Abstract
Genetic resistance is widely used to manage clubroot (Plasmodiophora brassicae) in canola (Brassica napus) and other brassica crops. Replicated field trials were conducted with three clubroot-resistant cultivars and one susceptible cultivar of either canola or napa cabbage (B. rapa) in southern Ontario in 2014 and 2015. These studies indicated that plant growth and development in resistant cultivars of both crops were often reduced at high concentrations of inoculum (resting spores) of the pathogen. In napa cabbage, leaf length was reduced by 31% in 2014 and 22% in 2015 at a site with high spore concentration relative to a site with lower concentration. In canola, height of resistant plants was reduced by 30%, biomass by 43%, and maturity was delayed at a site with high spore concentration relative to an adjacent site with lower spore concentration in 2014. However, there were no differences among sites in 2015. A growth room study demonstrated that plant height of a clubroot-resistant canola cultivar declined by 12–14% at high spore concentrations. Biomass of canola was also lower and maturity was delayed with increasing concentration of spores in some, but not all, field and laboratory studies. We suggest that the expression of resistance to clubroot in resistant cultivars can result in reduced plant growth and development in both B. rapa and B. napus, but that substantial reductions in plant growth and development occur only at high concentrations (> 106 g−1) of resting spores in soil.
Résumé
La résistance génétique est largement utilisée pour contrer la hernie (Plasmodiophora brassicae) chez le canola (Brassica napus) et d’autres plantes de la famille des brassicacées. En 2014 et 2015, dans le sud de l’Ontario, des essais en champ répétés ont été effectués avec trois cultivars résistants à la hernie et un cultivar réceptif de canola ou de chou champêtre (B. rapa). Ces études ont indiqué que, à de fortes concentrations d’inoculum (spores de réserve) de l’agent pathogène, la croissance et le développement chez les cultivars résistants des deux cultures étaient souvent réduits. Chez le chou champêtre, la longueur de la feuille était réduite de 31% en 2014 et de 22% en 2015, et ce, à un site qui affichait une forte concentration de spores comparativement à un site qui en affichait une plus faible. Chez le canola, la taille des plants résistants a été réduite de 30%, la biomasse, de 43% et l’atteinte de la maturité a été retardée à un site où la concentration de spores était élevée comparativement à un site adjacent qui en affichait une plus faible en 2014. Toutefois, il n’y avait aucune différence entre les sites en 2015. Une étude effectuée en chambre de croissance a démontré que, à de fortes concentrations de spores, la taille d’un cultivar de canola résistant à la hernie a diminué de 12 à 14%. La biomasse du canola était également plus faible et l’atteinte de la maturité retardait en fonction de l’augmentation de la concentration des spores chez certains, mais pas chez tous, en champ comme en chambre de croissance. Nous suggérons que l’expression de la résistance à la hernie chez les cultivars résistants peut entraîner la réduction de la croissance et du développement chez B. rapa et B. napus, mais qu’une réduction substantielle de ces paramètres ne se produit qu’à de fortes concentrations (> 106 g−1) de spores de réserve dans le sol.
Introduction
A metabolic cost of disease resistance is the negative effect on growth and development that occurs when a plant’s resources are reallocated toward defence in response to pathogen recognition (Brown & Rant Citation2013). Measurable levels of metabolic costs are most commonly incurred when pathogen attack stimulates induction of a resistance response (Bergelson & Purrington Citation1996), resulting in activation of previously inactive resistance mechanisms (Van Loon et al. Citation1998). There may be several physiological and genetic factors contributing to the overall cost of resistance (Brown & Rant Citation2013). The cost of disease resistance can be viewed as a trade-off against the greater cost of not expressing resistance in the presence of a virulent pathogen. A trade-off between plant growth and defence against pathogens involving the jasmonate-signalling pathway is a widely conserved defence strategy in angiosperms. Gibberellic acid activates defence and also mediates a delay in the degradation of growth-response proteins in rice (Oryza sativa L.) and Arabidopsis thaliana (L.) Heynh. (Yang et al. Citation2012).
Genetic resistance is essential for management of P. brassicae in canola (Cao et al. Citation2009). In the current suite of clubroot-resistant commercial cultivars, clubroot symptoms do not develop and resting spores are not produced in an incompatible interaction (Ludwig-Muller et al. Citation1999; Deora et al. Citation2012). Root hair infection, which plays an important role in recognition and induction of resistance to P. brassicae in canola (Siemens et al. Citation2002; Feng et al. Citation2012; McDonald et al. Citation2014), can occur in very young seedlings and continue until maturity, so the resistance response is expressed throughout the entire life of resistant plants.
A metabolic cost of resistance to plant pathogens has been identified in many crops. These costs are often attributed to a fitness cost associated with a specific gene for resistance (detrimental in the absence of challenge by the pathogen) or an opportunity cost associated with the plant allocating its physiological resources to defence response rather than to growth and development (Brown & Rant Citation2013). In canola, the fitness cost associated with genes for clubroot resistance in the current suite of cultivars appears to be low or non-existent, because their development and seed yield are similar to susceptible lines in the absence of the pathogen in standardized trials across the Canadian prairies (Canola Council of Canada Citation2016a). Indicators such as vegetative growth, leaf area and pod development are useful in the assessment of cost of resistance because they make important contributions to yield (Freyman et al. Citation1973; Krogman & Hobbs Citation1975; Campbell & Kondra Citation1978; Chongo & McVetty Citation2001).
