Abstract
Twig and blossom blight, and stem dieback, has become an impediment to highbush blueberry (Vaccinium corymbosum L.) production in British Columbia. Periodic assessment of disease symptoms and pathogenicity test confirmed Phomopsis vaccinii to be the major causal agent of the disease. Symptomatic tissues sampled at 7- to 10-day intervals in the 2014 and 2015 growing seasons were examined for presence of pycnidia, their developmental stages, and conidial presence in relation to phenological status of blueberry. Based on histological analysis, pycnidia were grouped into a series of developmental stages, referred to as pre-conidial, early-conidial, conidial, conidial-release and post-conidial stages. These different stages of development coincided with the phenological stages of blueberry plants during the growing season. Pycnidia at the conidial and conidial-release stages were predominantly found on symptomatic tissues sampled from bud-break to bloom; these pycnidia had high conidial contents of 1.0–2.0 × 104 conidia pycnidium−1. Pycnidia at the post-conidial stage were mostly found on symptomatic tissues sampled from green-fruit to post-harvest, under warm and dry weather conditions. Pycnidia at the pre-conidial stage were predominantly observed on symptomatic tissues sampled during latter parts of the growing season, from bud-set to leaf-fall. Mycelial growth of P. vaccinii isolates on potato dextrose agar was measured in response to temperatures from 4 to 32°C; the optimum growth occurred between 20 and 28°C. The number of pycnidia produced on quarter-strength potato dextrose agar at different incubation conditions varied amongst P. vaccinii isolates, and the optimum production was recorded at 16°C with a photoperiod of 14 h light/10 h dark or 14 h UV-A/10 h. Percentage germination of conidia of P. vaccinii isolate 11 BB 14 on water agar was higher at 16, 20 or 24°C than at 8 or 12°C. Conidia at 16°C reached 50% germination at ≥56 h incubation, whereas conidia at 20 and 24°C reached 50% germination at ≥72 h. Percentage germination of conidia at 8 and 12°C remained <50% during 104 h incubation. These findings provide an improved understanding of the role of pycnidia in the epidemiology of twig and blossom blight and stem dieback caused by P. vaccinii on highbush blueberry.
Résumé
En Colombie-Britannique, la brûlure des rameaux et des fleurs ainsi que le dépérissement des tiges sont devenus des obstacles à la production du bleuet en corymbe (Vaccinium corymbosum L.). L’évaluation périodique des symptômes de la maladie ainsi que des tests de pathogénicité ont confirmé que Phomopsis vaccinii était le principal agent causal de la maladie. Des tissus symptomatiques prélevés tous les 7 à 10 jours durant les saisons de croissance de 2014 et de 2015 ont été examinés pour détecter des pycnidies, évaluer leurs stades de développement et déceler des conidies en fonction du statut phénologique du bleuetier. En se basant sur l’analyse histologique, les pycnidies ont été regroupées en stades de développement désignés « pré-conidie », « conidie immature », « conidie mature », « libération des conidies » et « post-conidie ». Ces différents stades de développement correspondaient aux stades phénologiques des bleuetiers durant la saison de croissance. Aux stades de conidie mature et de libération des conidies, on trouvait les pycnidies principalement sur les tissus symptomatiques prélevés sur les bougeons qui débourrent et les fleurs; celles-ci affichaient un compte élevé de conidies, de l’ordre de 1.0–2.0 × 104 conidies pycnidie−1. Au stade de post-conidie, par temps sec et chaud, on trouvait les pycnidies surtout sur les tissus symptomatiques prélevés sur les fruits, tant verts que récoltés. Au stade de pré-conidie, on observait les pycnidies particulièrement sur les tissus symptomatiques prélevés vers la fin de la période de croissance, de l’apparition des bourgeons à la chute des feuilles. La croissance mycélienne des isolats de P. vaccinii sur de la gélose dextrosée à la pomme de terre a été évaluée en fonction de températures variant de 4 à 32°C: la croissance optimale se produisant de 20 à 28°C. Le nombre de pycnidies produites sur de la gélose dextrosée à la pomme de terre à 25% dans des conditions différentes d’incubation a varié selon les isolats de P. vaccinii, et la production optimale a été enregistrée à 16°C pour une photopériode de 14 heures de lumière/10 heures de noirceur, ou 14 heures d’UVA/10 heures de noirceur. Le taux de germination des conidies de l’isolat 11 BB 14 de P. vaccinii sur de l’eau gélosée était plus élevé à 16, 20 ou 24°C qu’à 8 ou 12°C. À 16°C, les conidies ont atteint un taux de germination de 50 % en 56 heures ou plus d’incubation, tandis que, à 20 et 24°C, le taux de germination de 50% a été atteint après 72 heures ou plus. À 8 et 12°C, le taux de germination des conidies est demeuré sous les 50% après 104 heures d’incubation. Ces résultats fournissent une meilleure compréhension du rôle des pycnidies dans l’épidémiologie de la brûlure des rameaux et des fleurs ainsi que du dépérissement des tiges causés par P. vaccinii chez le bleuetier en corymbe.
