Abstract
Pods of okra (Abelmoschus esculentus L Moench) ‘BARI Dherosh-1ʹ cultivated in a farmer’s field in Gazipur District, Bangladesh, were found rotted in December 2014. A fungus having fluffy mycelium and large sclerotia was isolated from affected pods. Combined results of morphological, molecular and pathological analyses identified the fungus as Sclerotinia sclerotiorum (Lib) de Bary. Inoculating the fungus on healthy okra pods reproduced the symptoms previously observed in the field. This is the first report of S. sclerotiorum causing disease of okra in Bangladesh.
Résumé
En décembre 2014, on a trouvé, dans le champ d’un cultivateur dans le district de Gazipur au Bangladesh, des gousses d’okra (Abelmoschus esculentus L Moench) ‘BARI Dherosh-1ʹ pourries. Un champignon produisant un mycélium duveteux et de gros sclérotes a été isolé à partir des gousses infectées. Les résultats combinés des analyses morphologiques, moléculaires et pathologiques ont permis d’identifier le champignon en tant que Sclerotinia sclerotiorum (Lib) de Bary. En inoculant des gousses saines d’okra avec le champignon, il a été possible de reproduire les symptômes observés au champ précédemment. Il s’agit de la première mention de S. sclerotiorum causant une maladie chez l’okra au Bangladesh.
Introduction
Okra (Abelmoschus esculentus L Moench) is one of the most important vegetable crops grown in Bangladesh (Saifullah & Rabbani Citation2009). It belongs to the family Malvaceae, originating from tropical and subtropical Africa (Sathish & Eswar Citation2013). Okra is cultivated throughout the year in Bangladesh but the yields can be as low as 1.33 tons ha−1 (BBS 2014). Diseases caused by different pathogens are the major reasons behind this low level of okra production in Bangladesh.
A new disease was observed on approximately 2% of okra pods in a farmer’s field located in Gazipur District, Bangladesh in 2014. Infected pods showed both internal and external large brown areas with necrotic tissues at the crown level. Fluffy white mycelia were evident on infected tissues. Some pods died prematurely and became bleached. Black sclerotia formed on the surface of infected tissues or were embedded within tissues. The objectives of the present study were to identify the pathogen involved in the okra pod rot in Bangladesh using morphological and molecular characterization and to verify the pathogenicity of this fungus.
Materials and methods
Isolation and characterization of the pathogen
Ten infected pods were collected from a farmer’s field in Gazipur District. They were washed in tap water and dried on sterile blotter paper. Symptomatic pod tissues were cut into 2–3-mm pieces, surface-sterilized with 70% ethanol for 30 s and then with 0.1% mercuric chloride solution for 3 min. After rinsing in sterile distilled water three times and subsequent drying on blotter paper, 30 tissue pieces were placed in Petri dishes containing potato dextrose agar (PDA) made from peeled potato infusion and amended with streptomycin sulphate (1.0 g L−1). Petri dishes were incubated at 25ºC in the dark for 5 days. For the morphological identification of the isolated fungus, single hyphal tips were transferred onto new PDA dishes. No signs of asexual spore formation were found on any of the fungal isolates while all the isolates produced large sclerotia.
Morphological characteristics of the teleomorph stages of 20 isolates were examined. Surface-sterilized sclerotia were placed in 9 cm diameter Petri dishes filled with sterile, wet sand and incubated at 4ºC for 5–6 weeks. Then the Petri dishes were transferred to another incubator and illuminated for 3–4 days at 20ºC under scattered fluorescent irradiation (260 µmol m−2 s−1) until apothecial discs were formed (Huang et al. Citation2005). Fifteen apothecia and the ascospores produced from sclerotia were examined under the microscope (40× and 100×) for morphological features.
