Abstract
Stripe rust of wheat (Triticum spp. L.) is an emerging problem in western Canada. The causal pathogen (Puccinia striiformis f. sp. tritici Eriks.) has the potential to overwinter, which is a concern because of green bridging leading to earlier establishment of rust epidemics. Resistance conferred by seedling/all-stage and adult plant genes is the most effective approach to manage this disease. However, many Canadian wheat varieties lack seedling or all-stage resistance. The effectiveness of Yr genes under field conditions was assessed by exposing differentials/varieties carrying Yr genes to natural inoculum in multiple environments. The differentials were planted at multiple Saskatchewan locations in 2013, 2014 and 2016 and significant stripe rust was detected at 12 site-years. Additionally, 33 winter wheat varieties and advanced breeding lines were assessed for stripe rust reaction in 2016 at Swift Current, Saskatchewan. Disease severity was assessed on flag and penultimate leaves between early milk to soft dough growth stages. The genes Yr5, Yr15, YrSP and those in ‘Yamhill’ are effective in Saskatchewan under field conditions. Genes/differentials were classified into two major groups based on cluster analysis, one with defeated genes and the other with partial resistant genes. Genes YrA, Yr6, Yr7, Yr8, Yr9, Yr27, Yr29, Yr31, Yr32 and YrSu were not effective in the field to SK races. At Swift Current, winter wheat varieties/lines carrying Yr17 were resistant in the field, whereas those carrying Yr10 were not.
Résumé
La rouille jaune du blé (Triticum spp. L.) est un problème émergent dans l’Ouest canadien. L’agent causal (Puccinia striiformis f. sp. tritici Eriks.) peut survivre à la saison froide sur le blé d’hiver qui agit comme « lien vert », permettant ainsi l’éclosion hâtive d’épidémies de rouille, ce qui soulève de nombreuses préoccupations. La résistance conférée aux semis, à tous les stades de croissance et aux adultes par les gènes de la plante demeure l’approche la plus efficace quant à la gestion de cette maladie. Toutefois, chez plusieurs variétés de blé canadien, la résistance fait défaut chez le semis ou aux autres stades de croissance. En champ, l’efficacité des gènes Yr a été évaluée en exposant des différentiels de blé et des variétés porteuses de gènes Yr à des inoculums naturels, et ce, dans différents milieux. En 2013, 2014 et 2016, les différentiels ont été semés à divers endroits en Saskatchewan et de graves cas de rouille ont été détectés à 12 sites annuellement. En outre, 33 variétés de blé d’hiver et lignées généalogiques avancées ont été testées pour leur réaction à l’égard de la rouille en 2016 à Swift Current, en Saskatchewan. La gravité de la maladie a été évaluée sur les feuilles paniculaires et les avant-dernières feuilles, et ce, du stade de croissance laiteux précoce au stade pâteux mou. En champ, en Saskatchewan, les gènes tels que Yr5, Yr15, YrSP et ceux du cultivar ‘Yamhill’ sont efficaces. En se basant sur l’analyse typologique, les gènes et les différentiels ont été classés en deux principaux groupes: un avec des gènes inopérants et l’autre, avec des gènes partiellement résistants. Les gènes YrA, Yr6, Yr7, Yr8, Yr9, Yr27, Yr29, Yr31, Yr32 et YrSu étaient inefficaces au champ quant aux races de la Saskatchewan. À Swift Current, les variétés de blé d’hiver et les lignées porteuses du gène Yr17 étaient résistantes au champ, tandis que celles portant le gène Yr10 ne l’étaient pas.
Introduction
Of three common wheat (Triticum spp. L.) rust diseases, namely stem rust, caused by Puccinia graminis f. sp. tritici Pers.:Pers, leaf rust, caused by Puccinia triticina Desmaz., and stripe rust, caused by Puccinia striiformis f. sp. tritici Eriks. (Pst), stem and leaf rust are generally well managed in Canada as a result of long-term resistance breeding programmes. This is not the case for stripe rust because this disease is relatively new to the wheat industry in Canada; it has become a major concern since 2000 (Chen Citation2005; Xi et al. Citation2015; Brar & Kutcher Citation2016a). Stripe rust inoculum arrives in western Canada from the USA with wind trajectories along the ‘Puccinia pathway’ and from the ‘Pacific Northwest (PNW)’ of the USA (Xi et al. Citation2015; Brar & Kutcher Citation2016a). Although stripe rust was a problem in irrigated soft white wheat crops in southern Alberta for many years, the emergence of a new strain in the USA in about the year 2000 changed the scenario and the disease became common across western Canada. The change in the pathogen population due to the new, more aggressive and warm temperature-adapted strain PstS1 and its variants (Milus et al. Citation2009) coincided with the defeat of resistance genes Yr8 and Yr9. These genes were common in hard and soft red winter wheat varieties in the USA east of the Rocky Mountains (Milus et al. Citation2015).
