Abstract
Fungi causing post-harvest decay of greenhouse-grown tomato fruits in British Columbia were recovered from diseased samples collected during 2010 and 2011. The most frequently isolated fungi were Penicillium olsonii, Botrytis cinerea, Rhizopus stolonifer and Alternaria alternata, and to a lesser extent Galactomyces geotrichum. Pathogenicity tests showed that R. stolonifer caused the greatest fruit decay, followed by P. olsonii. The remaining fungi also caused some fruit rot, and lesion development by all fungi was enhanced by wounding of the fruits. A post-inoculation incubation temperature of 21°C promoted much greater disease development compared with 13°C. To assess the composition of the mycoflora on the surface of ripening tomato fruits, swab samples were collected weekly over a 6–18 week period from two commercial greenhouses during 2011 and 2012 and streaked onto potato dextrose agar. The most commonly recovered fungi from the fruit surfaces were species of Penicillium, Cladosporium, Aspergillus, Rhizopus and Alternaria. The composition of fungal populations fluctuated from one week to the next in both greenhouses and over both years. Samples of leaf litter consisting of discarded leaf prunings harboured all of these fungi that were present on the fruit surface. Harvested fruit samples were subsequently incubated at 21°C to assess the level of disease development. The resulting fruit decay was caused primarily by species of Penicillium, Alternaria and Rhizopus, and a major source of inoculum originated from the stem and calyx tissues, which led to fruit infection near the stem end. Shipments of tomato fruits in refrigerated trucks were monitored over a 6–10 day period after harvest and showed variable temperature and humidity levels during transportation. Post-harvest decay of tomatoes is influenced by the composition of the fruit surface mycoflora, presence of fruit injury, storage temperatures and environmental conditions during shipping.
Résumé
Des champignons causant la pourriture post-récolte des tomates cultivées en serre en Colombie-Britannique ont été collectés en 2010 et 2011 sur des échantillons infectés. Les champignons les plus souvent isolés étaient Penicillium olsonii, Botrytis cinerea, Rhizopus stolonifer et Alternaria alternata et, à un degré moindre, Galactomyces geotrichum. Des tests de pathogénicité ont montré que R. stolonifer causait le plus de pourriture sur les fruits, suivi de P. olsonii. Les autres champignons ont également causé un certain degré de pourriture, et la formation de lésions causées par tous les champignons était accentuée par les blessures que les fruits avaient subies. Une incubation post-inoculation à 21°C a favorisé un développement plus fulgurant de la maladie par comparaison à une incubation à 13°C. Afin d’évaluer la composition de la mycoflore qui se développe à la surface des tomates qui murissent, des prélèvements ont été effectués hebdomadairement durant 6 à 18 semaines dans deux serres commerciales en 2011 et 2012, et déposés sur de la gélose dextrosée à la pomme de terre. Les champignons les plus souvent trouvés à la surface des fruits appartenaient à des espèces de Penicillium, de Cladosporium, d’Aspergillus, de Rhizopus et d’Alternaria. La composition des populations fongiques variait d’une semaine à l’autre, et ce, dans les deux serres et durant les deux années. Des échantillons de la litière de feuilles provenant des feuilles enlevées et jetées hébergeaient tous ces champignons qui poussaient à la surface des tomates. Les échantillons de fruits collectés ont été par la suite incubés à 21°C pour évaluer le degré de développement de la maladie. La pourriture qui en a résulté était causée principalement par des espèces de Penicillium, d’Alternaria et de Rhizopus, et une source majeure d’inoculum provenait des tissus de la tige et du calice, ce qui engendrait l’infection des fruits près de l’extrémité pédonculaire. Les expéditions de tomates dans des camions réfrigérés ont été suivies de près durant 6 à 10 jours après la récolte et les conditions ont révélé différentes valeurs de température et d’humidité durant le transport. La pourriture post-récolte des tomates est influencée par la composition de la mycoflore colonisant la surface des fruits, la présence ou l’absence de blessure, les températures d’entreposage et les conditions de milieu durant l’expédition.
Introduction
Greenhouse vegetable production in Canada consists of tomatoes, cucumbers, bell peppers and lettuce, with tomatoes (Solanum lycopersicum L.) representing over half of total sales, or $500 million annually. British Columbia (BC) is the second-largest producer of tomatoes in Canada (22% of total production) after Ontario (Fruit and Vegetable … Citation2009; Crop profile … Citation2011), with about 62 million kg of greenhouse tomatoes, valued at $141 million, produced under 116 ha of growing space. The tomatoes are branded as ‘tomato-on-the-vine’ or ‘vine-ripened’ and sold locally or shipped to the USA and Asia. Plants are initiated in late January–early February and grown until November. The industry utilizes mostly soil-less hydroponic culture, but a small percentage (c. 2%) of growers use soil-grown organic production methods. Fruits are harvested starting in late April, packed directly into cardboard cartons and shipped to a distribution centre, after which they are sent to retail outlets. The shipment from the greenhouse to the distribution centre and to the final destination may take 4–12 days, requiring controlled environmental conditions for up to a week. The post-harvest handling conditions are critical to maintaining optimal quality of the fruits, which should ideally be rapidly cooled after harvest and kept in a temperature controlled environment (13°C) with 70–75% relative humidity (Jones et al. Citation1991).
