Abstract
Verticillium wilt of sugar beet is a disease that has received very little attention, but which has been reported to reduce sugar quality. A survey of sugar beet fields with wilt symptoms was conducted in 2007 (5 roots from each of 40 fields) and 2008 (5 roots from each of 45 fields) in Idaho. Verticillium dahliae was isolated from all root samples. From a collection of 106 V. dahliae sugar beet isolates, all were of the MAT1-2 mating type. The vegetative compatibility grouping (VCG) was evaluated for 93 of these isolates and 95, 3, 1 and 1% were VCG 4A, VCG 2B, VCG 4B and non-compatible, respectively. All the VCG 4A isolates had the same mitochondrial haplotype based on sequencing of cox3 to nad6 and cox1 to rnl loci, while the VCG 2B isolates had two haplotypes. Pathogenicity tests on sugar beet cultivar ‘Monohikari’ revealed that the VCG 4A isolates produced more foliar symptoms (P < 0.0001) than VCG 1, 1A, 2A, 2B, 3 and 4B isolates, but none of the VCGs consistently reduced root and foliage weight. Since V. dahliae VCG 4A isolates have also been reported as a pathogen of potato in North America, rotating sugar beet fields with potato could be a concern.
Résumé
La flétrissure verticillienne de la betterave à sucre est une maladie dont on s’est peu préoccupé, mais qui est reconnue pour altérer la qualité du sucre. Une étude des champs de betterave à sucre affichant des symptômes de la flétrissure a été menée en Idaho en 2007 (5 racines dans chacun des 40 champs) et en 2008 (5 racines dans chacun des 45 champs). On a obtenu des isolats de Verticillium dahliae de tous les échantillons de racines. À partir d’une collection de 106 isolats de V. dahliae obtenus de la betterave à sucre, il a été possible de conclure que tous appartenaient au type sexuel MAT1-2. Le groupement de compatibilité végétative (GCV) a été évalué pour 93 de ces isolats et 95, 3 et 1 % appartenaient au GCV 4A, au GCV 2B et au GCV 4B, respectivement, et 1 % étaient incompatibles. En se basant sur le séquençage des locus cox3 à nad6 et cox1 à rnl, tous les isolats du GCV 4A avaient le même haplotype mitochondrial, tandis que les isolats du GCV 2B en avaient deux. Des tests de pathogénicité menés sur le cultivar de la betterave à sucre ‘Monohikari’ ont révélé que les isolats du GCV 4A produisaient plus de symptômes foliaires (P < 0.0001) que les isolats des GCV 1, 1A, 2A, 2B, 3 et 4B, mais qu’aucun ne réduisait substantiellement le poids du feuillage et de la racine. Étant donné qu’on a rapporté que les isolats du GCV 4A s’attaquent également à la pomme de terre en Amérique du Nord, le fait d’alterner, selon un plan de rotation, betterave à sucre et pomme de terre dans un même champ, devrait préoccuper les producteurs.
Introduction
Verticillium wilt of sugar beet (Beta vulgaris L.) is a disease that can reduce sucrose production and purity, but has not been reported to reduce root weight (Gaskill & Krentzer Citation1940). On sugar beet, the disease was initially attributed to Verticillium albo-atrum Reinke & Berthier in 1940 (Gaskill & Krentzer Citation1940), but more recent reports suggest Verticillium dahliae Kleb. is the causal agent (Puhalla Citation1979; Strausbaugh et al. Citation1992; Karadimos et al. Citation2000; Strausbaugh & Camp Citation2007a, Citation2007b, Citation2007c; Brantner et al. Citation2008; Harveson Citation2009). However, the causal agents may have been the same in all the studies, since Verticillium comprises a complex taxonomic group, which can lead to confusion over species identification (Inderbitzin & Subbarao Citation2014). Although reports of V. dahliae on sugar beet are limited, this fungus is known to infect over 200 plant species and cause considerable economic impact (Pegg & Brady Citation2002). In the Pacific North-west region (Idaho, Oregon and Washington), V. dahliae is one of the primary pathogens of concern in both mint and potato production (Strausbaugh Citation1993; Dung et al. Citation2013; Johnson & Cummings Citation2015).
Idaho is the most important state for potato production in the USA and ranks second for sugar beet production based on statistics from the National Ag Statistics Service (NASS), but only Verticillium wilt of potato has been investigated previously in Idaho. After Verticillium wilt of sugar beet was initially reported by Gaskill & Kreutzer in 1940, the only other study to address Verticillium wilt on this host in the USA was based on 10 isolates found in the Red River Valley (Brantner et al. Citation2008). Thus, there is limited previous literature on Verticillium wilt of sugar beet.
Long-term survival of V. dahliae as an asexually reproducing fungus is through microsclerotia produced on plant tissues which persist in the soil. This fungus has also been shown to potentially be heterothallic with two mating type idiomorphs, MAT1-1 and MAT1-2 (Usami et al. Citation2009, Citation2012). The primary idiomorph identified to date in the USA has been MAT1-2 (Inderbitzin et al. Citation2011b; Dung et al. Citation2013). However, the frequency of V. dahliae mating types has not been widely studied in most crops. In the coastal agricultural region of California (Salinas Valley), 30% of the V. dahliae population was MAT1-1 (Inderbitzin & Subbarao, unpublished data) as mentioned by Atallah et al. (Citation2010). Since significant gene flow has been measured among the various geographic and host sampling groups of V. dahliae, recombination has potentially occurred (Atallah et al. Citation2010).