Similar to other wheat rusts, genetic resistance is a preferred management option for stripe rust and is either due to seedling resistance or adult plant resistance (APR) (Brar Citation2015). Seedling resistance is expressed throughout the life of the plant, and is believed to be due to race-specific resistance genes, which are subject to breakdown. Adult plant resistance genes are ineffective at the seedling stage, but expressed at the adult plant stage and are race-non-specific, and thus are usually found to provide durable resistance to stripe rust. Additive APR genes, such as Yr18/Lr34, Yr29/Lr46, Yr30 and other unnamed genes confer partial, durable and non-race specific resistance (Milus et al. Citation2015). Of these genes, Yr18 is the most commonly used rust resistance gene in wheat breeding programmes in western Canada because it also confers resistance to leaf and stem rust, and powdery mildew (Randhawa et al. Citation2012, Citation2013). Additionally, availability of accurate molecular markers for Yr18 facilitates routine selection in Canadian wheat breeding programmes (Randhawa et al. Citation2013).
For successful stripe rust management, regular monitoring and virulence characterization of the pathogen are important (Brar Citation2015; Brar & Kutcher Citation2016a). Virulence characterization should be based on host genetics to serve the needs of wheat breeders and growers (McIntosh Citation2010). Sometimes a pathogen isolate is virulent when screened under controlled conditions, but may have a different phenotype under field conditions. This was exemplified in the case of the interaction of Sr21 and Puccinia graminis f. sp. tritici (McIntosh Citation2010). Thus, virulence characterization under controlled conditions should be coupled with phenotyping under field conditions to provide complete information to wheat breeders and growers. Host–pathogen interactions in the wheat-stripe rust pathosystem are greatly affected by climatic conditions: temperature, precipitation and relative humidity, which affect disease development in the field (Chen Citation2005). Stripe rust development in Canada depends on the inoculum load coming from the USA, climatic conditions, and the growth stage of the wheat crop at the time of inoculum arrival (Xi et al. Citation2015). If the inoculum arrives late in the season, the disease does not cause appreciable economic loss. However, overwintering of the pathogen in western Canada is expected to increase disease pressure at an early growth stage of the crop (Kumar et al. Citation2013). Thus, to address the needs of resistance breeding programmes and successfully manage this disease, the infection resulting from the host–pathogen interaction under field conditions, as well as controlled conditions, needs to be studied.
Resistance gene expression may differ between assessment made in controlled conditions and assessment in the field, and vary with climatic conditions among geographic locations. An excellent study by Xi et al. (Citation2015) examined effectiveness of Yr genes in central Alberta and Creston, British Columbia from 2007–2012. However, the pathogen is capable of gaining virulence to previously effective genes in a short period of time, rendering resistant varieties ineffective (Brar Citation2015). Also, long-distance dispersal of stripe rust can result in epidemics in areas where the disease is sporadic and occurs at low levels, such as the 2016 epidemic in Ontario, Canada (Alireza Navabi, personal communication). Despite the lack of a physical barrier, and the proximity of the province of Saskatchewan to Alberta, subtle differences in race distribution and diversity are reported (Brar & Kutcher Citation2016a). Thus, it is imperative for rust pathologists to regularly monitor and assess the effectiveness of Yr genes in their regions. In an effort to understand the effectiveness of Yr genes in Saskatchewan, the Cereal & Flax Pathology research group at the University of Saskatchewan conducted disease surveys and assessed stripe rust virulence on differential genotypes at 6–8 locations every year since 2013.
The objective of the present study was to assess the effectiveness of Yr genes on differential wheat lines exposed to natural inoculum of stripe rust in the province of Saskatchewan. Additionally, some common triticale varieties were included in the study. To accomplish this objective, stripe rust incidence and severity was assessed from 2013–2016 on wheat and triticale genotypes that differed in terms of Yr genes at multiple locations in the province.