Inoculum pressure, which for P. brassicae is quantified as resting spore concentration in soil, can have a strong impact on infection and symptom development. In a susceptible cultivar, 1000 spores g−1 soil is commonly cited as the threshold for consistent infection (Donald & Porter Citation2009; Faggian & Strelkov Citation2009). However, the effect of resting spore concentration on the severity of clubroot symptoms can vary depending on other factors, including temperature (Sharma et al. Citation2011a, Citation2011b), pH (Colhoun Citation1953; Gossen et al. Citation2013; Dalton Citation2015) and plant age (Hwang et al. Citation2011b). Root hair infection declined above pH 6.5 (Myers & Campbell Citation1985; Webster & Dixon Citation1991; Donald & Porter Citation2004). Development of primary plasmodia was inhibited at pH ≥ 7.2 prior to secondary zoospore release (Myers & Campbell Citation1985). Severe clubbing occurred at spore concentrations as low as 103 at pH 6.3, but spore concentration was positively correlated with clubroot incidence at pH 7.8 (Colhoun Citation1953).
The objectives of this research were to quantify the effect of concentration of P. brassicae resting spores on growth and development of clubroot-resistant canola and napa cabbage to determine if there was a measurable metabolic cost of resistance.
Materials and methods
Field trials
Field trials were conducted in 2014 and 2015 at adjacent sites (about 50 m apart) at the Muck Crops Research Station of the University of Guelph, Holland Marsh, ON to compare the growth of clubroot-resistant canola and napa cabbage cultivars. Each year, the sites were on organic soil (a typic humisol-muck), had a similar cropping history, and had received the same agronomic treatments, e.g. tillage and fertilization, the previous year. Recommended levels of N, P, K and micro-nutrient fertilizers were broadcast onto the entire study area, including all of the sites, in early spring each year. Although there were small differences in soil nutrient status among the sites based on individual soil tests (), levels of all of the nutrients assessed at each site were adequate to support strong crop growth (Canola Council of Canada Citation2016b). Also, canola plants develop a large, deep taproot on muck soil, and so can utilize nutrients from deep within the soil profile; therefore, small differences in nutrient status among sites should not have affected crop development. The main difference among the sites was the concentration of resting spores of P. brassicae; one site was heavily infested and the other had no recent history of clubroot infestation (). The experimental design at each site was a randomized complete block design with four replicates.
Table 1. Soil properties and estimated concentration of resting spores of Plasmodiophora brassicae in soil at the study sites at the Muck Crops Research Station at Holland Marsh, Ontario in 2014 and 2015.
Soil samples for assessment of resting spore concentration were collected on 13 June 2014 and 21 May 2015 using a 25-cm-long soil core sampler. Three cores were taken within 1 m2 at two places in each trial. The cores were air-dried at room temperature, bulked within place, pulverized using a clean, sterile ceramic mortar and pestle, and mixed thoroughly. DNA was extracted from the bulked soil sample using the PowerSoil DNA isolation kit (MO BIO Laboratories Inc., Carlsbad, CA). Three technical replicates were conducted per bulked sample. For each replicate, 0.1 g of soil was added directly into microbead tubes provided with the kit. DNA extraction and purification were performed according to the manufacturer’s instructions. The resting spore concentration in each sample was determined using a multiplexed TaqMan qPCR assay with a competitive internal positive control (CIPC) to adjust for differential inhibition among the samples (Deora et al. Citation2015).
One trial compared the growth of three clubroot-resistant canola cultivars, ‘45H29ʹ (Pioneer Hi-Bred Ltd, Chatham, ON), ‘73-67ʹ (Monsanto Canada Inc., Winnipeg, MB) and ‘73-77ʹ (Monsanto Canada Inc.), and a susceptible canola line, ACS-N39 (AAFC, Saskatoon SK) (Gludovacz Citation2013). The sites were direct seeded at about 18 seeds per m of row using an Earthway push seeder fitted with an Earthway 1002–9 disc (Earthway Products, Bristol, IN) on 26 June 2014. Each plot was a single 6-m-long row, with 26 cm between rows. The trial was seeded in early summer to optimize soil temperature for pathogen development (Gossen et al. Citation2013; Cranmer Citation2015).
A representative sample of 10 plants was selected in each plot for repeated measurement at 7-day intervals, starting 4 weeks after seeding. Plant growth was assessed by measuring plant height from the hypocotyl to the shoot apex. At 9 weeks after planting, the developmental stage (vegetative, bud, flowering, pod development) of each plant was assessed. Plants were harvested and weighed, and roots were assessed for clubroot incidence (%) and severity using a standard 0–3 rating scale, where 0 = no clubbing, 1 < 1/3 of root clubbed, 2 = 1/3–2/3 of roots clubbed and 3 > 2/3 of roots clubbed (Strelkov et al. Citation2006). A disease severity index (DSI) was calculated as follows (Crête et al. Citation1963):
In a companion trial, three clubroot-resistant napa cabbage cultivars, ‘China Gold’ (Sakata Seed Corporation, Morgan Hill, CA), ‘Yuki’ (Sakata Seed Corporation) and ‘Emiko’ (Bejo Seeds Inc. Oceano, CA), and the susceptible ‘Mirako’ (Bejo Seeds Inc.) were sown on 16 July 2014. Each plot was 3 m in length, and plants were thinned to about 25 cm apart within rows. Plant growth was assessed by measuring the length of the third and fourth youngest leaves. Plant developmental stage or seed production could not be assessed in napa cabbage because they do not flower in the first year after planting. Seed yield was not assessed in canola because pest issues at this site (especially Swede midge) make seed yield too variable to be a reliable indicator of crop growth.
Both trials were repeated in 2015, with an additional site added to each trial where the spore concentration was below detectable levels (). All plots were 6 m in length, and each plot for both canola and napa cabbage was seeded on 9 July 2015 to facilitate comparison across crop species. Also, the susceptible napa cabbage ‘Mirako’ was replaced with ‘Suzuko B-2961ʹ (Bejo Seeds, Inc.) due to the unexpected discontinuation of ‘Mirako’.
