Abstract
Diseases of wasabi (Wasabia japonica) are the most important reason for crop failure in commercial greenhouses, and expanding disease issues highlight the importance of identifying the causal agents. Diseased wasabi leaves were collected during 2013–2015 from greenhouses in the Fraser Valley of British Columbia. Isolations from plants showing symptoms of leaf spot and blight yielded a Botrytis sp. and a Colletotrichum sp. when plated onto PDA. In addition, pustules containing sporangiospores of an Albugo sp. were observed on naturally infected leaves. Molecular identification using the ITS1–ITS4 region of rDNA revealed the pathogens isolated from wasabi leaves were B. cinerea and C. higginsianum, while the Albugo species was A. candida. The B. cinerea isolates were shown to be weakly pathogenic, infecting only succulent and senescing or wounded leaves. Inoculation studies with C. higginsianum showed that it caused lesions on Brassica juncea (mustard) leaves and on wasabi, but not on Medicago sativa (alfalfa). In culture, fastest growth occurred at 25 and 30°C, and the highest conidial production after 7 days occurred under continuous darkness. Isolates of A. candida collected from naturally infected Capsella bursa-pastoris (shepherd’s purse) plants were identical to those from wasabi plants. These previously unreported pathogens on wasabi in Canada will continue to provide challenges to commercial producers and further research into disease control methods is warranted.
Résumé
Les maladies du wasabi (Wasabi japonica) sont les principales causes des récoltes déficitaires dans les serres commerciales, et les problèmes relatifs à l’expansion de ces maladies mettent en évidence l’importance d’en identifier les agents causaux. De 2013 à 2015, des feuilles infectées de wasabi ont été collectées dans des serres de la vallée du Fraser, en Colombie-Britannique. Lorsque placés sur de la gélose dextrosée à la pomme de terre, les tissus de plants affichant des symptômes de la tache des feuilles et de la brûlure helminthosporienne ont produit une espèce Botrytis et une de Colletotrichum. De plus, des pustules contenant des sporangiospores d’une espèce d’Albugo ont été observées sur des feuilles naturellement infectées. L’identification moléculaire basée sur la région de l’ITS (amorces ITS1-ITS4) de l’ADNr a permis de conclure que les agents pathogènes isolés à partir des feuilles de wasabi étaient B. cinerea et C. higginsianum, tandis que l’espèce d’Albugo était A. candida. Les isolats de B. cinerea se sont avérés faiblement pathogènes, infectant seulement les feuilles succulentes et sénescentes ou encore les feuilles blessées. Des études d’inoculation réalisées avec C. higginsianum ont montré qu’il causait des lésions sur les feuilles de Brassica juncea (moutarde) et le wasabi, mais pas sur Medicago sativa (luzerne). En culture, la croissance la plus rapide s’est produite à 25 et 30°C, et la production conidienne la plus élevée s’est manifestée au bout de sept jours d’obscurité totale. Les isolats d’A. candida collectés sur des plants de Capsella bursa-pastoris (bourse-à-pasteur) naturellement infectés étaient identiques à ceux provenant des plants de wasabi. Ces agents pathogènes non rapportés antérieurement sur le wasabi au Canada continueront de constituer un défi pour les producteurs commerciaux et, en conséquence, des recherches plus poussées concernant les méthodes de lutte contre cette maladie sont justifiées.
Introduction
Wasabia japonica (Miq.) Matsumura (syn. Eutrema japonicum Matsum.) (wasabi) is a perennial plant belonging to the Brassicaceae family that is native to Japan, where it grows on the shaded banks of cool streams and springs (Adachi Citation1987). It is cultivated primarily for its valuable rhizome which is a used as a freshly ground condiment eaten with fish or noodle dishes (Hodge Citation1974; Chadwick et al. Citation1993). Wasabi is cultivated in Japan (Follett Citation1986; Chadwick et al. Citation1993), Taiwan (Lo & Wang Citation2000), China, Korea (Choi et al. Citation2014), New Zealand (Palmer Citation1990), the USA (Chadwick et al. Citation1993) and Canada (Rodríguez & Punja Citation2007, Citation2009). While wasabi can be cultivated in soil, the rhizomes are generally considered to be of inferior quality compared with those grown in soilless media (Chadwick et al. Citation1993; Sultana et al. Citation2003). In British Columbia (BC), most commercial wasabi growers use hydroponic or semi-hydroponic systems, with river rock as the planting substrate () and overhead misting systems to provide a cool, moist environment (authors, personal observations). All of the production takes place inside greenhouses comprised of double polyethylene plastic (polyhouses) or glass. The plants are harvested after 12–18 months and new plantings are often initiated from vegetative cuttings taken from the axillary shoots, but tissue culture propagation is becoming increasingly popular (authors, personal observations). The two main cultivars grown are ‘Mazuma’ and ‘Daruma’; however, a third cultivar ‘Greenthumb’, has recently been introduced (authors, personal observation).