Materials and methods
A set of wheat stripe rust differential genotypes, including common wheat varieties, were used in the experiment and included 21 near-isogenic differential lines in the ‘Avocet’ background (Wellings et al. Citation2004), including ‘Avocet (Null)’ as a susceptible check (). Supplementary differentials included some wheat and triticale varieties (). Triticale varieties were included because triticale germplasm has previously been shown to have good resistance to stripe rust in Canada and Australia (Randhawa et al. Citation2012; Wellings et al. Citation2012; Brar Citation2015). Field trap nurseries were established at various locations in Saskatchewan from 2013–2016 to evaluate the effectiveness of Yr genes at the adult plant stage (). In 2016, a reduced number of differentials were seeded at various locations: ‘Avocet’ near-isogenic lines carrying no gene (null), YrA, Yr1, Yr5, Yr8, Yr10, Yr15, Yr18, Yr24, Yr26, Yr29, Yr32, YrSP and ‘Suwon92*Omar’, ‘Hybrid 46ʹ, ‘Nord Desprez’, ‘Produra’, ‘Heines VII’ and the triticale varieties: ‘Brevis’, ‘AC Certa’, ‘Ultima’, ‘Pronghorn’ and ‘Bunker’ (). The differentials were seeded in hills of 10–20 seeds per hill, with the test surrounded by a border of a susceptible variety. Nursery sites were established each year and stripe rust was observed at: Indian Head, Melfort and Scott in 2013; Indian Head, Swift Current, Prince Albert and Saskatoon in 2014; and Yorkton, Outlook, Prince Albert, Melfort and Saskatoon in 2016 (). Due to dry weather in 2015, stripe rust was not observed at any of the sites. In addition, a few locations with only trace levels of disease were excluded from the study. Consequently, conclusions are drawn from a total of 12 site-years (). The disease ratings were performed at the adult plant stage between early milk and soft dough stages using percentage incidence (number of flag or penultimate leaves showing stripe rust infection among plants), disease severity (per cent leaf area covered averaged for all infected plants), and infection response (host response combining incidence and severity) following the modified Cobb scale (Peterson et al. Citation1948).
Table 1. Per cent incidence, severity and infection response of stripe rust caused by Puccinia striiformis f. sp. tritici (Pst) on differential wheat genotypes across Saskatchewan in 2013 at Indian Head, Melfort and Scott.
Fig. 1 Map showing field sites in Saskatchewan, Canada, where stripe rust differentials, wheat varieties and triticale genotypes were seeded from 2013–2016.
Winter wheat varieties and advanced breeding lines were assessed in 3.66 m2 plots in randomized complete block design of three replicates at Swift Current in 2016. Disease rating was performed twice, first on 10 June and the second rating on 17 June, on flag and penultimate leaves using a 0–5 scale as follows: 0 – clean or flecks; 1 – a few isolated pustules on at least a few leaves; 2 – intermediate reaction with many pustules on at least a few leaves; 3 – a few to many pustules on most leaves; 4 – many pustules on all leaves with leaf tip necrosis or necrosis of leaves in general; 5 – susceptible reaction with all leaves covered with pustules, may include leaf necrosis. The ratings of 0–1, 2–3 and 4–5 are equivalent to R-MR, MR-MS (intermediate) and MS-S, respectively.
Correlation coefficients among sites were calculated from the average disease severity using the cor.test implemented in the R environment (R Core Team Citation2016). Cluster analysis based on average disease severity (of all site-years) of common differentials was performed in the Multivariate Statistical Package (MVSP) ver. 3.1. This analysis calculated the Gower’s simple matching similarity coefficient among clusters utilizing the similarity coefficient calculated with unweighted pair-groupings from the mathematical average (UPGMA) algorithm.
Results and discussion
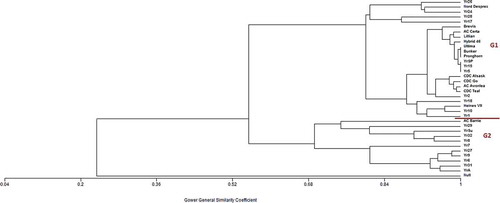
Significant levels of stripe rust were observed in all site-years based on disease severity; however, there was variability in disease development among years (–). Strong and significant correlations among sites (except for Indian Head – Swift Current in 2014) may be indicative of the close association in inoculum distribution among sites (–). Cluster analysis was used to group lines based on the average disease reaction at the adult plant stage based on all sites in all years (). Cluster analysis is an objective approach to classify the quantitative disease reaction of genotypes into groups based on similarity coefficients. Wheat lines were differentiated largely into two major groups (). Group 1 carried effective or partially effective resistance genes. Group 2 lines were susceptible; an effective resistance gene was absent.
