Abstract
Clubroot, caused by Plasmodiaphora brassicae, is an important soil-borne disease of canola (Brassica napus) and other brassica crops worldwide. Temperature and soil moisture are known to affect clubroot development. To understand the influence of weather on development of clubroot, replicated small-plot seeding date trials were conducted from 2011 to 2014 at the Muck Crops Research Station in southern Ontario. Each year, canola cultivar ‘InVigor 5030ʹ was seeded at 2-week intervals, and plants were sampled and assessed weekly for clubroot incidence and severity using a 0–3 scale. A base temperature of 14°C provided the best fit for degree day assessments, based on the assessments in 2011 and 2012. For modelling, data from previous trials on Chinese flowering cabbage (B. rapa subsp. chinensis var. utilis) from the same site were included in the data set, following assessment to ensure that both host species responded similarly across a range of environments. Temperature and rainfall affected clubroot initiation and development, but the results indicated that when conditions exceeded a threshold of temperature and soil moisture, clubroot quickly developed to high levels, irrespective of additional heat units or moisture. This indicated that clubroot initiation and development at sites where resting spores occur at high levels occurred rapidly across a wide range of soil temperature and moisture conditions above a low threshold around 14°C.
Résumé
La hernie, causée par Plasmodiophora brassicae, est une maladie terricole importante qui frappe le canola (Brassica napus) et d’autres plantes de la famille des Brassica partout dans le monde. Nous savons que la température et l’humidité du sol influencent le développement de la hernie. Pour comprendre l’influence du temps sur le développement de la hernie, des essais répétés sur l’effet de la date des semis ont été menés dans de petites parcelles, de 2011 à 2014, à la Station de recherche sur la culture des terres noires, dans le sud de l’Ontario. Chaque année, le cultivar de canola ‘InVigor 5030ʹ a été semé à deux semaines d’intervalle et les plants ont été échantillonnés et évalués hebdomadairement, sur une échelle de 0 à 3, en fonction de l’incidence et de la gravité de la hernie. Une température de base de 14°C a convenu le mieux aux évaluations des degrés-jours, en se référant aux évaluations de 2011 et de 2012. Pour la modélisation, des données issues d’essais précédents menés sur le pak-choï à fleurs (B. rapa subsp. chinensis var. utilis) sur le même site ont été incluses dans l’ensemble de données, après évaluation, pour s’assurer que les deux espèces réagissaient similairement à un éventail d’environnements. La température et la pluie ont influencé le déclenchement et le développement de la maladie, mais les résultats ont indiqué que, quand les conditions franchissaient un seuil de température et d’humidité du sol, la hernie se développait rapidement, indépendamment d’un accroissement du nombre de degrés-jours ou de l’humidité. Cela a montré que le déclenchement et le développement de la hernie, aux endroits où il y a de forts taux de spores de réserve, se produisaient rapidement, et ce, indépendamment d’une vaste gamme de températures et de conditions d’humidité du sol, même lorsqu’elles dépassaient le bas seuil d’environ 14°C.
Introduction
Clubroot, caused by Plasmodiophora brassicae Woronin, is a destructive soil-borne pathogen of brassica crops. Management is difficult because the resting spores are long-lived once a field is infested (Wallenhammar Citation1996; Peng et al. Citation2015). Inoculum pressure (resting spore concentration in soil) (Donald & Porter Citation2009), pH (Gossen et al. Citation2013) and plant age (Hwang et al. Citation2011) can have a strong impact on infection and symptom development. In addition, temperature (Sharma et al. Citation2011a, Citation2011b; Gossen et al. 2012b) and soil moisture (Dixon Citation2014; Gossen et al. Citation2016) can have an important influence on infection and development of P. brassicae.
Early studies reported that resting spores required at least 14°C for germination (Chupp Citation1917) and 18°C for symptom development (Colhoun Citation1953). At 14°C, variable levels of clubroot severity were observed in cabbage, Chinese cabbage, mustard and radish (Thuma et al. Citation1983) and little or no clubroot developed on Shanghai pak choy at temperatures ≤17°C (Sharma et al. Citation2011b; Gossen et al. Citation2012b). Subsequent studies showed that clubroot development was optimal between 20–26°C (Sharma et al. Citation2011a, Citation2011b; Gossen et al. Citation2013). At 25°C, secondary symptom development was visible at as early as 10 days after inoculation (Sharma et al. Citation2011b). Temperatures above 35°C limit cortical infection and symptom development (Wellman Citation1930), but are also unfavourable for crop development. Seeding early or late in the season, when soil temperatures are low, can provide effective management of clubroot in short-season crops (McDonald et al. Citation2004; Gossen et al. Citation2012a). In canola (Brassica napus L.), clubroot development was reduced and yield slightly increased in early-seeded canola sown in clubroot-infested soil (Hwang et al. Citation2012a), but early seeding generally produces higher yields even without a P. brassicae interaction (Christensen et al. Citation1985).
Degree days above a base (minimum) temperature can be used to predict a pathogen’s development based on the accumulated temperature of its environment over time (Wilson & Barnett Citation1983; McMaster & Wilhelm Citation1997). In one study of clubroot on radish (Raphanus sativus L.) grown on muck (very high organic matter, peat-based) soil, cumulative day degrees of soil temperature above 12°C for the 6th week of growth and the cumulative rainfall for the first 2 weeks were the best predictors of clubroot (Thuma et al. Citation1983). In another study on muck soil, clubroot incidence and severity of Shanghai pak choy (B. rapa subsp. chinensis (Rupr.) var. cummunis Tsen and Lee) and Chinese flowering cabbage (B. rapa subsp. chinensis var. utilis Tsen and Lee) were most closely correlated with air temperature during the last 10 days before harvest (McDonald & Westerveld Citation2008). None or very little clubbing developed at temperatures below a mean of 14°C. Studies under controlled conditions have demonstrated that clubroot symptoms do not develop in infected plants at temperatures below 17°C (Gossen et al. Citation2012a).