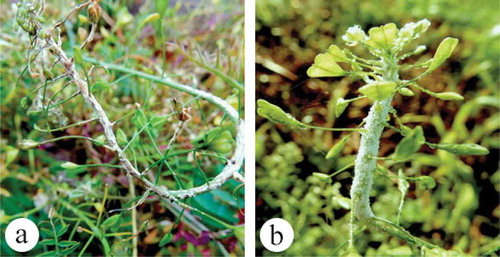
Fig. 1 (Colour online) Symptoms of botrytis leaf blight on wasabi plants in a commercial greenhouse. (a) Healthy plant. (b) Initial yellowing and necrosis on leaf margins. (c) Small flecking on upper leaves of plant. (d) Severe marginal necrosis under conditions of high moisture. (e) Blighting, stunted plants and plant death resulting in gaps in the plant stand. (f) Symptoms on wasabi leaf resulting from inoculation with Botrytis isolate. (g) Isolate growing on PDA, with sclerotial formation at the colony margins. (h) Branched conidiophore with conidia from PDA culture. Scale bar = 100 µm.
The biggest issues confronting wasabi growers are diseases caused by fungi and bacteria, which are favoured by the cool and humid growing environment inside wasabi greenhouses. The first diseases to be reported on wasabi grown in BC were root rot, caused by Pythium dissotocum Drechsler and P. intermedium de Bary, and internal vascular blackening of the rhizomes caused by Pectobacterium carotovorum (Jones) Waldee subsp. carotovorum (Jones) Bengey et al. (Rodríguez & Punja Citation2007, Citation2009). Subsequently, phoma leaf spot caused by Phoma wasabiae Yokogi (Leptosphaeria biglobosa Shoemaker & H. Brun subsp. occiaustralensis Vincenot et al.) was identified on wasabi (Punja et al. Citation2017). With the expanding wasabi production industry in BC, the incidence and severity of other pathogens are also beginning to increase (authors, personal observation). In this study, the occurrence of three previously unreported diseases affecting wasabi in Canada is summarized. The diseases were observed on wasabi plants over a period of 2 years and the symptoms and results from pathogenicity studies using isolated pathogens are described.
Materials and methods
Botrytis leaf blight
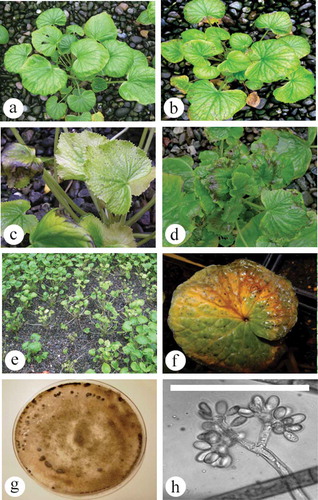
Wasabia japonica ‘Mazuma’ leaves showing symptoms of yellowing and marginal necrosis were collected from polyethylene greenhouses located at Maple Ridge and Agassiz, British Columbia in June 2013 and July 2015 (, c). Small pieces of diseased leaf tissue (5–10 mm2) were surface-sterilized in 70% ethanol for 30 s, followed by a dip in sterile distilled water for 1 min before being blotted dry and incubated on potato dextrose agar containing 5 drops of lactic acid per L (APDA) at 25°C for 7 days under ambient light. Ten Petri dishes, each containing three leaf pieces, were used for isolation. The resulting colonies were identified as a Botrytis sp. based on colony morphology and characteristics of conidia and conidiophores when examined using a Nikon Eclipse Ci microscope at 400× magnification (). To determine the pathogenicity of two selected isolates, separate conidial suspensions containing 2.0–4.0 × 105 conida mL−1 prepared from 7-day old cultures grown on PDA were sprayed onto each of six potted ‘Mazuma’ plants until runoff. Control plants were misted with sterile distilled water. The plants were kept in a humidity chamber at room temperature under natural daylight conditions, and assessed regularly for symptom development. After 1 week, symptomatic leaves were collected and pathogen isolation was conducted as previously described. The two representative isolates were deposited in the Canadian Collection of Fungal Cultures (CCFC), Ottawa (DAOMC 250 508 and DAOMC 250509).
Anthracnose leaf spot
Leaves from a commercial wasabi crop grown in a greenhouse in Maple Ridge, BC showing symptoms of leaf spot and blight were collected in June 2014 (). Small tissue pieces were excised, surface-sterilized and placed on APDA as described previously. Twelve Petri dishes, each containing three leaf pieces, were used for isolation. Resulting colonies were identified as a Colletotrichum sp. based on colony morphology and characteristics of the conidia, acervuli and setae in 10-day-old cultures.