Table 2. Per cent incidence, severity and infection response of stripe rust caused by Puccinia striiformis f. sp. tritici (Pst) on differential wheat genotypes across Saskatchewan in 2014 at Indian Head, Swift Current, Prince Albert and Saskatoon.
Table 3. Per cent incidence, severity and infection response of stripe rust caused by Puccinia striiformis f. sp. tritici (Pst) on differential wheat genotypes across Saskatchewan in 2016 at Yorkton, Outlook, Prince Albert, Melfort and Saskatoon.
Fig. 2 (Colour online) Cluster analyses of wheat differentials based on stripe rust severity, using the unweighted pair-grouping from mathematical average (UPGMA) algorithm, at common site-years in 2013, 2014 and 2016 in Saskatchewan. The x-axis of the plot shows the similarity among the genes based on Gower’s coefficient. Group 1 (G1) consisted of genes/differentials which are effective or partially effective to existing stripe rust races in Saskatchewan whereas Group 2 (G2) consisted of defeated genes/differentials.
Genes Yr5, Yr15, YrSP and those carried by ‘Yamhill’ conditioned resistance to stripe rust across all site-years in Saskatchewan (–). The genes Yr5 and Yr15 condition stripe rust resistance in wheat genotypes elsewhere in North America (Xi et al. Citation2015). Races virulent on YrSP are reported only in Alberta, but at extremely low frequency, indicating it is effective in western Canada (Su et al. Citation2003). Virulence to gene Yr1 was not reported in Saskatchewan in 2013 (), although virulence was reported in Alberta previously (Brar & Kutcher Citation2016a). Severe stripe rust on Yr1 was observed at two sites in 2014 and at one site (Saskatoon) in 2016 ( and ), indicating the existence of virulent races in the Saskatchewan pathogen population. Virulence on Yr1 was not reported in the western USA until 2007, and races virulent on Yr1 did not spread to Idaho, USA until 2011 (Wan & Chen Citation2012), and to Alberta a few years later. Virulence on Yr1 appeared in Saskatchewan in 2014, indicating the short time required for stripe rust to spread across geographic regions. None of the Canadian wheat varieties are reported to carry Yr1, but given the existence of virulent pathogen population (as indicated in 2014), the deployment of Yr1 is discouraged as it can result in resistance breakdown with time due to selection pressure.
The wheat genotype, ‘Yamhill’ (carrying Yr4) was resistant to stripe rust at all four locations in Saskatchewan in 2014 (); however, isolates of Pst collected from Alberta between 2009–2011 were all virulent on ‘Yamhill’ when tested under controlled conditions (Holtz et al. Citation2013). This could be attributed to the proximity of Alberta to the PNW, where ‘Yamhill’ is commonly cultivated and where most races are virulent on this variety (Chen Citation2005). Another reason could be attributed to the infrequent existence of Pst races in Saskatchewan carrying virulence to Yr4 (Brar and Kutcher Citation2016a). These results are also supported by the cluster analyses where Yr4 lines clustered together (). Similarly, Yr2 conditioned resistance to stripe rust in Saskatchewan under field conditions, opposite to what was observed under controlled conditions (Holtz et al. Citation2013; Xi et al. Citation2015; Brar & Kutcher Citation2016a). This could be due to lack of selective advantage against this gene or other unknown factors. However, this gene is not effective in the USA and thus the use of this gene in wheat breeding programmes may not be of benefit over the long term.
Genes YrA, Yr7, Yr27 and Yr31 were susceptible or moderately susceptible at most of the site-years, indicating they are defeated by pathogen populations prevalent in western Canada (–). Prince Albert and Indian Head in 2014 were exceptions to this as these sites had low disease severity on differentials carrying Yr7 (–). This could be attributed, in part, to lower inoculum levels as indicated by very low severity on the susceptible check at Indian Head in 2014 (). However, this is not true for Prince Albert where the susceptible check was severely infected (100%) and the reason for the apparent resistance on these genes could be attributed to avirulence. The ineffectiveness of these genes at most site-years agrees with the finding that Pst isolates collected from across western Canada between 2009–2013 overcame these genes (Xi et al. Citation2015; Brar & Kutcher Citation2016a). These genes are clustered in Group 2, which contains genotypes with defeated genes to existing races (). The ineffectiveness of Yr27 could also be attributed to its presence in ‘Selkirk’ wheat that was widely cultivated in western Canada about 30 years ago, and selection pressure may have induced a gain in virulence towards Yr27 (McCallum & DePauw Citation2008).
