Abstract
Infection of greenhouse pepper plants (Capsicum annuum L.) by Fusarium oxysporum was observed in two commercial operations in Ontario, causing plant death and yield losses. The pathogen was identified based on cultural, morphological and pathogenicity tests supplemented by PCR amplification of TEF primers. Following inoculation, symptoms of mild stunting occurred within 5–6 weeks, followed by more severe stunting later. Other symptoms, such as chlorosis, wilting and necrosis of the lower foliage, did not become readily evident until about 60 days after inoculation. At 77 days after inoculation, there was considerable brown-black discolouration and decay of crown tissue but little internal stem discolouration or damage beyond the crown portion was observed. Roots were dark brown to black, severely decayed, and easily separated from the surrounding rock wool medium. The fungus was not pathogenic to greenhouse tomato, cucumber or eggplant, nor to field crops such as bean, chickpea or zucchini squash. There were no significant differences in virulence among isolates but differences in resistance/susceptibility among pepper cultivars were observed. The fungicides Medallion® (fludioxonil) and Senator® (thiophanate-methyl) were as effective as bio-control products Mycostop® (Streptomyces griseoviridis) and Prestop® (Gliocladium catenulatum) in limiting Fusarium crown and root rot in greenhouse pepper. Aspects of an integrated disease management strategy are discussed.
Résumé
L’infection du piment de serre (Capsicum annuum L.), causée par Fusarium oxysporum et provoquant la mort des plants ainsi que des pertes de rendement, a été observée dans deux exploitations commerciales en Ontario. L’agent pathogène a été identifié en se basant sur des tests culturaux et morphologiques ainsi que sur des tests de pathogénicité, complétés par l’amplification par PCR d’amorces TEF. Au bout de 5 à 6 semaines après inoculation, des symptômes de léger rabougrissement sont apparus, suivis par la suite de rabougrissement plus grave. D’autres symptômes, comme la chlorose, le flétrissement et la nécrose des feuilles du bas, ne se sont pas manifestés de manière évidente avant une soixantaine de jours après inoculation. Au bout de 77 jours après inoculation, de larges taches brun-noir et de la pourriture sont apparues sur les collets, mais on pouvait observer peu de taches à l’intérieur des tiges ou de dommage au-delà des collets. Les racines qui étaient gravement pourries, et dont la couleur variait de brun à noir, se séparaient facilement de la laine minérale utilisée comme milieu de croissance. Le champignon n’était pas virulent à l’égard des tomates, des concombres et des aubergines cultivés en serre ni des haricots, des pois chiches ou des courgettes cultivés en champ. Il n’y avait pas de différences significatives quant à la virulence des isolats, mais des différences ont été observées sur le plan de la résistance ou de la réceptivité des cultivars de piment. Pour ce qui est de limiter l’action de la pourriture du collet et des racines, causée par le Fusarium chez le piment de serre, les fongicides MedallionMD (fludioxonil) et SenatorMD (thiophanate-méthyl) ont été aussi efficaces que les agents de lutte biologique MycostopMD (Streptomyces griseoviridis) et PrestopMD (Gliocladium catenulatum). Les divers aspects d’une stratégie intégrée de gestion de la maladie sont à l’étude.
Introduction
Greenhouse peppers were grown on 247 ha in Ontario in 2011, representing 65% of Canadian production which has a farm gate value of $300 million nationally. A significant portion of the greenhouse pepper production was targeted to export markets (Agriculture & Agri-Food Canada, http://www.agr.gc.ca/pmc-cropprofiles). In 2013, greenhouse pepper growers at two different sites in Essex County, Ontario reported a disease that was affecting their plants but the disease did not show any recognizable symptoms associated with other well-known diseases (Menzies & Jarvis Citation1994b; Black Citation2003). At one 7.6 ha site, there was 12–13% plant mortality, with a yield loss of 10 weeks of production for 25 000 pepper plants and an estimated loss of $300 000 in cost of seedlings and establishment (G. Ferguson, pers. comm.). Infection by Fusarium sp. such as F. solani (Martius) Appel & Wollenweber emend. Snyder & Hansen or F. oxysporum (Schlecht. emend. Snyder & Hansen) was suspected based upon disease symptoms on roots and crown of infected plants. Fusarium solani causes frequent losses in greenhouse pepper production in Ontario (Cerkauskas Citation2001; Ontario Ministry of Agriculture and Food Citation2005) while Lomas-Cano et al. (2014, 2016) reported major losses in nursery-grown sweet pepper in Spain attributed to a formae specialis of Fusarium oxysporum.
This research was initiated to: (i) identify the pathogen infecting pepper plants using cultural, morphological and molecular methods; (ii) determine if other greenhouse vegetables such as tomato, eggplant and cucumber were susceptible; (iii) examine whether differences in resistance/susceptibility were present in greenhouse pepper cultivars; and (iv) investigate means of disease management using chemical fungicides and biological control organisms.
Materials and methods
Diseased plant samples
Diseased plant samples were obtained in 2013 from two commercial greenhouse pepper operations near Leamington, ON. Plants were cut about 15 cm above the crown and a longitudinal cut was made from the top of the cut portion to the base of the crown. The plant tissue pieces, about 5 mm long and 2–3 mm thick, were cut from the margin of the inner crown area where slight browning was evident. They were surface-disinfested in 70% ethanol for 3–5 s and subsequently in 10% Clorox (0.5% NaOCl) for 4 min. The pieces were placed onto acidified potato dextrose agar (aPDA) in Petri dishes and incubated under fluorescent light at room temperature for 5 days. The plates were observed for any contamination using a Wild Leitz stereo microscope and transfers made onto new aPDA dishes. Isolates were assigned based on location, HsP and TriB, and cultivar of greenhouse pepper, ‘Healey’ and ‘Bentley’, from which the isolates were obtained.
Pathogen characterization – cultural and morphological observations
Morphological and cultural features were examined using cultures on aPDA Petri dishes that were incubated on a lab bench at 22°C for 10–12 days under continuous fluorescent illumination. Spores from the colony of each isolate were mounted in water and the length, width and shape of 25 conidia with each of 0, 1, 2, 3 and 4 septa and 10 chlamydospores were examined at 630× magnification using a Wild Leitz microscope. There were nine isolates in total with three isolates from site TriB from an unknown pepper variety (TriB-P1-Iso 1, 2, 3), and six from site HsP with four and two isolates from ‘Bentley’ (HsP-Bentley-P2-S1, S2, S3, S4) and ‘Healey’ (HsP-Healey-P1-S1, S2) pepper cultivars, respectively, used in the subsequent studies.