Soil moisture is also important for infection by P. brassicae. Zoospores released from germinating resting spores swim toward root hairs in the water film around soil particles (Dixon Citation2014), attracted by a nutrient gradient of root exudates (Friberg et al. Citation2005, Citation2006). The minimum level of soil moisture necessary for P. brassicae infection is not well understood. There is wide variation in the literature among the soil moisture values that have been found to limit disease development. In an early study, clubroot incidence (CI) increased when soil moisture levels were between 40–70% gravimetric soil moisture, with 70% being most favourable for clubroot development (Colhoun Citation1953). In a subsequent study, a gravimetric soil moisture content of 80% favoured clubroot development, with substantial reductions in clubroot levels at 40–60% (Narisawa et al. Citation2005). In another study, clubroot development in mineral soil required gravimetric soil moisture levels of only 9%, while in organic soils, moisture levels of at least 60% were needed (Hamilton & Crête Citation1978). These differences are likely due to differences in bulk density (Gossen et al. Citation2013) and pH. At the other end of the spectrum, high levels of infection can also develop in soil maintained for long periods at near-saturation (Gossen et al. Citation2013).
In the study on clubroot on radish, severity increased as a function of soil temperature or moisture (Thuma et al. Citation1983). On Shanghai pak choy and Chinese flowering cabbage, mean air temperature during crop development (range: 15–22°C) was positively correlated with clubroot incidence and severity (McDonald & Westerveld Citation2008). Also, clubroot severity was strongly correlated with season-long rainfall (range 68–173 mm) in Shanghai pak choy and Chinese flowering cabbage (Gossen et al. Citation2012a).
Knowledge of the relative impact of temperature and soil moisture on clubroot development could be used to identify locations where conditions are outside the range for clubroot development. It could help explain differences in the rate of clubroot spread in some areas. A climate model was used to predict the spread of clubroot when the disease was first identified on canola on the Canadian prairies (Turkington et al. Citation2004). The model identified temperature and soil moisture as critical factors affecting the distribution of clubroot in the prairie region. Clubroot has spread beyond the areas of high risk (Gossen et al. Citation2015) predicted by that initial effort at modelling, which indicates that additional study of these components would be useful.
The objective of this research was to examine the relationship between weather conditions (temperature and rainfall) and clubroot development on canola and brassica vegetables under field conditions at a site in Ontario and, if possible, develop a regression model to describe clubroot development using parameters that are readily available to growers.
Materials and methods
Study design and management
Trials were conducted each year from 2011 to 2014 at the Muck Crops Research Station, Holland Marsh, Ontario on organic soil (pH ~6.7, organic matter ~81%) naturally infested with P. brassicae pathotype 6. Each trial was planted to canola cultivar ‘Invigor 5030 LL’ (Bayer CropScience, Guelph, ON), which is moderately susceptible to pathotype 6 (Deora et al. Citation2012), at about 18 seeds per m of row. The trials each year were laid out as a randomized complete block design with four replications. Each plot consisted of seven 5-m long rows with 20 cm between rows. Seeding dates were spaced at ~2-week intervals each year, generally starting as early as possible in the spring. The seeding dates for each year were: (2011) 25 May, 10 June, 22 June, 6 July; (2012) 1 May, 15 May, 29 May, 13 June, 28 June, 10 July; (2013) 3 May, 15 May, 30 May, 12 June, 26 June, 9 July, 24 July, 7 August; and (2014) 8 May, 22 May, 4 June, 18 June, 3 July, 16 July, 30 July, 15 August.
The trial in each year was positioned at approximately the same location as the previous year’s trial. The plot area was rototilled several times prior to seeding to thoroughly mix the soil and provide (as much as possible) a uniform disease pressure throughout the study area. Previous seeding date trials (McDonald & Westerveld Citation2008; Adhikari Citation2010) were conducted in approximately the same location as the current study. Estimates based on manual extraction (Dhingra & Sinclair Citation1985) showed that there were 1.3 × 106 resting spores g−1 soil in 2009, 9.0 × 106 resting spores g−1 soil in 2010 (Saude et al. Citation2012), and 2.4 × 107 resting spores g−1 soil in 2011 (Kasinathan Citation2012). Estimates of resting spore numbers at this site using real-time qPCR in 2013 were as high as 4.7 × 107 resting spores g−1 soil.
The canola seed was pretreated with a mixture of carbathiin (50 g L−1), clothianidin (285.7 g L−1), metalaxyl (5.36 g L−1) and trifloxystrobin (7.14 g L−1) at 1.4 L per 100 kg of seed to reduce damage from flea beetles and damping-off. An Earthway® push seeder (Earthway Products Inc. Bristol, IN) fitted with an Earthway® 1002–9 mustard disc was used for planting. No irrigation was applied, or necessary, prior to seeding in 2011 to 2014. Weeds were managed according to recommended commercial practices (OMAFRA Citation2013), and flea beetles (family Chrysomelidae) and Swede midge (Contarinia nasturtii (Kieff)) were managed (when required) using label rates of Actara® 240SC and Movento® 240 SC. In 2013, Swede midge caused damage in the 26 June, 9 July and 24 July seeding dates, causing many plants to die prematurely. Despite this, there were still enough plants to sample at the 4- and 6-week harvest dates.