In the 2 weeks following seeding of canola in 2014, the total rainfall was 6 mm with no more than 4 mm in any single day. Mean daily air temperature was 29°C maximum and 14°C minimum. In contrast, in the 2 weeks following napa cabbage seeding in 2014, the total rainfall was 37 mm, of which 35 mm occurred over 2 days. In 2015, both canola and napa cabbage were seeded at the same date. In the 2 weeks following seeding, the total rainfall was 8 mm with no more than 4 mm in any single day. Mean daily air temperature was 27°C maximum and 14°C minimum.
Large pot studies – outdoors
An outdoor study was conducted using large plastic pots (30 cm × 28 cm diam.) in 2015 in a randomized complete block design with four replicates at a mineral soil site near the Muck Crops Research Station. Each experimental unit consisted of two pots, with five plants per pot. Canola ‘45H29ʹ (resistant) and breeding line ACS-N39 (susceptible) were seeded into 200-cell plug trays and grown in a greenhouse. The lines were seeded on 9 June 2015. The seedlings were transplanted 2 weeks later into pots containing 75% mineral soil from the site (dark grey gleysol, Granby sandy loam soil) thoroughly mixed with 25% soil-less media (Sunshine Mix #4, Sun Gro Horticulture Canada Ltd. Agawam, MA) to reduce compaction and promote normal root growth. The pH of the soil in each pot was lowered from 7.8 to 6.4 by application of 7.3 g sulphur chips (90%), 2.9 g nitrogen sulphate and 1.0 g magnesium sulphate in 400 mL water adjusted to pH 4.0 with phosphoric acid. After transplanting, the pots were watered once per week with water adjusted to pH 6.0 using commercial vinegar and N-P-K (20–20–20) solution.
Inoculum of P. brassicae pathotype 6 was prepared following a standard protocol (Sharma et al. Citation2011a). Briefly, clubbed roots collected the previous year from the Muck Crops Research Station where pathotype 6 predominates, were washed and soaked in deionized water, and 10 g of clubbed root tissue was homogenized in 300 mL deionized water for 2 min in a blender. The mixture was filtered through eight layers of cheesecloth and a hemocytometer was used to estimate the resting spore concentration. The resulting spore suspension was diluted with deionized water to create the required treatment concentrations.
Each pot was inoculated at transplanting with 450 mL of spore suspension of pathotype 6 to produce a final concentration of 0, 1 × 103, 1 × 104, 1 × 105, 1 × 106 and 1 × 107 resting spores g−1 soil in the top 15 cm of the pot. The study was repeated, seeded on 24 June 2015. In the 2 weeks following transplanting, the mean daily air temperature was 23°C maximum and 15°C minimum in the first repetition and 27°C maximum and 14°C minimum in the second repetition.
The height of each resistant plant was assessed at weekly intervals starting at 2 weeks after inoculation (WAI). At 8 WAI, the developmental stage (vegetative, bud, flowering, pod development) of each plant was assessed. Plants were then harvested, weighed and roots assessed for clubroot incidence (%) and severity using the 0–3 rating scale. Biomass was assessed on one pot (five plants) per experimental unit.
Controlled environment study – canola
Growth room studies were conducted using a randomized complete block design with four replicates. Two seeds of clubroot-resistant canola ‘45H29ʹ were sown in each tall narrow plastic pot (conetainers, Stuewe and Sons Inc., Corvallis, OR) filled with soil-less media (Sunshine Mix #4, Sun Gro Horticulture Canada Ltd, Agawam, MA) and thinned to one seedling per pot, with 10 pots per experimental unit. Canola line 'ACS-N39ʹ was used as a susceptible control. The study was conducted in a growth room at 25°/20°C day/night, with 16-h photoperiod and 65% relative humidity. The plants were watered with deionized water adjusted to pH 6.0 using commercial white vinegar and fertilized with 1 g L−1 N-P-K (20-20-20) and 1 g L−1 magnesium sulphate solution at 2–3 day intervals.
The treatments were inoculation with resting spore suspension at the following concentrations: 0, 1 × 104, 1 × 105, 1 × 106, 1 × 107 and 1 × 108 spores mL−1. Inoculum was prepared as described previously. Each seedling was inoculated by applying 5 mL of spore suspension to the base of the stem at 9 days after seeding.
The height of each plant from hypocotyl (at soil surface) to shoot apex was assessed at 7-day intervals from 2 to 6 weeks after inoculation. At 10 weeks after seeding, plants were harvested and weighed, and roots were assessed for clubroot incidence (%) and severity using the standard 0–3 rating scale and a disease severity index was calculated. Note that when the trial was repeated, the height measurement for week 5 was omitted by mistake.
Controlled environment study – canola and napa cabbage
In a growth room study to assess the impact of moderate inoculum pressure on growth of clubroot-resistant canola lines, three clubroot-resistant canola cultivars, ‘45H29ʹ, ‘73-67ʹ and ‘73-77ʹ and the susceptible line ACS-N39 were sown in tall narrow plastic pots filled with mineral soil from Elora Research Station, University of Guelph, Elora, ON (4% organic matter, pH 7.2). Two seeds were planted per pot and later thinned to one seedling per pot. The plants were maintained in a growth room as described previously, arranged in a randomized complete block design with four replicates and 10 pots per experimental unit. Each 10-day-old seedling was inoculated with 5 mL of resting spore suspension of 1 × 106 resting spores mL−1 of P. brassicae as described previously. The height of each plant was assessed at 7-day intervals, starting 2 weeks after inoculation, and biomass and clubroot severity were assessed as described previously.
In companion studies, three clubroot-resistant napa cabbage cultivars, ‘China Gold’, ‘Yuki’ and ‘Emiko’, and the susceptible ‘Mirako’ were assessed using the same protocol, except that leaf length was assessed instead of plant height. Leaf length was measured from the base of the blade to the tip of the blade for the third and fourth youngest leaves on each plant.