Many fungal pathogens cause post-harvest decay of tomato fruits grown under field and greenhouse conditions (Snowdon Citation1990; Jones et al. Citation1991; Howard et al. Citation1994; Bartz et al. Citation2002; Blancard Citation2012). These fungi may infect the fruits prior to and/or during harvest and subsequently develop during storage and shipping. Many remain undetected until environmental conditions are conducive for fungal growth. The range of pathogens that may cause post-harvest losses on greenhouse-grown tomatoes in BC are not well-characterized, and the sources of inoculum and conditions promoting fruit decay, including the importance of wounding, have not been studied.
The objectives of this study were to determine: (i) the range of fungi infecting greenhouse tomato fruits sampled over a 3-year period and the potential sources of inoculum; (ii) the influence of wounding and post-harvest temperatures on disease development; and (iii) the composition of fruit surface mycoflora and their potential role in disease development.
Materials and methods
Recovery of pathogens
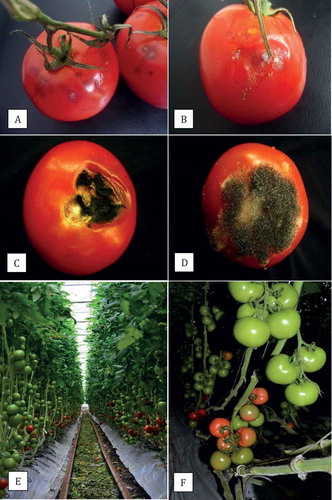
Tomato fruits displaying a range of symptoms, from small necrotic flecks to larger sunken lesions (–), were obtained from the storage facilities located on-site at two commercial greenhouse operations located in Delta and Langley, BC. In addition, fruit samples were obtained after they were shipped from the greenhouse to the distributor/retail outlets. Additional samples were obtained from several retail outlets throughout the lower Fraser Valley region. All fruits were of the vine-ripened TOV (tomato-on-the-vine) brand with the calyx attached (). Sampling was conducted from April–July, 2010 and repeated again during May–August, 2011. A minimum of 80 fruits were collected in each year of sampling. Individual fruits were placed inside a plastic bag, transported to the laboratory, and left at room temperature (21–23°C) overnight. Tissue pieces (~ 0.25 mm2) were cut from lesioned areas and dipped for 30 s in 70% ethanol followed by immersion in 0.5% NaOCl for 30 s, and rinsed thrice with sterile distilled water. Excess moisture was blotted on sterile filter paper and tissue pieces were placed on V8 juice agar and potato dextrose agar (PDA). Dishes were incubated under ambient laboratory conditions (21 ± 2°C) until fungal growth was observed (5–7 days), at which time mycelial transfers using 5-mm diameter discs were made onto fresh PDA. For morphological identification to the genus level, cultures grown on PDA were examined for macroscopic and microscopic features and compared with those of common fungal pathogens previously described from tomato fruits (Snowdon Citation1990; Howard et al. Citation1994; Blancard Citation2012). Representative isolates of different genera were selected, and hyphal-tip cultures were initiated from colonies growing on 1% water agar, transferred to PDA, and stored at 4°C. Further molecular identification to species level was conducted by the University of Guelph Laboratory Services, Agriculture and Food Laboratory, Guelph, ON using PCR with ITS primers ITS1 – 5ʹ-CTTGGTCATTTAGAGGAAGTAA-3ʹ and ITS4 – 5ʹ-TCCTCCGCTTATTGATATGC-3ʹ according to established protocols.
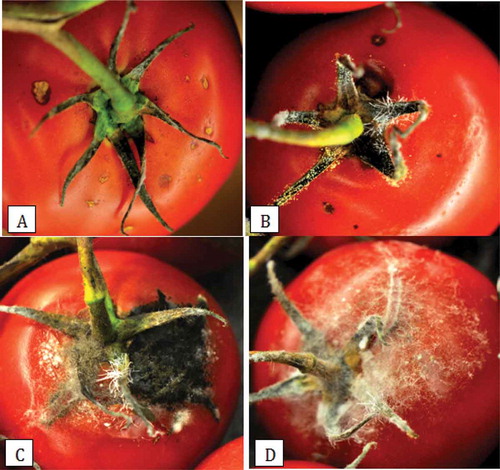
Fig. 1 (Colour online) Symptoms of fungal infection on tomato fruits and sampling for fruit surface mycoflora. (a, b) Initial symptoms of Penicillium fruit rot caused by P. olsonii; (c) Black mould caused by Alternaria alternata; (d) Rhizopus soft rot caused by R. stolonifer; (e) Fruit clusters of tomato in proximity to leaf litter left in the alleyway; (f) Fruit cluster at the breaker stage of ripening (bottom) that was used to sample for the presence of fruit surface mycoflora.