Assessment
Clubroot incidence and severity was assessed using a 0–3 rating scale, where 0 = no clubbing, 1 < 1/3 of roots with clubbing, 2 = 1/3 to 2/3 of roots with clubbing, and 3 > 2/3 of roots with clubbing. In 2011, assessments were started when roots started to display clubbing symptoms. In each plot, 50 plants were uprooted and each root was assessed for clubroot incidence and severity at weekly intervals. In 2011, sampling started 3 weeks after seeding for the 25 May seeding date treatment, 4 weeks after seeding for the 10 June seeding and 5 weeks after seeding for the 22 June and 6 July seeding dates. In 2012, initiation of assessments was standardized to 4 weeks after seeding. Sampling ceased after eight assessments or when there were no more plants. In 2013 and 2014, plants were assessed at about 14-day intervals starting 4 weeks after seeding. Sampling continued until all of the plants in a treatment reached a disease severity rating of 3, plants senesced, or frost terminated plant growth. A standard disease severity index (DSI) was calculated with the following equation (Crête et al. Citation1963; Strelkov et al. Citation2006):
Weather data
Ambient air temperature was measured using a CR21X weather station (Campbell Scientific, Edmonton, AB) with a HMP35C probe positioned in the shade at 1.2 m above ground level in the field plots. The station is recalibrated at only extended (generally 3-year) intervals. Temperature was assessed every 5 min and hourly mean air temperatures were recorded. Daily mean temperatures were calculated as maximum plus minimum temperature each day divided by two. A TE35C tipping bucket rain gauge placed less than 100 m from the trial was used to measure rainfall, reported hourly. Cumulative rainfall over a given time period was calculated based on adding daily totals.
The degree day equation for air temperatures was:
Where TMax and TMin are the daily maximum and minimum temperatures, respectively, and TBase is the base temperature (McMaster & Wilhelm Citation1997). An initial base temperature of 14°C was used, as a compromise between 12°C, where there was no clubroot development (Monteith Citation1924), and 15°C, where some clubroot development does occur (Sharma et al. 2012).
Soil moisture
In 2012, a ML2x ThetaProbe (Delta-T Devices Ltd., Cambridge, UK) soil moisture sensor was installed in the middle of the experimental plot and volumetric soil moisture data were collected hourly from the first seeding date until the final sampling date of the trial. The ThetaProbe measures the dielectric constant of soil, which is primarily a function of soil water content (Topp et al. 1980; Whalley Citation1993; White et al. Citation1994). A value of 0.3 m3 m−3 or higher represents wet to saturated soil. Soil moisture (m3 m−3) was calculated with the following formula:
where V is the ThetaProbe output in volts, a0 = 1.26, and a1 = 6.53. Constants a0 and a1 had been developed previously for the muck soil in the Holland Marsh (Kora Citation2004).
Soil moisture was not assessed in 2013. In 2014, soil volumetric water content was estimated from the dielectric constant using a EC-5 Soil Moisture Smart Sensor (Onset Computer Corporation, Bourne, MA, USA).
Statistical analysis and model development
Data on Chinese flowering cabbage (B. rapa subsp. chinensis (Rupr.) var. utilis Tsen and Lee) from 1999–2002 (McDonald & Westerveld Citation2008) were included in the analysis, and data from 2008 and 2009 (Adhikari Citation2010) were also included in the models. Both of these studies were conducted at the Muck Crops Research Station. This added 32 additional data points for the calibration and validation of the stepwise regression analyses. Plots in one study (McDonald & Westerveld Citation2008) were irrigated before seeding when the soil was dry. It is likely, however, that the high water-holding capacity of the muck soil resulted in sufficient soil moisture for clubroot development throughout the growing season.
Data on Shanghai pak choy grown at the Muck Crops Research Station (McDonald & Westerveld 2008) were initially also included in these analyses, but were subsequently excluded because the pattern of response appeared to be different from that for canola and Chinese flowering cabbage, with Shanghai pak choy consistently exhibiting higher severity than the other two crops.
The data on canola and Chinese flowering cabbage were pooled and partitioned into two subsets using the random function in Excel. One subset was used to produce the stepwise regression models. The significance level to enter the model was set at P = 0.15, and the significance level to stay in model was P = 0.10. The second subset was used to validate the efficacy of the models produced based on the first subset.
All of the statistical analyses were performed with SAS software (version 9.2 SAS Institute, Cary, NC). A mixed model analysis of variance was conducted using PROC MIXED, where seeding date and sampling week were the fixed effects, and year and block were random effects. Mean comparisons of clubroot incidence and severity were performed using Tukey’s test. Clubroot levels in one block (repetition) of the 2012 trial were consistently and significantly lower than in the other three blocks. This may have been due to differences in drainage or uneven distribution of inoculum, so the data were excluded from subsequent analysis.
Pearson correlations were calculated to examine the relationships among clubroot incidence and severity versus weather parameters based on specific time intervals using PROC CORR. These parameters were mean daily air temperature, cumulative degree days, cumulative daily rainfall, and mean daily volumetric soil moisture (when available). Parameters were calculated at selected time points (0- and 1-week delays, 5–10, 5–15, 7–14, 10–15 and 14–21 days after seeding or before harvest) to account for a potential lag in biological response to changing abiotic variables at different time points throughout the disease cycle. Correlations between clubroot levels at 6 weeks after seeding and these parameters were calculated with seeding dates from 2011–2014 (n = 25). Seeding date trials of Chinese flowering cabbage from 1999–2002 (n = 15) (McDonald & Westerveld Citation2008) and 2008–2009 (n = 10) (Adhikari Citation2010) were also included in the analysis to provide a wider range of years and environments.
Stepwise regression was used to identify the parameters for inclusion in the clubroot incidence/severity model using the same environmental parameters as in the correlation analysis. There were 11 environmental parameters that had a high positive auto-correlation from the correlation analysis and these were removed before the stepwise regression was conducted. Data of canola and Chinese flowering cabbage were pooled and randomly partitioned into two subsets using the random function in Microsoft® Excel 2008. One subset, which consisted of 35 seeding date treatments, was used to produce the stepwise regression models, while the other subset of 15 treatments was used to validate the model. Stepwise regression significance levels were set at P = 0.15 to enter the model and P = 0.10 to stay.