Pathogen characterization – pathogenicity tests
‘Fascinato’ pepper (Syngenta, Guelph, ON) plants were initiated from seeds sown in rock wool plugs in the greenhouse, then transplanted into 7.6 × 7.2 × 6.5 cm rock wool blocks (Fibrgro Horticultural Products, Sarnia, ON) and after 21 days they were placed onto ‘Fibrgro’ rock wool slabs, 45 cm long × 15 cm wide, in a double poly greenhouse at HRDC – AAFC. There were 2 plants per slab with 3 slabs per row and 4 rows. Plants were fertilized as per nutrient film technique and maintained under recommended growing conditions for greenhouse pepper (Ontario Ministry of Agriculture and Food Citation2005). Pepper plants were 29 days old at the time of inoculation.
A spore suspension of each isolate, as noted previously, was prepared by adding 10 mL of double distilled water to a 12-day-old aPDA culture of the fungus and the surface was agitated with a sterile rubber policeman. The spore suspension was filtered through four layers of cheesecloth and adjusted to 5 × 105 spores mL−1 using a hemocytometer slide. Ten mL of suspension/isolate/plant was poured onto the stem base of each ‘Fascinato’ pepper plant. Two rock wool slabs were inoculated with 10 mL distilled water to serve as checks. Symptoms were monitored for 77 days. Pepper plants were then cut 12–18 cm above the crown and removed from the rock wool slabs. Four disease severity ratings were conducted using a 0–5 rating scale as follows: (1) for overall plant symptoms prior to cutting, (2) for overall root health, (3) for crown decay and discolouration symptoms, and (4) for extent of internal stem discolouration and decay. The overall plant rating was as follows: 0 = no symptoms, 1 = minor plant stunting, wilting or drooping of a few leaves, 2 = plant stunting becoming evident; chlorosis and some necrosis of foliage, 3 = plant stunting obvious, drooping/wilting leaves with chlorosis and some necrosis, 4 = extreme plant stunting and/or wilting of foliage with chlorosis and necrosis, stem showing external brown discolouration, and 5 = death of plant. To evaluate the roots, each rock wool block was separated from the rock wool slab and the rock wool fibres around the roots were peeled off and the extent of root discolouration was noted. Roots were rated as follows: 0 = no symptoms, 1 = minor root tip discolouration, rock wool block fibres cannot be separated from the root mass, and extensive healthy development; 2 = root discolouration extending beyond root tips and some rock wool fibres can be separated from root mass when pulled and good root development; 3 = prominent dark root discolouration, smaller roots more severely affected or missing and rock wool fibres separate fairly easily from roots; 4 = smaller, secondary roots are black or missing, most of rock wool fibres easily separated from roots; 5 = only tertiary roots remaining with brown/black colouration, roots easily separated from entire rock wool block.
To evaluate the crown and stem, two perpendicular cuts were made in the crown tissue and any discolouration was noted. Condition of the crown was rated as follows: 0 = no discolouration, 1 = minor brown discolouration of internal crown region; 2 = moderate brown discolouration; 3 = extensive brown discolouration; 4 = severe dark brown discolouration; 5 = entire crown tissue rotted, secondary decay present. The stem tissue was evaluated by making a longitudinal cut and rated as follows: 0 = no discolouration; 1 = minor brown discolouration of inner stem tissue, about 5 mm in length; 2 = brown discoloured inner stem tissue extending between 5–20 mm; 3 = brown discoloured inner stem tissue extending 20–40 mm; 4 = brown/black discoloured inner stem extending > 40 mm with some external discolouration evident also; 5 = stem necrotic with extensive dark brown discolouration.
The disease severity index (DSI) was computed for each plant by adding the individual ratings comprising overall plant, root, crown and stem ratings: Disease Severity Index = Root + Plant + Stem + Crown ratings. After ratings were conducted, the crown tissue from each plant was stored at 4°C, and subsequently used for isolations on aPDA as previously described to confirm that disease symptoms were due to F. oxysporum. Resulting fungal cultures from 5 crown pieces per plant were examined 5–7 days later for cultural and morphological characteristics consistent with those of F. oxysporum. The experiment was repeated once.
Further pathogenicity tests were conducted on ‘Fraiser’ cucumber and ‘Carmen’ eggplant (Rijk Zwaan, De Lier, the Netherlands), ‘Trust’ tomato and ‘Fascinato’ pepper. Seeds were sown as described above except the rock wool plugs were transplanted into 10×10×10 cm rock wool blocks (Grodan Delta, Milton, ON) and after 21 days they were placed onto ‘Grodan’ rock wool slabs, 50 cm long × 20 cm wide, in a double poly greenhouse at HRDC – AAFC. There were 2 replicate rows per host with 11 slabs per row consisting of 2 plants per slab. Each slab represented a different treatment including a water control, uninoculated control, and one of the isolates, as noted previously. The treatments were randomly assigned in each row. The cucumber, eggplant, tomato and pepper plants were 28, 24, 28 and 31 days old, respectively, at the time of inoculation for the first trial and 29, 46, 32 and 46 days old, respectively, at the time of inoculation for the second trial. The inoculation was as described above. Disease development was monitored for 77 days and then four disease severity ratings were conducted and DSI calculated for each plant as described above. The isolations from the crown tissue of each plant were conducted as described previously. The experiment was repeated once.