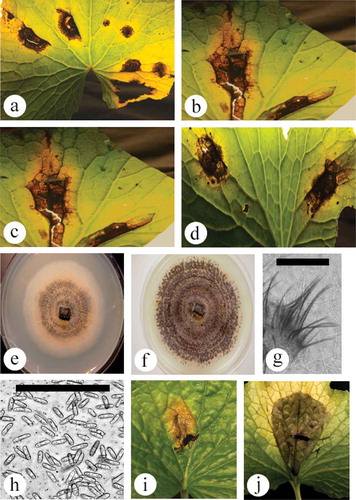
Fig. 2 (Colour online) Symptoms of anthracnose leaf spot on wasabi and pathogen characteristics. (a–d) Range of symptom development showing dark brown irregular-shaped lesions with visible yellowing. Some lesioned tissues have dropped out, leaving a shot-hole appearance. (e) 10-day-old colony on PDA, showing salmon colour and black centre. (f) One-month-old colony on PDA, showing black concentric rings of setae. (g) Close-up of setae. Scale bar = 100 µm. (h) Conidia of Colletotrichum from PDA culture. Scale bar = 100 µm. (i, j) Development of anthracnose lesions following inoculation of young wasabi leaves and incubation under high humidity conditions for 7 days. Chlorotic areas can also be seen developing around the lesions. Scale bar = 100 µm.
Five wasabi leaves were excised from healthy plants, and the centre of the leaf above the petiole was inoculated with a conidial suspension containing 8.4–8.6 × 104 conidia mL−1 mixture of two selected isolates to determine pathogenicity. The experiment was repeated on abaxial and adaxial leaf surfaces, and on wounded and non-wounded leaves. Wounding was performed by piercing a sterile dissecting needle through the centre of the leaf at the inoculation site. An equal number of control leaves were treated the same, but with sterile distilled water instead of a conidial suspension. Each leaf was placed in a Petri dish lined with moistened filter paper and incubated at 25 ± 2°C and observed for symptom development. Pathogen reisolation was completed as described previously and the resulting colonies were compared with the original isolate. A representative isolate was deposited in the CCFC (DAOMC 250510).
Five-mm-diameter mycelial plugs taken from PDA cultures were transferred from the colony margins to fresh PDA dishes, which were then kept on a thermal gradient plate (TGP) in darkness to assess the effect of temperature on colony growth of Colletotrichum sp. The TGP (built by Agriculture and Agi-Food Canada, Saskatoon) was comprised of 176 individually controlled cells with potential ranges of 0–40°C, and controlled by individual thermoelectric pumps. A range of 0–35°C was used for this experiment. After 7 days, maximum colony diameters were measured from five replicate dishes. The experiment was repeated three times. To determine the effect of light on spore production, PDA plates were incubated in each of three Conviron units set at 0, 12 or 24 h photoperiod (8000 ± 250 lux; 25°C). After 7 and 14 days, colony diameters were measured and spore numbers assessed from five replicate dishes using a hemocytometer. The experiment was repeated three times. Spore number experiments were analysed independently and completed in SAS University Edition (v3.4), using log-transformed mean values in a one-way ANOVA (Tukey’s HSD, P = 0.05).
A host differentiation study was conducted to differentiate between the closely related species C. destructivum O’Gara and C. higginsianum Sacc. Mustard (Brassica juncea (L.) Czern) ‘Southern Giant Curled’ and alfalfa (Medicago sativa L.) were selected for inoculation studies. Colletotrichum destructivum is reported to be pathogenic to members of Fabaceae (alfalfa) but not Brassicaceae (mustard), and vice versa for C. higginsianum (Damm et al. Citation2014). Ten mustard and 10 alfalfa plants were seeded in pots and grown in the greenhouse for 5 weeks then inoculated with a conidial suspension containing 1.8 × 105 conidia mL−1. An equal number of control plants received sterile distilled water. Each inoculated plant was misted with water, and covered with a humidity dome for 3 days. The plants were kept in a greenhouse with a targeted temperature setting of 25°C (set points: heating at 23°C and passive venting at 27°C). Pathogen isolation from developing lesions was conducted as previously described after 3 weeks and the resulting cultures examined after 7 days.
White blister rust
Wasabi plants ‘Daruma’ showing blister formation and white sori on the abaxial side of leaves were collected from a research polyhouse located in Agassiz, BC in October 2015 (). A severely diseased leaf was used to prepare slides for photomicrography and obtain measurements of sporangia at 400× magnification (Nikon Eclipse Ci microscope, Nikon DS-Fi2 camera). Naturally infected plants of Capsella bursa-pastoris (L.) Medic (shepherd’s purse) () with typical symptoms of white rust (Alexander & Burdon Citation1984) were collected from two field sites, one adjacent to the polyhouse in Agassiz and one in a field in Abbotsford, BC. A sporangia solution (4.0 × 105 spores mL−1) from each host was prepared by suspending sori in sterile distilled water and each was then misted onto four separate healthy, 9-month-old ‘Mazuma’ plants in 10 cm diameter pots until runoff, and then covered with a transparent humidity dome. An equal number of control plants were misted with sterile distilled water. After 3 days, the humidity domes were removed and disease incidence and symptoms were assessed after 7 days. Each inoculation experiment was also repeated on four field-collected shepherd’s purse plants that did not have symptoms of white blister rust.