The genetic variation found in V. dahliae populations has been assessed through vegetative compatibility groupings (Puhalla & Hummel Citation1983; Joaquim & Rowe Citation1990, Citation1991; Strausbaugh et al. Citation1992; Strausbaugh Citation1993; Rowe Citation1995). Vegetative compatibility groups (VCG) 1 through 6 have been established for V. dahliae, with a number of subgroups also mentioned within these VCGs (Puhalla & Hummel Citation1983; Joaquim & Rowe Citation1990, Citation1991; Strausbaugh et al. Citation1992; Strausbaugh Citation1993; Bhat et al. Citation2003; Collado-Romero et al. Citation2006, Citation2008; Jiménez-Díaz et al. Citation2006; Jiménez-Gasco et al. Citation2014). In many instances, these VCGs have been shown to be correlated with virulence on a specific host, such as VCG 1 or 1A strains infecting chrysanthemum and defoliating strains in cotton. Additionally, VCG 2 or 2A may be associated with race 1 strains in tomato; VCG 2B strains occur on artichoke and mint; VCG 4 or 4B may be associated with artichoke, race 2 strains infecting tomato and potato in Israel; and VCG 4A strains occur on potato and sunflower in North America (Joaquim & Rowe Citation1991; Strausbaugh et al. Citation1992; Strausbaugh Citation1993; Daayf et al. Citation1995; Dobinson et al. Citation1998; Omer et al. Citation2000; Douhan & Johnson Citation2001; Korolev et al. Citation2001; Tsror et al. Citation2001; Jiménez-Díaz et al. Citation2006; Qin et al. Citation2006; Alkher et al. Citation2009; Göre Citation2009; Dung et al. Citation2013; El-Bebany et al. Citation2013). The genetic diversity in V. dahliae populations has also been assessed using molecular methods (Dobinson et al. Citation1998, Citation2000; Bhat & Subbarao Citation1999; Pantou et al. Citation2006; Qin et al. Citation2006; Collado-Romero et al. Citation2008; Atllah et al. Citation2010, Citation2011; Martin Citation2010; Berbegal et al. Citation2011; Inderbitzin et al. Citation2011a; Jiménez-Díaz et al. Citation2011; Dung et al. Citation2013; Gurung et al. Citation2014; Jiménez-Gasco et al. Citation2014; Gharbi et al. Citation2015).
Disease observations and pathogen isolations made from sugar beet cultivars in a 2006 commercial and experimental cultivar trial located in Heyburn, ID indicated that wilt symptoms were primarily associated with V. dahliae (Strausbaugh & Camp Citation2007a, Citation2007b, Citation2007c), but some Fusarium oxysporum Schltdl. isolates could also be recovered. Previous work on wilt in sugar beet has identified F. oxysporum f. sp. betae as the causal agent, with pathogenic isolates being reported from Colorado, Michigan, Minnesota, Montana, North Dakota, Oregon, Texas, Washington and Wyoming (Ruppel Citation1991; Harveson & Rush Citation1997; Windels et al. Citation2005; Hanson Citation2006; Hanson et al. Citation2009; Hill et al. Citation2011; Burlakoti et al. Citation2012; Webb et al. Citation2012, Citation2013; Covey et al. Citation2014).
In southern Idaho and south-eastern Oregon, wilt symptoms can be observed in most fields with an incidence of 50% or higher in a few fields. Since these sugar beet wilt symptoms have been a cause of concern for both growers and seed companies, a survey was conducted during the 2007 and 2008 growing seasons to establish the cause. The only fungus isolated from all the symptomatic sugar beet plants was V. dahliae; therefore, this study focused on characterizing the V. dahliae isolates for vegetative compatibility, pathogenicity and genetic diversity and comparing them with tester and historical strains.
Materials and methods
Wilt survey
Commercial sugar beet plants exhibiting typical wilt symptoms in a 2006 cultivar trial (Strausbaugh & Camp Citation2007a, Citation2007b, Citation2007c) yielded isolates of V. dahliae; therefore, a survey was conducted in 2007 to collect roots from symptomatic plants (5 plants per field with necrotic and/or yellow sectors on leaves; and b). Samples from 40 commercial sugar beet fields (200 root samples) arbitrarily selected over the Amalgamated production area in southern Idaho (Magic Valley and Treasure Valley) and south-eastern Oregon (fields around Nyssa, OR) were obtained. Two additional commercial sugar beet fields were visited, but no plants with wilt symptoms were found. The geographic origins of the sampled fields are provided in Supplemental . The survey was repeated in 2008 by collecting symptomatic plants from 45 commercial sugar beet fields (5 roots per field; 225 root samples total) arbitrarily selected across the same production area.
Table 1. Summary of vegetative compatibility (VCG) and mitochondrial haplotypes for 97 V. dahliae isolates from sugar beet.
Isolations
Isolations were conducted by dissecting tissues from the vascular region of the root, surface sterilizing for 1 min in 10% bleach (containing 5% NaOCl), rinsing with sterile reverse osmosis (RO) water, blotting dry, removing the surface tissue, and placing on potato-dextrose agar (PDA; Becton Dickinson & Co., Sparks, MD) amended with streptomycin (200 mg L−1) (Strausbaugh Citation1993). Spores from conidiophores developing on PDA plates were streaked onto water agar amended with streptomycin (200 mg L−1). Single spores were transferred to PDA plates with the aid of a dissecting scope with green back lighting. If other fungi such as Fusarium were isolated from the vascular tissues, they were also recorded and cultures were saved. Isolates of V. dahliae were maintained on sterile barley kernels at −80°C as follows. The barley kernels were soaked in tap water for 24 h and then autoclaved in Erlenmeyer flasks on consecutive days for 1 h at 121°C. Mycelial plugs from 3 to 4 week old PDA cultures were used to inoculate the barley kernels, which were incubated in the dark at 21°C for ~6 weeks. The kernels were then placed in cryovials and frozen at −80°C.
Vegetative compatibility grouping
The vegetative compatibility grouping of V. dahliae from sugar beet was determined using 20 isolates from the 2006 cultivar trials (Strausbaugh & Camp Citation2007a, Citation2007b, Citation2007c) and 73 isolates from the 2007 and 2008 field surveys which provided the best geographic coverage for the Magic and Treasure Valley production areas ( and ; Supplemental ). The V. dahliae tester strains (70–21, 115, BB, MT, PH, S39, T9, V44) established in previous studies (Joaquim & Rowe Citation1990, Citation1991; Strausbaugh et al. Citation1992) were obtained from J.K.S. Dung at Washington State University, Pullman (). The isolates of V. dahliae were tested for VCG by pairing nit1 and nitM mutants on minimal media as described previously (Strausbaugh Citation1993). Mycelial plugs were placed 1.5 cm from the tester plug and evaluated 4 weeks after pairing. Successful complementation resulted in dense mycelial growth (sporulation and microsclerotia were also frequently present) at the mycelial interface. Weak complementation was noted when only scattered mycelial tuffs occurred at the interface.
Table 2. Mitochondrial haplotype sequence data from five loci was evaluated for nine representative V. dahliae isolates (F series numbers) from sugar beet in Idaho and eight VCG tester strains.