Virulence on Yr6, Yr7, Yr8 and Yr9 is common in Saskatchewan (–). However, there are some exceptions as low disease severity in the most northern site (Prince Albert) was observed on most differentials, although this could be attributed to a reduced amount of inoculum with distance from the source (Mundt et al. Citation2009). The low disease severity on most of the differentials at Indian Head (eastern Saskatchewan) could be attributed to a greater distance from the PNW compared with other sites and the relatively narrow virulence spectrum of pathogen races/inoculum moving north on the ‘Puccinia Pathway’, and the relatively warmer temperatures at this site (Brar Citation2015; Brar & Kutcher Citation2016a). These genes were contained in Group 2 in the cluster analysis (). The severity of leaf rust was more common on differentials than stripe rust at Indian Head in most years as leaf rust can tolerate higher temperatures (data not shown; personal observation).
Gene Yr17 was not effective against Pst isolates evaluated under controlled conditions at the seedling stage (Brar & Kutcher Citation2016a), but was effective under field conditions at the adult plant stage ( and ). This could be because the gene may be linked to an HTAP gene (Milus et al. Citation2015), but the more likely reason is an interaction of Yr17 with temperature and the physiology of the plant (Hickey et al. Citation2012; Milus et al. Citation2015). The Yr17 gene conditions resistance at higher temperatures (>18°C) and high light intensity, and a susceptible reaction at lower temperatures under low light intensity. This may explain the resistant reaction of winter wheat under field conditions in western Canada as the average temperatures during summers are >18°C. However, the effectiveness of Yr17 at the seedling stage could be assessed by using virulent, avirulent and partially virulent races along with right incubation temperature and rating both first and second leaves, as suggested by Milus et al. (Citation2015). This gene clustered with other effective genes, Yr24 and Yr18 in Group 1 (). Wheat genotypes carrying the genes YrSu, Yr32 and those carried by ‘Nord Desprez’, ‘Produra’ and ‘Paha’ exhibited moderately susceptible to susceptible reactions at a few site-years, suggesting these genes will not be effective in Saskatchewan in the future (–). ‘Nord Desprez’ also clustered with Yr24/26, along with Yr17 and Yr28 representing a sub-group in Group 1, indicating partial resistance to existing races in pathogen populations (). Genes Yr32 and YrSu are clustered in Group 2, which contains differentials with genes ineffective against existing races of Pst in Saskatchewan ().
Similar to Yr17, gene Yr28 also confers resistance at warm temperatures as reported by Singh et al. (Citation2000). Like Yr17, Yr28 was ineffective to all existing Pst races at the seedling stage in indoor screening as reported by Brar and Kutcher (Citation2016a). The resistance of Yr28 under field conditions in Saskatchewan may be attributed to warm temperatures during the growing season. It is important to note that the genes Yr17 and Yr28 are susceptible to moderately susceptible in the PNW of the USA, as the growing season is cooler in that region compared with western Canada (Xi et al. Citation2015; http://striperust.wsu.edu). The use of Yr17 might be favoured in warm growing conditions as it carries additional resistance genes, such as Lr37 (against leaf rust), Sr38 (against stem rust), Pm4b (against powdery mildew), Cre5 (against cereal cyst nematode), Rkn3 (against root rot nematode) and an unknown resistance gene(s) to wheat blast (Briana and McIntosh Citation1994; Cruz et al. Citation2016). The gene Yr17 originated from the Triticum ventricosum 2NS chromosome and a wheat derivative ‘VPM 1', carrying 25–38 cM of the 2NS/AS translocation segment, which was widely used in wheat breeding programmes around the world due to associated agronomic and disease resistance merits (Briana & McIntosh Citation1994; Cruz et al. Citation2016).
The Canadian wheat varieties included in the differential set were either resistant or moderately resistant to stripe rust as most of them carry adult plant resistance genes, such as Yr18 or Yr29 (), which reduce infection efficiency, increase latent period and decrease lesion size resulting in the slow-rusting type reaction (Milus et al. Citation2015). This agrees with the results of Randhawa et al. (Citation2012), except for ‘AC Barrie’, which had variable reactions to stripe rust in Saskatchewan compared with Alberta and British Columbia. ‘AC Barrie’ is considered susceptible to stripe rust in Saskatchewan (Anonymous Citation2016); however in our study, under field conditions, it was assessed as resistant to moderately resistant in 2013, but moderately susceptible to susceptible in the following years (–). The resistant reaction of ‘AC Barrie’ at the three sites in 2013 may be attributed to lower disease/inoculum pressure in eastern Saskatchewan (particularly at Indian Head). However, in cluster analysis, ‘AC Barrie’ was clustered in Group 2 representing defeated genes/differentials (). Additionally, the lower similarity coefficient value of Group 2 could be indicative of its placement between resistant and susceptible reactions.
