Abstract
There is abundant prior published information on antibiosis, one of the most studied biocontrol mechanisms for plant pathogens. Depending on their concentration, antibiotics can have various effects on target organisms, a phenomenon known as hormesis. Under complex soil conditions where subinhibitory concentrations of antibiotics are thought to prevail, the mechanism of action responsible for disease reduction through antibiosis is often overlooked, where it is generally assumed that biocontrol occurs through mortality of the pathogen. This concept of dose-dependent response must be taken into account to better understand antibiosis and how it can contribute to biocontrol in various ways. This review aims to focus on how antibiotics can operate and persist in soil, act as signalling molecules and enable interactions between soil microbial communities. It also aims to pinpoint specific examples where low, subinhibitory concentrations of antibiotics, which are widespread under natural soil conditions, can reduce disease symptoms by modulating the pathogen’s transcriptome, rather than by toxicity and death. This highlights the need to better understand and characterize as much as possible the mode of action of antibiosis under various complex environmental conditions, in order to anticipate future development of resistance and loss of efficiency through changes in environmental conditions.
Résumé
Beaucoup d’information a déjà été publiée sur l’antibiose, un des mécanismes de lutte biologique les plus étudiés pour combattre les agents pathogènes des plantes. Selon leur concentration, les antibiotiques peuvent avoir divers effets sur les organismes ciblés, un phénomène connu sous le nom d’« hormèse ». Dans des conditions complexes de sol où des concentrations subinhibitrices d’antibiotiques soi-disant prévalent, le mécanisme responsable de la réduction de la maladie par antibiose est souvent négligé, alors qu’on assume généralement que la lutte biologique se manifeste par la mort de l’agent pathogène. Ce concept de réaction dose-dépendante doit être considéré pour mieux comprendre l’antibiose et comment elle peut contribuer, de différentes façons, à la lutte biologique. Cet article vise à attirer l’attention sur la capacité qu’ont les antibiotiques d’agir et de persister dans le sol, d’agir en tant que molécules de signalisation et de permettre les interactions entre les communautés microbiennes du sol. Il vise également à mettre en évidence des exemples précis dans lesquels des concentrations subinhibitrices d’antibiotiques, qui sont presque omniprésentes naturellement dans le sol, peuvent atténuer les symptômes de la maladie par modulation du transcriptome de l’agent pathogène plutôt que par toxicité et, enfin, sa mort. Cela met en évidence le besoin de mieux comprendre et de caractériser autant que possible le mode d’action de l’antibiose sous différentes conditions environnementales complexes afin d’anticiper les futurs développements de la résistance et la perte d’efficacité en raison de modifications des conditions environnementales.
Introduction
The production of antibiotics by microorganisms involved in the biological control of plant pathogens has long been seen as an important contributing mechanism to the reduction of disease symptoms, particularly under soil conditions (Fravel Citation1988; Handelsman and Stabb Citation1996; Whipps Citation2001; Raaijmakers et al. Citation2002; Haas and Défago Citation2005). In this context, the term antibiosis relates to this mechanism through which microbially produced antibiotics are able to control plant pathogens and the diseases they cause. Many other biocontrol mechanisms, including competition for space and nutrients, induction of a systemic resistance in plants, and parasitism, have also been identified and can act in combination with the production of antibiotics by a particular biocontrol agent (Handelsman and Stabb Citation1996). Several soilborne microorganisms are well known for their biocontrol abilities, including numerous strains of fluorescent Pseudomonas and Bacillus bacteria (Weller Citation1988) and Trichoderma species (Benítez et al. Citation2004), among others. Some also have the ability to promote plant growth in addition to suppressing diseases (Fernando et al. Citation2006; Siddiqui Citation2006), making them even more interesting from a commercial point of view.
In complex soil ecosystems, it can be difficult to pinpoint an exact mechanism of action to explain disease suppression as several organisms are interacting with the plant and with each other (Borneman and Becker Citation2007; Janvier et al. Citation2007). The root-soil interface, and adjacent rhizosphere in particular, is an intricate environment where the plant can interact with and modulate its surroundings through the production of exudates and hormones (Whipps Citation2001; Oldroyd Citation2013). It constitutes a very competitive nutrient-rich area of the ecosystem where bacteria, fungi (including mycorrhizae), oomycetes, viruses, archaea, nematodes, invertebrates and many others co-exist (Lavelle Citation1997; Philippot et al. Citation2013). The shift in balance in any of these constituents can have consequences on plant health as the composition of beneficial, neutral and deleterious organisms changes. It is with this image in mind that we see how the presence of antibiotics in this environment, of varying types, localizations and concentrations, can be difficult to clearly link to disease reduction. However, in order to favour the development of biological control as an economically and environmentally sound method of disease reduction, it is crucial that the mechanisms involved are studied and understood in order to favour the conditions that will maintain that treatment’s efficiency. Knowing how a particular biocontrol agent is able to suppress a pathogen’s ability to cause disease can assist in anticipating the possible development of resistance. It also provides key information to prevent other components of the ecosystem interfering with the interaction leading to biocontrol. For example, it may provide guidance to better control the impact that abiotic factors such as temperature, soil type, water, nutrient and mineral content, may have on treatment efficiency.