Mitochondrial haplotype analysis
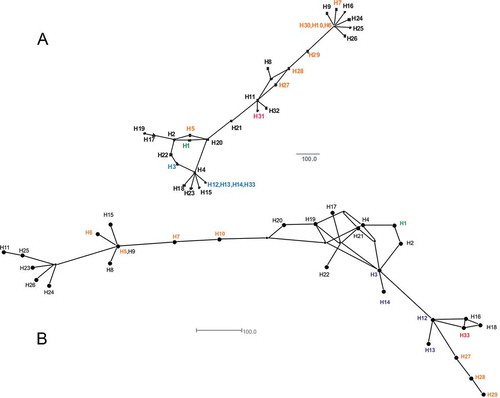
Mitochondrial haplotype analysis was conducted on 97 sugar beet isolates based on DNA sequences from region 3 (cox3 and nad6) and region 5 (cox1 and rnl) in the mitochondrial genome (GenBank accession DQ351941) of V. dahliae (Martin Citation2010; Martin, personal communication; and ). These two regions were included since 13 of the original 15 haplotypes found could be delineated by sequencing these specific regions (Martin Citation2010). For the tester strains and nine representative isolates from sugar beet (), three additional regions were sequenced: region 1 (nad3 to nad1), region 2 (apt6 to rns), and region 4 (cob to cox1) (Martin Citation2010). To provide a more comprehensive analysis of the relationship between VCG and mitochondrial haplotype, regions 3 and 5 were also sequenced for 32 historical strains () of V. dahliae (Puhalla & Hummel Citation1983; Joaquim & Rowe Citation1990, Citation1991; Strausbaugh et al. Citation1992; Strausbaugh Citation1993; Dung et al. Citation2013; Jiménez-Gasco et al. Citation2014). The isolates were grown in potato dextrose broth (PDB; Becton Dickinson & Co., Sparks, MD) in shake culture (100 rpm) at 21°C for about 1 week until a ~10-mm diameter fungal colony was generated from a 5-mm hyphal plug. The PDB was decanted and the tissue was placed in a sterile 2-mL microcentrifuge tube and stored at −80°C. Frozen tissue in individual tubes was freeze-dried and then pulverized using a Retch MM301 mixer mill (Retch Inc., Newton, PA) with 5-mm stainless steel beads. DNA was extracted using a DNeasy Plant Mini Isolation Kit (Qiagen Inc., Valencia, CA) following standard protocols suggested by the manufacturer. The DNA quality was assessed via gel electrophoresis (1.5% agarose gel) and quantified using a BioPhotometer (Eppendorf AG, Hamburg, Germany). The DNA was stored at −20°C. The DNA templates were amplified using the primers described in and performed in volumes of 30 µL in accordance with the manufacturer’s instructions: 14.35 µL molecular grade water (5 Prime Inc., Gaithersburg, MD), 6 µL 5× Green GoTaq buffer (pH 8.5 with 7.5 mM MgCl2; Promega Corp., Madison, WI), 0.8 µL of 25 mM MgCl2 (Applied Biosystems, Forster City, CA), 0.6 µL 10 mM dNTPs (Promega Corp.), 3 µL of 3 µM each primer (Integrated DNA Technologies, Coralville, IA), 0.25 µL GoTaq Taq DNA polymerase (Promega Corp.) and 2 µL (~10 ng) of V. dahliae DNA. The amplification consisted of 3 min at 95°C followed by 35 cycles of 95°C for 45 s, 57°C for 45 s and 72°C for 90 s, which was followed by 5 min at 72°C and a holding temperature of 4°C. Amplification products were electrophoresed through agarose gels (1.8% w/v) supplemented with ethidium bromide (0.01 mg mL−1) in Tris borate EDTA buffer (TBE, 89 mM Tris base, 89 mM boric acid and 2 mM EDTA). Amplicons were sent to TACGen (Richmond, CA) for polymerase chain reaction (PCR) product cleanup and sequencing in both directions. Sequences were analysed using BioEdit version 7.1.3.0 (Hall Citation1999) and consensus sequences were generated. Sequences were aligned and trimmed to match the same regions sequenced previously (Martin Citation2010) and deposited in GenBank (). After the original 15 haplotypes were identified (Martin Citation2010), additional sequencing has established haplotypes H16 through H26 which have been deposited in GenBank. These sequences were included in the analysis ( and ). Haplotypes were determined using DnaSP 5.10 ver. 5.10.01 (Librado & Rozas Citation2009). Once the analysis of individual regions was completed, the sequences were concatenated. Prior to analysis, DNA sequences were aligned using ClustalX Ver. 2.0 (Larkin et al. Citation2007). To graphically illustrate differences among haplotypes, concatenated datasets were analysed using SplitsTree4 version 4.13.1 (Huson & Bryant Citation2006). Given that insertions/deletions are a common mutation associated with haplotype differences, these trees should be interpreted as graphically showing the extent of differences among haplotypes rather than phylogenetic relationships.
Table 3. Vegetative compatibility (VCG) and mitochondrial haplotypes of 32 V. dahliae historical strains.
Table 4. Primers used to amplify and sequence the mitochondrial spacer regions which established the mitochondrial haplotypes for the V. dahliae isolates.
Table 5. GenBank accession numbers for spacer regions representing V. dahliae mitochondrial haplotype classification.
Table 6. Pathogenicity tests comparing 10 isolates from sugar beet (F series numbers) and eight tester strains of V. dahliae versus a non-inoculated water control on sugar beet cultivar ‘Monohikari’ in the greenhouse.
Table 7. Pathogenicity tests comparing the vegetative compatibility groups of 18 V. dahliae isolates and tester strains versus a non-inoculated water control on sugar beet cultivar ‘Monohikari’ in the greenhouse.
Table 8. V. dahliae mitochondrial haplotype classification.
Amplification of mating type idiomorphs
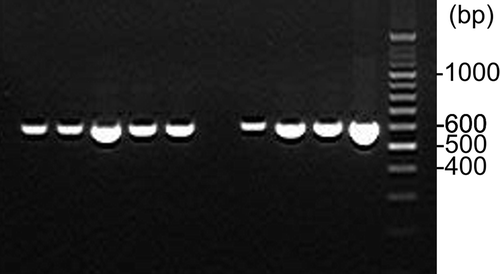
The DNA was extracted from mycelium, quantified, and stored as mentioned above. Mating type of the V. dahliae isolates was determined using primer pairs VdMAT1-1a/VdMAT1-1b and VdMAT1-2a/VdMAT1-2b (~400 and 600 bp amplicons, respectively), which amplify the MAT1-1 and MAT1-2 idiomorphs (Usami et al. Citation2009). Amplification of templates was performed in 20 µL final volume containing 8.8 µL molecular grade water (5 Prime Inc.), 4 µL 5× Green GoTaq buffer (pH 8.5 with 7.5 mM MgCl2; Promega Corp.), 0.6 µL of 25 mM MgCl2 (Applied Biosystems), 0.4 µL 10 mM dNTPs (Promega Corp.), 2 µL of 3 µM each primer (Integrated DNA Technologies), 0.2 µL GoTaq Taq DNA polymerase (Promega Corp.) and 2 µL (~10 ng) of V. dahliae DNA. The amplification consisted of 3 min at 95°C followed by 35 cycles of 95°C for 30 s, 65°C for 30 s and 72°C for 1 min, which was followed by 5 min at 72°C and a holding temperature of 4°C. All reactions in the experiment were repeated once. Amplification products were visualized by electrophoresing through 1.8% agarose gels in 1× TBE buffer.