Further host range studies were conducted using untreated seed of ‘Nautica’ bean (Phaseolus vulgaris L.), ‘Pioneer’ field cucumber (Cucumis sativus L.), chickpea (Cicer arietinum L.) from BulkBarn (Amherstburg, ON), and zucchini squash (Cucurbita pepo L.) from OSC Seeds (Waterloo, ON). Plants were grown in 10-cm-diameter Fibre pots in a soil mix (Fox sandy loam: peat moss, 3:2, v:v) with one plant per pot and maintained in a greenhouse. A spore suspension was prepared, as described earlier, using 18-day-old cultures of isolates TriB-P1 Iso 2, TriB-P1 Iso 3, HsP-Healey-P1- S1, HsP-Healey-P1- S2, HsP-Bentley-P2-S1, HsP-Bentley-P2-S4. Each replicate consisted of the six isolates and two control checks (water only), prepared as described previously. Ten mL of spore suspension of each isolate was poured onto the soil at the base of the plant and in contact with the stem. Isolate inoculations were randomly arranged within each replicate, with three replicates for each host plant per trial. Plants were 18 and 48–50 days old at the time of inoculation and disease assessment, respectively. Disease assessment was based upon whole plant/foliar, root and crown symptoms using a 0–5 scale. For root and crown evaluations, the soil was carefully washed from the roots and a longitudinal cut was made at the crown to observe the interior portion of the crown tissue. For the whole plant/foliar assessment this consisted of the following rating: 0 = no disease symptoms; 1 = trace symptoms such as slight wilting or slight chlorosis of foliage; 2 = some wilting with some foliar chlorosis; 3 = moderate wilting, possible minor stunting and some foliar chlorosis; 4 = extensive wilting with foliar chlorosis/necrosis; 5 = death of plant. Root rating evaluations included: 0 = healthy roots, no discolouration; 1 = minor brown discolouration near/at root tips; 2 = some brown discolouration of roots; 3 = moderate brown discolouration of roots; 4 = extensive brown discolouration with moderate root decay; 5 = extensive root decay. The crown tissue was assessed using the following scale: 0 = white, healthy crown tissue; 1 = trace brown discolouration within interior crown tissue; 2 = some brown discolouration of interior crown tissue and trace exterior brown discolouration; 3 = moderate brown discolouration of interior crown tissue and some exterior brown discolouration; 4 = severe brown discolouration occupying a significant portion of the crown region with external brown discolouration; 5 = decayed internal crown portion. The experiment was repeated once.
Pathogen characterization – molecular identification
The original isolates as well as an isolate each of F. oxysporum f. sp. lycopersici (Sacc.) W.C. Snyder & H.N. Hans and F. oxysporum f. sp. radicis-lycopersici W.R. Jarvis & Shoemaker were cultured and mycelia collected. Extraction of DNA was performed using liquid nitrogen and the procedure in the Norgen Plant/Fungi DNA Isolation Kit (Norgen Biotek Corp., Thorold, ON, Canada). Amplification of the transcription elongation factor (TEF) gene (Geiser et al. Citation2004) was done using the following primers: ef1 forward primer: 5ʹ ATG GGT AAG GAG GAC AAG AC 3ʹ, and ef2 reverse primer: 5ʹ GGA GGT ACC AGT GAT CAT GTT 3ʹ. For each isolate, one Illustra PuReTaq Ready-To-Go PCR Bead Tube® (GE Healthcare, Ottawa, ON) was used and the following was added: 1 µL of forward primer, 1 µL of reverse primer, 2–4 µL of template DNA (dependent on concentration of DNA) and 19–21 µL of RT-PCR Grade Water (Ambion™, Carlsbad, CA). The following PCR conditions were used: delay for 30 s to allow the heated lid to warm up, hold at 95°C for 5 min, repeat the following 30 times: 95°C for 1 min, 53°C for 1 min, 72°C for 1.5 min, and finally hold at 72°C for 5 min. Gel electrophoresis was then conducted and the bands containing the PCR product were extracted from the gel using the QIAquick Gel Extraction Kit (standard protocol) (Qiagen Inc., Valencia, CA, USA).
The samples were sent to the London Regional Genomics Centre, London, ON for DNA sequencing. A BLAST search was performed on the DNA sequences using the NCBI (National Center for Biotechnology Information) (Bethseda, MD, USA) database (http://blast.ncbi.nlm.nih.gov/Blast.cgi) as well as the Fusarium-ID database (http://isolate.fusariumdb.org/index.php) to compare with sequences of known Fusarium species. The percentage match was recorded and sequences were compared to each other using Clustal W2 Multiple Sequence Alignment program (http://www.ebi.ac.uk/Tools/msa/clustalw2/). Samples 1 and 2 were excluded because they were the (known species) controls, F. oxysporum f. sp. lycopersici and F. oxysporum f. sp. radicis-lycopersici, respectively.
Greenhouse pepper cultivar studies
Pepper cultivars ‘Bentley’ and ‘Healey’ (Syngenta Canada Inc., Guelph, ON), ‘Redwing’, ‘Crosby’, ‘Gilmour’, ‘35–237ʹ, ‘35–238ʹ (Rijk Zwaan USA, Inc., Salinas, CA), and ‘E 41.3561ʹ (Enza Zaden USA, Inc., Salinas, CA) were evaluated for resistance/susceptibility to F. oxysporum. Twenty-five seeds of each cultivar were planted in 10 × 10 × 10 cm rock wool blocks (Grodan Delta, Milton, ON) with 1 seed/block and covered with vermiculite. The plants were grown until roots began penetrating the base of the blocks, about 6–7 weeks and then transferred onto a 50 × 20 cm rock wool slab (Grotop Expert, Grodan, Milton, ON), where each slab held two plants. For inoculation, a spore suspension was prepared as described previously from a 13-day-old aPDA culture of one of three F. oxysporum isolates, TriB P1 Iso 2, HsP P1S2 Healey or HsP P2S4 Bentley. Ten mL of inoculum was dispensed onto the stem base of each pepper plant. The pepper plants were maintained in a greenhouse using standard greenhouse growing procedures (Ontario Ministry of Agriculture and Food Citation2005) and observed for symptom development for 77 days before rating. Ratings were made at 77 days post-inoculation based on the appearance and severity of the disease symptoms in roots, crown, stem and entire plant and the DSI was calculated as described previously. The experiment was repeated once.
Evaluation of fungicides and bio-controls
Seven-week-old pepper plants ‘Redwing’ growing on rock wool slabs (two plants per slab) were used in these experiments. Plants were fertilized as per nutrient film technique regime and maintained under recommended greenhouse growing conditions (Ontario Ministry of Agriculture and Food Citation2005). Each row in the greenhouse contained seven slabs representing one replicate with each slab receiving a separate treatment. There were four replicates in the experiment and the experiment was repeated twice. Five different reduced-risk materials (g L−1) were tested – three fungicide treatments consisting of Medallion® (fludioxonil, Novartis, Dorval, QC, 0.075), Senator® (thiophanate-methyl, Engage Agro Corp., Guelph, ON, 0.75) and Caramba® (metconazole, BASF, Mississauga, ON, 0.7 mL L−1), and two biological controls, consisting of Mycostop® (Streptomyces griseoviridis, Plant Products, Leamington, ON, 5) and Prestop® (Gliocladium catenulatum, Plant Products, Leamington, ON, 10). Positive control plants were treated with distilled water and inoculated with F. oxysporum while negative control plants were treated with distilled water and not inoculated. Five days after transferring the blocks to slabs, 120 mL of each reduced-risk material or distilled water was poured onto the stem at the base of the plant and this was repeated 10 days later. Caramba® was applied only once, during the second application time, as the first time it was applied, the rate used was too high (7 mL L−1) and phytotoxicity occurred resulting in leaf wilting. The plants were replaced and the rate was lowered during the second application to 0.7 mL L−1.