Although there is an abundance of literature demonstrating that the production of a given antibiotic is essential to biocontrol, very few studies describe how the antibiotic in question actually operates, especially in soil. In general, the assumption is made that the disease pressure is lifted by reducing populations of the pathogen through bactericidal or fungicidal activity. Raaijmakers and Mazzola (Citation2012) review this elegantly by stating that antibiotics are often assumed to act as weapons, when in reality few studies have proven that antibiotics are produced in soil, and often in quantities not sufficient to induce pathogen mortality.
The goal of this paper is not to review all instances of biocontrol where antibiosis occurs, but rather to focus on how these antibiotics can operate and persist in soil, and pinpoint specific examples where low concentrations of antibiotics, which are thought to be widespread under natural soil conditions (Davies et al. Citation2006), can contribute to biocontrol of soil pathogens by means other than direct killing or toxicity.
Defining antibiotics and hormesis
Antibiotics are low-molecular weight compounds that can include volatile compounds, lytic agents, enzymes and other toxic compounds (Fravel Citation1988). Waksman (Citation1947) defined antibiotics as: ‘inhibiting the growth or the metabolic activities of bacteria and other microorganisms by a chemical substance of microbial origin’. A vital part of understanding the effects of antibiotics on a target microorganism is accounting for the concentration to which it is exposed. It has been shown that antibiotics that are applied at a concentration below the deadly minimal inhibitory concentration (MIC) can increase or decrease transcriptional gene expression of up to 5% of bacterial promoters (Goh et al. Citation2002) and can specifically target certain processes such as colonization capacity and virulence (Li et al. Citation2016). Here, virulence is defined as the relative capacity to cause symptoms, or a measure of the degree of pathogenicity, itself defined as the ability to cause disease (D’Arcy et al. Citation2001). This differential dose-responsive physiological effect is termed hormesis (). It has been put forward that this concentration-dependent effect is due to an adaptation of the targeted organism to stress and disruption of homeostasis, which can result in a beneficial or harmful response (Calabrese and Baldwin Citation2002). From a biocontrol point of view, a beneficial response for the pathogen is harmful for disease control (such as an increase in antibiotic-degrading compounds (Schouten et al. Citation2004)), while a harmful response for the pathogen will improve disease control.
Fig. 1 Visualization of hormesis, a dose-dependent phenomenon where high concentrations of an antibiotic can result in cell death in the target organism, while low concentrations, below the minimal inhibitory concentration (MIC), induce changes in gene expression (GE).
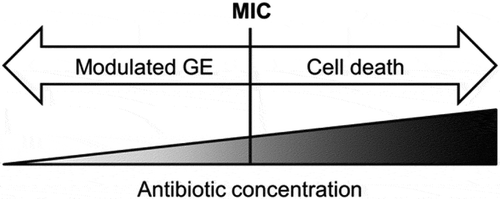
Although many of the studies on the hormetic response to antibiotics focus largely on microorganisms that are relevant and problematic in a clinical setting, under very controlled conditions, these types of responses are also expected to occur under environmental conditions, especially in soil (Thomashow et al. Citation2008; Raaijmakers and Mazzola Citation2012). In cases of biocontrol where the production of an antibiotic was proven to be linked to disease reduction, but where no significant reduction in pathogen populations occurred, it becomes essential to verify if subinhibitory concentrations of the antibiotic resulted in physiological changes in the pathogen that reduced its capacity to cause disease.
Antibiotics in plant agroecosystems
In order to gain a mechanistic understanding of how antibiotics can contribute to disease control in soil, several questions must be asked: (1) where and how are the antibiotics produced and found? (this can be very broad, such as an area of soil, or very specific, such as a small root portion); (2) which antibiotics are being produced?; (3) which microorganisms are producing them?; (4) to what extent is a particular targeted pathogen exposed, in terms of antibiotic concentration and composition? Keeping in mind that several microorganisms can produce multiple antibiotics, and are part of a diverse community of other microorganisms (Thomashow et al. Citation2008), this last question is the most complex and cannot be fully discussed in the context of this review. It represents the most challenging area of research moving forward in our understanding of the hormetic effect associated with antibiotics. Evidently, there is also the question of whether a particular pathogen is susceptible or resistant to a given antibiotic, but for the purpose of this review on instances where disease is reduced, we will focus on situations where susceptibility prevails.