Inoculum production
To produce inoculum for pathogenicity tests, spores were washed with RO water from monoconidial cultures grown on PDA amended with streptomycin (200 mg L−1) in the dark at 22°C. The spores were spread as a lawn on ¼ PDA (one-fourth strength PDA supplemented with 7 g L−1 Bacto agar and amended with streptomycin at 200 mg L−1). Dishes were incubated in the dark at 22°C. After 2 weeks, the conidia were washed from the ¼ PDA medium using RO water, counted with a hemacytometer, and adjusted with RO water to a concentration of 106 conidia mL−1 to be used as inoculum.
Pathogenicity tests
Ten isolates from sugar beet and eight strains representing the different VCGs () of V. dahliae were inoculated in the greenhouse on the sugar beet cultivar ‘Monohikari’ (Seedex Inc., Sheridan, WY) and compared with a non-inoculated water control. The experimental design was a randomized complete block design with six replications. There was one plant per pot (experimental unit) used for each isolate/strain. Plants were grown from seed in 10.2-cm square plastic pots with Sunshine Mix No. 1 (Sun Gro Horticulture, Bellevue, WA) that contained 70–80% Canadian sphagnum peat moss, perlite, dolomitic limestone, gypsum and a wetting agent. The plants were fertilized weekly with 20–10–20 (N–P–K) general-purpose fertilizer at 200 ppm N. The greenhouse was set to hold 27°C, with day length extended to 13 h with metal halide lamps (250 µmole s−1m−2 measured at plant top). At the four-leaf growth stage, plants were removed from the pots, the roots were rinsed in sterile RO water, and the root system was soaked in a spore suspension (106 spores mL−1) for 5 min. The non-inoculated control plants were soaked in RO water. After soaking, the plants were replanted. Three weeks after inoculation, the plants were visually evaluated for the percentage of foliage with symptoms (vein delimited sectors on leaves with chlorosis [] or necrotic tissue []) and the fresh weight of foliage and roots were recorded. Isolations were conducted from the roots of the non-inoculated water controls and 10 symptomatic plants on PDA amended with streptomycin (200 mg L−1). The experiment was repeated once.
Data analysis
The SAS (Version 9.2, SAS Institute Inc., Cary, NC) Univariate procedure was used to test for normality and Levene’s test was used to determine homogeneity of variance. Data were also subjected to analysis of variance using the SAS generalized linear mixed models procedure (Proc GLIMMIX). In the model statement, the fixed effects were experiment and isolate or VCG. The random effects were block and the block by experiment interaction. In the model statement, the denominator degrees of freedom were calculated using the DDFM = KENWARDRODGER option. Mean comparisons were conducted using least square means (α = 0.05) while using the ‘Lines’ output option.
Results
V. dahliae isolation from roots
Isolations from all 200 roots collected in 2007 from commercial sugar beet fields (plants had leaves with yellow and necrotic sectors; and b) yielded V. dahliae. In 2008, V. dahliae was isolated from all 225 roots from symptomatic plants.
Vegetative compatibility grouping and mating type
A total of 88 V. dahliae isolates were strongly compatible with the VCG 4A tester, three isolates were strongly compatible with the VCG 2B tester, one isolate was compatible with the VCG 4B tester, and one isolate was not compatible with any of the testers ( and ; Supplemental ). The 13 isolates not tested for compatibility failed to regrow from storage. Within the 4A isolates, 21 isolates were also weakly compatible with the 4B tester and four were weakly compatible with both 2B and 4B testers. All sugar beet isolates produced a 600 bp product, indicating they were mating type MAT1-2 (). Twenty-three of the historical strains were also mating type MAT1-2. Nine of the historical strains (115, AF1-10, AF10-4, CA, CF, J6-10, T8A, PCW and V44) did not produce any band with all primer sets, and their mating type remains unknown.
Fig. 2 Polymerase chain reaction (PCR) mating type assay using primer pairs VdMAT1-2a/VdMAT1-2b (600 bp product for MAT1-2 idiomorph). Lanes 1–5 (left to right) represent V. dahliae isolates F647, F652, F654, F658 and F662. Lane 6 is a negative control. Lanes 7–10 represent V. dahliae isolates F603, F611, F615 and F643. Lane 11 is the O’RangeRuler 100 bp ladder (Thermo Fisher Scientific Inc., Waltham, MA).
Pathogenicity tests
Results from the two pathogenicity tests are presented separately in , since they were significantly different (P < 0.0001) based on symptoms, foliage weight and root weight for individual isolates/strains. Based on foliar symptoms, 10 isolates (including all seven 4A isolates) were pathogenic in both tests () and the non-inoculated control plants showed no symptoms. When evaluating foliage and root weights, there were significant differences in both tests, but no consistent trends were evident between tests. Isolations established that V. dahliae was present in symptomatic plants (10 plants per test; 20 plants total) and not present in the non-inoculated water controls (6 plants per test; 12 plants total).
When comparing isolates/strains grouped by VCG in , results for the two pathogenicity studies are presented separately, since analysis based on symptoms, foliage weight and root weight indicated the two studies differed (P = 0.0002, <0.0001 and <0.0001, respectively). Based on foliar symptoms, VCG 4A was the only group significantly different from the non-inoculated water control in both tests. In Test 1, VCG 1 and 2B were also significantly different from the non-inoculated water control, but not in Test 2. In both tests, all VCG ranked the same based on foliar symptoms. Based on foliage and root weight, there were significant differences in both tests, but no consistent trends were evident between tests.