Pathogenicity studies
Fungal isolates recovered from tomato fruits were selected to represent different genera recovered in each of the two years. Healthy tomatoes (without any visible blemishes or infection) of the cultivars ‘Endeavour’ or ‘Kommett’, provided by the commercial greenhouse grower, were washed in water, surface-sterilized by dipping them in 70% ethanol for 30 s, and left to air-dry prior to inoculation. To prepare fungal inoculum, isolates were grown on PDA for 2 weeks under ambient laboratory conditions (21 ± 2°C). Mycelial plugs, 0.5 cm in diameter, were removed from the colony margin and carefully placed mycelial side down, on the fruit near the shoulder region. On one set of fruits, a small wound was created by inserting a sterile dissecting needle gently into the fruit to a depth of 3 mm and the plug was placed over the wound. Controls were either wounded or not and received a plug of PDA. There were 16 replicate fruits for each treatment (wounded, non-wounded) and a total of 40 isolates were evaluated. Fruits were placed inside a plastic container (20 × 12 × 8 cm) lined with moist paper towel and misted with sterile distilled water. One set of eight fruits from each treatment/isolate was placed in a Conviron incubator set at 13°C and 80% relative humidity (RH), while a second set was placed at 21°C and the same RH. The fruit were monitored daily for disease development and infection was rated after 10 days of incubation. The extent of fungal growth (colonization) of the fruit surface away from the mycelial plug, inclusive of any softening of the fruit, was measured in two directions perpendicular to each other. Reisolations were made by plating diseased tissues onto PDA and the resulting colonies identified. The experiment was conducted twice. Data were analysed using the Statistical Analysis System (SAS Institute, version 9.1, Cary, NC). Data from repeated trials were homogeneous based on Bartlett’s test for homogeneity of variance, and therefore were pooled prior to analysis. Analysis of variance (ANOVA) was performed initially using PROC ANOVA, and means associated with each treatment and fungal species were separated using Tukey’s HSD test when the ANOVA indicated significance (P ≤ 0.05).
Monitoring presence of fruit surface mycoflora
Two commercial tomato greenhouses located in Delta and Langley, BC, were selected to monitor fruit surface mycoflora over two consecutive years (2011, 2012). Greenhouse 1 used a conventional hydroponic production system, in which plants were propagated on rockwool blocks in sawdust bags, and provided with appropriate nutrient solution according to the recommendations for commercial tomato production (BCMAFF Citation1997). Greenhouse 2 used an organic production system, in which plants were propagated in steam-sterilized soil, and provided with organic sources of fertilizer (bone meal, chicken litter). All plants were pruned and trained on supporting wires as per standard production practices (, ). Sampling of fruit surface mycoflora was initiated midway through the growing season in 2011 (15 June) for a 6-week period. In 2012, sampling was initiated on 10 July for an 18-week period, ending on 26 November. Two rows, each ~100 m long, were set aside for the sampling and managed by the grower using the same practices as for the entire greenhouse. Clusters of fruit () were harvested between 8:00–11:00 am from both greenhouses and brought to the laboratory. Samples consisted of 10 clusters (replicates) of five fruits each, taken from different plants within the 100 m rows, and were at the commercial industry breaker stage of ripening (Jones et al. Citation1991). From each replicate cluster, two fruits were chosen arbitrarily and the surface (~2 cm2 area around the shoulder region) and the calyx were swabbed with a sterile Q-tip. The Q-tip was streaked onto the surface of PDA, for a total of 10 dishes per sampling time, which were incubated at ambient temperature (range of 21–24°C) and 12 h of fluorescent lighting in the laboratory. One week later, fungal colonies with similar morphology were enumerated on each dish. Representative colonies were subcultured onto PDA, identified morphologically to genus level, and subsequently identified to species level using PCR as described earlier for the fungi recovered from symptomatic tomato fruits. The total numbers of each fungal species from the 10 Petri dishes per treatment per sampling date for each greenhouse were averaged. Simple linear regression analysis and Pearson’s r-value (Kenney & Keeping Citation1962) were used to establish the trend between numbers of fungal colonies and time of sampling. In addition to fruit samples, leaf litter was collected from the alleyways between the plant rows where they were left to dry (). The leaf samples were placed inside plastic bags for several days under ambient laboratory conditions and examined microscopically for the types of fungi present. Small leaf segments were surface-sterilized and plated onto PDA to recover and identify the fungi as described previously. Pathogenicity tests were conducted for 2–3 isolates of each of the different fungal genera recovered from the fruit surface and leaf litter as described previously.
Disease development
The 10 fruit clusters (replicates), each with five fruits, used for the fruit surface mycoflora assay, were split into two groups after the swabbing was completed and each was placed in a cardboard carton, misted with sterile distilled water, and a rubbish bag was placed over the top, loosely covering the carton. The fruits were placed inside an incubator set either at 13°C or at 21°C, both with 75% RH for 12–16 days. Disease incidence (number of fruit infected/25) and disease severity were rated using a scale of 0 to 4 for each fruit individually, where: 0 = no infection of fruit; 1 = minor spotting, with < 10% of fruit surface area infected; 2 = 10–25% of fruit surface area infected, large lesions visible; 3 = 26–50% of fruit surface infected, decay visible; 4 = > 50% of the fruit affected, advanced decay, fruit almost destroyed. The fruit samples were collected weekly over a 6-week period in 2011 and an 18-week period in 2012. The relationship between disease incidence and disease severity was determined using simple linear regression analysis and determination of Pearson’s r-value.