During the 2016 survey of wheat crops in Saskatchewan, 29 common and durum wheat varieties were identified (Brar & Kutcher Citation2016b). The most common durum wheat varieties were: ‘Strongfield’, ‘Transcend’, ‘Brigade’, ‘CDC Verona’ and ‘Eurostar’, and all these varieties are designated as moderately resistant or resistant to stripe rust in the Saskatchewan Seed Guide (Anonymous Citation2016). The most common bread wheat varieties were: ‘Cardale’, ‘AAC Brandon’, ‘Goodeve’, ‘Lillian’, ‘CDC Utmost’ and ‘Carberry’. ‘Lillian’ is designated as resistant to stripe rust as it carries at least three APR genes (Yr18, Yr36, Yr30), and ‘Carberry’ (Yr18 and Yr29), and ‘AAC Brandon’ (possibly Yr18+) are moderately resistant (Randhawa et al. Citation2013). Although ‘Cardale’ is resistant to other diseases, it is susceptible to stripe rust as it does not carry any known Yr gene(s) (Randhawa et al. Citation2013; Anonymous Citation2016). ‘Goodeve’ and ‘CDC Utmost’ carry Yr18 and are intermediate in resistance to stripe rust (Randhawa et al. Citation2013; Anonymous Citation2016).
The reaction of APR genes Yr18 (MR-MS) and Yr29 (MS-S) were similar at all sites in 2013, but disease severity was greater on Yr29 than Yr18 (). This might be attributed to the stronger effect of Yr18 as an APR gene (Martinez et al. 2001, cited in Hickey et al. Citation2012; Milus et al. Citation2015). As the genes condition partial resistance and are additive, the resistance is usually not sufficient in epidemic years as reported in 2011, when the stripe rust epidemic in western Canada was intense (Kutcher et al. Citation2012). Thus, the genes do not always provide complete protection in stripe rust ‘hot spots’ such as the PNW (Xi et al. Citation2015). In that case, pyramiding of Yr18 with other APRs or HTAP resistance genes might be beneficial under Canadian growing conditions. The HTAP gene Yr36 could be a good target to pyramid with Yr18, as that gene is linked to the Gpc-B1 gene for high grain protein content, favoured by wheat breeders (Uauy et al. 2006, cited in Randhawa et al. Citation2013).
All triticale varieties were highly resistant under field conditions at the four sites in 2014. ‘Brevis’, the only triticale variety tested in 2013 and 2016, was highly resistant (–). This is also evident in cluster analyses, where all triticale varieties are closely associated and placed in Group 1 along with genes Yr5, Yr15, YrSP and those carried by ‘Hybrid 46' (). This is in agreement with Randhawa et al. (Citation2012), who reported similar results for other varieties. However, contrary to Randhawa et al. (Citation2012), under controlled conditions, many Pst isolates were reported to be virulent on ‘Brevis’ at the seedling stage (Brar & Kutcher Citation2016a). Even single pustule isolates collected from Outlook in 2016 were virulent on ‘Brevis’ at the seedling stage (data not shown). These results can be attributed to the presence of unknown adult plant resistance genes in triticale germplasm in Canada.
Most of the winter wheat varieties or the advanced breeding lines derived from the cultivated varieties in western Canada carry Yr17 and some carry Yr10 (). Although Yr17 was effective at the adult plant stage in winter wheat, there was a subtle variation in infection response (evident from the second disease rating), which could be attributed to the fact that expression of this gene was also affected by genotypic background to some extent (Briana & McIntosh Citation1994). This intermediate infection response of 2–3 was similar to some winter wheat lines in the USA as reported by Milus et al. (Citation2015). They attributed this intermediate response on lines carrying Yr17 as the progressive increase in virulence of the pathogen population. This progressive increase was further speculated to be associated with heterozygosity at avirulence loci in the pathogen population, which produces intermediate infection levels on Yr17 wheat genotypes, as compared with pathogen isolates which are either homozygous virulent or homozygous avirulent. The Yr10 gene in advanced breeding lines is mainly derived from ‘AC Radiant’ as one of the parents (pedigree data not shown). The lines susceptible to stripe rust carry an unknown source of resistance (), which means that either the lines do not carry any resistance gene(s) or the gene(s) carried are not effective against existing races. The winter wheat genotypes ‘W541' and ‘W579' were resistant to stripe rust; however, the resistance gene(s) are unknown (). The lines carrying Yr10 were not effective against most of the existing Pst races in population in Saskatchewan, as indicated by the susceptible reactions of ‘W576', ‘W577' and ‘W578' (). This was also apparent from the susceptible reaction of ‘AC Radiant’ (Yr10) in Outlook in 2016 (Brar et al. Citation2017).