Molecular identification
Total genomic DNA was extracted as described by Toda et al. (Citation1999). Polymerase chain reaction (PCR) with forward primer ITS-1 (5ʹ-TCC GTA GGT GAA CCT GCG G-3ʹ) and reverse primer ITS-4 (5ʹ-TCC TCC GCT TAT TGA TAT GC-3ʹ) was used to amplify rDNA-ITS regions of the fungal isolate PSLF1 (White et al. Citation1990) and was conducted following the method described by Hayakawa et al. (Citation2006). At least two complete sequences were obtained for each ITS region and the BLAST search program was used to search for nucleotide sequence homology in GenBank. Highly homologous sequences were aligned using Clustal-X version 2.0.11 and manually adjusted as required. Neighbour joining trees were generated using MEGA version 6.06 (Tamura et al. Citation2013; Islam et al. Citation2016). Bootstrap replication (1000 replications) was used as a statistical support for the nodes in the phylogenetic trees. The adjusted alignment consisted of eight taxa including Magnaporthe oryzae Ar2 (KJ850437) as an outgroup taxon.
Pathogenicity tests
Pathogenicity of isolate PSLF1 used in the molecular analysis was tested. The okra variety ‘BARI Dherosh-1ʹ was used throughout the study. PDA plugs excised from actively growing margins of fungal colonies were placed in wounds made with a sterile blade in the outer tissue layer of okra pods and sealed with moistened cheesecloth. Wounded pods inoculated with sterile PDA plugs served as controls. The inoculated pods were subsequently covered with plastic bags and maintained in a growth chamber in dark for 48 h at 23–25°C. Twenty pods were inoculated with isolate PSLF1, and 20 pods were included as controls. Pathogenicity of the isolate was also tested on 4-week-old whole plants by placing a mycelial plug of the fungus onto an incised stem surface in a similar manner. Re-isolation of the fungus was done from symptomatic tissues to compare and confirm that the original and the re-isolated fungus were the same.
Results and discussion
Isolation and characterization of the pathogen
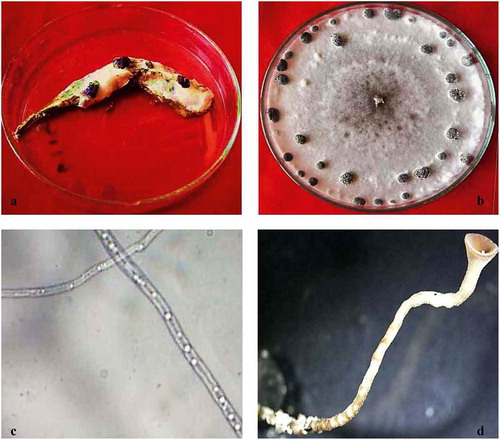
One fungus was consistently isolated from symptomatic tissues of okra pods (). On PDA, colonies were fast growing with white, floccose, aerial mycelia and reverse salmon-buff colour (). Hyphae were hyaline, branched and multinucleate (). No conidia or conidiophores were produced. Sclerotia developed in culture at the growing margins of the colonies, forming concentric rings and radiating lines (). Sclerotia were variable in shape, but mostly globose to cylindrical with a black outer rind and a white inner cortex. Individual sclerotia were 4–8 mm long and 3–5 mm wide. Apothecia were produced at 6 weeks of incubation. Individual to several apothecia arose from a sclerotium and were tan to amber coloured. Receptacles were broad, slightly concave when young and convex at maturity often with a central depression (). Fully mature apothecia were 4–6 mm in diameter with a stipe length of 3–5 mm. Asci from apothecia were cylindrical and eight-spored. Ascospores were uniseriate, single celled, hyaline and ellipsoid. Paraphyses were abundant. These characteristics are consistent with those described for Sclerotinia sclerotiorum (Lib.) de Bary (Chang & Kim Citation2003; Jeon et al. Citation2006).
Fig. 1 (Colour online) Symptoms of field-infected okra pods and the isolated fungus. (a) Infected pod displaying white mycelium growth and sclerotial development. (b) Pure culture of the isolated fungus showing white fluffy mycelium and rings of sclerotia. (c) Hyaline mycelium of S. sclerotiorum. (d) Stalk of mature apothecium.