Monitoring of temperature and humidity conditions during shipping
To monitor post-harvest conditions during shipping, temperature and humidity data were collected using HOBO® U12-013 thermocouples (Onset Computer Corporation, Mourne, MA) connected to data loggers which were placed inside two replicate cardboard cartons of fruits shortly after harvest and prior to shipment from greenhouse 1. A total of four shipments, all to Delaware, USA, were monitored during September–October 2012. The data loggers were set to record at 5 min intervals until the fruits reached their destination, which was followed by storage on-site as required (a total of 6–10 days). At that time, the data loggers were retrieved prior to shipment of the fruits to retail outlets. Graphs of temperature and relative humidity over time during transport and delivery and storage at the distribution centre were plotted after downloading the data into the HOBOware Lite v. 3.3 (MicroDAQ), and subsequently exported to Microsoft Excel (2007).
Results
Recovery of fungi and pathogenicity testing
Fruit samples collected during 2010–2011 exhibited fungal growth (–) when placed inside a plastic bag and incubated overnight at room temperature. Five different genera and species were recovered (). The most frequently isolated fungus was Penicillium olsonii Bainier & Sartory in both years of the study. The second-most frequently isolated fungus was Botrytis cinerea Per.:Fr., followed by Alternaria alternata Fr. Keissl., Rhizopus stolonifer (Enhren.) Vuill. and Galactomyces geotrichum (Butler & Petersen) Redhead & Malloch (). The frequency of recovery of P. olsonii was higher earlier during the growing season (April–June) compared with July–August; for the remaining fungi, recovery was higher later during the season (July–August). In pathogenicity tests conducted on non-wounded and wounded fruits, R. stolonifer and P. olsonii caused the greatest extent of decay, followed by B. cinerea (). Wounding of the fruits increased the extent of colonization by all fungi tested. Fruits incubated at 21°C had greater overall disease compared with 13°C.
Table 1. Frequency of recovery of fungi from symptomatic tomato fruits collected during 2010 and 2011.
Table 2. Fungal colonization of tomato fruits (expressed as mycelial growth plus fruit softening) 10 days after inoculation with five fungi with or without wounding and incubation at two temperatures.
Monitoring presence of fruit surface mycoflora
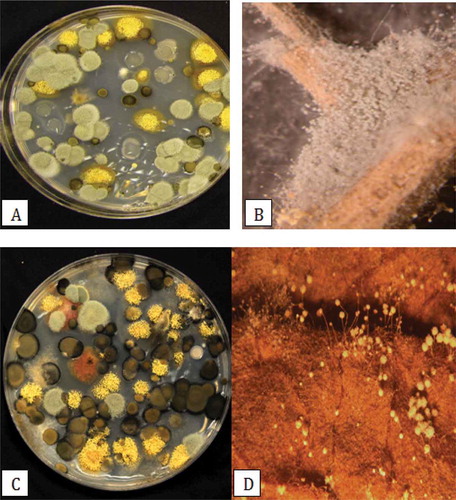
The mean colony-forming units of different fungi recovered on PDA over a 6-week period in 2011 from greenhouses located in Delta (greenhouse 1) and Langley, BC (greenhouse 2) are shown in . The most frequently recovered genus was Penicillium, followed by Cladosporium in greenhouse 1 and Aspergillus in greenhouse 2. In greenhouse 1, fungal populations were generally low over the first 3 weeks of sampling, and increased rapidly in the following 3 weeks. In greenhouse 2, total fungal population levels were generally higher than in greenhouse 1, and Aspergillus populations increased rapidly after 4 weeks. Very few bacterial colonies were observed growing on the plates. For 2012, the data are shown as total fungal colonies recovered (inclusive of Aspergillus, Cladosporium, Penicillium, Rhizopus and others) and those of Penicillium are represented as a sub-set of the total (). In greenhouse 1, there was a trend towards declining fungal populations over time, while in greenhouse 2, overall populations generally remained high and were not reduced over time. These trends were more obvious when total populations were plotted as a function of time (). A comparison of the morphology of fungal colonies recovered on PDA at two different times during the 2012 sampling period is shown in . The colonies representing different genera appeared very distinct on PDA and could be enumerated readily. They belonged to at least six different genera and species (based on subsequent molecular identification), and included Penicillium olsonii, Cladosporium herbarum (Pers.) Link (and C. cladosporioides (Fresen.) G.A. de Vries), Aspergillus ochraceus K. Wilh. (and A. niger van Tieghem), Alternaria alternata, Rhizopus stolonifer and Epicoccum nigrum Link. In pathogenicity tests, P. olsonii, A. alternata and R. stolonifer caused lesions on the fruit; the remainder of the fungi did not cause fruit infection (data not shown).
Fig. 2 (Colour online)Colony-forming units of four genera of fungi recovered from swabs of tomato fruit surfaces plated onto potato dextrose agar. Two greenhouses in Delta and Langley, BC were sampled over a 6-week period in 2011. Pen = Penicillium; Asp = Aspergillus; Clad = Cladosporium; Rhiz = Rhizopus.