Sources of antibiotics in soil
Firstly, where could antibiotics be found in soil? The production of antibiotics requires adequate and sufficient sources of carbon, nitrogen and other nutrients. The relatively poor nutrient content of bulk soils results in low microbial metabolic activity, whereas the higher availability of substrates in the richer regions of the rhizoplane and the rhizosphere make them likely areas for antibiotic production, in addition to other metabolic activities (Thomashow et al. Citation2008). The rhizosphere is also one of the most dynamic and complex environments hosting several multitrophic interactions (Philippot et al. Citation2013). In this environment, plants themselves produce rhizodeposits which can contain fungicidal and bactericidal compounds, such as phytoalexins, to select particular microbial communities which are able to thrive despite the presence of these molecules (Bais et al. Citation2006). Other sources of antibiotics in the environment include pharmaceutical antibiotics from animal or human waste (Thiele-Bruhn Citation2003; Kemper Citation2008). However, the major producers of antibiotics in the rhizosphere are the microorganisms present, whether they are pathogenic or beneficial.
Antibiotic production is part of the secondary metabolism of microorganisms, which produce metabolites that are not essential for their growth, but can have implications in survival, fitness and competitiveness. It occurs during late growth phases, and as such is dependent on environmental conditions (Martin and Demain Citation1980) and requires a critical mass of signal-producing cells (quorum-sensing) for its initiation (Haas and Keel Citation2003; Williams Citation2007). This suggests that even in an environment favouring antibiotic production, such as the rhizosphere, production is limited to specific areas or pockets where all the conditions and the threshold populations needed are attained. This means that the concentration of a particular antibiotic can vary greatly, even within a distance of a few millimetres of roots, depending on the microorganisms that are present in a specific area. Moreover, as antibiotic extraction and detection from soil requires several grams of sample (Thomashow et al. Citation2008), precise localized measurements of antibiotic concentrations are very difficult to obtain. Even though antibiotics are non-essential molecules for growth, natural selection pressures have been shown to generally conserve their production. For example, in the pathogen Cephalosporium gramineum Y. Nisik. & Ikata, even though its pathogenicity is not dependent upon it, evidence suggests that antibiotic production may offer other advantages in ecological niches (Bruehl et al. 1969).
In the context of disease control, it has been shown that soils which are suppressive to disease often owe their activity to the presence of an array of plant-beneficial microorganisms with diverse microbial activities, including nutrient cycling, degradation of harmful compounds, but also and most importantly, production of antibiotics (Janvier et al. Citation2007). As stated above, specific suppressiveness, or biological control, of particular diseases, has also been associated with the production of antibiotics by beneficial microorganisms. The identity of these antibiotics has become clearer with time, and include a diverse array of molecules and producing microorganisms (), such as phenazines (phenazine-1-carboxylic acid (PCA), phenazine-1-carboxamide (PCN), pyocyanin (PYO)), 2,4-Diacetylphloroglucinol (DAPG), iturin A, bacillomycins, gliotoxins, pyoluteorins, pyrrolnitrins, etc. Several genera of microorganisms have also been recognized as multiple producers of antibiotics, particularly bacteria in the genus Pseudomonas (Raaijmakers et al. Citation2002).
Table 1. Some antibiotics involved in biocontrol of plant pathogens in soil and the microbial producers.
The fate of antibiotics in soil
A major influence on the lifespan of antibiotics in soil is their degradation by soil microorganisms (Gottlieb Citation1976; Costa et al. Citation2015), which in some cases can even utilize them as nutrients (Dantas et al. Citation2008). Inactivation might also occur through adsorption to soil particles depending on pH, water content, solubility, and the composition of soil in clay, sand, silt, minerals and organic matter (ter Laak et al. Citation2006; Wegst-Uhrich et al. Citation2014). This results in relatively low half-lives (a few days) for some known antibiotics of biocontrol interest (), highlighting the importance of consistent production to ensure their sufficient presence in soil to be effective against plant pathogens. While several studies following the fate of clinical antibiotics have been done to estimate their persistence in the environment (Thiele-Bruhn Citation2003), few have analysed the fate of antibiotics produced by soil microorganisms involved in plant disease control.
The persistence of antibiotics in soil is evidently dependent on their chemical structure and physicochemical properties, as it affects their mobility and sorption in soil and water (Thiele-Bruhn Citation2003). Furthermore, adsorption to soil particles does not always inactivate antibiotics, as has been shown for two antibiotics used in the animal production industry (tetracycline and tylosin), which remained active despite being strongly adsorbed to clay particles (Chander et al. Citation2005). This highlights how information about antibiotic persistence in soil has important implications with regards to its role in the soil ecosystem.
The function of antibiotics in soil
Beyond the toxic effects of antibiotics observed at high concentrations, it has become clear that at subinhibitory concentrations, these molecules can operate in diverse manners. Under soil conditions, where the concentration of antibiotics is very likely below the MIC (Davies et al. Citation2006; Martínez Citation2008), antibiotics are thought to have natural functions which are mostly involved in signalling and microbial community interactions (Davies Citation2006; Romero et al. Citation2011; Raaijmakers and Mazzola Citation2012), as well as communication with plants (Venturi and Keel Citation2016). For many antibiotics, these exact signalling roles remain a mystery, but there are some for which some information has surfaced.