Introduction
British Columbia is one of the largest producers of highbush blueberry (Vaccinium corymbosum L.) in North America, where most of the production is concentrated in the south-western region of the Fraser Valley. In the Pacific North-west of North America, commonly cultivated varieties are ‘Bluecrop’, ‘Duke’, ‘Elliott’ and ‘Reka’ followed by ‘Liberty’ and ‘Draper’ as well as other minor varieties such as ‘Aurora’ (http://productionguide.agrifoodbc.ca/). With the introduction of many different varieties and rapid expansion of blueberry cultivation in the Fraser Valley, diseases caused by bacteria, fungi and viruses have become more prevalent. Amongst the diseases caused by fungal pathogens, mummy berry (Monilinia vacinnii-corymbosi Reade), anthracnose (Colletotrichum acutatum Simmonds), grey mould (Botrytis cinerea Pers.) and stem canker (Godronia cassandrae Peck.) have been known to cause substantial damage to blueberry production.
Fungal species belonging to the genus Phomopsis (Sacc.) Bubák (teleomorph Diaporthe Shear.) are versatile and cosmopolitan in nature, and they are known to be associated with a number of plant genera, including Vaccinium, as pathogens, endophytes and saprophytes (Uecker Citation1988; Webber Citation2009). In general, Phomopsis spp. are capable of causing many different diseases, such as cankers, root and crown rots, blights, leaf spots, and fruit and seed rots on a wide range of host plants (Smith et al. Citation2005). Symptoms associated with Phomopsis vaccinii Shear were first observed in commercial blueberry fields in the eastern USA (Wilcox Citation1939). Historically, P. vaccinii has been considered to be a relatively minor pathogen of blueberry, primarily causing stem canker and twig blight on susceptible cultivars in the north-eastern regions of North America (Wilcox Citation1940; Parker & Ramsdell Citation1977; Baker et al. Citation1995). However, in recent years, symptoms of twig and blossom blight, and stem die-back of highbush blueberry have become more prevalent in the Fraser Valley, resulting in substantial crop damage and yield losses. Particularly, young, non-bearing plants (2–3 years after planting) appear to be more susceptible to the disease than mature plants. Although P. vaccinii is known to cause diseases on both lowbush and highbush blueberries in North America (Polashock et al. Citation2017), the observed symptoms of twig and blossom blight, and stem dieback on blueberry in the Pacific North-west, particularly in the Fraser Valley, appear to be more damaging than the typical symptoms caused by P. vaccinii as previously described (Wilcox Citation1939; Parker & Ramsdell Citation1977).
Current knowledge of the biology and disease epidemiology of P. vaccinii on cultivated Vaccinium spp., i.e. blueberries and cranberries, is based on previous studies conducted exclusively in the north-eastern regions of the USA (Wilcox Citation1939; Demaree & Wilcox Citation1947; Weingartner & Klos Citation1975; Parker & Ramsdell Citation1977). These studies may not adequately explain the nature and interaction of the pathogen with the host plant in the north-western regions of North America since genotypic/phenotypic variations amongst the pathogen populations and their interactions with the host plant may vary due to geographic separation and climatic variations between the eastern and western regions of North America. Based on the preliminary observations and existing literature on Phomopsis, we hypothesize that Phomopsis is the major pathogen responsible for the observed symptoms of twig and blossom blight, and stem dieback on highbush blueberry in British Columbia.