Each study was repeated, except that 25% soil-less media (Sunshine Mix #4, Sun Gro Horticulture Canada Ltd, Agawam, MA) was mixed into the mineral soil prior to planting to reduce compaction. Also, the soil mixture was saturated with water adjusted to pH 4.0 using phosphoric acid to lower the pH of the soil mixture at 2 weeks prior to planting.
Statistical analysis
All analyses were conducted using SAS version 9.3. The data for field trials and the controlled environment study of canola and napa cabbage were analysed using a mixed model analysis of variance. Single degree of freedom contrasts and the slice option were used to examine the interaction between cultivar and site (spore concentration). Homogeneity of variance across sites in the field trials was assessed using Levene’s test. The variances in biomass and maturity of resistant canola across sites were homogeneous within each year. Variance in plant height was homogeneous across sites in 2015 but not in 2014. There was homogeneity of variance within CI and DSI for the susceptible canola line among sites within year. Variance was not homogeneous across years for any variable, so each year was analysed separately. Means separation was conducted using Tukey’s test at P = 0.05. The fixed effects in the mixed model analysis were cultivar, crop and spore concentration, and random effects were block and repetition.
The data for the controlled environment study of canola and the large pot outdoor study were analysed using a mixed model analysis of variance supplemented with single-degree of freedom contrasts. Spore concentration was a fixed effect and block and repetition were random effects. Plant height measurements were compared at individual time-points. Growth over time was assessed using the area under the disease progress stairs concept (Simko & Piepho Citation2012). This approach was used to produce a summary statistic of area under the growth stairs (AUGS) where equal weighting of the growth at each time-point summarized season-long growth into a single value, calculated as follows:
where Yi is plant height in cm at the ith observation, ti is time in days after inoculation at the ith observation, and n is the total number of observations.
Results
Field trials
The estimated concentrations of resting spores of P. brassicae in soil at the study sites in 2014 and 2015 are presented in . In 2014, spore concentration at the ‘low site’ was 7 × 105 spores g−1 soil, which was 90% lower compared with the 7 × 106 spores at the ‘high site’, but clubroot levels were near 100% in the susceptible canola line at both sites (). For napa cabbage, the susceptible line had 23% CI and 13% DSI at the low site, and 90% CI and 44% DSI at the high site (). There were no clubbing symptoms in resistant canola or napa cabbage on either site in 2014.
Table 2. Clubroot incidence (CI) and severity (disease severity index, DSI) in clubroot-resistant and susceptible canola and napa cabbage lines in field trials at adjacent sites at Muck Crops Research Station that differed in levels of resting spore concentration of Plasmodiophora brassicae in the soil (high, low and below detection limit, BDL) in 2014 and 2015.
In 2015, clubroot incidence was 100% in the susceptible canola line at both the high and low sites, where the spore concentration at the low site was 3 × 106. Even at the site where spore concentration was below detectable limits, incidence was 45%. DSI showed a similar pattern of response (). All three clubroot-resistant canola lines developed low levels of clubroot symptoms at all sites in 2015. In napa cabbage, no clubroot symptoms were observed on ‘Suzuko B-2961ʹ, which had been selected to replace the susceptible cultivar used in 2014. No clubbing symptoms were observed on any of the napa cabbage cultivars in 2015.
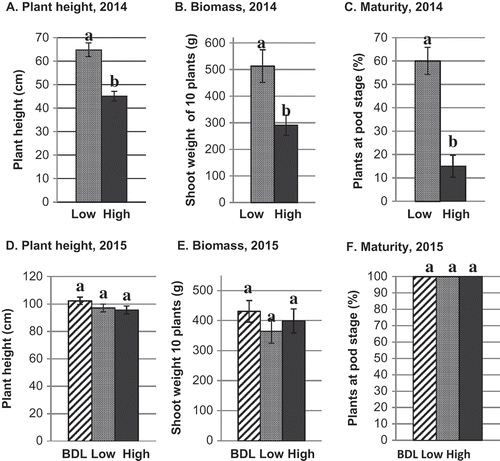
All of the resistant canola cultivars showed the same pattern of response to spore concentration in the field trials over time (). Reductions in plant height were apparent at 8 weeks after seeding in field trials in 2014. At this time, growth and development were reduced at the high site compared with the low site; plant height was reduced by 30% (P < 0.0001), biomass by 43% (P = 0.006), and the proportion of plants with pods was reduced by 75% (P < 0.0001, A–C). Area under the growth stairs (AUGS) of resistant canola was reduced in the high spore site compared with the low site in 2014 (P = 0.009). In 2015, however, there was no effect of spore concentration on plant height, biomass, maturity or AUGS (D–F).
Fig. 1 Mean plant height of three resistant canola cultivars at sites with high, low and below detectable levels (BDL, 2015 only) of resting spore concentrations of Plasmodiophora brassicae, Muck Crops Research Station, Ontario, 2014 and 2015. Capped lines = Standard error.
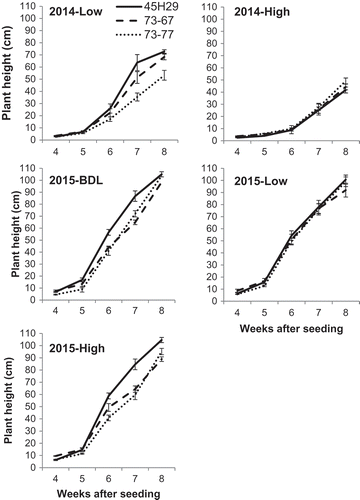
Fig. 2 Mean growth and development of clubroot-resistant canola cultivars at 8 weeks after seeding at adjacent sites at Muck Crops Research Station in 2014 and 2015 with high, low and below detection limit (BDL, 2015 only) of resting spores of Plasmodiophora brassicae in the soil. Bars with the same letter do not differ at P = 0.05 based on Tukey’s multiple means comparison test. Capped lines = Standard error.