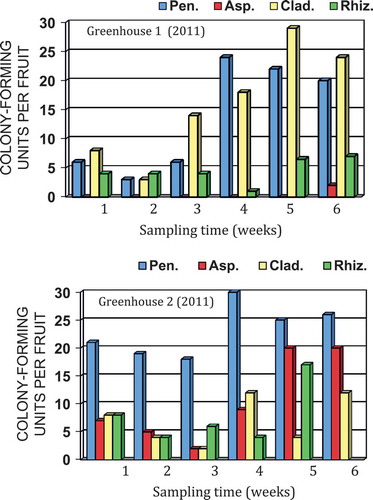
Fig. 3 Colony-forming units of total fungi and Penicillium species recovered from Q-tip swabs of tomato fruit surfaces plated onto potato dextrose agar. Two greenhouses in Delta and Langley, BC were sampled over an 18-week period in 2012.
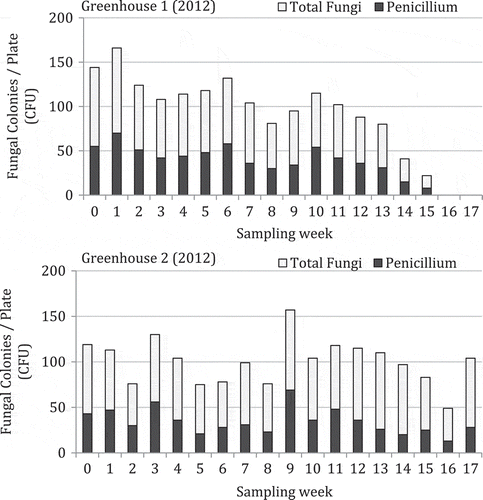
Fig. 4 Colony-forming units of total fungi recovered from tomato fruit surface swabs over an 18-week period in two greenhouses in Delta and Langley, BC sampled in 2012. The relationship was determined by simple linear regression analysis and the Pearson’s r-values are shown.
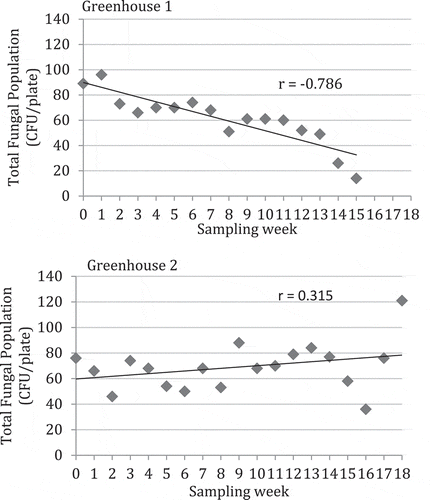
Fig. 5 (Colour online)Colonies of different species of fungi recovered on potato dextrose agar following Q-tip swabs of tomato fruit surfaces and observed growing on leaf litter. (a) Colonies of species of Penicillium (green) and Aspergillus (yellow) observed early in the growing season; (b) Profuse sporulation of Penicillium species on the surface of a dried tomato leaf; (c) Colonies of Cladosporium (black), Aspergillus (yellow) and Penicillium (green) species observed later in the growing season; (d) Conidiophores of Aspergillus species growing on the surface of a dried tomato leaf.
Monitoring of temperature and humidity conditions during shipping
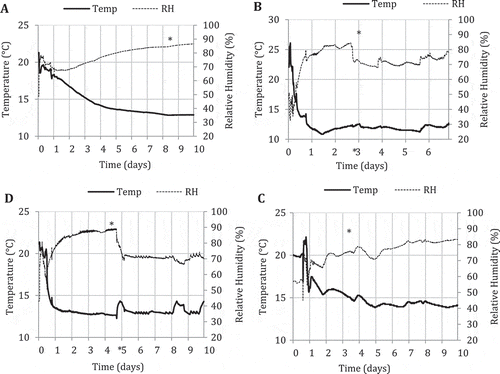
The temperature and humidity data collected for four shipments of tomato fruits originating from greenhouse 1 to Delaware, USA in refrigerated trucks is shown in . At the loading point in the greenhouse facility, the temperature range was 20–23°C and the humidity fluctuated between 40% and 50%. Within one day after refrigerated transport, the temperatures were ~18°C (), 12°C (), 16°C () or 14°C (), depending on the shipment. The shipment that required the longest time to reach 14°C is shown in . The relative humidity in all shipments was in the range of 65–75% after 24 hours and was maintained at 80–85% up to the time of arrival at the distribution centre (shown with an asterisk). At arrival time, the temperatures in the truck were in the range of 13–15°C. The opening of the doors of the truck caused a marked drop in the relative humidity to around 70% in three shipments; once in storage in the distribution centre, the humidity increased to 80% (, ) or remained at 70% (). With regard to temperature, the unloading of the cartons caused only a 0.5–1°C increase in temperature or it remained unchanged (). Once in storage, the temperature range was maintained at 13–15°C.
Fig. 6 Measurements of temperature (°C) and relative humidity (%) in four loads of tomato fruits (A to D) originating from Delta, BC (time 0) that were shipped to Delaware, USA over a 6–10 day period in refrigerated trucks. Asterisks mark the point at which the storage compartment was opened to deliver the fruits to the warehouse/distribution centre.