Fluorescent Pseudomonas spp. offer many examples of these signalling functions. Studies have shown that pyoluteorin and DAPG act as signals for their own auto-induction (Schnider-Keel et al. Citation2000; Brodhagen et al. Citation2004). Furthermore, a recent study has discovered that an intermediate in the production of DAPG, phloroglucinol, has a broad effect on the transcriptome of P. protegens Pf5 (over 200 genes) depending on its concentration, and also regulates the production of pyoluteorin (Clifford et al. Citation2016). In addition to modulating microbial responses, DAPG (along with Fusarium-produced zearalenone) has also been shown to affect plant roots, significantly altering the production (both in quantity and composition) of root exudates in several crops, which has ecological implications, particularly for soil microbial community composition (Phillips et al. Citation2004). Other well studied Pseudomonas-produced antibiotics are the phenazines. These redox-active antibiotics have been implicated in the regulation of biofilm formation (Mavrodi et al. Citation2013), and one type of phenazine, pyocyanin, has been found able to bind DNA (Das et al. Citation2013), perhaps suggesting a direct role in transcriptional modulation.
In addition to these specific examples, the general presence of antibiotics in soil has been thought to contribute to soil suppressiveness, where plants do not develop disease symptoms despite the presence of pathogens and suitable conditions for disease development. This lack of activity from soil pathogens in suppressive soils was linked to their state of biostasis (Garbeva et al. Citation2011) (fungistasis or bacteriostasis), where growth is prevented, particularly spore germination and the growth of fungal hyphae, without killing the microorganism (Watson and Ford Citation1972; Agrios Citation1978). It is suggested that the presence of antibiotics in soil, linked to a depletion of nutrients for pathogens, could explain this stasis (Garbeva et al. Citation2011).
Cases of biocontrol of plant pathogens through reduced virulence
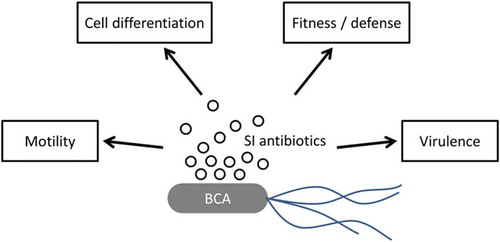
The idea of suppressing pathogens by targeting their virulence has been gaining traction in recent years, whether through the production of subinhibitory concentrations of antibiotics, or inhibiting intercellular signals (quorum-quenching) (Mahmoudi et al. Citation2011; Zhu and Kaufmann Citation2013), in a variety of different environments including clinical (Gerber et al. Citation2008; Goneau et al. Citation2015), animal (Haas and Grenier Citation2016), wastewater (Bruchmann et al. Citation2013) and to a lesser extent, soil. In addition to microbial antibiotics which can contribute to biocontrol, it is also interesting to note that plants themselves can also produce compounds which specifically target bacterial virulence (Silva et al. Citation2016). Several physiological processes can be altered by the presence of subinhibitory concentrations of antibiotics which result in reduced disease-causing abilities in a targeted plant pathogen (), including the suppression of motility, cell differentiation, fitness and specific virulence factors. Here we present specific studies which sought to characterize the mechanisms of action involved in the biocontrol of plant diseases operating through the production of antibiotics, and resulting in reduced pathogen virulence through one or several of these repressed physiological processes.
Fig. 2 (Colour online) How subinhibitory (SI) concentrations of antibiotics can achieve disease reduction in soil through the alteration of several physiological aspects in a given phytopathogen by modulating gene expression. Examples include a reduction in (1) motility, which for example is essential for virulence in Pseudomonas syringae (Ichinose et al. Citation2003), (2) cell differentiation, which encompasses the formation of secondary structures such as hyphae and spores which are essential to pathogen propagation and survival, (3) fitness (competitiveness) or defence against predators, which includes the formation of biofilms which can protect cells from the environment (Davey and O’Toole Citation2000) and (4) virulence, which will result in reduced symptom severity.
Control of common scab of potato through phenazine-1-carboxylic acid (PCA) production
A good example of antibiosis through subinhibitory antibiotic concentrations (in this case PCA), is the control of common scab of potato by Pseudomonas fluorescens LBUM223 under controlled (Arseneault et al. Citation2013) and field conditions (Arseneault et al. Citation2015). In these experiments, PCA production by LBUM223 was essential to disease control (demonstrated by developing an isogenic phzC− mutant incapable of producing PCA), and the mechanisms responsible for disease reduction were further investigated. The geocaulosphere soil populations (adjacent to the potato tuber) of the pathogen, Streptomyces scabies, and the expression of a gene (txtA) essential to its production of a major pathogenicity and virulence factor required for tuber symptom development, thaxtomin A, were studied. The results showed that the geocaulosphere soil populations of the pathogen did not decrease in the presence of P. fluorescens LBUM223, but the expression of txtA was reduced by at least half. As other key genes involved in virulence and infection might also be transcriptionally altered by PCA, the same research team also performed a whole-transcriptome RNA-seq analysis of S. scabies exposed to P. fluorescens LBUM223, its PCA-deficient mutant, and pure PCA to determine how PCA affects gene expression in the pathogen (Arseneault et al., unpublished). Results showed that PCA (pure or LBUM223-produced) altered a significant portion of S. scabies’ transcriptome (14% and 12% of genes, respectively) while the PCA-deficient mutant had virtually no effect. Furthermore, the genes affected by PCA are mainly secondary metabolism-related, affecting both virulence and sporulation, thereby decreasing the risk of the pathogen being able to sustain itself in soil over long periods and affecting its capacity to infect potato tubers.