Mitochondrial haplotype analysis
Four haplotypes were identified among the V. dahliae isolates, with haplotype 13 being the predominant haplotype ( and ; Supplemental ). Haplotype 13 was always associated with VCG 4A isolates. Two of the four haplotypes (27 and 28) from the sugar beet isolates were new haplotypes and were associated with VCG 2B isolates. When tester strains and historical strains were evaluated, five additional haplotypes (H29 to H33) were identified (). When comparing relationships among the haplotypes, the two region sequence analysis () did not provide as much separation as the five region sequence analysis (). In , the haplotypes associated with VCG 1 and 2B fell into separate areas, while haplotypes associated with VCGs 2A, 4A, 4B and 4A/B were clustered together. Haplotype 33 (associated with a VCG 3 strain) also was placed close to the cluster containing VCG 4A. An updated listing of the sequence types for each locus for the 33 known mitochondrial haplotypes is presented in .
Fig. 3 (Colour online) Relationships among V. dahliae mitochondrial haplotypes based on three (a) and 5 (b) loci concatenated, and then analysed and visualized as a median-joining network using SplitsTree4 Ver. 4.13.1. The different haplotypes are represented by H followed by a number designation. The vegetative compatibility groups (VCG) associated with the haplotypes were VCG 1 and 1A (green) = H1; VCG 2A (blue) = H3 and H12; VCG 2B (orange) = H5, H6, H7, H10, H27, H28, H29 and H30; VCG 3 (blue) = H33; VCG 4A (blue) = H3 and H13; VCG 4A/B (blue) = H3, H12 and H13; VCG 4B (blue) = H3, H12 and H14; and VCG 5 (red) = H31; and the remaining haplotypes have unknown VCG association (black).
Discussion
This investigation represents the first major study conducted on wilt of sugar beet where production of potatoes and sugar beet are centred in the same geographic region. Data from this survey also represent the first major investigation into Verticillium wilt in sugar beet since it was originally reported in 1940. The survey of wilt symptomatic plants from 85 fields in the Amalgamated production area established that all plants were infected by V. dahliae. Diversity testing showed that 95% of the isolates evaluated for vegetative compatibility were VCG 4A with the same mitochondrial haplotype and MAT1-2 mating type. Based on the five mitochondrial region analysis, all VCG 4A isolates were haplotype 13, while the VCG 4B isolates were haplotype 3. The VCG 2B isolates were variable for region 5, leading to a number of novel haplotypes among both the sugar beet isolates as well as the historical strains. The pathogenicity tests indicated that the VCG 4A group was the only VCG group significantly different from the non-inoculated water control based on foliar symptoms. Based on these findings, growers and seed companies should consider inclusion of sugar beet cultivars with resistance to the V. dahliae VCG 4A group when planting or selling seed in areas where sugar beet and potatoes are grown in close rotation.
Demonstrating that 95% of isolates from sugar beet were VCG 4A is consistent with a previous report from eastern North Dakota (Brantner et al. Citation2008) and for V. dahliae strain 277 isolated from sugar beet in Washington State (Puhalla Citation1979; Strausbaugh et al. Citation1992). Finding only one or a few VCG of V. dahliae associated with one crop in an area has also been reported for other crops and regions (Joaquim & Rowe Citation1991; Strausbaugh et al. Citation1992; Strausbaugh Citation1993; Elena Citation2000; Korolev et al. Citation2000, Citation2001; Bhat et al. Citation2003; Jiménez-Díaz et al. Citation2011). In the pathogenicity tests, the VCG 4A group was the most pathogenic on sugar beet, which also holds true for potatoes grown in Idaho and other areas of North America (Joaquim & Rowe Citation1990, Citation1991; Strausbaugh et al. Citation1992; Strausbaugh Citation1993; Omer et al. Citation2000, Citation2008; Dung et al. Citation2013). Dung et al. (Citation2013) showed that VCG 4A isolates from Beta (including isolates F608, F611, F612 and F616 collected as part of the 2007–2008 sugar beet survey in the current study) were pathogenic on potato, while the VCG 2B haplotype containing the F625 Idaho Beta isolate (collected as part of the 2007–2008 survey) was not pathogenic on potato. On Scotch spearmint, the VCG 2B isolate was found to be pathogenic, while the isolate from the VCG 4A haplotype was not pathogenic (Dung et al. Citation2013). The only VCG 2B isolates recovered in the 2007–2008 survey were found near Nampa, ID in furrow irrigated sugar beet fields with a history of mint production. Thus, the VCG, mating type and pathogenicity of the five isolates from the 2007–2008 survey which were included by Dung et al. (Citation2013) support the data presented in this study.
Over the 2-year period of this study, 98% of the sugar beet fields (85 out of 87 fields) had wilt symptomatic plants in an area where sugar beet and potato production are centred. The exact cropping histories of the fields are not known, but potatoes and sugar beet are frequently the first crops planted when converting to sprinkler irrigation to help recover the higher cost of the conversion. Over the last 25 years, most Idaho fields in sugar beet production have converted from furrow irrigation to sprinkler irrigation, if not already being irrigated with sprinklers (Panella et al. Citation2014). Ninety-three per cent of the sugar beet fields sampled as part of this study were under sprinkler irrigation.
In both the field survey and greenhouse assays, plants infected by V. dahliae were never killed by the pathogen. This observation is consistent with the early report of Verticillium wilt in sugar beet in which the disease only affected sucrose production and purity, but did not influence root weight (Gaskill & Krentzer Citation1940). The greenhouse pathogenicity results also support this observation, since no consistent trends could be established with foliage and root fresh weights when comparing both strains and VCGs. During the 2007–2008 survey period, two or three fields were observed each year with as many as 50% of the plants showing wilt symptoms.
Since V. dahliae was isolated from all wilt symptomatic sugar beet plants, the present study focused on this pathogen. However, the recovery of F. oxysporum from 19 to 21% of the sugar beet plants implies it could also have contributed to symptoms. Twelve isolates (10 collected in 2007 and two in 2008) of F. oxysporum were evaluated in the greenhouse on the susceptible commercial sugar beet cultivar ‘Monohikari’ and shown to be non-pathogenic (data not shown). Webb et al. (Citation2013) also reported that two of these isolates (F597 and F598) were VCG B and non-pathogenic on sugar beet, dry bean and onion. These observations do not rule out that Fusarium could also be an important component of the wilt complex in the Idaho production area. In fact, most previous work on wilt in sugar beet has attributed F. oxysporum f. sp. betae as the causal agent, with pathogenic isolates recovered from Colorado, Michigan, Minnesota, Montana, North Dakota, Oregon, Texas, Washington and Wyoming (Ruppel Citation1991; Harveson & Rush Citation1997; Windels et al. Citation2005; Hanson Citation2006; Hanson et al. Citation2009; Hill et al. Citation2011; Burlakoti et al. Citation2012; Webb et al. Citation2012, Citation2013; Covey et al. Citation2014). To fully assess the importance of F. oxysporum on sugar beet in Idaho, additional studies are required.