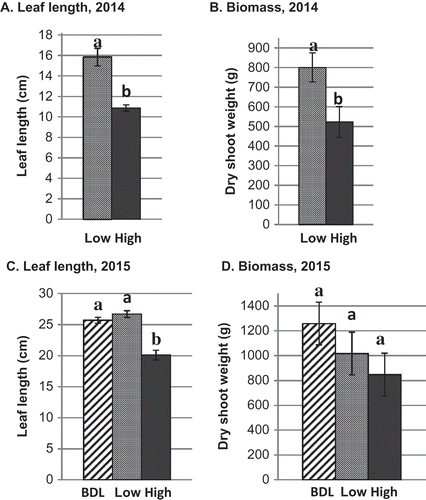
In the resistant napa cabbage cultivars in 2014, leaf length was reduced by 31% (P < 0.0001) and biomass by 34% (P = 0.007) in the high site compared with the low site (). In 2015, leaf length was reduced by 22% (P < 0.0001) in the high site compared with the low site, and there were no differences between the low site and the site below detectable limits (). AUGS of napa cabbage was reduced in the high site compared with sites with lower concentrations of spores in 2014 (P < 0.0001) and 2015 (P < 0.0001). There were no differences in biomass in 2015, but the trend in biomass was similar to that for leaf length in 2014 ().
Fig. 3 Leaf length and biomass of clubroot-resistant napa cabbage cultivars at adjacent sites at Muck Crops Research Station, Ontario in 2014 and 2015 with high, low and below detection limit (BDL, 2015 only) of resting spores of Plasmodiophora brassicae in the soil. Bars with the same letter do not differ at P = 0.05 based on Tukey’s multiple means comparison test. Capped lines = Standard error.
Large pot studies – outdoors
Clubroot levels in the susceptible canola line ACS-N39 were high in both repetitions, with a mean incidence of 99% and severity of 88 DSI. A low incidence of clubbing was observed on ‘45H29ʹ in both repetitions of the study, indicating a breakdown of resistance. These plants were removed from the biomass assessments when the trial was repeated, but could not be removed from the plant height measurements in either repetition of the study. There was no effect of inoculation on plant height, maturity or biomass among the treatments (data not shown).
Controlled environment study
Clubroot levels in the susceptible canola line ‘ACS-N39ʹ were 100% in both repetitions, with mean severity of 71 DSI in the initial study and 95 DSI in the repetition. No clubroot symptoms were observed in resistant canola '45H29ʹ in either repetition.
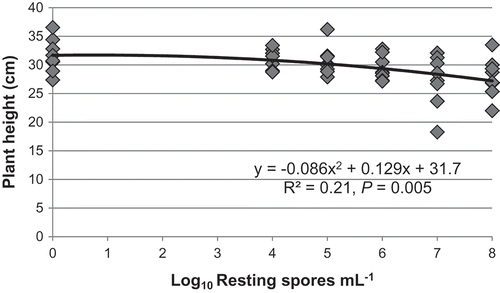
In the initial analysis of plant height of resistant canola at 6 weeks after inoculation, there was no repetition effect, so the data were analysed across repetitions. Height declined in a quadratic relationship (y = −0.086x2 + 0.129x + 31.7, R2 = 0.21, P = 0.005) with increasing concentration of resting spores (). Height was reduced by 12–14% at high concentrations of spores at 5 and 6 weeks after inoculation. However, there was no effect of inoculum level on height at 2 to 4 weeks after inoculation or on AUGS.
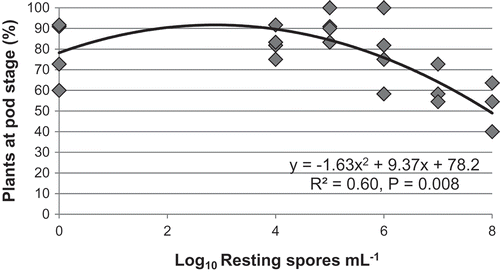
Fig. 4 Plant height of clubroot-resistant canola at 6 weeks after inoculation in response to increasing concentrations of Plasmodiophora brassicae resting spores under controlled conditions (two repetitions, n = 8).
There was a substantial difference between the repetitions of assessments of plant biomass (dry shoot weight) and maturity of ‘45H29ʹ at 8 weeks after inoculation, so each repetition was analysed separately. In the first repetition, biomass declined slightly with increasing spore concentration (y = 0.189x + 6.47, R2 = 0.07, P = 0.03), as did the proportion of plants with pods (y = −1.63x2 + 9.37x + 78.2, R2 = 0.60, P = 0.007) (). In the second repetition, clubroot symptoms in the susceptible 'ACS-N39ʹ were more severe, where 25% soil-less media was incorporated into field soil and a lower pH was maintained using acetic acid. However, spore concentration did not affect biomass or maturity.
Controlled environment study – canola and napa cabbage
Clubroot incidence and severity were low in the susceptible canola cultivar (CI = 18%, 6 DSI) and low to moderate in the susceptible napa cabbage (CI = 39%, 20 DSI) in the first repetition of the study, and low to moderate in both canola (CI = 63%, 35 DSI) and napa cabbage (CI = 30%, 21 DSI) in the second repetition.
In the resistant cultivars of napa cabbage, inoculation with P. brassicae reduced leaf length at 5 weeks after inoculation by 11% (P = 0.005) across repetitions. There was no effect of inoculation on plant height, AUGS or biomass of the resistant napa cabbage or canola cultivars (data not shown).
Discussion
Previous research under controlled conditions has provided indications that there may be a metabolic cost associated with expression of resistance to clubroot in canola (Hwang et al. Citation2011a; Deora et al. Citation2012, Citation2013; Peng et al. Citation2014). In the current study, growth of resistant canola and napa cabbage cultivars was examined at low and high inoculum levels in field and pot trials. In canola, plant height and maturity were delayed at high inoculum levels in 2014 but not in 2015 in field studies. In resistant napa cabbage, growth was reduced at the high concentration of resting spores in field trials in both 2014 and 2015. The responses for both species were highly variable in pot trials, with no clear pattern of effect. To our knowledge, this is the first study to directly assess the metabolic cost of resistance to P. brassicae.
