snap bean in Michigan. Plant Dis. 92:201–209.
- Golden JK, van Gundy SD. 1975. A disease complex of okra and tomato involving the nematode Meloidogyne incognita and the soil-inhabiting fungus, Rhizoctonia solani. Phytopathology. 65:265–273.
- Granke LL, Quesada-Ocampo L, Lamour KH, Hausbeck MK. 2012. Advances in research on Phytophthora capsici on vegetable crops in the United States. Plant Dis. 95:1588–1600.
- Hausbeck MK, Lamour KH. 2004. Phytophthora capsici on vegetable crops: research progress and management challenges. Plant Dis. 88:1292–1303.
- Jonathan EI, Sivakumar M, Padmanabhan D. 1996. Interaction of Meloidogyne incognita and Phytophthora palmivora on betelvine [Piper betle, India]. Nematol Mediterr. 24:341–343.
- Leonian L. 1922. Stem and fruit blight of peppers caused by Phytophthora capsici. Phytopathology. 12:401–408.
- Maheswari UT, Sharma SB, Reddy DD, Haware MP. 1995. Co-infection of wilt-resistant chickpeas by Fusarium oxysporum f.sp. ciceri and Meloidogyne javanica. J Nematol. 27:649–653.
- Mai WF, Abawi GS. 1987. Interactions among root-knot nematodes and Fusarium wilt fungi on host plants. Annu Rev Phytopathol. 25:317–338.
- McLean KS, Lawrence GW. 1993. Interrelationship of Heterodera glycines and Fusarium solani in sudden-death syndrome of soybean. J Nematol. 25:434–439.
- Miller CR. 1968. Interaction of Meloidogyne javonica and Phytophthora parasitica var. nicotianae on Nicotiana tabacum ‘NC 95ʹ. Phytopathology. 58:553(abstr.).
- Navas-Cortés JA, Landa BB, Rodríguez-López J, Jiménez-Díaz RM, Castillo P. 2008. Infection by Meloidogyne artiellia does not break down resistance to races 0, 1A, and 2 of Fusarium oxysporum f. sp. ciceris in chickpea genotypes. Phytopathology. 98:709–718.
- Palloix A, Daubeze AM, Pochard E. 1988. Time sequences of root infection and resistance expression in an artificial inoculation method of pepper with Phytophthora capsici. J Phytopathol. 123:12–24.
- Perry RN, Moens M, Starr JL. 2009. Meloidogyne species - a diverse group of novel and important plant parasites. In: Perry RN, Moens M, Starr JL, editor. Root-knot nematodes. CABI, Wallingford, UK.
- Powell NT. 1971. Interactions between nematodes and fungi in disease complexes. Annu Rev Phytopathol. 9:253–274.
- Powell NT, Nusbaum CJ. 1960. The black shank-root knot complex in flue-cured tobacco. Phytopathology. 50:899–906.
- Prot J. 1993. Biochemical and genetic basis of fungus-nematode interactions. In: Khan MV, editor. Nematode interactions. New York (NY): Chapman and Hall; p. 273–285.
- Ristaino JB. 1990. Intraspecific variation among isolates of Phytophthora capsici from pepper and cucurbit fields in North Carolina. Phytopathology. 80:1253–1259.
- Ros C, Lacasa CM, Martínez V, Bielza P, Lacasa A. 2014. Response of pepper rootstocks to co-infection of Meloidogyne incognita and Phytophthora spp. Eur J Hortic Sci. 79:22–28.
- Sanogo S, Ji P. 2012. Integrated management of Phytophthora capsici on solanaceous and cucurbitaceous crops: current status, gaps in knowledge, and research needs. Can J Plant Pathol. 34:479–492.
- Sanogo S, Ji P. 2013. Water management in relation to control of Phytophthora capsici in vegetable crops. Agri Water Manag. 129:113–119.
- Sholberg PL, Walker MC, O’Gorman DT, Jesperson GD. 2007. First report of Phytophthora capsici on cucurbits and peppers in British Columbia. Can J Plant Pathol. 29:153–158.
- Sikora RA, Fernández E. 2005. Nematode parasites of vegetables. In: Luc M, Sikora RA, Bridge J, editors. Plant parasitic nematodes in subtropical and tropical agriculture. Wallingford: CABI Publishing; p. 319–392.
- Thies JA, Ariss JJ, Kousik CS. 2011. Response of pepper (Capsicum annuum) genotypes to co-infection by Phytophthora capsici and Meloidogyne incognita. Phytopathology. 101:S176(abstr.).
- Thies JA, Davis RF, Mueller JD, Fery RL, Langston DB, Miller G. 2005. Host resistance and metam sodium for managing root-knot nematodes in a pepper-cucumber rotation. HortScience. 40:2080–2082.
- Trujillo-Viramontes F, Zavaleta-Mejía E, Rojas-Martínez RI, Lara J. 2005. Inoculation time and inoculum density are determining factors in induction of breaking of resistance to Phytophthora capsici in chili pepper (Capsicum annuum) by Nacobbus aberrans. Nematropica. 35:37–44.
- Welty RE, Barker KR, Lindsey DL. 1980. Effects of Meloidogyne hapla and M. incognita on Phytophthora root rot of alfalfa. Plant Dis. 64:1097–1099.
- Yin J, Jackson KL, Candole BL, Csinos AS, Langston DB, Ji P. 2012. Aggressiveness and diversity of Phytophthora capsici on vegetable crops in Georgia. Ann Appl Biol. 160:191–200.