Populations of V. dahliae are traditionally characterized by VCGs, with the assumption being that genetically related isolates correlate with clonal lineages. However, strains belonging to a single VCG were frequently only compatible with a sub-set of the other strains included in the same VCG (Strausbaugh et al. Citation1992; Strausbaugh Citation1993). These incompatibilities between strains within a VCG could be utilized to establish subgroups within VCGs, but the value of subdivisions is not established from either a genetic or biological perspective. Inconsistencies among VCG data and more recent molecular studies draw into question whether V. dahliae VCGs always represent clonal lineages. For example, phylogenetic relationships based on sequences of the intergenic spacer region (IGS) of the ribosomal DNA and six single-nucleotide polymorphisms (SNPs) indicated that some V. dahliae VCG such as 2B comprise a genetically heterogeneous group of strains that are phylogenetically distant (Jiménez-Gasco et al. Citation2014). In the present study, six mitochondrial haplotypes were found among the eight VCG 2B isolates/strains evaluated, providing evidence of the diversity observed previously among VCG 2B strains. Split network analysis based on five regions also provides evidence for diversity among the VCG 2B isolates, since the network splits the isolates into two groups. The haplotypes associated with VCG 2A, 3, 4A, 4A/B and 4B all were grouped closely in the network analysis. A graphical representation of mitochondrial relationships among V. dahliae VCG in previous work (Martin Citation2010), also showed a close association of VCG 4B and VCG 2A isolates separate from VCG 2B isolates. The haplotype H1 associated with VCG 1 and 1A strains was distinctly grouped from other haplotypes with known VCG in this study. Using principal coordinate analysis of V. dahliae microsatellite haplotypes, Dung et al. (Citation2013) were able to establish similar relationships. They showed that VCG 1 genotypes grouped by themselves, while the VCG 2A, 3, 4A, 4A/B and 4B genotypes tended to cluster together ( in Dung et al. Citation2013). They also showed the 2B genotypes were clustered separately ( in Dung et al. Citation2013).
Mitochondrial haplotypes were previously shown to be a useful tool to differentiate isolates of V. dahliae, but the limited data on the VCG for the isolates examined previously did not allow for conclusions about the relationship between VCG and mitochondrial haplotype (Martin Citation2010). The data from the present study, while not exhaustive, provides additional information that indicates there is overlap in haplotypes with VCG 2A and VCG 4 (representative of H3 and H12 in both VCGs) that are reflective of their close phylogenetic relationship (Martin Citation2010; Dung et al. Citation2013). However, overlap in haplotypes was not observed for VCG 1, VCG 2B and VCG 3; each had unique haplotypes. Studies with additional isolates from geographically diverse regions are needed to confirm if mitochondrial haplotype analysis is consistent with VCG and would be useful in a broader application as a diagnostic tool.
Populations of V. dahliae have been shown to contain the MAT1-1 and MAT1-2 idiomorph similar to other species of Ascomycota (Usami et al. Citation2009; Atallah et al. Citation2010; Inderbitzin et al. Citation2011b). In a study by Inderbitzin et al. (Citation2011b), most of the V. dahliae strains carried a MAT1-2 idiomorph and only 11 strains were MAT1-1, while all V. longisporum isolates were MAT1-1. In a study with V. dahliae lettuce isolates, the MAT1-2-1 idiomorph was found in 99% of the isolates (Gurung et al. Citation2014). Only the MAT1-2 idiomorph was found among 286 isolates from mint, potato and other hosts (Dung et al. Citation2013). In the present study, all 106 isolates from sugar beet were the MAT1-2 idiomorph, which supports and confirms data by Dung et al. (2103).
To help reduce Verticillium wilt in sugar beet, rotations with hosts susceptible to V. dahliae VCG 4A strains, such as potato or hosts able to harbour 4A strains (Malcolm et al. Citation2013), should be avoided. Previous work indicates that utilizing resistant cultivars is a valuable alternative (Strausbaugh & Camp Citation2007a, Citation2007b, Citation2007c). However, if seed companies did not purposely screen parental lines to ensure resistance is uniform throughout the lines, then cultivars could potentially be segregating for resistance after the hybrid cross is made to produce seed. Several of the cultivars evaluated as potential susceptible cultivars in the pathogenicity test were found to be segregating for resistance (authors, unpublished data). Seed companies should therefore consider stabilizing the resistance to V. dahliae in commercial sugar beet cultivars sold in Idaho and other areas with a crop rotation that includes potato.
Disclaimer
Mention of trade names or commercial products in this article is solely for the purpose of providing scientific information and does not imply recommendation or endorsement by the US Department of Agriculture.
Supplemental Table 1
Download MS Word (24.6 KB)Acknowledgements
This work was supported by the United States Department of Agriculture, CRIS project 5368-21220-004-00D. We acknowledge the Snake River Sugar Company, Beet Sugar Development Foundation, and Snake River sugar beet growers for supporting our research work. We also gratefully acknowledge the technical support provided by J. Reed.
Supplemental material
Supplemental data for this article can be accessed online here: http://dx.doi.org/10.1080/07060661.2016.1260639
Additional information
Funding
References
- Alkher H, El Hadrami A, Rashid KY, Adam LR, Daayf F. 2009. Cross-pathogenicity of Verticillium dahliae between potato and sunflower. Eur J Plant Pathol. 124:505–519.
- Atallah ZK, Maruthachalam K, Toit L, Koike ST, Davis RM, Klosterman SJ, Hayes RJ, Subbarao KV. 2010. Population analyses of the vascular plant pathogen Verticillium dahliae detect recombination and transcontinental gene flow. Fungal Genet Biol. 47:416–422.
- Atallah ZK, Maruthachalam K, Vallad GE, Davis RM, Klosterman SJ, Subbarao KV. 2011. Analysis of Verticillium dahliae suggests a lack of correlation between genotypic diversity and virulence phenotypes. Plant Dis. 95:1224–1232.
- Berbegal M, Garzón CD, Ortega A, Armengol J, Jiménez-Díaz RM, Jiménez-Gasco MM. 2011. Development and application of new molecular markers for analysis of genetic diversity in Verticillium dahliae populations. Plant Pathol. 60:866–877.
