Abstract
Passion fruit (Passiflora edulis Sims.) has become an important commercial fruit with large-scale cultivation in China. A disease affecting the stems, fruits, leaves and tendrils was observed on passion fruit vines in Sanming, Fujian Province, China, in August 2015. The typical symptoms included lesions of oval to irregular shapes, brown to brownish black in colour, with sunken cavities. Acervuli and dark setae were observed within the lesions. The disease incidence varied from 25 to 60% in different fields. The causative pathogen was identified as Colletotrichum brevisporum on the basis of morphological characteristics and sequence analysis of the internal transcribed spacer (ITS), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), beta-tubulin 2 (Tub2) and actin (ACT) genes. Pathogenicity was confirmed by fulfilling Koch’s postulates on plants and detached fruit. To our knowledge, this is the first report of anthracnose disease on passion fruit caused by C. brevisporum worldwide.
Résumé
La passiflore (Passiflora edulis Sims.) est devenue un fruit important commercialement en Chine et elle y est cultivée extensivement. En août 2015, une maladie s’attaquant aux tiges, aux fruits, aux feuilles et aux vrilles a été observée sur les plants grimpants de la passiflore à Sanming, dans la province du Fujian, en Chine. Les symptômes typiques incluaient des lésions dont la forme variait d’ovale à irrégulière, brunes à brun noir, produisant des cavités. Dans ces dernières, des acervules et des sétules foncées ont été observées. L’incidence de la maladie variait de 25 à 60%, selon les champs. Sur la base des caractéristiques morphologiques et de l’analyse de la séquence de l’espaceur transcrit interne (ITS) ainsi que des gènes de la glycéraldéhyde-3-phosphate déshydrogénase, de la ß-tubuline 2 (Tub2) et de l’actine (ACT), l’agent causal a été identifié en tant que Colletotrichum brevisporum. La pathogénicité a été confirmée en satisfaisant aux postulats de Koch appliqués à des plants et à des fruits détachés. À notre connaissance, il s’agit de la première mention d’anthracnose chez la passiflore, causée par C. brevisporum, et ce, dans le monde.
Introduction
Passion fruit (Passiflora edulis Sims), family Passifloraceae, is a tropical and subtropical perennial evergreen vine, which has become an important commercial fruit with large-scale cultivation in Guangdong, Fujian, Yunnan, Guangxi and other provinces in China. Passion fruit is beneficial to humans because of its high content of vitamins, amino acids and essential mineral salts (Matsuzaki et al. Citation2013). The large-scale cultivation of passion fruit has attracted increased attention in recent years (Lin et al. Citation2015).
A severe disease affecting the stems, fruits, leaves and tendrils was observed in several passion fruit plantations in Sanming, Fujian Province, China, in August 2015. The typical symptoms included lesions of oval to irregular shapes, brown to brownish black in colour, with sunken cavities. Acervuli and dark setae were observed within the lesions. The disease incidence varied from 25 to 60% in different fields, causing high yield loss. The purpose of this study was to identify the causal pathogen associated with this disease on passion fruit through morphological, molecular approaches and pathogenicity tests.
Materials and methods
Sampling and isolation
Diseased stems, fruits, leaves and tendrils with typical lesions were collected from nine infected plants from three plantations of passion fruit cultivar ‘Zhixiang No.1' in Sanming, during August to September 2015. Infected tissues were cut into 3–5 mm pieces, and surface-sterilized by immersion in 75% (v/v) ethanol for 30 s, followed by 1% NaClO for 1.5 min. The surface-sterilized tissues were rinsed three times in sterile distilled water, and then incubated on potato dextrose agar (PDA) amended with 100 µg mL−1 rifampicin at 28°C in the dark for 3 days. Mycelia growing from the tissues were transferred to fresh PDA dishes by transferring 5 mm diameter agar pieces from the margins of the colony. Isolates were purified using the hyphal tip method. Eleven pure cultures were obtained and incubated on PDA at 28°C. The morphological characteristics of 7–15-day-old cultures of each isolate were observed by light microscopy (Nikon E100, Japan) under 400× magnification. The shape, length and width of each of 100 conidia, conidial appressoria, asci and ascospores produced from perithecia, and the shape and diameter of 60 acervuli and perithecia were measured.