A mixture of the original isolates of putative F. oxysporum consisting of TriB-P1-Iso 1, 2, 3, HsP-Bentley-P2-S1, S2, S3, S4 and HsP-Healey-P1-S1, S2 were used for inoculation. They were grown at room temperature on aPDA under continuous fluorescent light for 24 days. Four days after the second treatment application, a 10 mL solution containing 5 × 105 spore suspension consisting of an equal mixture of the isolates was carefully poured onto the rock wool block around the base of the plant but not in direct contact with the stem. All plants were inoculated except for the negative controls. Following inoculation, the plants were grown in the greenhouse for 77–85 days with set points of 20°C and 33°C for lower and upper temperatures, respectively. The ratings of the roots, crown, stem and entire plants were conducted, the DSI was calculated, and isolations from crown tissue performed, as described previously.
Data analysis
Analysis of variance of DSI values was done using the ANOVA (SAS Institute Inc.,Citation2000) and Duncan’s Multiple Range Test (Steel & Torrie Citation1960). Results were considered significant at P < 0.05. The Hartley test for equality of variances was conducted prior to combining DSI results of the three fungicide/biological control experiments (Neter et al. Citation1985).
Results
Pathogen characterization – cultural and morphological tests
Isolates HsP-Bentley-P2-S1, TriB-P1-Iso 1, 2 and HsP-Healey-P1-S1 growing on aPDA had sometimes sparsely developed colony growth and arachnoid in appearance with pale salmon colouration, somewhat flat appressed and pionnotal. Isolate HsP-Healey-P1-S2 from the same sources had more floccose mycelium that was white or light mauve colour (Fig. 1a). Sporulation was abundant with all isolates. All older cultures had abundant white or mauve mycelial growth and some black stromatic bodies scattered in the culture plate. No perithecia were observed at any time nor in culture plates after 155 days. Microconidia, 1- or 2-celled, were oval with occasional obovoid shape and were produced on short, subcylindrical monophialides sometimes arising laterally on the hyphae (, red arrows) or in false heads but not in chains. Macroconidia were straight to slightly curved with thin walls. The apical cells were slightly curved and tapered. The basal cells had a slight foot shape in some cases (). Sporodochia were present. Mean length and width of 1-celled microconidia ranged from 7.07 to 9.0 μm and from 2.95 to 3.24 μm, respectively, with overall mean dimension of 8.03 μm (±0.49 standard error) and 2.94 ± 0.13 μm, respectively. Two-celled microconidia ranged from 12.20 to 18.00 μm and from 3.29 to 4.28 μm for length and width, respectively, with overall mean dimension of 14.82 ± 0.91 μm and 3.52 ± 0.18 μm, respectively. Three-celled macroconidia ranged from 17.36 to 23.99 μm and from 3.65 to 4.18 μm for length and width, respectively, with overall mean dimension of 19.55 ± 1.38 μm and 3.78 ± 0.11 μm, respectively. Four-celled macroconidia ranged from 21.42 to 34.61 μm and from 3.60 to 4.97 μm for length and width, respectively, with overall mean dimension of 25.31 ± 2.99 μm and 4.15 ± 0.35 μm, respectively. The overall mean percentage of 1-, 2-, 3- and 4-celled conidia for all isolates was 50, 14, 16, and 20, respectively. Chlamydospores of isolates were smooth, globose, usually single, light yellow-brown colour with thick walls, immersed in the culture media rather than superficial with range in diameter from 6.21 to 8.53 μm with mean diameter of 7.3 ± 0.63 μm.
Fig. 1 (Colour online) Colonies, conidia and development of F. oxysporum on greenhouse ‘Fascinato’ pepper following inoculation. (a) 14-day-old cultures of F. oxysporum on aPDA. (b) Hypha and lateral subcylindric monophialides with conidia (arrows), scale bar = 8 μm, (c) 1, 2, 3, and 4-celled conidia, scale bar = 8 μm, (d) Greenhouse ‘Fascinato’ pepper plants: healthy, uninoculated (left) and plants with severe symptoms (right) (red arrows), 77 days after inoculation. The affected plants are stunted, the leaves are wilted and necrotic. (e) Foliar chlorosis originates from the leaf apex and in leaf vein tissue of lower leaves. (f) Later stage of disease development includes foliar necrosis starting from the lower leaves. (g) Crown tissue from control, water-inoculated ‘Fascinato’ pepper plants shows no internal stem damage, healthy white roots and no crown damage. (h) Crown tissue from ‘Fascinato’ pepper plants inoculated with Fusarium oxysporum shows significant black and brown discolouration (red arrow), roots that are dark brown or black and in many cases decayed (red arrows) and minor internal stem discolouration. (i) Dark brown, external stem discolouration (red arrow) at the base of the ‘Fascinato’ pepper plant.
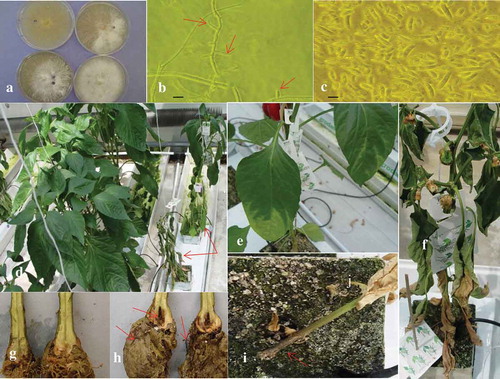
Pathogen characterization – pathogenicity tests
Healthy, water-inoculated greenhouse ‘Fascinato’ pepper plants showed no stunting (), with white roots that could not easily be removed from surrounding rock wool substrate. The crown tissue was cream-coloured () with no internal or external stem discolouration and a mean DSI = 0.62. In contrast, F. oxysporum-infected pepper plants initially showed mild stunting 5–6 weeks after inoculation, followed by more severe stunting later (). Other symptoms, starting on the lower foliage, such as foliar chlorosis (), wilting and finally foliar necrosis () were not readily evident until about 60 days after inoculation. When internal crown and stem tissue was examined 77 days after inoculation, there was considerable brown-black discolouration and decay of crown tissues including cortex and vascular tissue (), with secondary bacterial decay evident but little internal stem discolouration or damage beyond the crown portion (i.e. root/stem transition area). Roots were dark brown to black (), severely decayed, weak and easily separated from the surrounding rock wool fibres. Plants that were necrotic or nearly necrotic had dark brown external stem discolouration at the base of the rock wool block (). No fungal fruiting structures were observed on any stem tissues. The mean DSI for isolates from HsP Healey, HsP Bentley and TriB were 8.62, 10.18 and 10.95, respectively. The fungus was re-isolated from diseased crown tissue of inoculated plants but not from healthy check plants. Symptoms on inoculated plants were similar to those observed in commercial greenhouses and the recovered isolates had the same cultural and morphological characteristics as the original isolates, thus fulfilling Koch’s postulates.