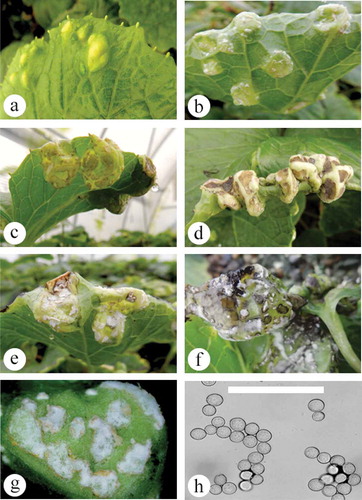
Fig. 3 (Colour online) Albugo white rust symptom development on naturally infected leaves of wasabi. (a) Initial puckering of the upper leaf surface with yellowing. (b) Swellings on the underside of leaves with white mycelium. (c) Large swellings that produce gall-like symptoms. (d) Severe infection at the leaf margin, showing leaf curling and development of galls. (e) Close-up of swollen tissues with white sporulation on surface. (f) White spore masses with black sori. (g) Close-up of leaf swelling with spore masses. (h) Sporangiospores as viewed under the microscope. Scale bar = 100 µm.
Molecular identification of pathogen isolates
Two cultures each of Botrytis and Colletotrichum isolates, as well as Albugo-infected wasabi and shepherd’s purse leaf and stem samples, were sent to the University of Guelph Laboratory Services, Agriculture and Food Laboratory, Guelph, ON where they were subjected to PCR using the primers ITS1F-ITS4 (ITS1-F CTTGGTCATTTAGAGGAAGTAA and ITS4 TCCTCCGCTTATTGATATGC). The corresponding sequences were compared with ITS1-5.8S-ITS2 sequences of Botrytis cinerea Pers. (teleomorph Botryotinia fuckeliana (de Bary) Whetzel) (11 isolates), Colletotrichum destructivum O’Gara (8 isolates), Colletotrichum higginsianum Sacc. (5 isolates), Colletotrichum truncatum (Schwein.) Andrus & W.D. Moore (5 isolates) and Albugo candida (Pers. ex Lev) Kuntze (13 isolates) from the National Center for Biotechnology Information (NCBI) GenBank database. Multiple sequence alignment of the respective isolates of each species were made using CLUSTAL W program (http://www.genome.jp/tools/clustalw). The sequences were subsequently subjected to the neighbour joining (NJ) method analysis (Saitou & Nei Citation1987; Tamura et al. Citation2004) using the software MEGA v. 5 (Tamura et al. Citation2011). A bootstrap consensus tree was inferred from 1000 replicates to represent the distance. The outgroup used was Sclerotinia sclerotiorum (Lib.) de Bary (M96382) and Hyaloperonospora parasitica (Pers.) Constant (AY531452). Branches corresponding to partitions reproduced in less than 50% of bootstrap replicates were collapsed. The percentages of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) were shown next to the branches as described by Felsenstein (Citation1985).
Results
Botrytis leaf blight
Initial symptoms on naturally infected plants were a general yellowing of leaves, small necrotic flecking on the upper leaves, and marginal leaf necrosis (–e). With progressive development of the disease, larger necrotic areas developed on the leaves and young plants showed symptoms of blighting and were stunted. Light grey to white mycelial growth was observed under high humidity, together with masses of grey conidia. Over time, infected plants died, leaving gaps in the stand (). Two isolates (DAOMC 250508, DAOMC 250509) developed grey- to brown-coloured, fast-growing colonies on PDA, with masses of conidia borne on branched conidiophores (, h). The two isolates produced conidia which measured 14.0 µm in length × 8.2 µm in width (8.1–18.7 × 6.2–11.1) and 12.9 µm × 7.6 µm (9.2–17.5 × 5.3–9.9), respectively, and were single-celled, colourless and typically oval in shape. Black sclerotia developed on the colonies at the edges of the Petri dish (). These features were used to identify the cultures as Botrytis sp. Following inoculation of wasabi leaves, a general yellowing was observed with subsequent flecking and marginal necrosis on wounded or senescing leaves, and abundant grey sporulation characteristic of Botrytis was observed (). Reisolation from diseased tissues yielded morphologically identical colonies to those used for inoculation.
Colletotrichum leaf spot
Symptoms observed on naturally infected plants included dark brown lesions that varied in size and shape, and were frequently surrounded by a chlorotic margin (–d). Concentric rings of varying shades of brown were present in some lesions. Colonies growing on PDA were initially light salmon in colour with black masses of concentric rings, presumed to be of conidia production (, f). Acervuli were produced that were cream to apricot coloured, measuring 170–790 µm in diameter, and with abundant setae, which were dark brown to black, 65–236 µm in length (). Masses of cylindrical-shaped conidia were produced, which were obtuse, hyaline and granulose and measured 14.2 µm in length × 4.3 µm in width (8.1–22.3 × 2.8 – 5.6) (). These observations were consistent with the description of O’Gara (Citation1915), Higgins (Citation1917) and Damm et al. (Citation2014) of Colletotrichum species. Leaf inoculations with a conidial suspension of a mixture of two isolates resulted in symptoms that were similar to those on naturally infected plants (Fig. 2i, j). There were no differences in inoculations regardless of whether they were done on the adaxial or abaxial surface, or whether leaves were wounded or not. After 3 days, small brownish-black lesions formed at the site of inoculation and lesions enlarged and coalesced by 8 days. A yellow chlorotic halo developed in the leaf tissues surrounding the lesions. Cultures obtained from inoculated leaves were morphologically identical to the original culture.