Disease development
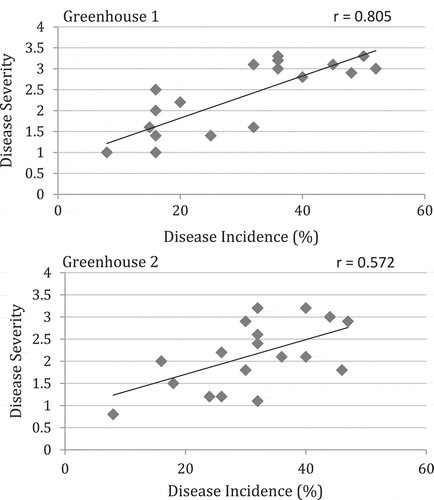
Fruit samples incubated at 21°C developed more disease than samples placed at 13°C at all sampling times in both greenhouses 1 and 2 (). There was considerable variation in the level of disease observed from one week to the next. The highest disease levels in both greenhouses were observed in weeks 6, 13 and 15 (). Overall disease levels were higher in greenhouse 2 compared with greenhouse 1, especially towards the end of the growing season (weeks 13–18). With regard to storage time, there was more disease after 16 days compared with 12 days (). There was a positive relationship between disease incidence and disease severity for fruits sampled from both greenhouses (). Symptoms of fruit infection at the calyx end are shown in . Mycelium and spore production of Penicillium, Alternaria and Rhizopus species were observed on the calyx and stem tissues and resulted in fruit infection and extensive decay. Additional fungi observed on the green tissues were Asp. ochraceus and Hirsutella sp.
Fig. 7 Disease incidence on tomato fruit samples originating from two greenhouses in Delta and Langley, BC and stored at 21°C or 13°C for 12 days. A second batch of samples was stored at 21°C for 12 or 16 days. The number of fruits showing symptoms of disease was rated from a total of 25 fruits in each treatment. Vertical bars represent standard errors from five replicates.
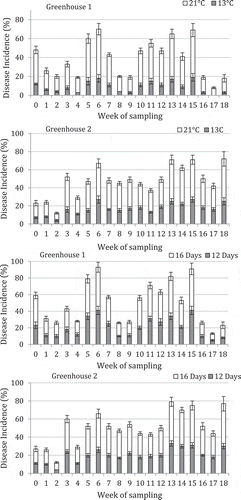
Fig. 8 Relationship between disease incidence and disease severity in tomato fruit samples collected weekly over an 18-week period from two greenhouses in Delta and Langley, BC Samples were stored at 21°C for 7–10 days after harvest and rated for disease. The relationship was determined by simple linear regression analysis and the Pearson’s r-values are shown.
Discussion
The fungal species recovered from diseased tomato fruits over two years of sampling have all been previously reported as pathogens of field-grown and greenhouse tomatoes (Snowdon Citation1990; Jones et al. Citation1991; Howard et al. Citation1994; Bartz et al. Citation2002; Blancard Citation2012; Bourett et al. 2013). In particular, P. olsonii was first identified as a new post-harvest pathogen of greenhouse tomato fruits in 2011 (Chatterton et al. Citation2012) and was the most prevalent of all the fungi recovered in this study from diseased fruits and also from the surface of healthy developing fruits in the greenhouse. Wounding of the fruit surface significantly increased the extent of infection by all fungi tested, confirming previous reports on the importance of wounding in promoting post-harvest decay of tomatoes caused by B. cinerea (Barkai-Golan Citation2001), R. stolonifer (Kwon et al. Citation2001), A. alternata (Hall et al. Citation1980) and G. geotrichum (Bartz et al. Citation2002). Cracking of the fruit surface cuticle is known to occur in ripening tomato fruit (Dorais et al. Citation2004) and was shown to promote infection by P. olsonii (Chatterton et al. Citation2012) and by R. stolonifer (Kwon et al. Citation2001).
Penicillium species that cause fruit decay of apples, pears, oranges (Amiri & Bompeix Citation2005; Korsten & Louw Citation2014; Palou Citation2014), or found epiphytically on plant debris, have been reported to be present at high levels in the air and also represent a significant component of air-borne mycoflora in greenhouses (Rodolfi et al. Citation2003; Hansen et al. Citation2012), packinghouses (Amiri & Bompeix Citation2005; Oliveri et al. Citation2007) and indoor environments (Cooley et al. Citation1998; Ren et al. Citation1999; Reboux et al. Citation2009). Our study is the first to demonstrate the occurrence of high levels of Penicillium spores on the surface of developing tomato fruits, representing potential inoculum for disease development under favourable environmental conditions. Penicillium olsonii is a common saprobic species found on decaying vegetation, with the potential to be a secondary pathogen (Pitt Citation2001). This species has been recovered, together with Cladosporium species, from air samples in tomato greenhouses and a botanical greenhouse (Rodolfi et al. Citation2003; Hansen et al. Citation2012). Li & LaMondia (Citation2010) reported the presence of Aspergillus/Penicillium, Trichoderma, Cladosporium and Botrytis species in air samples from two greenhouses containing ornamental plants. They observed dramatic differences in population levels over time, and spore levels were positively correlated with temperature, relative humidity, dew point, heat index and light and negatively correlated with air movement (Li & LaMondia Citation2010). The high Trichoderma spore levels were associated with the application of biocontrol products for disease management in the greenhouse. In the present study, neither Trichoderma nor Botrytis was recovered in the Petri dish assay of swabs of tomato fruit surfaces.