Pathogenicity test
Pathogenicity was tested on fruits and one-year-old plants of passion fruit cultivar ‘Zhixiang no. 1'. Fruits were surface-disinfested with 75% (v/v) ethanol, rinsed with sterilized water and wounded randomly with a sterile needle at four locations. Conidial suspensions were prepared by flooding dishes of 10-day-old cultures grown on PDA at 28°C with sterile distilled water, gently dislodging the conidia, and adjusting the conidial concentration to 1 × 106 spores mL−1 using a hemocytometer. For each isolate, five fruits were inoculated by spraying with 25 mL of the conidial suspension. Control fruits were sprayed with distilled water. All fruits were incubated in a plastic bag at 28°C. The experiment was repeated three times.
A second experiment involved the spraying of plants with a conidial suspension containing 1 × 106 spores mL−1 of each of the 11 isolates. Control plants were sprayed with sterilized water. Five stems, 10 leaves and 10 tendrils were inoculated for each isolate. The inoculated and control plants were incubated in a greenhouse under natural daylight and maintained at 22–25°C at night and 25–28°C during the day with RH >90%. Plants were observed daily for symptoms. Fungi were isolated from any resulting lesions, and the cultural and morphological characteristics of these fungi were compared with the original isolates.
Molecular identification
Genomic DNA of all 11 isolates was extracted from pure mycelium using the HP Plant DNA Kit (OMEGA Biotech, Guangzhou, China) following the manufacturer’s instructions. The internal transcribed spacer (ITS) region of ribosomal DNA, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), beta-tubulin 2 (Tub2) and actin (ACT) genes were amplified. Primers used for PCR amplification and sequencing are shown in . Amplification reactions were performed in a 25 µL reaction volume containing 2.5 µL 10×PCR buffer (Toyobo, Japan), 2.0 µL dNTP mix (2.5 mM µL−1), 0.2 µL Taq polymerase (5U µL−1), 1 µL genomic DNA (50 ng µL−1), 0.5 µL each primer (50 pmol µL−1), and total volume was adjusted to 25 µL with ddH2O. PCR reactions were conducted in a C1000TM thermal cycler (Bio-Rad, CA) with the following profile: 94°C for 5 min, 35 cycles at 94°C for 45 s, 45 s annealing at 56°C for ITS and ACT, 54°C for GAPDH and Tub2, 72°C for 45 s, and a final extension at 72°C for 10 min. The PCR-amplified products were checked on 1% agarose gels and visualized under a UV transilluminator. PCR products were purified with a gel extraction kit (Aidlab Biotech, Beijing, China) and sequenced at Sangon Biotech (Shanghai, China). Resultant sequences were compared with those available in NCBI’s GenBank database (http://www.ncbi.nlm.nih.gov). Sequences were aligned using the multiple sequence alignment program, Clustal W software and the phylogenetic analysis was performed using MEGA5.1 with the neighbour-joining method and Tajima-Nei distance model (Tamura et al. Citation2011). Magnaporthe grisea was used as an outgroup.
Table 1. Primers used for PCR amplification and sequencing in this study.
Results
Disease symptoms in the field
Initial symptoms on stems and tendrils appeared as many small, slightly sunken, water-soaked, irregular, pale greenish to greyish spots. With expansion, the lesions merged together to form a strip lesion (4.5–13 × 0.3–0.6 cm in size) with greyish white in the centre and yellow at the margin (, ). Leaf spots appeared as dark brown to brownish black, oval to irregular shapes (). Initial symptoms on fruits appeared as many small water-soaked, oval to irregular pale greenish spots. As the disease progressed, the lesions became medium brown, sunken, and 0.6–1.2 cm in diameter (). Ultimately, the fruits became shrivelled. At later stages, numerous acervuli and dark setae appeared on the lesions of stems, tendrils, leaves and fruits.
Fig. 1 (Colour online) Symptoms of anthracnose on passion fruit and morphological features of the pathogenic fungus Colletotrichum brevisporum (teleomorph: Glomerella sp.). (a–d) Symptoms of anthracnose on stems (a), tendrils (b), fruits (c), and leaves (d) in the field. (e–f) Purified upper colony (e) and reverse colony (f) of C. brevisporum grown on PDA. (g–h) conidia (g) and conidial appressoria (h) (scale bar = 20 μm). (i–j) acervuli (i) and perithecia (j) (scale bar = 50 μm). (k) asci (scale bar = 40 μm). (l) ascospores (scale bar = 20 μm). (m–p) symptoms observed on fruits (m), stems (n), tendrils (o) and leaves (p) 14 days after inoculation with a conidial suspension of C. brevisporum.

Morphological identification
Eleven isolates with similar colony morphology were obtained from the diseased tissues.