Control of Pythium spp. with 2,4-diacetylphloroglucinol (DAPG)
A study by de Souza et al. (Citation2003) sought to characterize the specific effects of DAPG on 14 different strains of Pythium spp., for which its production by fluorescent Pseudomonas was shown as essential for biocontrol in planta. The goal was to identify the mode of action by which DAPG can affect different Pythium spp. throughout their life cycle, from mycelium, zoosporangia, zoospore cysts and zoospores, in order to reduce disease severity. A range of DAPG concentrations was tested on the different cell structures and varying sensitivity among the different strains of Pythium was shown. Among the Pythium spp. structures, zoospores were identified as the most sensitive to the antibiotic. Interestingly, while mycelial growth of the pathogen occurred at low antibiotic concentrations, it resulted in the disorganization of cellular components in the hyphal tips. Although not validated in soil, these findings are interesting as they account for the different conditions under which DAPG may operate, including varying life stages of the pathogen, varying pH and the presence or absence of wheat root exudates, and offer a comprehensive view of the possible mechanisms of action of this antibiotic.
Biocontrol through reduced virulence where the responsible antibiotic(s) was not identified
In some studies, the biocontrol of a given plant pathogen clearly occurs to some extent through reduction of the pathogen’s virulence; however, the exact molecule(s) responsible for this effect were not identified. This was the case for an arbuscular mycorrhizal fungus able to down-regulate gene expression involved in the production of trichothecene toxins by a Fusarium sambucinum pathogen (Ismail et al. Citation2011). It was also demonstrated that Brevibacillus laterosporus was able to reduce symptoms of brown strip of rice (caused by Acidovorax avenae) through reduction of biofilm formation, motility and expression of virulence genes (Kakar et al. Citation2014). Future research should determine the active molecules responsible for these effects.
Concluding remarks
The quest to understand the roles and mechanisms of action of antibiotics under natural soil conditions, particularly with regards to the control of crop plant diseases, remains a challenge. This paper aimed at bringing to light several notions that have enabled clinical researchers to advance their understanding of antibiotics and move forward in finding solutions for the antibiotic-resistant pathogen strains plaguing them, so that as plant pathologists we may apply them in our research and adopt a wider ecosystem-based approach to understand antibiosis in soils. We have access to ever-evolving analytical and molecular tools which will enable us to better characterize dose-dependent responses to antibiotics in plant pathogens and identify the associated global transcriptional patterns, providing clues as to the metabolic pathways targeted. This will be essential in order to better anticipate the future effects of a biocontrol treatment reliant upon antibiotic production, in particular with regards to the development of resistance and the looming threat of climate change which will modulate soil and environmental conditions. Gaining knowledge on how specific antibiotics can modulate pathogen virulence under agricultural soil conditions is the first step that will enable the filling in of pieces of the larger picture that constitutes soil health and suppressiveness to plant diseases in agroecosystems.
Acknowledgements
We would like to thank the Canadian Phytopathological Society (CPS) for the opportunity to present at the 2016 conference.
Additional information
Funding
References
- Agrios GN. 1978. Plant pathology. 2nd ed. New York (NY): Academic Press; p. 673–686.
- Arseneault T, Goyer C, Filion M. 2013. Phenazine production by Pseudomonas sp. LBUM223 contributes to the biological control of potato common scab. Phytopathology. 103:995–1000.
- Arseneault T, Goyer C, Filion M. 2015. Pseudomonas fluorescens LBUM223 increases potato yield and reduces common scab symptoms in the field. Phytopathology. 105:1311–1317.
- Asaka O, Shoda M. 1996. Biocontrol of Rhizoctonia solani damping-off of tomato with Bacillus subtilis RB14. Appl Environ Microbiol. 62:4081–4085.
- Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM. 2006. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol. 57:233–266.
- Benítez T, Rincón AM, Limón MC, Codón AC. 2004. Biocontrol mechanisms of Trichoderma strains. Int Microbiol. 7:249–260.
- Borneman J, Becker JO. 2007. Identifying microorganisms involved in specific pathogen suppression in soil. Ann Rev Phytopathol. 45:153–172.