The levels of the fruit surface mycoflora varied significantly over time in the present study, probably due to variable temperature, humidity and cultural management practices in the greenhouse, as reported by Li & LaMondia (Citation2010). Studies on monitoring levels of fungal spores in the air (aerobiology) or on the surface of fruits have shown that correlations between spore levels and disease development can be demonstrated (Blanco et al. Citation2006; Spotts et al. Citation2008, Citation2009). In the present study, it was not possible to correlate peaks of spore levels to subsequent disease development in the following week due to high variation in the data, as well as variable and undefined environmental conditions. In addition, not all of the fungal species recovered as part of the fruit surface mycoflora were pathogenic, with a large component representing saprophytic species. The practice of leaving leaf prunings on the greenhouse floor, in the walkways in particular, can result in a large build-up of fungal populations, of which Penicillium, Aspergillus and Cladosporium species are major constituents (Domsch et al. Citation1993; Hansen et al. Citation2012). In greenhouse 1, the removal of the dried leaf litter was conducted every 7–10 days during the 2012 growing season; in greenhouse 2, the leaf litter was removed at week 17. The overall levels of mycoflora on the fruit surface were higher in greenhouse 2 compared with greenhouse 1, and there was a gradual increase in total populations of fungi over time when compared with greenhouse 1, in which there was a gradual decline. This suggests that the removal of leaf litter can reduce overall fungal population build-up in the greenhouse and subsequently on the developing tomato fruits. Following the leaf litter removal on week 17 in greenhouse 1, there was a significant increase in fruit surface mycoflora levels the following week, which was attributed to the release of spores during the disturbance of the leaf litter. Hansen et al. (Citation2012) observed that spore levels of Penicillium species in air samples were significantly higher during harvest periods and when clearing of plant debris was undertaken in tomato greenhouses.
Monitoring of the temperature and humidity conditions in four shipments of tomato fruits revealed differences in the rate at which the recommended storage and shipping temperature of 13°C was reached, as well as revealing variation in the range of relative humidity levels attained. Post-harvest shipment of tomato fruits at temperatures above 13°C increases the likelihood of fungal infection from resident inoculum on the fruit surface; hence, rapid cooling is recommended (Sommer Citation1982). At the time of arrival of the shipments at their destination, the ideal temperature range of 13–15°C had been reached, and the relative humidity was in the optimal range of 80–85%. The storage environment after unloading of the fruits was also shown to be in the optimal range. Therefore, the most significant variable in the shipments was the rate at which fruits were cooled from the initial temperature of 20–23°C at harvest time. Incubation of tomatoes at a temperature of 21°C significantly increased the extent of decay in this study compared with 13°C, highlighting the importance of cooler storage temperatures. Most post-harvest infections occurred at the calyx end of the fruit, suggesting a build-up of fungal spores on the green tissue was taking place. Previous studies have not implicated the attached green calyx and stem tissue on fresh market tomatoes as a possible source of inoculum for post-harvest decay. A longer duration of storage (16 days) resulted in more decay compared to 12 days. On apple fruit, prolonged periods of storage (6 months) were shown to increase the density of Penicillium species on the fruit surface from 10–50 spores cm−2 at 1 month to 300–400 spores cm−2 (Amiri & Bompeix Citation2005). The results from this study highlight the presence of a diverse mycoflora on greenhouse tomato fruit surfaces, and the potential interactions between these populations and storage temperature and duration, as well as shipping conditions, on development of post-harvest fruit decay.
Acknowledgements
This research was funded by the Natural Sciences and Engineering Research Council of Canada, Discovery Grants program and Engage Grants program, as well as a grant from the Growing Forward 1 initiative sponsored through Agriculture and Agri-Food Canada, the BC Greenhouse Growers Association, and the BC Ministry of Agriculture and Food. We thank Village Farms International, Delta, BC and OriginO Farms, Langley, BC, for their generosity in providing access to growing facilities for sampling and assistance with various aspects of the research.
Additional information
Funding
References
- Amiri A, Bompeix G. 2005. Diversity and population dynamics of Penicillium spp. on apples in pre- and postharvest environments: consequences for decay development. Plant Pathol. 54:74–81.
- Barkai-Golan R. 2001. Postharvest diseases of fruits and vegetables: development and control. Amsterdam, Netherlands: Elsevier Science.
- Bartz JA, Sargent SA, Mahovic M 2002. Guide to identifying and controlling postharvest tomato diseases in Florida. University of Florida, Institute of Food and Agricultural Sciences, Extension Bull. HS866. [ cited 2015 Mar 26]. Retrieved from: http://edis.ifas.ufl.edu/hs131
- Blancard D. 2012. Tomato diseases: Identification, biology and control: a colour handbook. 2nd ed. London: Manson Publishing.
- Blanco C, Santos B, Romero F. 2006. Relationship between concentrations of Botrytis cinerea conidia in air, environmental conditions, and the incidence of grey mould in strawberry flowers and fruits. Eur J Plant Pathol. 114:415–425.
- Bourret TB, Kramer EK, Rogers JD, Glawe DA. 2013. Isolation of Geotrichum candidum pathogenic to tomato (Solanum lycopersicum) in Washington State. North Amer Fungi. 8:1–7.