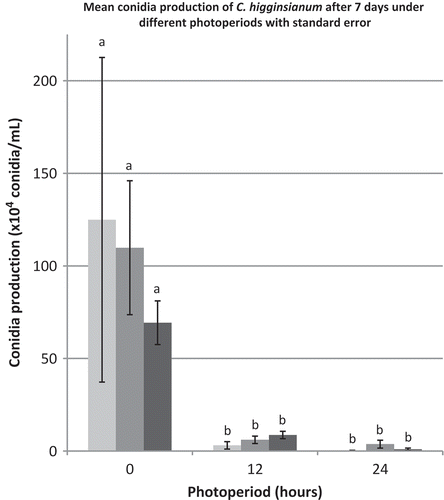
Colonies grew best at 25 and 30°C, and growth was reduced at higher and lower temperatures (). At 5°C, colonies showed very little growth. Photoperiod had no effect on colony diameter; however, morphology of colonies was different under varying light regimes. Colonies were white under darkness, a deep salmon colour under 12 h light per day, and a faded salmon colour under 24 h light per day. Conidia production was affected by different photoperiods after 7 days. In the presence of light (12 and 24 h photoperiods), fewer conidia were produced, while colonies grown under darkness produced the most conidia (). However, after 14 days, there was no difference in conidial production under different light regimes (data not shown).
Fig. 5 Mean diameter of colonies of C. higginsianum after 7 days when grown on PDA at 5, 10, 15, 20, 25, 30 and 35°C. Data are from three different experiments (n = 35) with standard deviation.
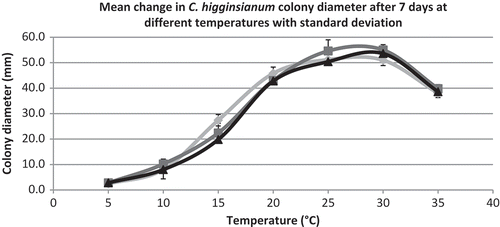
Fig. 6 Mean conidia production in C. higginsianum cultures after 7 days when grown on PDA under 0, 12 and 24-h photoperiods. Data are from three different experiments (n = 15) except experiment 2 (dark grey shade) where one significant outlier (value = 643) was excluded from the 24-h photoperiod. Each letter represents a significance difference in the Log-transformed values in a one-way ANOVA (Tukey’s, P = 0.05).
Lesions developed on three of the 10 mustard plants, but not on any alfalfa plants. Following isolations from diseased tissues, a Colletotrichum sp. was identified on three of the six dishes.
Albugo white blister rust
About 50% of wasabi plants naturally developed symptoms of white blister rust, which ranged from small (<1 cm) swellings with no chlorosis, with sori developing on the underside, to large swellings and distortions enveloping the circumference of a leaf (–e). Frequently, a black rot, with cream-coloured sori and blistering on the abaxial surface, developed with chlorotic symptoms on the adaxial leaf surface (, g). Sporangia appeared white to the naked eye. Sporangiospores were globose or sub-globose and hyaline with a cell wall of uniform thickness, and measured 15.8 µm × 17.1 µm (12.4–18.9 × 14.3–19.9) (). The symptoms and microscopic observations matched those of wasabi white blister rust caused by Albugo sp. in Korea (Choi et al. Citation2014). After 7–10 days, the abaxial side of a single wasabi leaf inoculated with sporangiospores collected from shepherd’s purse produced blisters. These were similar to those observed on naturally infected leaves, although they were much smaller in size, possibly due to the environmental conditions during infection. Inoculations using sporangiospores collected from wasabi did not confirm pathogenicity on shepherd’s purse or wasabi plants.
Molecular identification of pathogen isolates
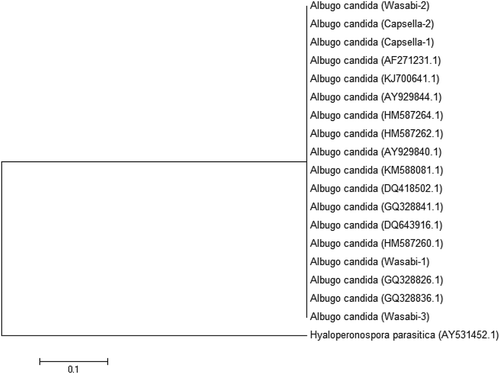
The two isolates of Botrytis were identified as B. cinerea (). They grouped together with isolates from a range of substrates and hosts and sequences of the ITS1-5.8S-ITS2 rDNA region were 99–100% identical. The isolates of the Colletotrichum sp. from wasabi were grouped within a large cluster of isolates identified as C. destructivum (). These isolates were also grouped together with C. higginsianum and could not be separated from them in the phylogenetic analysis. However, isolates of C. truncatum formed a separate group (). The isolates of the Albugo sp. from wasabi and shepherd’s purse collected from two field sites, one near the wasabi polyhouse, were identified as A. candida and grouped together with isolates from a range of hosts and geographic regions (). One isolate collected from shepherd’s purse 60 km from the polyhouse was identified as Hyaloperonospora parasitica (), the causal agent of downy mildew.