Additional correlation analyses on the full data set (n = 50) were conducted using the following parameters: the number of days with a maximum temperature over 12, 14 and 17°C, and the number of days with the mean temperature below 12, 14 and 17°C to assess the impact of base temperature on the relationship between clubroot levels and temperature.
Results
Weather
The mean monthly air temperature was consistently higher than the long-term average over the course of the study. It was 1–2°C higher in 2011, 0–3°C higher in 2012, 1°C higher in 2013 and 0.4°C higher in 2014 (). Rainfall was more variable. In 2011, precipitation was generally above normal and the site did not experience more than 2 weeks between rainfall events during the growing season. In 2012, heavy rainfall immediately after the first seeding date flooded part of the study area. That event was followed by 3 weeks without rain, and precipitation remained low until mid-July. In 2013, rainfall was above average in every month except September. In 2014, rainfall was above average for June and September, below average for May and August and normal for July (). There were no more than 10 days between rainfall events during the growing season in both 2013 and 2014.
Table 1. Mean monthly air temperature and rainfall during the canola seeding date trials at Holland Marsh, ON, 2011–2014.
Following an early-season rain event in 2012, volumetric soil moisture dropped from 75% to 45% before the next rainfall. Soil moisture did not increase to 60% until mid-July. The lowest volumetric soil moisture experienced in this trial was 43% on 29 May, and the highest experienced was 78% on 5 May.
Clubroot incidence and severity
As expected, clubroot levels increased over time for almost every seeding date each year. In 2011, clubroot incidence at 6 weeks after seeding was highest in the 22 June seeding (30%), intermediate in the 25 May (25%) and 10 June (22%) seeding dates, and lowest for the 6 July seeding (17%) (). The low incidence of clubroot in the 6 July seeding treatment was associated with a severe infestation of Swede midge, which killed most of the plants by 5 weeks after seeding. Clubroot levels were substantially higher in 2012 than in 2011. Clubroot incidence at 6 weeks after seeding was higher in the seeding dates from late May until the end of June than from early May or July. In 2013, clubroot incidence at 6 weeks after seeding was highest from early May to mid-June (31–33%), and lower in July (8–21%). The decline in clubroot incidence in the 26 June seeding was likely due to stunted growth of the plants during peak Swede midge damage around 24 July. In 2014, environmental conditions were conducive for clubroot development. Clubroot incidence at 6 weeks after seeding was highest on 3 July and 16 July (100%; ).
Fig. 1 (Colour online) Clubroot incidence on canola planted at about 2-week intervals in naturally infested muck soil at the Muck Crops Research Station, Holland Marsh, ON in 2011, 2012, 2013 and 2014.
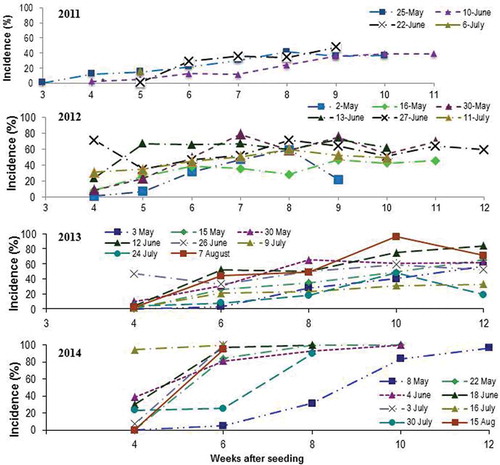
Chinese flowering cabbage was seeded and harvested at the same time as canola in 2014. Plants seeded earlier in the season developed less severe clubroot than those planted later. Flowering cabbage had a lower incidence of clubroot (range 5–95%, mean = 55%) than canola ‘InVigor 5030ʹ (range 5–100%, mean = 74%) at 6 weeks after seeding (data not shown), as has been reported previously (Adhikari Citation2010; Deora et al. Citation2012), but clubroot incidence (r = 0.91) and severity (r = 0.87) of the two crops were strongly correlated. In an initial analysis, a linear adjustment factor created by obtaining an average difference between the two crops was used to adjust clubroot incidence and severity on flowering cabbage to be similar to canola (not shown). Correlations and regression parameters were not improved, so the adjustment was omitted from the model.
Only six seeding dates were identified with low levels of clubroot at 6 weeks after seeding (incidence < 5%, DSI ≤ 5), and these were generally seeded in early May. They were exposed to low average temperatures at 7–21 days after seeding (treatment mean = 16.3°C, season mean = 18.8°C), total air degree days (108°D, mean 203°D) and near-normal total rainfall (118 mm, mean 111 mm).
Between 1999 and 2009, clubroot incidence in Chinese flowering cabbage never exceeded 70% (mean = 28%) or 50 DSI (mean = 18 DSI) at 6 weeks after seeding. In 2013, it had a mean incidence of 73% and 67 DSI, and 94% incidence and 90 DSI in 2014. Clubroot levels on canola ‘InVigor 5030ʹ showed a similar pattern, with the highest incidence at 6 weeks of 30% in 2011, 67% in 2012, 52% in 2013 and 100% in 2014.
Model calibration and results
Harvesting 6 weeks after seeding was chosen as the optimum time to assess clubroot incidence and severity based on the results of earlier studies (Thuma et al. Citation1983; Gossen et al. Citation2012a). The use of clubroot ratings taken at 8 weeks after seeding was assessed, but resulted in weaker correlations (data not shown). Therefore, 6 weeks after seeding was used to assess the effects of environment parameters on clubroot development.