- British Columbia Ministry of Agriculture, Fisheries and Food. 1997. Greenhouse vegetable production guide for commercial growers. Victoria, BC: British Columbia Ministry of Agriculture, Food and Fisheries.
- Crop profile for Greenhouse. 2011. Crop profile for greenhouse tomato in Canada, 2011. Agriculture and Agri- Food Canada, Pesticide Risk Reduction Program. AAFC Publication no. 1199E. Ottawa, ON.
- Chatterton S, Wylie AC, Punja ZK. 2012. Fruit infection and postharvest decay of greenhouse tomatoes caused by Penicillium species in British Columbia. Can J Plant Pathol. 34:524–535.
- Cooley JD, Wong WC, Jumper CA, Straus DC. 1998. Correlation between the prevalence of certain fungi and sick building syndrome. Occupation Environ Medicine. 55:579–584.
- Domsch KH, Gams W, Anderson T-H. 1993. Compendium of soil fungi. IHW Verlag: Eching; 1264 p.
- Dorais M, Demers DA, Papadopoulos A, Van Ieperen W. 2004. Greenhouse tomato fruit cuticle cracking. Horticul Rev. 30:163–184.
- Fruit and Vegetable Production. 2009. Fruit and vegetable production. Catalogue no. 22-003-X; Statistics Canada. Greenhouse, Sod and Nursery Industries, Catalogue no. 22-202-X, 2010.
- Hall DH, Teviotdale BL, Paulus AO 1980. Blackmold of ripe tomato fruit. University of California, Vegetable Research and Information Center. Leaflet 21154. [cited 2015 Mar 28]. Accessed from: http://vric.ucdavis.edu/pdf/TOMATO/tomato_blackmold
- Hansen VM, Meyling NV, Winding A, Eilenberg J, Madsen AM. 2012. Factors affecting vegetable growers’ exposure to fungal bioaerosols and airborne dust. Ann Occup Hyg. 56:170–181.
- Howard R J, Garland J A, Seaman W L (editors). 1994. Diseases and pests of vegetable crops in Canada: an illustrated compendium. Ottawa: Canadian Phytopathological Society and the Entomological Society of Canada. 554 p.
- Jones JB, Stall RE, Zitter TA. 1991. Compendium of tomato diseases. St. Paul, MN: American Phytopathological Society Press.
- Kenney JF, Keeping ES. 1962. Mathematics of Statistics. In: Linear regression and correlation. 3rd ed. Princeton, NJ: Van Nostrand; p. 252–285.
- Korsten L, Louw JP. 2014. Penicillium rot of apples and pears. Hortgro Sci Tech. Oct.-Nov. 2014 74–75. [cited 2015 Mar 26]. Retrieved from: http://www.hortgro-science.co.za/
- Kwon J-H, Kang S-W, Kim J-S, Park C-S. 2001. Rhizopus soft rot on cherry tomato caused by Rhizopus stolonifer in Korea. Mycobiology. 29:176–178.
- Li D, LaMondia J. 2010. Airborne fungi associated with ornamental plant propagation in greenhouses. Aerobiologia. 26:15–28.
- Oliveri C, Campisano A, Catara A, Cirvilleri G. 2007. Characterization and AFLP genotyping of Penicillium strains from postharvest samples and packinghouse environments. J Plant Pathol. 89:29–40.
- Palou L. 2014. Penicillium digitatum, Penicillium italicum in citrus fruit (green mold, blue mold). In: Bautista-Banos S, editor. Postharvest decay: control strategies. New York: Elsevier; p. 45–102.
- Pitt JI. 2001. A laboratory guide to common Penicillium species. 3rd ed. North Ryde, NSW: Food Science Australia; 197 p.
- Reboux G, Bellanger AP, Roussel S, Grenouillet F, Sornin S, Piarroux R, Dalphin JC, Millon L. 2009. Indoor mold concentration in Eastern France. Indoor Air. 19:446–453.
- Ren P, Jankun TM, Leaderer BP. 1999. Comparisons of seasonal fungal prevalence in indoor and outdoor air and in house dusts of dwellings in one Northeast American county. J Exp Analy Environ Epidemiol. 9:560–568.
- Rodolfi MM, Lorenzi E, Picco AM. 2003. Study of the occurrence of greenhouse microfungi in a botanical garden. J Phytopathol. 151:591–599.
- Snowdon AL. 1990. A color atlas of post-harvest diseases and disorders of fruits and vegetables. Vol. 2. Vegetables. CRC Press: Boca Raton, FL; 416 pp.
- Sommer NF. 1982. Postharvest handling practices and postharvest diseases of fruit. Plant Dis. 66:357–364.
- Spotts RA, Serdani M, Wallis KM, Walter M, Harris-Virgin T, Spotts K, Sugar D, Xiao C-L, Qu A. 2009. At-harvest prediction of grey mould risk in pear fruit in long-term cold storage. Crop Prot. 28:414–420.
- Spotts RA, Wallis KM, Serdani M, O’Gorman DT, Sholberg PL. 2008. Methodology for determining relationships between inoculum concentration of Botrytis cinerea and Penicillium expansum and stem end decay of pear fruit. Plant Dis. 92:451–455.