- Bhat RG, Smith RF, Koike ST, Wu BM, Subbarao KV. 2003. Characterization of Verticillium dahliae isolates and wilt epidemics of pepper. Plant Dis. 87:789–797.
- Bhat RG, Subbarao KV. 1999. Host range specificity in Verticillium dahliae. Phytopathology. 89:1218–1225.
- Brantner JR, Windels CE, Omer MA. 2008. Verticillium dahliae causes wilt on sugar beet following potato in eastern North Dakota. Plant Health Progress. doi:10.1094/PHP-2008-1212-01-BR
- Burlakoti P, Rivera V, Secor GA, Qi A, Del Rio-Mendoza LE, Khan MFR. 2012. Comparative pathogenicity and virulence of Fusarium species on sugar beet. Plant Dis. 96:1291–1296.
- Collado-Romero M, Mercado-Blanco J, Olivares-García C, Jiménez-Díaz RM. 2008. Phylogenetic analysis of Verticillium dahliae vegetative compatibility groups. Phytopathology. 98:1019–1028.
- Collado-Romero M, Mercado-Blanco J, Olivares-García C, Valverde-Corredor A, Jiménez-Díaz RM. 2006. Molecular variability within and among Verticillium dahliae vegetative compatibility groups determined by fluorescent amplified fragment length polymorphism and polymerase chain reaction markers. Phytopathology. 96:485–495.
- Covey PA, Kuwitzky B, Hanson M, Webb KM. 2014. Multilocus analysis using putative fungal effectors to describe a population of Fusarium oxysporum from sugar beet. Phytopathology. 104:886–896.
- Daayf F, Nicole M, Geiger J-P. 1995. Differentiation of Verticillium dahliae populations on the basis of vegetative compatibility and pathogenicity on cotton. Eur J Plant Pathol. 101:69–79.
- Dobinson KF, Harrington MA, Omer M, Rowe RC. 2000. Molecular characterization of vegetative compatibility group 4A and 4B isolates of Verticillium dahliae associated with potato early dying. Plant Dis. 84:1241–1245.
- Dobinson KF, Patterson NA, White GJ, Grant S. 1998. DNA fingerprinting and vegetative compatibility analysis indicate multiple origins for Verticillium dahliae race 2 tomato isolates from Ontario, Canada. Mycol Res. 102:1089–1095.
- Douhan LI, Johnson DA. 2001. Vegetative compatibility and pathogenicity of Verticillium dahliae from spearmint and peppermint. Plant Dis. 85:297–302.
- Dung JKS, Peever TL, Johnson DA. 2013. Verticillium dahliae populations from mint and potato are genetically divergent with predominant haplotypes. Phytopathology. 103:445–459.
- El-Bebany AF, Alkher H, Adam LR, Daayf F. 2013. Vegetative compatibility of Verticillium dahliae isolates from potato and sunflower using nitrate non-utilizing (nit) mutants and PCR-based approaches. Can J Plant Pathol. 35:1–9.
- Elena K. 2000. Vegetative compatibility among Verticillium dahliae isolates from watermelon in Greece. Phytoparasitica. 28:115–120.
- Gaskill JO, Krentzer WA. 1940. Verticillium wilt of sugar beet. Phytopathology. 30:769–774.
- Gharbi Y, Triki MA, Trabelsi R, Fendri I, Daayf F, Gdoura R. 2015. Genetic structure of Verticillium dahliae isolates infecting olive trees in Tunisia using AFLP, pathogenicity and PCR markers. Plant Pathol. 64:871–879.
- Göre ME. 2009. Vegetative compatibility and pathogenicity of Verticillium dahliae isolates from chrysanthemum in Turkey. Phytoparasitica. 37:87–94.
- Gurung S, Short DPG, Atallah ZK, Subbarao KV. 2014. Clonal expansion of Verticillium dahliae in lettuce. Phytopathology. 104:641–649.
- Hall T. 1999. BioEdit: a user-friendly biological science sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser. 41:95–98.
- Hanson LE. 2006. First report of Fusarium yellows of sugar beet caused by Fusarium oxysporum in Michigan. Plant Dis. 90:1554.
- Hanson LE, Hill AL, Jacobsen BJ, Panella L. 2009. Response of sugarbeet lines to isolates of Fusarium oxysporum f. sp. betae from the United States. J Sugar Beet Res. 46:11–26.
- Harveson RM. 2009. Verticillium wilt. In: Harveson RM, Hanson LE, Hein G, editors. Compendium of beet diseases and pests. 2nd ed. St. Paul (MN): American Phytopathological Society; p. 40–41.
- Harveson RM, Rush CM. 1997. Genetic variation among Fusarium oxysporum isolates from sugar beet as determined by vegetative compatibility. Plant Dis. 81:85–88.
- Hill AL, Reeves PA, Larson RL, Fenwick AL, Hanson LE, Panella L. 2011. Genetic variability among isolates of Fusarium oxysporum from sugar beet. Plant Pathol. 60:496–505.
- Huson DH, Bryant D. 2006. Application of phylogenetic networks in evolutionary studies. Mol Biol Evol. 23:254–267.
- Inderbitzin P, Bostock RM, Davis RM, Usami T, Platt HW, Subbarao KV. 2011a. Phylogenetics and taxonomy of the fungal vascular wilt pathogen Verticillium, with the descriptions of five new species. PLoS One. 6:e28341.
- Inderbitzin P, Davis RM, Bostock RM, Subbarao KV. 2011b. The Ascomycete Verticillium longisporum is a hybrid and a plant pathogen with an expanded host range. PLoS One. 6:e18260.
- Inderbitzin P, Subbarao KV. 2014. Verticillium systematics and evolution: how confusion impedes Verticillium wilt management and how to resolve it. Phytopathology. 104:564–574.
- Jiménez-Díaz RM, Mercado-Blanco J, Olivares-García C, Collado-Romero M, Bejarano-Alcázar J, Rodríguez-Jurado D, Giménez-Jaime A, García-Jiménez J, Armengol J. 2006. Genetic and virulence diversity in Verticillium dahliae populations infecting artichoke in eastern-central Spain. Phytopathology. 96:288–298.
- Jiménez-Díaz RM, Olivares-García C, Landa BB, Jiménez-Gasco MM, Navas-Cortés JA. 2011. Region-wide analysis of genetic diversity in Verticillium dahliae populations infecting olive in southern Spain and agricultural factors influencing the distribution and prevalence of vegetative compatibility groups and pathotypes. Phytopathology. 101:304–315.