One reason that the cost of resistance has not previously been examined in field trials is the difficulty of identifying suitable sites. The field sites in the current study were arranged to facilitate comparisons among resistant cultivars and the susceptible control within and between sites. In 2015, a site with a spore concentration below the detection limit was added as a clubroot-free control. Even though low levels of clubroot developed at that site, it had a much lower (>1000-fold) concentration of spores relative to other sites.
The biomass and plant height of resistant canola was reduced and crop maturity was delayed where spore concentration was 10-fold higher in adjacent field sites in 2014. However, spore concentration had no effect on plant height, biomass or maturity in 2015. Spore concentration in the high sites was similar in 2014 and 2015, but clubroot severity in the susceptible control was substantially lower in 2015 relative to 2014 (70 vs. 100 DSI), which indicates that the effective disease pressure was lower in 2015. Inoculum at the low site in both years was much higher than the 103 spores g−1 soil identified as the effective infection threshold for susceptible canola cultivars (Faggian & Strelkov Citation2009). Also, substantial levels of clubroot developed at the site where spore concentration was below detectable limits in 2015. Rainfall and air temperature in the 3 weeks after seeding were generally similar in both years.
One difference between years was that damage from Swede midge (Contarinia nasturtii) was higher in 2014 than in 2015. We suggest that growth reduction in canola is larger when other stresses are also present.
Under controlled conditions, the height of clubroot-resistant canola plants decreased by 12–14% at high spore concentration (> 106 spores). This decrease was consistent with observations from previous studies under controlled conditions (Hwang et al. Citation2011a; Deora et al. Citation2012). Similarly, biomass declined and maturity was delayed at 6 WAI in one of two repetitions. There was, however, no effect of resting spore concentration on plant height, biomass or maturity in the outdoor pot studies.
Reductions in plant height of resistant canola were often apparent at 5 to 6 weeks after inoculation (WAI) in indoor studies and 8 weeks after seeding (WAS) in field trials. In a previous study, restriction of pathogen development in inoculated, resistant canola occurred from 2–4 weeks after inoculation (Deora et al. Citation2013). In the current study, 4 weeks after inoculation corresponded with initiation of flowering. Canola is known to be highly sensitive to abiotic stresses such as drought from flowering through pod development (Champolivier & Merrien Citation1996), and abiotic and biotic stresses often activate similar pathways for plant response (Creelman & Mullet Citation1995).
Several mechanisms of resistance to clubroot have been identified (Gludovacz et al. Citation2014; Chu et al. Citation2015). Up-regulation of signalling, callose deposition and metabolism of jasmonic acid, ethylene, and indole-containing compounds occur in inoculated, clubroot-resistant plants. In one study, the salicylic acid defence pathway was not elevated, and auxin and a chitinase-like protein were down-regulated in B. rapa (Chu et al. Citation2015). In another study, clubroot-resistant canola cultivars responded to secondary infection by P. brassicae by forming a concentrated ring of reactive oxygen species (ROS) in the inner root cortex (Deora et al. Citation2013). ROS generation can react with proteins to decrease enzyme activity (Moller Citation2001; Apel & Hirt Citation2004), which may reduce respiration and plant growth (Clifton et al. Citation2005; Sevilla et al. Citation2015).
The metabolic basis for a cost of resistance to clubroot infection may be related to the stages of pathogen development during which the resistance response takes place and the metabolic pathways involved in host resistance. Recognition and induction of resistance takes place during root hair infection (McDonald et al. Citation2014). In resistant canola, little or no pathogen development was observed during cortical infection (Deora et al. Citation2012).
Growth of napa cabbage was reduced at sites with a high spore concentration in field trials and declined with increasing spore concentration under controlled conditions. In field trials in both 2014 and 2015, leaf length of resistant napa cabbage grown at a high concentration of resting spores was > 20% shorter relative to a site with a lower spore concentration. This was consistent with one of two studies under controlled conditions, where leaf length was reduced in inoculated, resistant napa cabbage. It is possible that there are differences in the metabolic cost of resistance to P. brassicae among Brassica species, which may explain the more consistent observation of cost of resistance in napa cabbage compared with canola. It is also possible that leaf length may be more strongly correlated with cost of resistance than plant height, because leaf area affects photosynthetic capacity, whereas plant height does not (Freyman et al. Citation1973; Krogman & Hobbs Citation1975; Campbell & Kondra Citation1978).
The studies involving inoculated soil generally did not exhibit a cost of resistance response. High plant-to-plant variability and fewer plants per experimental unit may have obscured the impact of cost of resistance in the pot trials. Also, the effect of resting spore concentration on resistant plants may have been influenced by soil compaction and reduced drainage in pots (Gossen et al. Citation2016). For example, moist soils are required for infection to occur, but excessive soil moisture may suppress pathogen development (Thuma et al. Citation1983; Cranmer Citation2015).
Another reason that the field trials provide a more consistent response to clubroot may have been associated with the inoculation technique. The spore suspension was applied to the soil surface of each pot. If most of the spores stayed near the soil surface, large parts of the root system of each plant would not have been challenged with P. brassicae. In contrast, the entire root system of plants in a heavily infested field soil is under constant pathogen pressure (Hwang et al. Citation2011b) because each new rootlet becomes infected as soon as it is produced and spores are dispersed deep into the soil profile over time (Cranmer et al. Citation2017).