Molecular identification
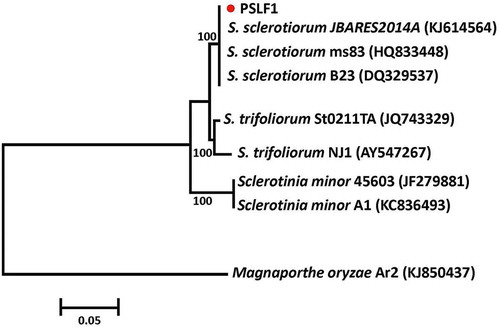
The ITS sequences obtained in this study were 586 bp in length. The sequence was submitted to NCBI GenBank, and was given accession no. KT452625. The sequence shared 100% nucleotide identity and 99% Query Coverage with the ITS sequences of different strains of S. sclerotiorum in the BLAST search, confirming that the isolated fungus is a member of this group. In the phylogenetic tree, the fungus isolated from okra pods grouped with S. sclerotiorum with good bootstrap support ().
Fig. 2 (Colour online) Phylogenetic tree constructed with the ITS-5.8S rDNA sequence of the isolate from this study (PSLF1), and other species of Sclerotinia retrieved from GenBank. Magnaporthe oryzae was used as the out-group taxon. The bar indicates nucleotide substitutions per site. Numbers of bootstrap support values ≥ 50% based on 1000 replicates.
Pathogenicity test
Pathogenicity testing of the candidate isolate on okra pods revealed that symptoms identical to those observed in the farmer’s field developed 10–14 days after inoculation. All inoculated pods were successfully infected by PLSF1 isolate. The inoculated pods were destroyed within 21 days. Similarly, all stem-inoculated plants had lesions by 5 days post-inoculation. The lesions progressed upward and downward on the stem and formed a distinct demarcation zone between healthy and infected tissue. Two weeks after inoculation, all plants wilted and died and stem breakage was also observed for some dead plants. Dark sclerotia were found after splitting the dead pods or stems. The pathogen was re-isolated from the infected tissues and identified as S. sclerotiorum, as previously described. Even though the pathogen can infect the stems of okra, this type of infection has not been observed in the field.
Morphological features of the fungus associated with disease symptoms on okra pods including hyphae, sclerotia and apothecial morphology, as well as partial gene sequences for ITS, identify the pathogen studied as S. sclerotiorum. In addition, Koch’s postulates demonstrated the pathogenicity of this fungus and its role as the causal agent of the new disease detected in Bangladesh. Sclerotinia sclerotiorum causes flower blights, stem rots, fruit rots, head blight, crown rots and basal stem infection of numerous hosts (Link & Johnson Citation2007). This species has a large distribution in many countries (Bolton et al. Citation2006). In Bangladesh, S. sclerotiorum was previously reported on hyacinth bean, marigold and jackfruit (Prova Citation2012; Prova et al. Citation2014; Rahman et al. Citation2015). The role of ascospores is important in initiating foliar infections on crops such as beans and canola (McDonald & Boland Citation2004) and could be involved in initiating pod infections on okra. To our knowledge, this is the first report of the occurrence of a disease caused by S. sclerotiorum on okra pod in Bangladesh, and it is the first detection using molecular data of this fungal pathogen in Bangladesh. The disease has previously been reported to occur on okra pods in Mie Prefecture in Japan (Kubota & Misawa Citation2014).
Acknowledgements
The authors would like to acknowledge the financial assistance from the Ministry of Science and Information & Communication Technology (MOSICT), Project No. 41/1(7), Government of the People’s Republic of Bangladesh. The authors are thankful to Prof. M. T. Islam, Bangabandhu Sheikh Mujibur Rahman Agricultural University, Gazipur-1706, Bangladesh for his contribution in obtaining molecular identification of the fungal pathogen.