- Jiménez-Gasco MM, Malcolm GM, Berbegal M, Armengol J, Jiménez-Díaz RM. 2014. Complex molecular relationship between vegetative compatibility groups (VCGs) in Verticillium dahliae: VCGs do not always align with clonal lineages. Phytopathology. 104:650–659.
- Joaquim TR, Rowe RC. 1990. Reassessment of vegetative compatibility relationships among strains of Verticillium dahliae using nitrate-nonutilizing mutants. Phytopathology. 80:1160–1166.
- Joaquim TR, Rowe RC. 1991. Vegetative compatibility and virulence of strains of Verticillium dahliae from soil and potato plants. Phytopathology. 81:552–558.
- Johnson DA, Cummings TF. 2015. Effect of extended crop rotations on incidence of black dot, silver scurf, and Verticillium wilt of potato. Plant Dis. 99:257–262.
- Karadimos DA, Karaoglanidis GS, Klonari K. 2000. First report of Verticillium wilt of sugar beet, caused by Verticillium dahliae, in Greece. Plant Dis. 84:593.
- Korolev N, Katan J, Katan T. 2000. Vegetative compatibility groups of Verticillium dahliae in Israel: their distribution and association with pathogenicity. Phytopathology. 90:529–536.
- Korolev N, Pérez-Artés E, Bejarano-Alcázar J, Rodríguez-Jurado D, Katan J, Katan T, Jiménez-Díaz RM. 2001. Comparative study of genetic diversity and pathogenicity among populations of Verticillium dahliae from cotton in Spain and Israel. Eur J Plant Pathol. 107:443–456.
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, et al. 2007. Clustal W and Clustal X version 2.0. Bioinformatics. 23:2947–2948.
- Librado P, Rozas J. 2009. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 25:1451–1452.
- Malcolm GM, Kuldau GA, Gugino BK, Jiménez-Gasco MM. 2013. Hidden host plant associations of soilborne fungal pathogens: an ecological perspective. Phytopathology. 103:538–544.
- Martin FN. 2010. Mitochondrial haplotype analysis as a tool for differentiating isolates of Verticillium dahliae. Phytopathology. 100:1231–1239.
- Omer MA, Johnson DA, Douhan LI, Hamm PB, Rowe RC. 2008. Detection, quantification, and vegetative compatibility of Verticillium dahliae in potato and mint production soils in the Columbia Basin of Oregon and Washington. Plant Dis. 92:1127–1131.
- Omer MA, Johnson DA, Rowe RC. 2000. Recovery of Verticillium dahliae from North American certified seed potatoes and characterization of strains by vegetative compatibility and aggressiveness. Am Potato Res. 77:325–331.
- Panella L, Kaffka SK, Lewellen RT, McGrath JM, Metzger MS, Strausbaugh CA. 2014. Yield gains in major U.S. field crops. Crop Science Society of America (CSSA) special publication 33. Madison (WI): CSSA. Chapter 13, Sugarbeet; p. 357–396.
- Pantou MP, Kouvelis VN, Typas MA. 2006. The complete mitochondrial genome of the vascular wilt fungus Verticillium dahliae: a novel gene order for Verticillium and a diagnostic tool for species identification. Curr Genet. 50:125–136.
- Pegg GF, Brady BL. 2002. Verticillium wilts. New York (NY): CABI Publishing; 552 pp.
- Puhalla JE. 1979. Classification of isolates of Verticillium dahliae based on heterokaryon incompatibility. Phytopathology. 69:1186–1189.
- Puhalla JE, Hummel M. 1983. Vegetative compatibility groups within Verticillium dahliae. Phytopathology. 73:1305–1308.
- Qin Q-M, Vallad GE, Wu BM, Subbarao KV. 2006. Phylogenetic analyses of phytopathogenic isolates of Verticillium spp. Phytopathology. 96:582–592.
- Rowe RC. 1995. Recent progress in understanding relationships between Verticillium species and subspecific groups. Phytoparasitica. 23:31–38.
- Ruppel EG. 1991. Pathogenicity of Fusarium spp. from diseased sugar beets and variation among sugar beet isolates of F. oxysporum. Plant Dis. 75:486–489.
- Strausbaugh CA. 1993. Assessment of vegetative compatibility and virulence of Verticillium dahliae isolates from Idaho potatoes and tester strains. Phytopathology. 83:1253–1258.
- Strausbaugh CA, Camp S. 2007a. Verticillium wilt in commercial sugar beet cultivars in Cassia County, ID, 2006. Plant Dis Manage Rep. 1:V112.
- Strausbaugh CA, Camp S. 2007b. Verticillium wilt in experimental sugar beet cultivars in Cassia County, ID, 2006. Plant Dis Manage Rep. 1:V113.
- Strausbaugh CA, Camp S. 2007c. Verticillium wilt in transgenic sugar beet cultivars in Cassia County, ID, 2006. Plant Dis Manage Rep. 1:V114.
- Strausbaugh CA, Schroth MN, Weinhold AR, Hancock JG. 1992. Assessment of vegetative compatibility of Verticillium dahliae tester strains and isolates from California potatoes. Phytopathology. 82:61–68.
- Tsror (Lahkim) L, Hazanovsky M, Mordechi-Lebiush S, Sivan S. 2001. Aggressiveness of Verticillium dahliae isolates from different vegetative compatibility groups to potato and tomato. Plant Pathol. 50:477–482.
- Usami T, Itoh M, Amemiya Y. 2009. Asexual fungus Verticillium dahliae is potentially heterothallic. J Gen Plant Pathol. 75:422–427.
- Usami T, Itoh M, Morii S, Miyamoto T, Kaneda M, Ogawara T, Amemiya Y. 2012. Involvement of two different types of Verticillium dahliae in lettuce wilt in Ibaraki Prefecture, Japan. J Gen Plant Pathol. 78:348–352.
- Webb KM, Case AJ, Brick MA, Otto K, Schwartz HF. 2013. Cross pathogenicity and vegetative compatibility of Fusarium oxysporum isolated from sugar beet. Plant Dis. 97:1200–1206.
- Webb KM, Covey PA, Hanson LE. 2012. Pathogenic and phylogenetic analysis of Fusarium oxysporum from sugarbeet in Michigan and Minnesota. J Sugar Beet Res. 49:38–56.
- Windels CE, Brantner JR, Bradley CA, Khan MFR. 2005. First report of Fusarium oxysporum causing yellows on sugar beet in the Red River Valley of Minnesota and North Dakota. Plant Dis. 89:341.