Fig. 7 A phylogenetic tree constructed with ITS1-5.8S-ITS2 rDNA sequence of the two Botrytis isolates from this study (‘wasabi-1’ and ‘wasabi-2’), and other isolates of Botrytis retrieved from GenBank. Sclerotinia sclerotiorum was used as the out-group taxon. Number of bootstrap support values ≥50% based on 1000 replicates.
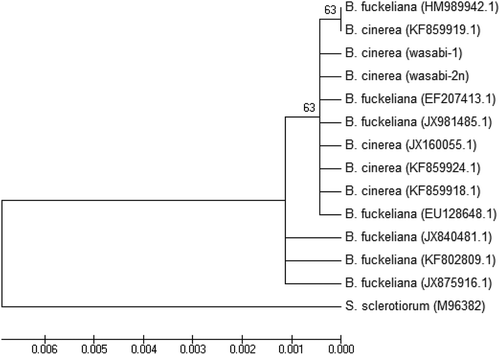
Fig. 8 A phylogenetic tree constructed with ITS1-5.8S-ITS2 rDNA sequence of the two Colletotrichum isolates from this study (‘wasabi-3’ and ‘wasabi-4’), and other isolates of Colletotrichum retrieved from GenBank. Sclerotinia sclerotiorum was used as the out-group taxon. Number of bootstrap support values ≥50% based on 1000 replicates.
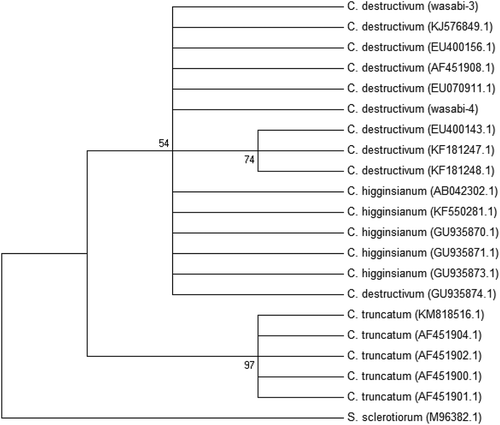
Fig. 9 A phylogenetic tree constructed with ITS1-5.8S-ITS2 5 rDNA sequence of the five Albugo isolates (‘Wasabi-1’, ‘Wasabi-2’, ‘Wasabi-3’, ‘Capsella-1’ and ‘Capsella-2’) from this study, and other isolates of Albugo retrieved from GenBank. Hyaloperonospora parasitica was used as the out-group taxon. Number of bootstrap support values ≥50% based on 1000 replicates.
Discussion
The high humidity environment required for commercial production of wasabi provides ideal conditions for the development and spread of plant pathogens. Grey mould caused by B. cinerea was observed as a weak pathogen on wasabi plants, causing yellowing, necrotic lesions and stunted growth under high humidity conditions. Botrytis cinerea is known to be an opportunistic necrotrophic pathogen that infects over 200 host plant species worldwide (Williamson et al. Citation2007). The pathogen can persist in plant debris as mycelia, conidia or sclerotia (Williamson et al. Citation2007). With the extended growing period required for wasabi (18 months), and the wide host range and abundance of Botrytis sp. in the environment, reducing disease incidence would require growers to clean and/or sterilize wasabi growing materials between crops in order to avoid losses to leaf blight. Interestingly, wasabi defensin genes have been the focus of a number of studies in transgenic crops which have been used to successfully introduce resistance or partial resistance to B. cinerea in potato and tobacco plants (Khan et al. Citation2006; Kiba et al. Citation2007; Hoshikawa et al. Citation2012). The fact that leaf blight has been observed at low levels consistently throughout the growing period in wasabi plantings reinforces the need to prevent leaf damage and avoid conditions which would allow Botrytis to establish in stressed plants (authors, personal observation).
The Colletotrichum sp. causing anthracnose symptoms on wasabi plants was grouped with previously identified isolates of C. destructivum or C. higginsianum in the phylogenetic analysis. Colletotrichum destructivum infects host plants from at least 11 different families (Damm et al. Citation2014), including alfalfa in Canada (Boland & Brochu Citation1989), while C. higginsianum has been implicated as the most common causal agent of Brassica anthracnose, and was tentatively named C. brassicae (Higgins Citation1917). Our sequence analysis using the ITS1-5.8S-ITS2 rDNA region placed the wasabi isolates within the same clade that contained C. destructivum as well as C. higginsianum, making it difficult to confirm the identity of the species causing anthracnose. If the host of origin has precedence on the species identification, the causal organism on wasabi would be C. higginsianum, which is a part of the C. destructivum species complex (Damm et al. Citation2014). The preliminary results of the mustard and alfalfa inoculations support the identification of the wasabi anthracnose as C. higginsianum, since it is known to be pathogenic on mustard but not alfalfa, and vice versa for C. destructivum. This agrees with a previous note of anthracnose on wasabi reported to be caused by C. higginsianum in New Zealand by Martin and Deo (Citation2000); however, their method for species identification was not provided .