- Brodhagen M, Henkels MD, Loper JE. 2004. Positive autoregulation and signaling properties of pyoluteorin, an antibiotic produced by the biological control organism Pseudomonas fluorescens Pf-5. Appl Environ Microbiol. 70:1758–1766.
- Bruchmann J, Kirchen S, Schwartz T. 2013. Sub-inhibitory concentrations of antibiotics and wastewater influencing biofilm formation and gene expression of multi-resistant Pseudomonas aeruginosa wastewater isolates. Environ Sci Pollut Res Int. 20:3539–3549.
- Bruehl GW, Millar RL, Cunfer B. 1969. Significance of antibiotic production by Cephalosporium gramineum to its saprophytic survival. Can J Plant Sci. 49:235–246.
- Calabrese EJ, Baldwin LA. 2002. Defining hormesis. Human Exp Toxicol. 21:91–97.
- Chander Y, Kumar K, Goyal SM, Gupta SC. 2005. Antibacterial activity of soil-bound antibiotics. J Environ Qual. 34:1952–1957.
- Chin-A-Woeng TFC, Bloemberg GV, van der Bij AJ, van der Drift KMGM, Schripsema J, Kroon B, Scheffer RJ, Keel C, Bakker PAHM, Tichy H-V, et al. 1998. Biocontrol by phenazine-1-carboxamide-producing Pseudomonas chlororaphis PCL1391 of tomato root rot caused by Fusarium oxysporum f. sp. radicis-lycopersici. Mol Plant Microbe Interact. 11:1069–1077.
- Clifford JC, Buchanan A, Vining O, Kidarsa TA, Chang JH, McPhail KL, Loper JE. 2016. Phloroglucinol functions as an intracellular and intercellular chemical messenger influencing gene expression in Pseudomonas protegens. Environ Microbiol. 18:3296–3308.
- Costa KC, Bergkessel M, Saunders S, Korlach J, Newman DK. 2015. Enzymatic degradation of phenazines can generate energy and protect sensitive organisms from toxicity. mBio. 6:e01520–15.
- D’Arcy CJ, Eastburn DM, Schumann GL. 2001. Illustrated glossary of plant pathology. The plant health instructor. St. Paul (MN): The American Phytopathological Society. doi:10.1094/PHI-I-2001-0219-01
- Dantas G, Sommer MOA, Oluwasegun RD, Church GM. 2008. Bacteria subsisting on antibiotics. Science. 320:100–103.
- Das T, Kutty SK, Kumar N, Manefield M. 2013. Pyocyanin facilitates extracellular DNA binding to Pseudomonas aeruginosa influencing cell surface properties and aggregation. PLoS One. 8:e58299.
- Davey ME, O’Toole GA. 2000. Microbial biofilms: from ecology to molecular genetics. Microbiol Mol Biol Rev. 64:847–867.
- Davies J. 2006. Are antibiotics naturally antibiotics? J Ind Microbiol Biotechnol. 33:496–499.
- Davies J, Spiegelman GB, Yim G. 2006. The world of subinhibitory antibiotic concentrations. Curr Opin Microbiol. 9:445–453.
- de Souza JT, Arnould C, Deulvot C, Lemanceau P, Gianinazzi-Pearson V, Raaijmakers JM. 2003. Effect of 2,4-diacetylphloroglucinol on Pythium: cellular responses and variation in sensitivity among propagules and species. Phytopathology. 93:966–975.
- Dong H, Gao B, Wang W, Fan L, Xu Y, Zhang X, Cao C. 2011. Experimental study on the determination and degradation of pyoluteorin in soil via CE with Soxhlet’s extraction and field-amplified sample stacking. Chromatographia. 73:609–612.
- Fernando WGD, Nakkeeran S, Zhang Y. 2006. Biosynthesis of antibiotics by PGPR and its relation in biocontrol of plant diseases. In: Siddiqui ZA, editor. PGPR: Biocontrol and Biofertilization. Dordrecht (NL): Springer; p. 67–109.
- Fravel DR. 1988. Role of antibiosis in the biocontrol of plant diseases. Annu Rev Phytopathol. 26:75–91.
- Garbeva P, Hol WHG, Termorshuizen AJ, Kowalchuk GA, De Boer W. 2011. Fungistasis and general soil biostasis – a new synthesis. Soil Biol Biochem. 43:469–477.
- Gerber M, Walch C, Löffler B, Tischendorf K, Reischl U, Ackermann G. 2008. Effect of sub-MIC concentrations of metronidazole, vancomycin, clindamycin and linezolid on toxin gene transcription and production in Clostridium difficile. J Med Microbiol. 57:776–783.
- Goh E-B, Yim G, Tsui W, McClure J, Surette MG, Davies J. 2002. Transcriptional modulation of bacterial gene expression by subinhibitory concentrations of antibiotics. Proc Nat Acad Sci USA. 99:17025–17030.