The degree day calculation was based on a threshold of TBase = 14°C, where temperatures below 14°C would not be suitable for clubroot development. Initially, the 14°C temperature was selected as a compromise among the estimates of a lower threshold from previous studies (Monteith Citation1924; Thuma et al. Citation1983; Sharma et al. Citation2011a, Citation2011b; Gossen et al. Citation2012a). Initial assessments indicated that a base of 14°C provided the strongest correlations between clubroot levels and environmental parameters (Gludovacz Citation2013), but degree day calculations did not improve these correlations relative to a base of 0°C (Cranmer Citation2015). Similarly, use of soil temperature estimates in place of air temperature did not improve these correlations (Cranmer Citation2015), so only the values associated with air temperature are presented. Simply counting the number of days in the first 6 weeks after seeding with the mean temperature below 14°C indicated when temperatures were not conducive for clubroot development. Similarly, counting the number of days when maximum temperatures meet or exceed 17°C can also indicate when temperatures are most conducive for clubroot development ().
Table 2. Correlations (r) between clubroot incidence/severity at 6 weeks after seeding vs. temperature, air degree days and rainfall during selected time intervals for 50 seeding dates of canola and Chinese flowering cabbage grown at Holland Marsh, ON and used for model development. Values in bold type are significant at P < 0.05.
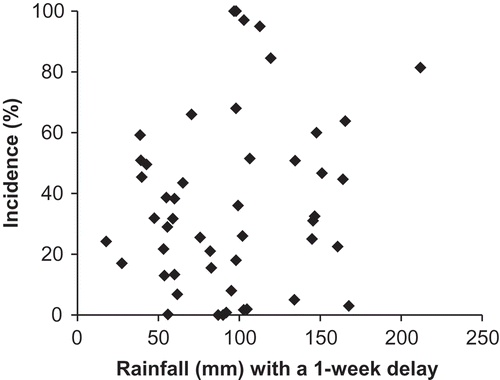
Clubroot incidence increased with increasing daily mean air temperature at 7–21 days after seeding and with season-long total rainfall with a 1-week delay. Mean air temperature at 7–21 days after seeding (range = 13.8–23.3°C) accounted for 22% of the variation ( and ) and season-long total rainfall with a 1-week delay (range = 18–212 mm) accounted for 9% of the variation (). Similarly, severity increased with mean air temperature at 14–21 days after seeding (15%) and with season total rainfall with a 1-week delay, which accounted for 11% of the variation in the model ( and ). The model for clubroot incidence was CI = −91.77 + 5.69 × (Air temperature 7–21 days after seeding) + 0.18 × (Total precipitation with a 1-week delay) and the model for clubroot severity was DSI = −70.50 + 4.11 × (Air temperature 7–21 days after seeding) + 0.17 × (Total precipitation with a 1-week delay).
Table 3. Stepwise regression of the effect of rainfall, air temperature degree days (°D), air temperature and soil moisture over selected time intervals on clubroot incidence (CI) and severity (DSI) over time on flowering cabbage and canola based on 35 seeding dates at Holland Marsh, ON.
Fig. 2 Relationship between mean air temperature at 7–21 days after seeding and clubroot incidence on canola and flowering cabbage at Holland Marsh, ON (n = 50).
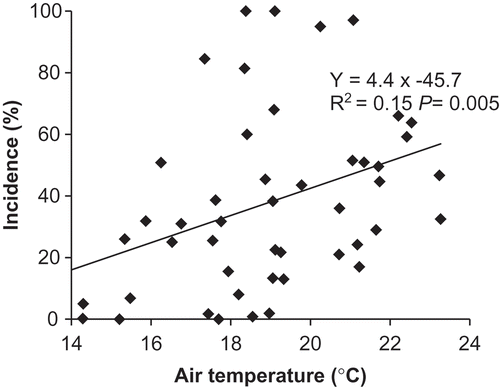
Fig. 3 Scatter plot of season total rainfall with a 1-week delay after seeding and clubroot incidence on canola and flowering cabbage at Holland Marsh, ON (n = 50).
There was a negative relationship between soil moisture and clubroot incidence (only available for 2012 and 2014). Soil moisture at 5–10 days after seeding was closely related to clubroot incidence (r = −0.60) and severity (r = −0.57). Soil moisture 2 and 3 weeks after seeding as well as at 5–15 and 10–15 days after seeding, were also significant for both incidence and severity (r = −0.52 to r = −0.57). The mean volumetric soil moisture was 0.35 m3 m−3 (range = 0.27–0.48 m3 m−3) in 2012 and 0.22 m3 m−3 (range = 0.11–0.29 m3 m−3) in 2014.
Model validation
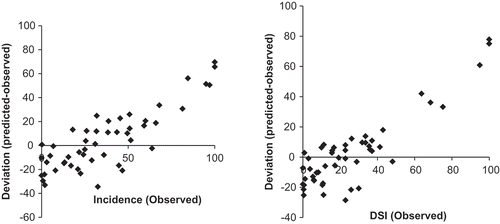
Thirty of 50 (60%) of deviations between predicted and observed clubroot incidence and severity were ± 20%. The model underestimated clubroot levels when clubroot levels were low and over-estimated when levels were high for both incidence and severity (). All deviations over +35% were treatments from 2014, where environmental conditions were very conducive for clubroot development.
Discussion
A multiple regression approach to modelling the effect of weather conditions on clubroot incidence and severity on spring-seeded crops of canola and Chinese flowering cabbage at the Muck Crops Research Station in southern Ontario identified temperature and precipitation as having an important effect on infection and symptom development. Clubroot levels were consistently low in treatments seeded in early May (planted into cool soil) or July (harvested after temperatures declined in autumn). For most of the remaining seeding dates, clubroot developed quickly and to high levels. The model accounted for only a relatively small proportion of the variation in incidence (31%) or severity (26%) and the deviations between the model and the validations set were quite large, often approaching ± 20%. Mean daily temperatures >14°C and <30°C, which have been identified as conducive for infection and development of P. brassicae under controlled conditions (Sharma et al. 2011a, 2011b), occurred throughout most of the growing season each year. Mean daily temperature never exceeded 30°C, and fell below 14°C only in the earliest seeding dates and at the end of the growing season. In practical terms, this indicated that conditions at this site were conducive for development of clubroot throughout most of the growing season for spring-seeded brassicas.