There was no significant difference among isolates of F. oxysporum in virulence as determined by DSI on ‘Fascinato’ pepper plants, with DSI values ranging from 4.5 to 13.5 (). The variability in the overall plant ratings was quite large and varied from minor to severe. This was unlike the ratings of the roots, stem and crown, which were much less variable (data not shown). All of the plants inoculated with isolates showed stunting and wilting.
There were no visible foliar symptoms on greenhouse cucumber, tomato and eggplant inoculated with isolates of F. oxysporum and only occasional trace symptoms on the respective roots, internal crown or stem tissues. Mean DSI values were 0.0, 0.40, 0.08 for cucumber, tomato and eggplant, respectively, for experiment one, and were 0.30, 0.25, 0.60, respectively, for experiment two. Inoculated pepper plants had stunting, foliar wilting followed by chlorosis and necrosis, severe root and crown decay and had mean DSI of 9.2 and 9.8 for experiments one and two, respectively. There were no differences in severity among isolates. Water-inoculated eggplant, tomato, pepper and cucumber did not show any symptoms with mean DSI of 0. The fungus was recovered from surface-sterilized symptomless internal crown tissue from 86%, 75%, 61% and 50% of inoculated pepper, eggplant, tomato and cucumber plants, respectively, in experiment one, and from 93%, 82%, 75% and 85% plants, respectively, in experiment two. There were no symptoms visible on ‘Nautica’ Phaseolus bean, ‘Pioneer’ field cucumber, chickpea, or zucchini squash 30–32 days after fungus inoculation.
Pathogen characterization – molecular identification
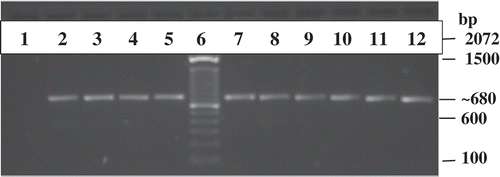
Putative F. oxysporum isolates (, lanes 4, 5, 7–12) displayed the same band at 680 bp as F. oxysporum f. sp. lycopersici (, lane 2) and F. oxysporum f. sp. radicis-lycopersici (, lane 3) when amplified using TEF primers. The results of the BLASTn (Madden Citation2002) search using the NCBI database showed a 95–98% match to F. oxysporum and 97.45–99.84% match using the Fusarium ID database (Geiser et al. Citation2004). The control isolates () are F. oxysporum, which indicates the results are reliable.
Fig. 2 (Colour online) Combined DSI from two experiments of ‘Fascinato’ pepper plants inoculated with various isolates of F. oxysporum. Error bars denote standard deviation (n = 4).
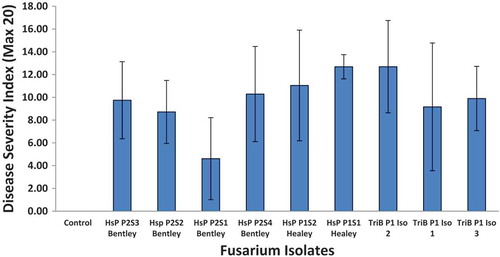
Fig. 3 Gel electrophoresis of PCR amplified greenhouse pepper isolates of F. oxysporum from the first site (TriB-P1- Iso 1, 2, 3) and five isolates from the second site (HsP-Bentley-P2- S1, S2, S3, S4; HsP-Healey-P1- S2) as well as an isolate each of F. oxysporum f. sp. lycopersici and F. oxysporum f. sp. radicis-lycopersici. DNA extracts of individual isolates were PCR amplified using TEF forward and reverse primers. PCR amplicons were run on a 1% agarose gel containing 8.5 μL of Invitrogen SYBR Safe DNA gel stain. The isolate lanes are as follows: 1 = blank, 2 = Fusarium oxysporum f. sp. lycopersici, 3 = Fusarium oxysporum f. sp. radicis-lycopersici, 4 = HsP P2S3 Bentley, 5 = TriB PI Iso 1, 6 = Check – 100 bp DNA Ladder, 7 = HsP P2S1 Bentley, 8 = HsP P2S2 Bentley, 9 = TriB P1 Iso 3, 10 = TriB P1 Iso 2, 11 = HsP P1S2 Healey, 12 = HsP P2S4 Bentley.
Greenhouse pepper cultivar studies
The most susceptible cultivars to F. oxysporum isolates HsP P2S4 Bentley and HsP P1S2 Healey, as measured by DSI, were red pepper fruit ‘Redwing’, ‘Healey’, and yellow pepper fruit line 35-238, while isolate TriB P1 Iso 2 was less severe on the same cultivars (Fig. 4). Most resistant cultivars to all three isolates were yellow pepper fruit ‘Crosby’ and line 35-237. The other cultivars were intermediate in response.
Evaluation of fungicides and bio-controls for control of fusarium crown and root rot of greenhouse pepper
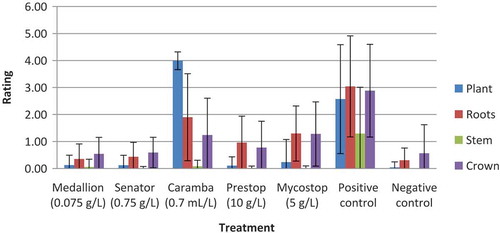
The fungicides Medallion (fludioxonil) and Senator (thiophanate-methyl) and the biocontrol agents Mycostop (S. griseoviridis) and Prestop (G. catenulatum) were the most effective materials at controlling infection by F. oxysporum on ‘Redwing’ peppers, with DSI of 1.08, 1.11, 2.83 and 1.86, respectively, which were significantly lower than the fungus-inoculated control at 9.79 DSI () and not different from the uninoculated control. Caramba (metconazole) at 0.7 mL L−1 had DSI of 6.91 that was significantly greater than any of the fungicides or biological controls but significantly less than the inoculated check (). The phytotoxicity observed after application of Caramba contributed to the DSI. Combined mean ratings from experiments 1–3 of individual components such as plant, roots, stem and crown from inoculated plants (Fig. 5) showed similar relationships as described above, with fungicide and biological control treatment ratings less than the inoculated (positive) control and with the Caramba treatment having the highest plant rating and high root rating, indicating considerable phytotoxicity that was present ().