Table 4. Stripe rust disease severity score (0–5 scale)† of winter wheat varieties and advanced breeding lines at Swift Current in 2016. Data are the mean of three replicates.
The disease rating of winter wheat on both dates was very similar in terms of determining resistance or susceptibility of the lines, although there was a slight increase in severity the week following the first rating (). An important point to note is that the disease level on 10 June was quite high, which is unusual. Stripe rust on winter wheat in western Canada is usually not observed before late June to July. The early detection of stripe rust is possible when the disease overwinters. Overwintering of stripe rust has been reported from central Alberta in the past few years (Kumar et al. Citation2013). The early detection of high levels of stripe rust in early June in Saskatchewan could be evidence of overwintering.
In conclusion, some stripe rust resistance genes were still effective to confer resistance to existing races in Pst population Saskatchewan under field conditions. Some genes, which are completely ineffective to stripe rust isolates in indoor screening, such as Yr2, Yr17 and Yr28 (Brar & Kutcher Citation2016a), conferred a high level of resistance in the field. This is also true for the triticale variety ‘Brevis’. Infection on Yr17 may be affected by various factors, including temperature and light intensity, and physiological stage of the plant at infection. Thus, assessing effectiveness of this gene depends on one’s ability to distinguish avirulent and virulent pathogen population/races. The intermediate infection response on Yr17 might result in high infection levels in future years. Although indoor screening is an important part of pathogen population characterization, it should be coupled with field screening of differentials or varieties as the host–pathogen interaction in the field is controlled by various factors. The correlated response of genes or varieties from indoor and field screening (to natural inoculum under existing environmental conditions) is valuable to wheat breeders, pathologists, and growers. The effective seedling resistance genes can potentially be used in wheat breeding programs and should be pyramided with APR or HTAP genes to reduce the risk of resistance breakdown.
Acknowledgements
The authors gratefully acknowledge the technical assistance from Jiafeng Liu, Jessica Taylor, Juan Lobo, Mallory Dyck, Paulina Cholango, Gursahib Singh, Greg Ford and Everett Boots.
Additional information
Funding
References
- Anonymous. 2016. Varieties of grain crops 2016. Government of Saskatchewan; [ accessed 2016 Oct 7]. http://publications.gov.sk.ca/documents/20/96889-Varieties%20of%20Grain%20Crops%202017.pdf
- Brar GS. 2015. Population structure of Puccinia striiformis f. sp. tritici, the cause of wheat stripe rust, in western Canada [M.Sc. Thesis]. Saskatoon (SK): University of Saskatchewan.
- Brar GS, Kutcher HR. 2016a. Race characterization of Puccinia striiformis f. sp. tritici, the cause of wheat stripe rust, in Saskatchewan and southern Alberta and virulence comparison with races from the United States. Plant Dis. 100:1744–1753.
- Brar GS, Kutcher HR. 2016b. SK disease situation report. 2016 SK Plant Disease Committee Meeting; November 29; Saskatoon, SK.
- Brar GS, Singh G, Cholango-Martinez P, Lobo J, Nabetani K, Knox R, Kutcher HR. 2017. Stripe rust of wheat in Saskatchewan in 2015 and 2016. Can Plant Dis Surv. 97:142–143.
- Briana HS, McIntosh RA. 1994. Characterization and origin of rust and powdery mildew resistance genes in VPM1 wheat. Euphytica. 76:53–61.
- Chen XM. 2005. Epidemiology and control of stripe rust [Puccinia striiformis f. sp. tritici] on wheat. Can J Plant Pathol. 27:314–337.
- Cruz CD, Peterson GL, Bockus WW, Kankanala P, Dubcovsky J, Jordan KW, Akhunov CF, Baldelomar FD, Valent B. 2016. The 2NS translocation from Aegilops ventricosa confers resistance to the Triticum pathotype of Magnaporthe oryzae. Crop Sci. 56:990–1000.