These results indicated that the thresholds of minimum temperature and moisture for clubroot development were low, with 14°C representing a useful low temperature threshold. Once these minimum thresholds were achieved, clubroot development was not dramatically affected by further increases in temperature and precipitation. For example, mean monthly temperatures and rainfall were both higher in 2013 than 2014, but clubroot levels were higher in 2014. This supports the results (but not all of the conclusions) of a previous study of the effect of weather on clubroot development (Thuma et al. Citation1983). A recent study of winter-seeded brassica vegetables in Brazil provided an important counter-point to this observation; little clubroot developed in the cauliflower crop despite high inoculum pressure when temperatures remained below this temperature threshold (da Bhering et al. Citation2017).
The results of seeding date trials on canola at the Muck Crops Research Station in southern Ontario were combined with existing data on Chinese flowering cabbage from previous studies at this site to model and validate the effect of temperature and precipitation on clubroot infection and development. Stepwise regression was used to examine the relationships between clubroot incidence and severity 6 weeks after seeding with weather parameters. Assessments at 6 weeks after seeding were selected for this analysis to provide compatibility with previous studies (e.g. Sharma et al. Citation2011a, Citation2011b), but also because differences among the treatments were largest and correlations with weather parameters were strongest at this time (Gludovacz Citation2013). The best predictive parameters for forecasting clubroot incidence and severity at 6 weeks were mean air temperature at 7–21 days after seeding and season total rainfall with a 1-week delay: clubroot incidence and severity increased with increasing air temperature in the 3 weeks after seeding (r = 0.29 to 0.32) and decreased with increasing soil moisture (r = −0.52 to −0.60). A negative association of clubroot development with rainfall and soil moisture was unexpected, but is consistent with a previous study (Thuma et al. Citation1983). In contrast, rainfall was not correlated with clubroot development on Chinese flowering cabbage in another study (McDonald & Westerveld Citation2008). Use of degree days, or soil temperatures instead of air temperatures, did not improve the correlation parameters or the fit of the regression model relative to air temperatures (Cranmer Citation2015). Instead, counting the days with mean temperature below 14°C or with maximum temperatures above 17°C are proposed as a simple and equally effective method of determining risk of clubroot development.
An increase in clubroot incidence with increasing mean temperature during the first 3 weeks after seeding is consistent with controlled environment studies of the effect of temperature on infection and development of P. brassicae. Under controlled conditions, primary zoospores infect root hairs at seedling emergence, and secondary infection can occur at 5 days after primary infection at 20–25°C (Sharma et al. Citation2011b). If canola and Chinese flowering cabbage seeds germinated 2 days after seeding and were infected immediately, cortical infection and development would begin about 7 days after seeding. Seedlings infected at emergence develop more severe clubroot than those where infection was delayed (Hwang et al. Citation2012a), so delaying infection in cold soil should reduce final clubroot levels.
The absence of a clear and consistent correlation between temperature and clubroot symptoms in this study may have resulted from the specific range of temperature assessed. Taken over all of the studies, 22 of the seeding dates fell entirely within the range of conducive temperatures (maximum temperature over 17°C) and 18 had 0 or 1 day with mean temperatures of 14°C or lower. Most of the remaining dates had extended time periods within the conducive zone. Seven of eight seeding dates with the lowest levels of clubroot were seeded in early May or late in the summer, but other seeding dates with higher clubroot levels had lower mean temperatures. This indicated that there may be an interaction with other factors such as soil moisture. The seeding date treatments were generally started as soon as possible in the spring, so earlier seeding to avoid clubroot infection was not an option. In contrast, a winter brassica crop (seeded in autumn for harvest in summer) would experience a long period of temperatures below the minimum threshold for clubroot development (da Bhering et al. Citation2017). A degree day analysis would likely be much more applicable for a winter brassica crop than for a spring-seeded crop.
High rainfall was associated with decreased clubroot development. For example, there was a negative correlation between total rainfall (r = −0.54) and clubroot incidence when data from 2013 and 2014 were analysed separately (Cranmer Citation2015). In a previous study, accumulated rainfall at 2 weeks after seeding was positively correlated (r = 0.59 and r = 0.33) with clubroot severity in radish grown in muck soil, but soil moisture over the season was negatively correlated (r = −0.25) with severity in one of two years (Thuma et al. Citation1983).
A negative relationship between clubroot and soil moisture is difficult to reconcile with the requirement for free water in the soil spaces to facilitate zoospore movement. One previous study reported that saturated conditions after seeding reduced infection (Dobson et al. Citation1982), but a more recent study indicated that there was little or no difference in infection in saturated or drained soils (Gossen et al. Citation2016). In an earlier study on mineral soil, the critical moisture level for infection was below 9% soil moisture (25% of water holding capacity). In muck soil, the threshold moisture level to cause clubroot was > 45% and >60% soil moisture (Hamilton & Crête Citation1978). At the study site at the Muck Crops Research Station, rainy weather and resulting high soil moisture were often negatively associated with temperature, so it is possible that low temperature was the environmental parameter that actually limited clubroot symptom development rather than a direct effect of precipitation. Alternatively, high soil moisture levels may dilute root exudates, which act as triggers for resting spore germination, and also increase plant growth, which may reduce early expression of symptoms (Hwang et al. Citation2012a). At some sites, excess moisture can cause stunting of crop roots, but this is not an issue in muck soils.
In the initial two years of the seeding date study, correlations with clubroot incidence were strongest when accumulated season total rainfall and degree days of soil temperature were assessed with a 1-week delay (Gludovacz Citation2013), but a delay did not strengthen the correlations in the final two years of the study (Cranmer Citation2015). Year-to-year variability was high. The weather in 2014 was clearly conducive for clubroot development, but inoculum concentration likely also increased over time because the canola seeding date trial was located in the same area each year to provide a uniform distribution of inoculum.