White blister rust was observed on wasabi in this study and has been previously reported to occur on wasabi. The causal pathogen was reported as either Albugo wasabiae Hara (Lo & Wang Citation2000; Joshi & Jeffries Citation2010) or A. candida (Choi et al. Citation2014). The sequence analysis of the isolates collected from wasabi and from shepherd’s purse in this study showed that A. candida was the causal agent. The species designation as A. wasabiae based on host of origin (wasabi) is not supported. Albugo candida is a commonly occurring pathogen of shepherd’s purse worldwide (Choi et al. Citation2007) and infects many other members of the Brassicaceae (Saharan & Verma Citation1992; Choi et al. Citation2009). However, most of the reports from Canada were restricted to crop species of economic importance in the genus Brassica (Pidskalny & Rimmer Citation1985; Rimmer et al. Citation2000). Spores collected from shepherd’s purse in this study, when inoculated onto wasabi leaves, caused small galls to form, although the susceptibility of shepherd’s purse plants to the wasabi isolate could not be confirmed. The wasabi production guide (Miles & Chadwick Citation2008) states that white rust infection by A. wasabiae occurs at 45–68°F (7–20°C) (Adachi Citation1987); therefore, our inoculation experiments may have been carried out under temperature conditions unsuitable for infection, and resources limited our ability to repeat the experiment under different conditions. It is conceivable that inoculum for initiating white blister rust on the wasabi plants originated from naturally infected shepherd’s purse plants that were growing in the vicinity of the greenhouse. Pathogen spread was rapid and wasabi plants developed severe disease symptoms, showing they were highly susceptible at all stages of growth. Recent outbreaks of white blister rust have been reported in several greenhouses during autumn 2016 (authors, unpublished observations), indicating this is a disease that has the potential to limit wasabi production.
An increased occurrence of diseases of wasabi is becoming apparent with increased intensity of wasabi production and use of vegetatively propagated plants that could harbour inoculum. The diseases that affect wasabi in British Columbia include some of the most common and destructive pathogens described in other parts of the world, including A. candida (Choi et al. Citation2014) and L. biglobosa (Punja et al. Citation2017), as well as this first report of Colletotrichum causing anthracnose of wasabi in Canada. The use of overhead misting, vegetative propagation, continual and long-term planting of a few cultivars, and use of recycled planting medium is probably contributing to the increased occurrence of these diseases. While ‘Daruma’ may be less susceptible to anthracnose than ‘Mazuma’, it is highly susceptible to white blister rust infection in addition to ‘Greenthumb’ (authors, personal observation).
Disease management practices for wasabi pathogens include fumigating of the growing medium between crops (Chadwick et al. Citation1993), restricting the use of offshoots to 2–3 generations (Adachi Citation1987), and using clean tissue cultured plants to repropagate a planting. In Canada, there currently are no fungicides registered for use on wasabi. Monitoring of Brassica plant species, both weedy species and cultivated crops, will become increasingly important, as observations from this study suggest that infected weedy species may produce inoculum that can spread to nearby wasabi plantings. While the range of pathogens capable of infecting wasabi therefore remains unknown, widespread diseases that affect Brassicaceae crops in BC, such as clubroot and alternaria blight, are likely to emerge on wasabi plants in the future.
Acknowledgements
This research was supported by funding from Agriculture & Agri-Food Canada and Simon Fraser University. We thank staff at the Agassiz Research & Development Centre, especially Markus Clodius, for providing space, time and materials for our research, Carol Koch and Erica Li-Leger for their assistance with the initial molecular identification, and James Nicholson and Seth Nussbaum for continued maintenance of the research plots. We also thank Brian Oates of Pacific Coast Wasabi for his help in establishing our research plots and providing ‘Mazuma’ wasabi plants, and Clearview Horticulture for providing ‘Daruma’ wasabi plants. We extend our thanks to Sarah Chen (Simon Fraser University) for conducting the phylogenetic analyses, and Denise Neilson (Summerland Research & Development Centre) for assisting with the statistical analyses.
Additional information
Funding
References
- Adachi S, editor. 1987. Wasabi sabai. In Shizuoka prefecture agricultural experimental station. Shizuoka (Japan).
- Alexander HM, Burdon JJ. 1984. The effect of disease induced by Albugo candida (white rust) and Peronospora parasitica (downy mildew) on the survival and reproduction of Capsella bursa-pastoris (shepherd’s purse). Oecologia. 64:314–318.
- Boland GJ, Brochu LD. 1989. Colletotrichum destructivum on alfalfa in Ontario and cultivar response to anthracnose. Can J Plant Pathol. 11:303–307.