Table 1. Efficacy of various fungicides and biological control products for control of Fusarium crown and root rot incited by Fusarium oxysporum measured using disease severity index (DSI) in greenhouse ‘Redwing’ peppera.
Fig. 4 (Colour online) Response of various greenhouse pepper cultivars and lines, as measured by disease severity index, to three isolates of Fusarium oxysporum. Disease Severity Index (DSI) = Root + Plant + Stem + Crown ratings. Each rating is a maximum value of 5 with four different ratings for a possible maximum disease severity rating of 20. Error bar represents the standard deviation.
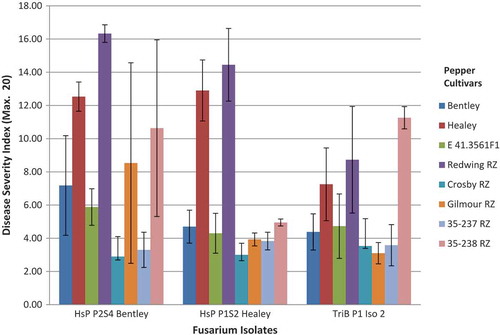
Fig. 5 (Colour online) Combined mean ratings of trials 1, 2 and 3 for plant, roots, stem and crown tissue of ‘Redwing’ greenhouse peppers following treatment with reduced-risk materials for control of F. oxysporum. Positive control = fungus-inoculated; Negative control = water only. Error bars denote standard deviation.
Discussion
This study represents the first report of Fusarium oxysporum affecting soilless greenhouse pepper production in Canada. At one commercial greenhouse pepper operation in southern Ontario, approximately 50% of plants of cultivar ‘Bentley’ and 10% of other cultivars were affected. The disease was also reported to affect more than seven million nursery-grown sweet pepper plants in Almeria, Spain (Lomas-Cano et al. Citation2014). Identification of Ontario isolates of F. oxysporum was based on cultural, morphological and molecular characteristics (Booth Citation1971; Gerlach & Nirenberg Citation1982; Geiser et al. Citation2004; Leslie & Summerell Citation2006). The production of microconidia on short monophialides, the shape of the micro and macroconidia and the dimensions of 1-, 2-, 3- and 4-celled conidia as well as the lack of perithecia in vitro or in vivo distinguish these isolates from F. solani, and the production of chlamydospores further distinguishes the current isolates from F. subglutinans (Wollenweber & Reinking) Nelson, Toussoun & Marasas (Leslie & Summerell Citation2006). Additionally, the molecular results confirmed the identity of the causal agent as F. oxysporum.
Disease symptoms on greenhouse pepper plants were similar to those reported on nursery-grown sweet pepper in Spain (Lomas-Cano et al. Citation2014) although wilting without yellowing of leaves was absent in the latter, and they observed the occasional presence of pinkish-white sporodochia on stem necroses (Lomas-Cano et al. Citation2016). They also reported that xylem necrosis was never observed in contrast to the Ontario observations. In both cases, roots and crowns were affected with no symptoms observed in the stem tissue. In Ontario, external brown-black stem discolouration was observed only on severely affected plants near death and likely the consequence of decay by secondary organisms. Rivelli (Citation1989) reported F. oxysporum infection of Tabasco pepper in Louisiana but cortical tissue remained intact with no external symptoms evident on the stem or major roots in contrast to the dark brown to black discolouration of the diseased roots seen in the current report. Rivelli (Citation1989) and Lomas-Cano et al. (Citation2016) reported that young pepper plants were more susceptible to F. oxysporum f. sp. radicis-capsici than older plants. Also macroconidia were considerably larger in length at 36.0 – 52.8 μm in the Louisiana isolates (Black Citation2003) than those reported here at 21.4–34.6 μm. Sporulation of F. oxysporum was not observed on pepper stems in this study in contrast to symptoms incited by F. solani where both sexual and asexual stages may be observed at the base of pepper stems and ascospores may be discharged from the perithecia produced (Cerkauskas Citation2001). Consequently, spread of aerial inoculum of F. oxysporum may be of less concern than with F. solani in soilless greenhouse pepper production. Dissemination of F. oxysporum inoculum from affected roots into recycled water-nutrient systems, reuse of soilless media such as rock wool slabs from affected plants, disinfestation of nutrient solutions of fungal pathogens involving methods such as UV radiation, ozonation, chlorination, heat treatment, membrane or slow filtration and other methods (Runia Citation1995; Wohanka Citation1995), and cleaning and disinfestation of irrigation lines is important to the introduction, survival and propagation of the fungus in greenhouse pepper production rather than airborne inoculum or aerial dispersal of the fungus. In contrast, sporulation on stems resulting in aerial dissemination of microconidia has been reported for F. oxysporum f. sp. lycopersici (Katan et al. Citation1997) and for F. oxysporum f. sp. radicis-lycopersici (Rekah et al. Citation2000) on greenhouse tomato under high temperature and humidity and by F. oxysporum Schlechtend.Fr. f. sp. radicis-cucumerinum D.J. Vakalounakis on greenhouse cucumber (Punja & Parker Citation2000).