It is possible that other environmental factors contributed to the variation observed among years. However, the impact of weather variables such as vapour pressure or solar radiation, which can affect crop growth and disease development in foliar pathogens, on the host-pathogen interaction of a soil-borne pathogen are expected to be relatively small. Similarly, factors in the soil environment such as pH, organic matter and micronutrients could have an important impact on clubroot levels (reviewed in Gossen et al. Citation2014), but are unlikely to change dramatically within a growing season or between years, and so are unlikely to have contributed substantially to the variability observed in the study. These results support previous suggestions (Turkington et al. Citation2004; Gossen et al. Citation2011) that increases in mean temperature associated with climate change are likely to increase the incidence and severity of clubroot over time.
In conclusion, this multi-year study demonstrated that when resting spore populations in soil are high, weather conditions have only a limited impact on final clubroot severity above low threshold values of temperature and precipitation on spring-seeded annuals on muck soils. These threshold values are similar, but not identical to, the minimum values required for rapid crop growth. Seeding susceptible brassicas as early as possible minimized clubroot severity, which supports the results of previous studies (McDonald & Westerveld Citation2008; Gossen et al. Citation2012a; Hwang et al. Citation2012a). Additional assessments of the impact of soil moisture on infection and development of clubroot on mineral soils are required.
Acknowledgements
The authors thank Dr A. Deora for help and advice, the staff at the Muck Crops Research Station for technical assistance with the field trials, and Dr S. Vail for providing seed of ACS-N39. Funding for the study was provided by the Canola Council of Canada and Agriculture and Agri-Food Canada as part of the Canola Science Cluster of Growing Forward 2.
Additional information
Funding
References
- Adhikari KC 2010. Effect of temperature, biofungicides and fungicides on clubroot on selected brassica crops [M.Sc. Thesis]. Guelph (ON): University of Guelph.
- Christensen JV, Hennig A, McKenzie JS, Legge W, DePauw G, Siemens RM, Thomas JB. 1985. Effect of seeding date, nitrogen and phosphate fertilizer on growth, yield and quality of rapeseed in northwestern Alberta. Can J Plant Sci. 65:275–284.
- Chupp C. 1917. Studies on clubroot of cruciferous plants. Bull Cornell Agric Exp Stn. 387:419–452. https://archive.org/details/studiesonclubroo00chup
- Colhoun BYJ. 1953. The study of the epidemiology of club-root disease of brassicae. Ann Appl Biol. 40:262–283.
- Cranmer TJ 2015. Vertical distribution of Plasmodiophora brassicae resting spores in soil and the effect of weather conditions on clubroot development [M.Sc. thesis]. Guelph (ON): University of Guelph.
- Crête R, Laliberté J, Jasmin JJ. 1963. Lutte chimique contre la hernie, Plasmodiophora brassicae Wor., des cruciferes en sols mineral et organique. Can J Plant Sci. 43:349–354.
- da Bhering AS, do Carmo MGF, de Matos TS, Lima ESA, do Amaral Sobrinho NMB. 2017. Soil factors related to the severity of clubroot in Rio de Janeiro, Brazil. Plant Dis. 101:1345–1353.
- Deora A, Gossen BD, McDonald MR. 2012. Infection and development of Plasmodiophora brassicae in resistant and susceptible canola cultivars. Can J Plant Pathol. 34:239–247.
- Dhingra OD, Sinclair JB. 1985. Detection and estimation of inoculum. In: Dhingra OD, Sinclair JB, editors. Basic plant pathology methods. Boca Raton (FL): CRC Press.
- Dixon GR. 2014. Clubroot (Plasmodiophora brassicae Woronin) – an agricultural and biological challenge worldwide. Can J Plant Pathol. 36(supp 1):5–18.
- Dobson R, Gabrielson RL, Baker AS. 1982. Soil water matric potential requirements for root‒hair and cortical infection of Chinese cabbage by Plasmodiophora brassicae. Phytopathology. 72:1598‒1600.
- Donald C, Porter I. 2009. Integrated control of clubroot. J Plant Growth Regul. 28:289–303.
- Friberg H, Lagerlöf J, Rämert B. 2005. Germination of Plasmodiophora brassicae resting spores stimulated by a non-host plant. Eur J Plant Pathol. 113:275–281.
- Friberg H, Lagerlöf J, Rämert B. 2006. Usefulness of nonhost plants in managing Plasmodiophora brassicae. Plant Pathol. 55:690–695.
- Gludovacz TV 2013. Clubroot development on canola and cabbage in relation to soil temperature and resistance [M.Sc. thesis]. Guelph (ON): University of Guelph.
- Gossen BD, Adhikari KKC, McDonald MR. 2012a. Effect of seeding date on development of clubroot in short-season brassica crops. Can J Plant Pathol. 34:516–523.
- Gossen BD, Adhikari KKC, McDonald MR. 2012b. Effects of temperature on infection and subsequent development of clubroot under controlled conditions. Plant Pathol. 61:593–599.
- Gossen BD, Deora A, Peng G, Hwang SF, McDonald MR. 2014. Effect of environmental parameters on clubroot development and the risk of pathogen spread. Can J Plant Pathol. 36(S1):37–48.
- Gossen BD, Kasinathan H, Cao T, Manolii VP, Strelkov SE, Hwang SF, McDonald MR. 2013. Influence of pH and temperature on infection and symptom development of clubroot in canola. Can J Plant Pathol. 35:294−303.
- Gossen BD, Kasinathan H, Deora A, Peng G, McDonald MR. 2016. Soil type, soil compaction and biofungicides affect clubroot (Plasmodiophora brassicae). Plant Pathol. 65:1238–1245.