Low levels of clubroot symptoms were observed in the resistant canola cultivars in 2015, but no symptoms had been noted in the resistant canola cultivars in 2014 or in the resistant napa cabbage cultivars in either year. Previous studies have demonstrated that selection of virulent lines of the pathogen can occur quickly in P. brassicae (Kiyosawa Citation1982; LeBoldus et al. Citation2012). It appears likely that a shift in the pathotype was occurring at Muck Crops Research Station that affected the clubroot reaction in canola cultivars but not napa cabbage.
In canola and napa cabbage, comparisons of the opportunity cost associated with the plant allocating physiological resources must be conducted between induced and non-induced plants to assess the cost of induced resistance, because isolines with and without knocked-out resistance pathways are not available (Purrington Citation2000). However, isolines with individual genes for resistance in both species are under development in several laboratories. These lines could be used to determine if individual resistance genes differ for the associated cost of resistance.
In summary, field studies indicated that there was a small but consistent cost of resistance to clubroot in napa cabbage, and cost of resistance was observed in canola in only one of two years. Growers need to be aware that growing a clubroot-resistant cultivar in a field with a high concentration of resting spores could result in a reduction in yield and delayed maturity, even if no clubroot symptoms develop. In western Canada, delayed maturity of canola is an important issue because of increased likelihood of frost damage, with associated loss in both quality and yield.
Acknowledgements
The authors thank Dr A. Deora for help and advice, the staff at the Muck Crops Research Station for technical assistance with the field trials, and Dr S. Vail for providing seed of ACS-N39. Funding for the study was provided by the Canola Council of Canada and Agriculture and Agri-Food Canada as part of the Canola Science Cluster of Growing Forward 2.
Additional information
Funding
References
- Apel K, Hirt H. 2004. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 55:373–399.
- Bergelson J, Purrington CB. 1996. Surveying patterns in the cost of resistance in plants. Amer Naturalist. 148:536–558.
- Brown JKM, Rant JC. 2013. Fitness costs and trade-offs of disease resistance and their consequences for breeding arable crops. Plant Pathol. 62:83–95.
- Campbell DC, Kondra ZP. 1978. Relationships among growth patterns, yield components and yield of rapeseed. Can J Plant Sci. 58:87–93.
- Canola Council of Canada. 2016a. Canola performance trials; [ cited 2016 Dec 21]. Available from: http://www.canolaperformancetrials.ca/
- Canola Council of Canada. 2016b. Mineral fertilizer. In Canola – Encyclopedia; [ cited 2016 Dec 21]. Available from: http://www.canolacouncil.org/canola-encyclopedia/fertilizer-management/
- Cao T, Manolii VP, Hwang SF, Howard RJ, Strelkov SE. 2009. Virulence and spread of Plasmodiophora brassicae [clubroot] in Alberta, Canada. Can J Plant Pathol. 31:321–329.
- Champolivier L, Merrien A. 1996. Effects of water stress applied at different growth stages to Brassica napus L. var. oleifera on yield, yield components and seed quality. Eur J Agric. 5:153–160.
- Chongo G, McVetty PBE. 2001. Relationship of physiological characters to yield parameters in oilseed rape (Brassica napus L.). Can J Plant Sci. 81:1–6.
- Chu M, Song T, Yu F, Peng G, Lahlali R, Liu X, Zhang X, Falk KC, Gossen BD. 2015. Fine mapping of Rcr1 and analyses of its effect on transcriptome patterns during infection by Plasmodiophora brassicae. BMC Genomics. 15:1166.
- Clifton R, Lister R, Parker KL, Sappl PG, Elhafez D, Millar AH, Day DA, Whelan J. 2005. Stress-induced co-expression of alternative respiratory chain components in Arabidopsis thaliana. Plant Mol Biol. 58:193–212.
- Colhoun J. 1953. A study of the epidemiology of club-root disease of Brassicae. Ann Appl Biol. 40:262–283.
- Cranmer TJ 2015. Vertical distribution of Plasmodiophora brassicae resting spores in soil and the effect of weather conditions on clubroot development [dissertation]. Guelph (ON): University of Guelph.
- Cranmer TJ, Gossen BD, Al-Daoud F, Deora A, McDonald MR. Forthcoming 2017. Vertical distribution of resting spores of Plasmodiophora brassicae in soil. Eur J Plant Pathol.
- Creelman RA, Mullet JE. 1995. Jasmonic acid distribution and action in plants: Regulation during development and response to biotic and abiotic stress. Proc Natl Acad Sci USA. 92:4114–4119.
- Crête R, Laliberté J, Jasmin JJ. 1963. Lutte chimique contre la hernie, Plasmodiophora brassicae Wor. des cruciferes en sols mineral et organique. Can J Plant Sci. 43:349–354.
- Dalton. 2015. Effect of Plasmodiophora brassicae resting spore concentration and crop rotation on growth of clubroot-resistant crops [dissertation]. Guelph (ON): University of Guelph.
- Deora A, Gossen BD, McDonald MR. 2012. Infection and development of Plasmodiophora brassicae in resistant and susceptible canola cultivars. Can J Plant Pathol. 34:239–247.
- Deora A, Gossen BD, McDonald MR. 2013. Cytology of infection, development and expression of resistance to Plasmodiophora brassicae in canola. Ann Appl Biol. 163:56–71.
- Deora AD, Gossen BD, Amirsadeghi S, McDonald MR. 2015. A multiplex qPCR assay for detection and quantification of Plasmodiophora brassicae in soil. Plant Dis. 99:1002–1009.
- Donald EC, Porter IJ. 2004. A sand—Solution culture technique used to observe the effect of calcium and pH on root hair and cortical stages of infection by Plasmodiophora brassicae. Australas Plant Pathol. 33:585–589.
- Donald EC, Porter IJ. 2009. Integrated control of clubroot. J Plant Growth Regul. 28:289–303.
- Faggian R, Strelkov SE. 2009. Detection and measurement of Plasmodiophora brassicae. J Plant Growth Regul. 28:282–288.