- Chadwick CI, Lumpkin TA, Elberson LR. 1993. The botany, uses and production of Wasabia japonica (Miq.) (Cruciferae) Matsum. Econ Bot. 47:113–135.
- Choi YJ, Han KS, Park YH, Shin HD. 2014. First report of white blister rust caused by Albugo candida on wasabi in Korea. Plant Dis. 98:1006.
- Choi YJ, Shin HD, Hong SB, Thines M. 2007. Morphological and molecular discrimination among Albugo candida materials infecting Capsella bursa-pastoris world-wide. Fungal Div. 27:11–34.
- Choi YJ, Shin HD, Thines M. 2009. The host range of Albugo candida extends from Brassicaceae through Cleomaceae to Capparaceae. Mycol Progress. 8:329–335.
- Damm U, O’Connell RJ, Groenewald JZ, Crous PW. 2014. The Colletotrichum destructivum species complex – hemibiotrophic pathogens of forage and field crops. Stud Mycol. 79:49–84.
- Felsenstein J. 1985. Phylogenies and the comparative method. Amer Naturalist. 125:1–15.
- Follett JM. 1986. Production of some traditional Japanese vegetables in Japan. Hamilton (New Zealand): Report of the Ruakura Agricultural Centre; p. 59.
- Higgins BB. 1917. A Colletotrichum leaf spot of turnips. J Agri Res. 10:157–162.
- Hodge WH. 1974. Wasabi - Native condiment plant of Japan. Econ Bot. 28:118–129.
- Hoshikawa K, Ishihara G, Takahashi H, Nakamura I. 2012. Enhanced resistance to gray mold (Botrytis cinerea) in transgenic potato plants expressing thionin genes isolated from Brassicaceae species. Plant Biotech. 29:87–93.
- Joshi V, Jeffries M. 2010. Diseases diagnosed on commercial crops submitted to the British Columbia Ministry of Agriculture and Lands (BCMAL) Plant Diagnostic Laboratory in 2009. Can Plant Dis Surv. 90:7–15.
- Khan RS, Nishihara M, Yamamura S, Nakamura I, Mii M. 2006. Transgenic potatoes expressing wasabi defensin peptide confer partial resistance to gray mold (Botrytis cinerea). Plant Biotech. 23:179–183.
- Kiba A, Nishihara M, Nakatsuka T, Yamamura S. 2007. Pathogenesis-related protein 1 homologue is an antifungal protein in Wasabia japonica leaves and confers resistance to Botrytis cinerea in transgenic tobacco. Plant Biotech. 24:247–253.
- Lo CT, Wang KM. 2000. Survey of fungal diseases on aboveground parts of wasabi in Taiwan. Plant Pathol Bull. 9:17–22.
- Martin RJ, Deo B. 2000. Preliminary assessment of the performance of soil-grown wasabi (Wasabia japonica (Miq.) Matsum.) in New Zealand conditions. NZ J Crop Hort Sci. 28:45–51.
- Miles C, Chadwick C. 2008. Growing Wasabi in the Pacific Northwest. Pullman (WA): Washington State University.
- O’Gara PJ. 1915. New species of Colletotrichum and Phoma. Mycologia. 7:38–41.
- Palmer J. 1990. Germination and growth of wasabi (Wasabia japonica (Miq.) Matsumara). NZ J Crop Hort Sci. 18:161–164.
- Pidskalny RS, Rimmer SR. 1985. Virulence of Albugo candida from turnip rape (Brassica campestris) and mustard (Brassica juncea) on various crucifers. Can J Plant Pathol. 7:283–286.
- Punja ZK, Chandanie WA, Chen X, Rodriguez G. Forthcoming 2017. Phoma leaf spot of wasabi (Wasabia japonica) caused by Leptosphaeria biglobosa. Plant Pathol. 66:480–489.
- Rimmer SR, Mathur S, Wu CR. 2000. Virulence of isolates of Albugo candida from western Canada to Brassica species. Can J Plant Pathol. 22:229–235.
- Rodríguez G, Punja ZK. 2007. Root infection of wasabi (Wasabia japonica) caused by Pythium species. Can J Plant Pathol. 29:79–83.
- Rodríguez G, Punja ZK. 2009. Vascular blackening of wasabi rhizomes caused by Pectobacterium carotovorum subsp. carotovorum. Eur J Plant Pathol. 124:483–493.
- Saharan GS, Verma PR. 1992. White rusts: a review of economically important species. Ottawa: International Development Research Centre.
- Saitou N, Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 4:406–425.
- Sultana T, Porter NG, Savage GP, McNeil DL. 2003. Comparison of isothiocyanate yield from wasabi rhizome tissues grown in soil or water. J Agri Food Chem. 51:3586–3591.
- Tamura K, Nei M, Kumar S. 2004. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Nat Acad Sci USA. 101:11030–11035.
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 28:2731–2739.
- Williamson B, Tudzynski B, Tudzynski P, Van Kan JAL. 2007. Botrytis cinerea: the cause of grey mould disease. Mol Plant Pathol. 8:561–580.