- Gossen BD, McDonald MR, Kalpana KC, Hwang SF, Strelkov SE, Peng G. 2011. Impact of climate change on clubroot of canola on the Canadian prairies. Can J Plant Pathol. 33:277 (abstr).
- Gossen BD, Strelkov SE, Manolii VP, Cao T, Hwang SF, Peng G, McDonald MR. 2015. Spread of clubroot on canola in Canada, 2003–2014. Old pathogen, new home. Can J Plant Pathol. 37:403–413.
- Hamilton HA, Crête R. 1978. Influence of soil moisture, soil ph, and liming sources on the incidence of clubroot, the germination and growth of cabbage produced in mineral and organic soils under controlled conditions. Can J Plant Sci. 58:45–53.
- Hwang SF, Ahmed HU, Strelkov SE, Gossen BD, Turnbull GD, Peng G, Howard RJ. 2011. Seedling age and inoculum density affect clubroot severity and seed yield in canola. Can J Plant Sci. 91:183–190.
- Hwang SF, Cao T, Xiao Q, Ahmed HU, Manolii VP, Turnbull GD, Gossen BD, Peng G, Strelkov SE. 2012a. Effects of fungicide, seeding date and seedling age on clubroot severity, seedling emergence and yield of canola. Can J Plant Sci. 92:1175–1186.
- Hwang SF, Strelkov SE, Feng J, Gossen BD, Howard RJ. 2012b. Plasmodiophora brassicae: A review of an emerging pathogen of the Canadian canola (Brassica napus) crop. Mol Plant Pathol. 13:105–113.
- Kasinathan H 2012. Influences of pH, temperature and biofungicides on clubroot of canola [M.Sc. thesis]. Guelph (ON): University of Guelph.
- Kora C. 2004. Etiology, epidemiology, and management of sclerotinia rot of carrot caused by Sclerotinia sclerotiorum (Lib.) be Bary. [Ph.D. thesis] Guelph (ON): University of Guelph.
- McDonald MR, Kornatowska B, McKeown AW. 2004. Management of clubroot of Asian brassica crops grown on organic soils. Acta Hort. 635:25–30.
- McDonald MR, Westerveld SM. 2008. Temperature prior to harvest influences the incidence and severity of clubroot on two Asian brassica vegetables. HortScience. 43:1509–1513.
- McMaster GS, Wilhelm W. 1997. Growing degree-days: one equation, two interpretations. Agric Forest Meteorol. 87:291–300.
- Monteith J. 1924. Relation of soil temperature and soil moisture to infection by Plasmodiophora brassicae. J Agric Res. 28:549–561.
- Narisawa K, Shimura M, Usuki F, Fukuhara S, Hashiba T. 2005. Effects of pathogen density, soil moisture, and soil pH on biological control of clubroot in Chinese cabbage by Heteroconium chaetospira. Plant Dis. 89:285–290.
- OMAFRA. 2013. Vegetable crop protection guide 2012–2013. Toronto (ON): Queen’s Printer for Ontario. Publication 838: 1–64.
- Peng G, Pageau D, Strelkov SE, Gossen BD, Hwang SF, Lahlali R. 2015. A > 2-year rotation reduces Plasmodiophora brassicae resting spores in soil and the impact of clubroot on canola. Eur J Agron. 70:78–84.
- Saude C, McKeown A, Gossen BD, McDonald MR. 2012. Effect of host resistance and fungicide application on clubroot pathotype 6 in green cabbage and napa cabbage. HortTechnology. 22:311–319.
- Sharma K, Gossen BD, McDonald MR. 2011a. Effect of temperature on primary infection by Plasmodiophora brassicae. Plant Pathol. 60:830–838.
- Sharma K, Gossen BD, McDonald MR. 2011b. Effect of temperature on cortical infection by Plasmodiophora brassicae and clubroot severity. Phytopathology. 101:1424–1432.
- Strelkov SE, Cao T, Manolii VP, Lange RM, Smith-Degenhardt E, Orchard D, Tewari JP. 2006. Incidence of clubroot of canola in Alberta in 2005. Can Plant Dis Surv. 86:91–93.
- Thuma BA, Rowe RC, Madden RV. 1983. Relationships of soil temperature and moisture to clubroot (Plasmodiophora brassicae) severity on radish in organic soil. Plant Dis. 67:758–762.
- Topp GC, Davis JL, Annan AP 1980. Electromagnetic determination of soil water content: Measurements in coaxial transmission lines. Water Resources Res. 16:574–582.
- Turkington T, Olfert OO, Weiss RM, Clear RM, Xi K, Tewari JP, Strelkov SE. 2004. Forecasting the potential distribution and abundance of plant diseases using CLIMEXTM modeling with historical and potential weather scenarios associated with climate change. In: Manitoba Agronomy Conference. p. 99–110. http://www.umanitoba.ca/afs/agronomists_conf/proceedings/2004/0turkington_forecasting_potential.pdf.
- Wallenhammar AC. 1996. Prevalence of Plasmodiophora brassicae in a spring oilseed rape growing area in central Sweden and factors influencing soil infestation levels. Plant Pathol. 45:710–719.
- Wellman FL 1930. Clubroot of crucifers. United States Department of Agriculture 181. http://books.google.ca/books?id=JecDE43ZSnAC&printsec=frontcover&source=gbs_ge_summary_r&cad=0#v=onepage&q&f=false
- Whalley WR. 1993. Considerations on the use of time-domain reflectometry (TDR) for measuring soil water content. J Soil Sci. 44:1–9.
- White I, Knight JH, Zegelin SJ, Topp GC. 1994. Comments on ‘Considerations on the use of time-domain reflectometry (TDR) for measuring soil water content’ by W.R. Whalley. Eur J Soil Sci. 45:503–508.
- Wilson LT, Barnett WW. 1983. Degree-days: an aid in crop and pest management. California Agric. 37:4–7.