- Feng J, Hwang SF, Strelkov SE. 2012. Analysis of expressed sequence tags derived from a compatible Plasmodiophora brassicae-canola interaction. Can J Plant Pathol. 34:562–574.
- Freyman S, Charnetski WA, Crookston RK. 1973. Role of leaves in the formation of seed in rape. Can J Plant Sci. 53:693–694.
- Gludovacz TV 2013. Clubroot in canola and cabbage in relation to soil temperature, plant growth and host resistance [dissertation]. Guelph (ON):University of Guelph.
- Gludovacz TV, Deora A, McDonald MR, Gossen BD. 2014. Cortical colonization by Plasmodiophora brassicae in susceptible and resistant cabbage cultivars. Eur J Plant Pathol. 140:859–862.
- Gossen BD, Kasinathan H, Cao T, Manolii VP, Strelkov SE, Hwang SF, McDonald MR. 2013. Influence of pH and temperature on infection and symptom development of clubroot in canola. Can J Plant Pathol. 35:294–303.
- Gossen BD, Kasinathan H, Deora A, Peng G, McDonald MR. 2016. Soil type, soil compaction and biofungicides affect clubroot (Plasmodiophora brassicae). Plant Pathol. 65:1238–1245.
- Hwang SF, Ahmed HU, Strelkov SE, Gossen BD, Turnbull GD, Peng G, Howard RJ. 2011b. Seedling age and inoculum density affect clubroot severity and seed yield in canola. Can J Plant Sci. 91:183–190.
- Hwang SF, Ahmed HU, Zhou Q, Strelkov SE, Gossen BD, Peng G, Turnbull GD. 2011a. Influence of cultivar resistance and inoculum density on root hair infection of canola (Brassica napus) by Plasmodiophora brassicae. Plant Pathol. 60:820–829.
- Kiyosawa S. 1982. Genetics and epidemiological modeling of breakdown of plant disease resistance. Annu Rev Phytopathol. 20:93–117.
- Krogman KK, Hobbs EH. 1975. Yield and morphological response of rape (Brassica campestris L. cv. Span) to irrigation and fertilizer treatments. Can J Plant Sci. 55:903–909.
- LeBoldus JM, Manolii VP, Turkington TK, Strelkov SE. 2012. Adaptation to Brassica host genotypes by a single-spore isolate and population of Plasmodiophora brassicae (clubroot). Plant Dis. 96:833–838.
- Ludwig-Muller J, Bennett RN, Kiddle G, Ihmig S, Ruppell M, Hilgenberg W. 1999. The host range of Plasmodiophora brassicae and its relationship to endogenous glucosinolate content. New Phytol. 141:4443–4458.
- McDonald MR, Sharma K, Gossen BD, Deora A, Feng J, Hwang SF. 2014. The role of primary and secondary infection in host response to Plasmodiophora brassicae. Phytopathology. 104:1078–1087.
- Møller IM. 2001. Plant mitochondria and oxidative stress: Electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu Rev Plant Biol. 52:561–591.
- Myers DF, Campbell RN. 1985. Lime and the control of clubroot of crucifers: Effects of pH, calcium, magnesium and their interactions. Phytopathology. 75:670–673.
- Peng G, Lahlali R, Hwang SF, Pageau D, Hynes RK, McDonald MR, Gossen BD, Strelkov SE. 2014. Crop rotation, cultivar resistance, and fungicides/biofungicides for managing clubroot (Plasmodiophora brassicae) on canola. Can J Plant Pathol. 36:99–112.
- Purrington CB. 2000. Costs of resistance. Curr Opin Plant Biol. 3:305–308.
- Sevilla F, Jimenez A, Lazarro JJ. 2015. What do the plant mitochondrial antioxidant and redox systems have to say under salinity, drought and extreme temperature? In: Gupta DK, Corpas FJ, Palma JM, editors. Reactive oxygen species and oxidative damage in plants under stress. Switzerland: Springer; p. 23–56.
- Sharma K, Gossen BD, McDonald MR. 2011a. Effect of temperature on primary infection by Plasmodiophora brassicae and initiation of clubroot symptoms. Plant Pathol. 60:830–838.
- Sharma K, Gossen BD, McDonald MR. 2011b. Effect of temperature on cortical infection by Plasmodiophora brassicae and clubroot severity. Phytopathology. 101:1424–1432.
- Siemens J, Nagel M, Ludwig-Müller J, Sacristan MD. 2002. The interaction of Plasmodiophora brassicae and Arabidopsis thaliana: Parameters for disease quantification and screening of mutant lines. J Phytopathol. 150:592–605.
- Simko I, Piepho HP. 2012. The area under the disease progress stairs: Calculation, advantage, and application. Phytopathology. 102:381–389.
- Strelkov SE, Tewari JP, Smith-Degenhardt E. 2006. Characterization of Plasmodiophora brassicae populations from Alberta, Canada. Can J Plant Pathol. 28:467–474.
- Thuma BA, Rowe RC, Madden LV. 1983. Relationships of soil temperature and moisture to clubroot (Plasmodiophora brassicae) severity on radish in organic soil. Plant Dis. 67:758–762.
- Van Loon LC, Bakker PA, Pieterse CM. 1998. Systemic resistance induced by rhizosphere bacteria. Annu Rev Phytopathol. 36:453–483.
- Webster MA, Dixon GR. 1991. Calcium, pH and inoculum concentration influencing colonization by Plasmodiophora brassicae. Mycol Res. 95:64–73.
- Yang D-L, Yao J, Mei C-S, Tong X-H, Zeng L-J, Li Q, Xiao LT, Sun TP, Li J, Deng XW, Lee CM. 2012. Plant hormone jasmonate prioritizes defense over growth by interfering with gibberellin signaling cascade. Proc Natl Acad Sci USA. 109:E1192–E1200.