- Goneau LW, Hannan TJ, MacPhee RA, Schwartz DJ, Macklaim JM, Gloor GB, Razvi H, Reid G, Hultgren SJ, Burton JP. 2015. Subinhibitory antibiotic therapy alters recurrent urinary tract infection pathogenesis through modulation of bacterial virulence and host immunity. MBio. 6:e00356–15.
- Gottlieb D. 1976. The production and role of antibiotics in soil. J Antibiot. 29:987–1000.
- Haas B, Grenier D. 2016. Impact of sub-inhibitory concentrations of amoxicillin on Streptococcus suis capsule gene expression and inflammatory potential. Pathogens. 5:37.
- Haas D, Défago G. 2005. Biological control of soil-borne pathogens by fluorescent Pseudomonads. Nat Rev Microbiol. 3:307–319.
- Haas D, Keel C. 2003. Regulation of antibiotic production in root-colonizing Peudomonas spp. and relevance for biological control of plant disease. Annu Rev Phytopathol. 41:117–153.
- Handelsman J, Stabb EV. 1996. Biocontrol of soilborne plant pathogens. Plant Cell. 8:1855–1869.
- Hu W, Gao Q, Hamada MS, Dawood DH, Zheng J, Chen Y, Ma Z. 2014. Potential of Pseudomonas chlororaphis subsp. aurantiaca strain Pcho10 as a biocontrol agent against Fusarium graminearum. Phytopathology. 104:1289–1297.
- Ichinose Y, Shimizu R, Ikeda Y, Taguchi F, Marutani M, Mukaihara T, Inagaki Y, Toyoda K, Shiraishi T. 2003. Need for flagella for complete virulence of Pseudomonas syringae pv. tabaci: genetic analysis with flagella-defective mutants ΔfliC and ΔfliD in host tobacco plants. J Gen Plant Pathol. 69:244–249.
- Ismail Y, McCormick S, Hijri M. 2011. A fungal symbiont of plant-roots modulates mycotoxin gene expression in the pathogen Fusarium sambucinum. PLoS One. 6:e17990.
- Janvier C, Villeneuve F, Alabouvette C, Edel-Hermann V, Mateille T, Steinberg C. 2007. Soil health through soil disease suppression: which strategy from descriptors to indicators? Soil Biol Biochem. 39:1–23.
- Kakar KU, Nawaz Z, Cui Z, Almoneafy AA, Zhu B, Xie G-L. 2014. Characterizing the mode of action of Brevibacillus laterosporus B4 for control of bacterial brown strip of rice caused by A. avenae subsp. Avenae RS-1. World J Microbiol Biotechnol. 30:469–478.
- Keel C, Schnider U, Maurhofer M, Voisard C, Laville J, Burger U, Wirthner P, Haas D, Défago G. 1992. Suppression of root diseases by Pseudomonas fluorescens CHA0: importance of the bacterial secondary metabolite 2,4-diacetylphloroglucinol. Mol Plant Microbe Interact. 5:4–13.
- Kemper N. 2008. Veterinary antibiotics in the aquatic and terrestrial environment. Ecol Indic. 8:1–13.
- Kwak Y-S, Bonsall RF, Okubara PA, Paulitz TC, Thomashow LS, Weller DM. 2012. Factors impacting the activity of 2,4-diacetylphloroglucinol-producing Pseudomonas fluorescens against take-all of wheat. Soil Biol Biochem. 54:48–56.
- Lavelle P. 1997. Faunal activities and soil processes: adaptive strategies that determine ecosystem function. In: Begon M, Fitter AH, editors. Advances in ecological research. London (UK): Academic Press; p. 93–132.
- Li B, Ge M, Zhang Y, Wang L, Ibrahim M, Wang Y, Sun G, Chen G. 2016. New insights into virulence mechanisms of rice pathogen Acidovorax avenae subsp. avenae strain RS-1 following exposure to ss-lactam antibiotics. Sci Rep. 6:22241.
- Mahmoudi E, Ahmadi A, Sayed-Tabatabaei BE, Ghobadi C, Akhavan A, Hasanzadeh N, Venturi V. 2011. A novel AHL-degrading rhizobacterium quenches the virulence of pectobacterium atrosepticum on potato plant. J Plant Pathol. 93:587–594.
- Martin JF, Demain AL. 1980. Control of antibiotic biosynthesis. Microbiol Rev. 44:230–251.
- Martínez JL. 2008. Antibiotics and antibiotic resistance genes in natural environments. Science. 321:365–367.
- Maurhofer M, Keel C, Haas D, Défago G. 1994. Pyoluteorin production by Pseudomonas fluorescens strain CHA0 is involved in the suppression of Pythium damping-off of cress but not of cucumber. Eur J Plant Pathol. 100:221–232.
- Mavrodi DV, Parejko JA, Mavrodi OV, Kwak Y-S, Weller DM, Blankenfeldt W, Thomashow LS. 2013. Recent insights into the diversity, frequency and ecological roles of phenazines in fluorescent Pseudomonas spp. Env Microbiol. 15:675–686.