Additional information
Funding
References
- Anonymous. 2014. Yearbook of agricultural statistics. 26th series. Bangladesh Bureau of Statistics (BBS), Statistics and Informatics Division (SID). Ministry of Planning, Government of the People’s Republic of Bangladesh; p. 130.
- Bolton MD, Thomma BPHJ, Nelson BD. 2006. Sclerotinia sclerotiorum (Lib.) de Bary: biology and molecular traits of a cosmopolitan pathogen. Mol Plant Pathol. 7:1–16.
- Chang SW, Kim SK. 2003. First report of Sclerotinia rot caused by Sclerotinia sclerotiorum on some vegetable crops in Korea. Plant Pathol J. 19:79–84.
- Hayakawa T, Toda T, Ping Q, Mghalu JM, Yaguchi S, Hyakumachi M. 2006. A new subgroup of Rhizoctonia AG-D, AG-D III, obtained from Japanese zoysia grass exhibiting symptoms of a new disease. Plant Dis. 90:1389–1394.
- Huang HC, Erickson RS, van Hezewijk B, de Clerck-Floate R. 2005. White mold of Houndstongue (Cynoglossum officinale) caused by Sclerotinia sclerotiorum in Canada. Plant Dis. 89:1013.
- Islam S, Akanda AM, Prova A, Islam MT, Hossain MM. 2016. Isolation and Identification of plant growth promoting rhizobacteria from cucumber rhizosphere and their effect on plant growth promotion and disease suppression. Front Microbiol. 6:1360.
- Jeon YJ, Kwon HW, Nam JS, Kim SH. 2006. Characterization of Sclerotinia sclerotiorum isolated from paprika. Mycobiology. 34:154–157.
- Kubota M, Misawa T. 2014. Gray mold and Sclerotinia rot of okra plants. Annu Rep Kanto-Tosan Soc. 61:43–46.
- Link HV, Johnson KB. 2007. White mold. The plant health instructor. [Internet]; [ cited 2007 Aug 29]. Available from: http://www.apsnet.org/edcenter/intropp/lessons/fungi/ascomycetes/Pages/WhiteMoldPortuguese.aspx
- McDonald MR, Boland GJ. 2004. Forecasting diseases caused by Sclerotinia spp. in eastern Canada: fact or fiction? Can J Plant Pathol. 26:480–488.
- Prova A. 2012. Isolation, identification and characterization of white mold disease in marigold (Tagetes officinales L.) and hyacinth bean (Lablab purpureus) casued by Sclerotinia sclerotiorum [dissertation]. Gazipur: Bangabandhu Sheikh Mujibur Rahman Agricultural University.
- Prova A, Akanda MAM, Islam S, Sultana F, Islam MT, Hossain MM. 2014. First report of stem and pod blight of hyacinth bean caused by Sclerotinia sclerotiorum in Bangladesh. J Plant Pathol. 96:603–611.
- Rahman MME, Dey TK, Hossain DM, Nonaka M, Harada N. 2015. First report of white mould caused by Sclerotinia sclerotiorum on jackfruit. Australasian Plant Dis Notes. 10:10.
- Saifullah M, Rabbani MG. 2009. Evaluation and characterization of okra (Abelmoschus esculentus L. Moench.) genotypes. SAARC J Agric. 7:92–99.
- Sathish D, Eswar A. 2013. A review on: Abelmoschus esculentus (Okra). Inter Res J Pharm Appl Sci. 3:129–132.
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA 6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 30:2725–2729.
- Toda T, Hyakumachi M, Suga H, Kageyama K, Tanaka A, Tani T. 1999. Differentiation of Rhizoctonia AG-D isolates from turfgrass into subgroups I and II based on rDNA and RAPD analysis. Eur J Plant Pathol. 105:835–846.
- White TJ, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. New York (NY): Academic Press; p. 315–322.