Anamorph: Colletotrichum sp. All isolates produced pale grey, dense aerial hyphae ((, f) and black structures similar to sclerotia on PDA (, f). The black structures were round to irregular, and semi-immersed, and sometimes associated with conidial masses and setae. Conidia were fusiform-elliptical, sometimes long obclavate to oblong-elliptical, obtuse or round ends, one celled, aseptate, hyaline, smooth-walled, guttulate, and 9.8–22.9 × 4.6–7.2 μm () (n = 100). Conidial appressoria were ovoid to irregular and 10.3–17.2 × 3.9–9.2 μm () (n = 100). Acervuli were black, containing smooth, dark setae and 55–286 μm in diameter () (n = 60). Morphological characteristics of the isolates were similar to descriptions of Colletotrichum brevisporum (Noireung et al. Citation2012).
Teleomorph: Glomerella sp. perithecia were globose, black and 93.5–223.0 μm in diameter () (n = 60). Asci were clavate, straight or slightly curved, 8-spored, and 51.8–75.7 × 10.5–14.2 μm () (n = 100). Ascospores were hyaline, aseptate, smooth-walled, ellipsoidal or reniform with rounded ends, and 10.6–23.1 × 3.6–7.2 μm () (n = 100).
Pathogenicity test
Symptoms similar to those observed on naturally infected plants in the field were observed on all of the inoculated fruits, stems, leaves and tendrils 14 days after inoculation (–p). No symptoms were observed on the control fruits, stems, leaves and tendrils. The fungi re-isolated from lesions on inoculated plant tissues showed the same morphological and cultural properties as the original isolates, thus fulfilling Koch’s postulates. The results of the three repeated experiments were identical.
Molecular identification
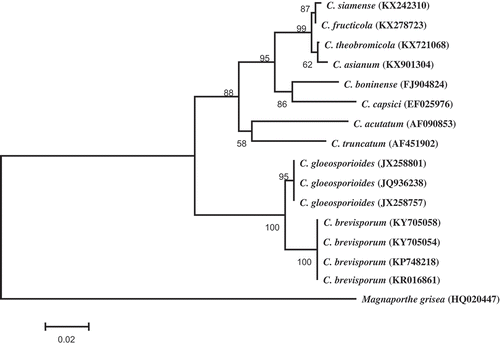
The amplicon size of ITS, ACT, GAPDH and Tub2 was 543, 276, 256 and 470 bp, respectively, from all 11 isolates. The sequences of two representative isolates were deposited in GenBank (accession nos. KY705054-KY705057 for ITS, ACT, GAPDH and Tub2 of isolate PF-1, KY705058-KY705061 for ITS, ACT, GAPDH and Tub2 of isolate PF-2, respectively). The ITS consensus sequence showed 100% identity to the ITS sequence of C. brevisporum (KP748218) and 99% identity with the ITS sequence of C. gloeosporiodes (MF076615). The other three consensus sequences revealed 99% homology with the corresponding sequences of C. brevisporum (JN050217 for ACT, KU315572 for GAPDH and KU319453 for Tub2). However, the GAPDH, Tub2 and ACT genes revealed 98%, 81% and 92% homology with the corresponding sequences of C. gloeosporiodes (KX426539, GU935900 and HQ846670, respectively). In the phylogenetic tree constructed based on ITS sequences, the two representative isolates were placed within a clade comprising two reference isolates of C. brevisporum (). The pathogen causing anthracnose on passion fruit was therefore identified as C. brevisporum based on cultural features, morphological characteristics, molecular data and pathogenicity.
Fig. 2 Phylogenetic tree obtained through Neighbour-joining method using MEGA 5.1 based on ITS rDNA sequences of two representative isolates C. brevisporum (KY705054 and KY705058), and 13 Colletotrichum spp. isolates retrieved from GenBank. The numbers above the branches indicate bootstrap values resulting from 1000 replicates. Magnaporthe grisea was used as an outgroup.
Discussion
Anthracnose caused by C. brevisporum has been previously recorded on numerous hosts, including Neoregelia sp. from Thailand (Noireung et al. Citation2012), papaya fruits (Vieira et al. Citation2013), Lycium chinense Mill (Paul et al. Citation2014), chayote fruits (Bezerra et al. Citation2016), pepper (Liu et al. Citation2016) and others. The conidial lengths of C. brevisporum in the present study were longer than those previously reported by Noireung et al. (Citation2012), but were consistent with those reported by Liu et al. (Citation2016) and Paul et al. (Citation2014). Anthracnose caused by Glomerella cingulata on passion fruit has been reported in Argentina (Wolcan & Larran Citation2000). Colletotrichum boninense, C. capsici and Glomerella sp. have been reported previously in Florida as causes of postharvest anthracnose on passion fruit during storage (Tarnowski & Ploetz Citation2010). Passion fruit has a desirable microclimate for proliferation of fungal pathogens owing to its high levels of nutrients and sugars (Al-Hindi et al. Citation2011). In China, stem rot caused by Fusarium solani and black spot disease caused by Alternaria passiflorae Simmons on passion fruit have been reported to occur at 3–14% incidence (Fang et al. Citation2008; Lin et al. Citation2012). To the best of our knowledge, this is the first report of C. brevisporum causing anthracnose on passion fruit worldwide. In addition, anthracnose caused by C. brevisporum on passion fruit in Sanming in 2015 occurred at 25–60% incidence, which resulted in significant yield loss.