- Oldroyd GED. 2013. Speak, friend, and enter: signalling systems that promote beneficial symbiotic associations in plants. Nat Rev Micro. 11:252–263.
- Philippot L, Raaijmakers JM, Lemanceau P, van der Putten WH. 2013. Going back to the roots: the microbial ecology of the rhizosphere. Nat Rev Microbiol. 11:789–799.
- Phillips DA, Fox TC, King MD, Bhuvaneswari TV, Teuber LR. 2004. Microbial products trigger amino acid exudation from plant roots. Plant Physiol. 136:2887–2894.
- Raaijmakers JM, Mazzola M. 2012. Diversity and natural functions of antibiotics produced by beneficial and plant pathogenic bacteria. Annu Rev Phytopathol. 50:403–424.
- Raaijmakers JM, Vlami M, de Souza JT. 2002. Antibiotic production by bacterial biocontrol agents. Antonie Van Leeuwenhoek. 81:537–547.
- Romero D, Traxler MF, López D, Kolter R. 2011. Antibiotics as signal molecules. Chem Rev. 111:5492–5505.
- Schnider-Keel U, Seematter A, Maurhofer M, Blumer C, Duffy B, Gigot-Bonnefoy C, Reimmann C, Notz R, Défago G, Haas D, et al. 2000. Autoinduction of 2,4-diacetylphloroglucinol biosynthesis in the biocontrol agent Pseudomonas fluorescens CHA0 and repression by the bacterial metabolites salicylate and pyoluteorin. J Bacteriol. 182:1215–1225.
- Schouten A, van den Berg G, Edel-Hermann V, Steinberg C, Gautheron N, Alabouvette C, de Vos CH, Lemanceau P, Raaijmakers JM. 2004. Defense responses of Fusarium oxysporum to 2,4-diacetylphloroglucinol, a broad-spectrum antibiotic produced by Pseudomonas fluorescens. Mol Plant Microbe Interact. 17:1201–1211.
- Siddiqui ZA. 2006. PGPR: Prospective biocontrol agents of plant pathogens. In: Siddiqui ZA, editor. PGPR: Biocontrol and biofertilization. Dordrecht: Springer Netherlands; p. 111–142.
- Silva LN, Zimmer KR, Macedo AJ, Trentin DS. 2016. Plant natural products targeting bacterial virulence factors. Chem Rev. 116:9162–9236.
- ter Laak TL, Gebbink WA, Tolls J. 2006. Estimation of soil sorption coefficients of veterinary pharmaceuticals from soil properties. Environ Toxicol Chem. 25:933–941.
- Thiele-Bruhn S. 2003. Pharmaceutical antibiotic compounds in soils - a review. J Plant Nutr Soil Sci. 166:145–167.
- Thomashow LS, Bonsall RF, Weller DM. 2008. Detection of antibiotics produced by soil and rhizosphere Mmcrobes in situ. In: Karlovsky P, editor. Secondary metabolites in soil ecology. Berlin, Heidelberg: Springer Berlin Heidelberg; p. 23–36.
- Thomashow LS, Weller DM, Bonsall RF, Pierson LS. 1990. Production of the antibiotic phenazine-1-carboxylic acid by fluorescent Pseudomonas species in the rhizosphere of wheat. Appl Environ Microbiol. 56:908–912.
- Venturi V, Keel C. 2016. Signaling in the rhizosphere. Trends Plant Sci. 21:187–198.
- Waksman SA. 1947. What is an antibiotic or an antibiotic substance? Mycologia. 39:565–569.
- Watson AG, Ford EJ. 1972. Soil fungistasis-a reappraisal. Annu Rev Phytopathol. 10:327–346.
- Wegst-Uhrich SR, Navarro DA, Zimmerman L, Aga DS. 2014. Assessing antibiotic sorption in soil: a literature review and new case studies on sulfonamides and macrolides. Chem Cent J. 8:5.
- Weller DM. 1988. Biological control of soilborne plant pathogens in the rhizosphere with bacteria. Annu Rev Phytopathol. 26:379–407.
- Whipps JM. 2001. Microbial interactions and biocontrol in the rhizosphere. J Exp Bot. 52:487–511.
- Wilhite SE, Straney DC. 1996. Timing of gliotoxin biosynthesis in the fungal biological control agent Gliocladium virens (Trichoderma virens). Appl Microbiol Biotechnol. 45:513–518.
- Williams P. 2007. Quorum sensing, communication and cross-kingdom signalling in the bacterial world. Microbiology. 153:3923–3938.
- Yu GY, Sinclair JB, Hartman GL, Bertagnolli BL. 2002. Production of iturin A by Bacillus amyloliquefaciens suppressing Rhizoctonia solani. Soil Biol Biochem. 34:955–963.
- Zhu J, Kaufmann GF. 2013. Quo vadis quorum quenching? Curr Opin Pharmacol. 13:688–698.