- Hickey LT, Wilkinson PM, Knight CR, Godwin ID, Kravchuk OY, Aitken EAB, Bansal UK, Bariana HS, DeLacy IH, Dieters MJ. 2012. Rapid phenotyping for adult-plant resistance to stripe rust in wheat. Plant Breed. 131:54–61.
- Holtz MD, Kumar K, Xi K. 2013. Virulence phenotypes of Puccinia striiformis in Alberta from 2009–2011. Can J Plant Pathol. 35:241–250.
- Kumar K, Holtz MD, Xi K, Turkington TK. 2013. Overwintering potential of the stripe rust pathogen (Puccinia striiformis) in central Alberta. Can J Plant Pathol. 35:304–314.
- Kutcher H, Randhawa H, Puchalski B, Wogsberg S, Graf R, Gaudet D. 2012. The 2011 stripe rust epidemic in western Canada. In: Chen WQ, editor. Proceedings of the 13th International Cereal Rusts and Powdery Mildews Conference; Aug 28–Sep 1; Beijing, China: China Agricultural Science and Technology Publishing House. (Abstr.), p. 22.
- McCallum BD, DePauw RM. 2008. A review of the wheat cultivars grown in the Canadian prairies. Can J Plant Sci. 88:649–677.
- McIntosh RA. 2010. What do we mean by virulence? Is it time to move on from 1920? [ accessed 2016 Oct 1]. http://www.globalrust.org/sites/default/files/mcintosh_race.pdf.
- Milus EA, Kristensen K, Hovmoller MS. 2009. Evidence for increased aggressiveness in a recent widespread strain of Puccinia striiformis f. sp. tritici causing stripe rust of wheat. Phytopathology. 99:89–94.
- Milus EA, Lee KD, Brown-Guedira G. 2015. Characterization of stripe rust resistance in wheat lines with resistance gene Yr17 and implications for evaluating resistance and virulence. Phytopathology. 105:1123–1130.
- Mundt CC, Sackett KE, Wallace LD, Cowger C, Dudley JP. 2009. Aerial dispersal and multiple-scale spread of epidemic disease. EcoHealth. 6:546–552.
- Peterson RF, Campbell AB, Hannah AE. 1948. A diagrammatic scale estimating rust intensity of leaves and stem of cereals. Can J Res Sec C. 26:496–500.
- R Core Team. 2016. R: a language and environment for statistical computing. Vol. 2016. Vienna (Austria): R Foundation for Statistical Computing.
- Randhawa H, Asif M, Pozniak C, Clarke JM, Graf RJ, Fox SL, Humphreys G, Knox RE, DePauw RM, Singh AK, et al. 2013. Application of molecular markers to wheat breeding in Canada. Plant Breed. 132:458–471.
- Randhawa H, Puchalski BJ, Frick M, Goyal A, Despins T, Graf RJ, Laroche A, Gaudet DA. 2012. Stripe rust resistance among western Canadian spring wheat and triticale varieties. Can J Plant Sci. 92:713–722.
- Singh RP, Nelson JC, Sorrells ME. 2000. Mapping Yr28 and other genes for resistance to stripe rust in wheat. Crop Sci. 40:1148–1155.
- Su H, Conner RL, Graf RJ, Kuzyk AD. 2003. Virulence of Puccinia striiformis f. sp. tritici, cause of stripe rust on wheat, in western Canada from 1984 to 2002. Can J Plant Pathol. 25:312–319.
- Wan AM, Chen XM. 2012. Virulence, frequency, and distribution of races of Puccinia striiformis f. sp. tritici and P. striiformis f. sp. hordei identified in the United States in 2008 and 2009. Plant Dis. 96:67–74.
- Wellings C, Snyman JE, Robake JE, Bansal U. 2012. Stripe rust resistance in triticale in Australia: pathogen change exposes genetic vulnerability. In: Chen WQ, editor. Proceedings of the 13th International Cereal Rusts and Powdery Mildews Conference; Aug 28–Sept 1; Beijing, China: China Agricultural Science and Technology Publishing House. (Abstr.), p. 38–39.
- Wellings CR, Singh RP, McIntosh RA, Pretorius ZA. 2004. The development and application of near isogenic lines for the stripe (yellow) rust pathosystem. In: Proceedings of the 11th International Cereal Rust and Powdery Mildew Conference; August 22–27; Norwich, UK. [Abstr.] A1.39, Cereal Rusts and Powdery Mildew Bull.
- Xi K, Kumar K, Holtz MD, Turkington TK, Chapman B. 2015. Understanding the development and management of stripe rust in central Alberta. Can J Plant Pathol. 37:21–39.