Fusarium oxysporum is widespread in agricultural and other ecosystems (Booth Citation1971; Domsch & Gams Citation1972; Gerlach & Nirenberg Citation1982; Leslie & Summerell Citation2006), with a very broad host range, although individual strains may be highly host-specific (Armstrong & Armstrong Citation1981). For example, F. oxysporum f. sp. lycopersici has a narrow host range (Rowe Citation1980) while F. oxysporum f. sp. radicis-lycopersici can infect more plant families (Menzies et al. Citation1990). Current evidence suggests that F. oxysporum is a complex of multiple species with species boundaries and extent of genetic exchange within this complex poorly defined (Laurence et al. Citation2014). Identification of pathogenic F. oxysporum isolates is based solely on pathogenicity and the correct identification of formae speciales requires inoculation onto a large number of plant species and cultivars. The use of molecular identification at this level is incomplete because the genetic basis of host specificity is poorly understood at this time (Lievens et al. Citation2008). Consequently the recognition of species boundaries in the F. oxysporum species complex is difficult because of the lack of distinct taxonomic characters, the wide range of strains/pathotypes, the broad geographic distribution, and the influence of agricultural practices on pathogenic and saprophytic populations of the fungus complex (Laurence et al. Citation2014). Also, horizontal transfer of pathogenicity elements in the F. oxysporum complex has been demonstrated between F. o. f. sp. lycopersici and a non-pathogenic F. oxysporum that rendered it pathogenic to tomatoes (Ma et al. Citation2010). This may explain the apparent origins of host specialization and the emergence of new pathogenic lineages. Epidemiologically, this is important for the development of adequate Fusarium management strategies in greenhouse vegetable production. The current study does not identify a formae speciales designation because a greater number of host pathogenicity tests and comparisons via molecular work with other formae speciales of F. oxysporum need to be conducted. In contrast Lomas-Cano et al. (2014, Citation2016) suggested a formae speciales for F. oxysporum isolated from greenhouse pepper seedlings in Spain based on pathogenicity tests of five hosts and molecular analysis that showed identical TEF sequences with an isolate of F. oxysporum f. sp. capsici.
Reisolation of F. oxysporum from symptomless crown tissue of inoculated cucumber, tomato and eggplant in the current study is consistent with other work involving endophytic colonization by the fungus (Armstrong & Armstrong Citation1948; Katan Citation1971; Kuldau & Yates Citation2000). Long-distance spread of F. oxysporum inoculum arising from use of colonized but symptomless greenhouse vegetable transplants or seedling plugs may also be an important factor due to the prolonged latency. Disease control measures for F. oxysporum in greenhouse pepper production must encompass epidemiological factors, such as symptomless colonization of various hosts by the fungus and occurrence of long-term survival structures such as chlamydospores. Therefore, removal of previous and current crop residue and weeds near the greenhouse, and proper sanitation practices are important. Lomas-Cano et al. (Citation2016) reported F. oxysporum f. sp. radicis-capsici could survive in soil up to 8 months after transplantation in 50% of their surveyed greenhouses although the disease was not observed during the subsequent crop year in the same infested soil.
Greenhouse pepper seed was not evaluated for incidence of seedborne F. oxysporum in this study although the association between the fungus and various seed hosts such as greenhouse tomato is well known but at low levels of incidence (Menzies & Jarvis Citation1994a) while Lomas-Cano et al. (Citation2016) did not find F. oxysporum on seed from six pepper cultivars that were tested. The current study demonstrates that some greenhouse pepper cultivars do have partial resistance to Fusarium crown and root rot incited by F. oxysporum. This is the first such report involving greenhouse peppers in Canada. The use of partially resistant cultivars or grafting of susceptible cultivars onto resistant rootstock could be an effective component of an integrated management strategy for the disease. In contrast, Lomas-Cano et al. (Citation2016) found 19 pepper cultivars tested, including three rootstocks, developed symptoms when inoculated with F. oxysporum f. sp. radicis-capsici.
Mihuta-Grimm et al. (Citation1990) demonstrated effective management of Fusarium crown and root rot of greenhouse tomato in rock wool substrate using benomyl. In the current study, fungicides such as fludioxonil and thiophanate-methyl and bio-controls such as Mycostop (S. griseoviridis) and Prestop (G. catenulatum) when applied preventively were effective in limiting root, crown and plant symptoms as well as combined symptoms associated with the disease in greenhouse pepper. Similarly, Rose et al. (Citation2003) reported that Prestop, but not Mycostop, applied preventively to rock wool blocks significantly reduced disease incited by F. oxysporum f. sp. radicis-cucumerinum in greenhouse cucumber. In addition, chemical amendments to the nutrient feed such as silicon (i.e. potassium metasilicate) have been effective in suppression of P. capsici in greenhouse pepper (Lee et al. Citation2004), in field pepper (French-Monar et al. Citation2010) and in reduction of disease symptoms incited by F. oxysporum f. sp. radicis-cucumerinum (Cerkauskas et al. Citation2004).
Finally, various biological control agents including non-pathogenic strains of Fusarium spp., Trichoderma spp., Gliocladium virens and bacteria have been reported to be effective in control of F. oxysporum f. sp. radicis-lycopersici (Lemanceau & Alabouvette Citation1991; Datnoff et al. Citation1995; Larkin & Fravel Citation1998) and may be similarly effective for control of F. oxysporum in greenhouse pepper production. Further efficacy trials involving preventive applications of other fungicides and biological controls, as well as longer term application regime, timing and frequency of application, and compatibility evaluation of use of multiple reduced-risk materials, need to be considered for an integrated management strategy for the disease.
Acknowledgements
The technical assistance of University of Waterloo Co-op term students A. Matysiakiewicz, J. Haribhakti, N. Chow and E. Luk is gratefully acknowledged.
Additional information
Funding
References
- Armstrong GM, Armstrong JK. 1948. Nonsusceptible hosts as carriers of wilt Fusaria. Phytopathology. 38:808–826.
- Armstrong GM, Armstrong JK. 1981. Formae speciales and races of Fusarium oxysporum causing wilt diseases. In: Nelson PE, Toussoun TA, Cook RJ, editors. Fusarium: diseases, biology, and taxonomy. University Park (PA): Penn. State Univ; p. 391–399.
- Black LL. 2003. Fusarium wilt. In: Pernezny K, Roberts PD, Murphy JF, Goldberg NP, editors. Compendium of pepper diseases. St. Paul (MN): APS Press; p. 14–15.
- Booth C. 1971. The genus Fusarium. Bucks (England): Commonwealth Agricultural Bureaux.
- Cerkauskas R. 2001. Fusarium stem and fruit rot of greenhouse pepper. AgDex 294/638, Ontario Ministry of Agriculture, Food & Rural Affairs. Toronto (ON): Queen’s Printer forOntario.
- Cerkauskas RF, Brown J, Meyers C. 2004. Efficacy of potassium silicate as a hydroponic amendment in control of fusarium crown and root rot of greenhouse cucumber. Proceedings of the 15th International Plant Protection Congress; Beijing, China; p. 539.
- Datnoff LE, Nemec S, Pernezny K. 1995. Biological control of Fusarium crown and root rot of tomato in Florida using Trichoderma harzianum and Glomus intraradices. Biol Control. 5:427–431.
- Domsch KH, Gams W. 1972. Fungi in agricultural soils. London (England): Longman Group Limited.