The occurrence of the disease indicates that anthracnose caused by C. brevisporum could pose a serious threat to yield and quality of passion fruit in China, and these results will be helpful for developing an integrated strategy to control this disease.
Acknowledgements
This research was supported by the Seed Project of Fujian Province (Grant No. FJZZZY-1526), and Construction of Scientific and Technological Innovation of Fujian Province (Grant No. 2009N2005).
Additional information
Funding
References
- Al-Hindi RR, Al-Najada AR, Mohamed SA. 2011. Isolation and identification of some fruit spoilage fungi: screening of plant cell wall degrading enzymes. Afr J Microbiol Res. 5:443–448.
- Bezerra JP, Ferreira PV, Barbosa LF, Ramos R, Pinho DB, Rels A, Assunção IP, Lima GSA. 2016. First report of anthracnose on chayote fruits (Sechium edule) caused by Colletotrichum brevisporum. Plant Dis. 100:217.
- Carbone I, Kohn LM. 1999. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia. 91:553–556.
- Fang BN, Luo WJ, Zhou JP, Pei XQ. 2008. The occurrence and integrated control measures of black spot on passion fruit. Guangxi Plant Protec. 21:24–25.
- Lin H, Lin ZQ, Chen Y. 2015. Passion fruit and its high yield cultivation techniques. Mod Horticult. 1:39–40.
- Lin SH, Huang TJ, Fu CY. 2012. Occurrence and control of stem rot on passion fruit. South China Fruits. 41:82.
- Liu FL, Tang GT, Zheng XJ, Li Y, Sun XF, Qi XB, Zhou Y, Xu J, Chen HB, Chang XL, et al. 2016. Molecular and phenotypic characterization of Colletotrichum species associated with anthracnose disease in peppers from Sichuan Province, China. Sci Rep. 6:32761.
- Matsuzaki Y, Tsujisawa T, Nishihara T, Nakamura M, Kakinoki Y. 2013. Antifungal activity of chemotype essential oils from rosemary against Candida albicans. Open J Stomatol. 3:176–182.
- Noireung P, Phoulivong S, Fang L, Cai L, Eric HCM, Ekachai C, Jones EBG, Ali HB, Hyde DK. 2012. Novel species of Colletotrichum revealed by morphology and molecular analysis. Cryptogamie Mycol. 33:347–362.
- Paul NC, Lee HB, Lee JH, Shin KS, Ryu TH, Kwon HR, Kim YK, Youn YN, Yu SH. 2014. Endophytic fungi from Lycium chinense mill and characterization of two new Korean records of Colletotrichum. Int J Mol Sci. 15:15272–15286.
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA 5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 28:2731–2739.
- Tarnowski TLB, Ploetz RC 2010. First report of Colletotrichum boninense, C. capsici, and a Glomerella sp. as causes of postharvest anthracnose of passion fruit in Florida. Plant Dis. 94: 786–787.
- Templeton MD, Rikkerink EHA, Solon SL, Crowhurst RN. 1992. Cloning and molecular characterization of the glyceraldehyde-3-phosphate dehydrogenase encoding gene and cDNA from the plant pathogenic fungus Glomerella cingulata. Gene. 122:225–230.
- Vieira WAS, Nascimento RJ, Michereff SJ, Hyde KD, Câmara MPS. 2013. First report of papaya fruit anthracnose caused by Colletotrichum brevisporum in Brazil. Plant Dis. 97:1659.
- White TJ, Bruns T, Lee S, Taylor JW. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. New York (NY): Aademic Press; p. 315–322.
- Wolcan S, Larran S. 2000. First report of anthracnose caused by Glomerella cingulata on passion fruit in Argentina. Plant Dis. 84:706.
- Woudenberg JHC, Aveskamp MM, Gruyter JD, Spiers AG, Crous PW. 2009. Multiple Didymella teleomorphs are linked to the Phoma clematidina morphotype. Persoonia. 22:56–62.