- French-Monar RD, Rodrigues FA, Korndörfer GH, Datnoff LE. 2010. Silicon suppresses phytophthora blight development on bell pepper. J Phytopathol. 158:554–560.
- Geiser DM, Jimenez-Gasco M, Kang S, Makalowska I, Veeraraghavan N, Ward TJ, Zhang N, Kuldau GA, O’Donnell K. 2004. FUSARIUM-ID v. 1.0: a DNA sequence database for identifying Fusarium. Eur J Plant Path. 110:473–479.
- Gerlach W, Nirenberg H. 1982. The genus Fusarium – a pictorial Atlas. Berlin (Germany): Biologische Bundesanstalt für Land- und Forstwirtschaft.
- Katan J. 1971. Symptomless carriers of the tomato Fusarium wilt pathogen. Phytopathology. 61:1213–1217.
- Katan T, Shlevin E, Katan J. 1997. Sporulation of Fusarium oxysporum f. sp. lycopersici on stem surfaces of tomato plants and aerial dissemination of inoculum. Phytopathology. 87:712–719.
- Kuldau GA, Yates IE. 2000. Evidence for Fusarium endophytes in cultivated and wild plants. In: Bacon CW, White JFJr, editors. Microbial endophytes. New York (NY): Marcel Dekker; p. 85–117.
- Larkin RP, Fravel DR. 1998. Efficacy of various fungal and bacterial biocontrol organisms for control of Fusarium wilt of tomato. Plant Dis. 82:1022–1028.
- Laurence MH, Summerell BA, Burgess LW, Liew ECY. 2014. Genealogical concordance phylogenetic species recognition in the Fusarium oxysporum species complex. Fungal Biol. 118:374–384.
- Lee JS, Seo ST, Wang TC, Jang HI, Pae DH, Engle LM. 2004. Effect of potassium silicate amendments in hydroponic nutrient solutions to suppress phytophthora blight (Phytophthora capsic) in pepper (Capsicum annuum). Plant Pathol J. 20:277–282.
- Lemanceau P, Alabouvette C. 1991. Biological control of fusarium diseases by fluorescent Pseudomonas and non-pathogenic Fusarium. Crop Prot. 4:279–286.
- Leslie JF, Summerell BA. 2006. The Fusarium laboratory manual. Ames (IA): Blackwell Publishing.
- Lievens B, Rep M, Thomma BPHJ. 2008. Recent developments in the molecular discrimination of formae speciales of Fusarium oxysporum. Pest Manag Sci. 64:781–788.
- Lomas-Cano T, Boix-Ruiz A, Cara-García M, Marín-Guirao J, Palmero-Llamas D, Camacho-Ferre F, Tello-Marquina J. 2016. Etiological and epidemiological concerns about pepper root and lower stem rot caused by Fusarium oxysporum f. sp. radicis-capsici f. sp. nova. Phytoparasitica. 44:283–293.
- Lomas-Cano T, Palmero-Llamas D, Cara M, Garcia-Rodriguez C, Boix-Ruiz A, Camacho-Ferre F, Tello-Marquina JC. 2014. First report of Fusarium oxysporum on sweet pepper seedlings in Almeria, Spain. Plant Dis. 98:1435.
- Ma L-J, van der Does HC, Borkovich KA, Coleman JJ, Daboussi M-J, Di Pietro A, Dufresne M, Freitag M, Grabherr M, Henrissat B, Houterman PM. 2010. Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium. Nature. 464:367–373.
- Madden T. 2002. Chapter 16, The BLAST sequence analysis tool. In: McEntyre J, Ostell J, editors. The NCBI handbook. Bethesda (MD): National Center for Biotechnology Information (US).
- Menzies JG, Jarvis WR. 1994a. The infestation of tomato seed by Fusarium oxysporum f. sp. radicis-lycopersici. Plant Pathol. 43:378–386.
- Menzies JG, Jarvis WR. 1994b. Greenhouse peppers – fungal diseases. In: Howard RJ, Garland JA, Seaman WL, editors. Diseases and pests of vegetable crops in Canada. Ottawa (ON): The Canadian Phytopathological Society and the Entomological Society of Canada; p. 333–334.
- Menzies JG, Koch C, Seywerd F. 1990. Additions to the host range of Fusarium oxysporum f.sp. radicis-lycopersici. Plant Dis. 74:569–572.
- Mihuta-Grimm L, Erb WA, Rowe RC. 1990. Fusarium crown and root rot of tomato in greenhouse rock wool systems: sources of inoculum and disease management with benomyl. Plant Dis. 74:996–1002.
- Neter J, Wasserman W, Kutner MH. 1985. Applied linear statistical models. 2nd ed. Homewood (IL): Richard D. Irwin, Inc.
- Ontario Ministry of Agriculture & Food. 2005. Growing greenhouse vegetables. Publication 371. Toronto (ON): Queen’s Printer for Ontario.
- Punja ZK, Parker M. 2000. Development of fusarium root and stem rot, a new disease on greenhouse cucumber in British Columbia, caused by Fusarium oxysporum f. sp. radicis-cucumerinum. Can J Plant Pathol. 22:349–363.
- Rekah Y, Shtienberg D, Katan J. 2000. Disease development following infection of tomato and basil foliage by airborne conidia of the soilborne pathogens Fusarium oxysporum f. sp. radicis-lycopersici and F. oxysporum f. sp. basilica. Phytopathology. 90:1322–1329.
- Rivelli VC. 1989. A wilt of pepper incited by Fusarium oxysporum f. sp. capsici f. sp. nov. [MS dissertation]. Baton Rouge (LA): Louisiana State University.
- Rose S, Parker M, Punja ZK. 2003. Efficacy of biological and chemical treatments for control of Fusarium root and stem rot on greenhouse cucumber. Plant Dis. 87:1462–1470.
- Rowe RC. 1980. Comparative pathogenicity and host ranges of Fusarium oxysporum isolates causing crown and root rot of greenhouse and field-grown tomatoes in North America and Japan. Phytopathology. 70:1143–1148.
- Runia WTH. 1995. A review of possibilities for disinfection of recirculation water from soilless cultures. Acta Hortic. 382:221–229.
- SAS Institute Inc. 2000. SAS/STAT© user’s guide, version 8.1 [computer program]. Cary (NC): SAS Institute, Inc.
- Steel RGD, Torrie JH. 1960. Principles and procedures of statistics. New York (NY): McGraw-Hill.
- Wohanka W. 1995. Disinfection of recirculating nutrient solutions by slow sand filtration. Acta Hortic. 382:246–255.