The objectives of this study were to: (1) assess the occurrence and impact of Phomopsis-associated diseases on highbush blueberry grown in the Fraser Valley; (2) isolate and characterize Phomopsis species associated with symptoms of blossom and twig blight, and stem dieback, and confirm their pathogenicity on blueberry; and (3) determine the key aspects of the biology of the pathogen in relation to host plant phenology and climatic conditions in the Fraser Valley, British Columbia.
Materials and methods
Field sampling
Periodic field inspections were carried out during the growing seasons of 2014 and 2015 to understand the occurrence, distribution and progression of disease symptoms associated with Phomopsis on northern highbush blueberry (Vaccinium corymbosum L.), cultivated in the south-western regions (generally referred to as the Fraser Valley) of British Columbia. During the growth phases, blueberry plants undergo a series of phenological changes – bud-swell, bud-break, green-tip and blossom in spring, fruit-set, green fruit and ripening in summer, and bud-set and leaf-fall in autumn. Bushes with symptoms of twig and blossom blight, stem dieback and bush decline were inspected and recorded periodically. Observations were also made on any correlation between disease symptoms/severity and different cultivars grown in the Fraser Valley. To further understand the disease progress and its impact, 20–30 blueberry plants in each of four commercial fields, expressing symptoms of twig or blossom blight, and stem dieback, were flagged and monitored periodically during the growing seasons of 2014 and 2015. Representative tissue samples from those symptomatic plants were collected during the growing seasons and brought to the laboratory for analysis. Additional information on Phomopsis-related diseases of blueberry available in the internal database of the Plant Health Laboratory, Ministry of Agriculture, British Columbia, was also reviewed and recorded.
Isolation of pathogens from tissues and identification
Symptomatic tissue samples collected from commercial fields were first examined under a stereo dissection microscope to verify the presence of pycnidia. If pycnidia were present, they were examined under a compound microscope (200×) to tentatively identity the pathogens by their spore morphology. Tissue segments 0.5–1.0 cm long, cut from samples representing different symptoms, were surface-sterilized in a 0.5% NaClO solution for one minute and washed three times in sterile distilled water. The tissues were cut into 3–4 mm segments and placed on 9 cm Petri plates, 3–4 segments per plate, containing acidified quarter-strength potato-dextrose-agar (¼PDA) (Difco Laboratories) or acidified water-2% agar (aWA) (Difco Laboratories) medium. Acidified medium was prepared by supplementing 2 mL of 25% lactic acid to 998 mL of ¼PDA or WA. The plates were incubated in the dark at 22°C until growth of fungal colonies from the tissues was apparent. Representative fungal colonies with similar colony characteristics on ¼PDA or WA were transferred to PDA, and maintained in the dark at 22°C. Over 98% of the isolates obtained from symptomatic blueberry tissues were identified as Phomopsis based on the colony characteristics and morphology of alpha and beta conidia, as described previously (Shear et al. Citation1931; Farr et al. Citation2002; Polashock et al. Citation2017). These isolates were further identified to species by PCR amplification of the internal transcribed spacer region of ribosomal DNA with the universal fungal primers ITS1 and ITS4 (White et al. Citation1990) and primers T1 (O’Donnell & Cigelnik Citation1997) and Bt-2b (Glass & Donaldson Citation1995) to amplify part of the β-tubulin gene. DNA was extracted from the fungal mycelia and subjected to PCR amplification (Lee Citation1990). The DNA amplifications were sequenced and compared by BLAST analysis with the GenBank nucleotide database of the National Center for Biotechnology Information (NCBI).
Pathogenicity testing of Phomopsis on blueberry
Since Phomopsis species are known for their ability to cause diseases or exist as non-pathogenic endophytes or saprophytes on a wide range of plant species, three representative isolates of P. vaccinii, 12 BB 10 from ‘Draper’ and 11 BB 14 and BB Gp 2.2 from ‘Liberty’ isolated from symptomatic plant tissues () were tested for pathogenicity on blueberry plants.
Table 1. Assessment of pathogenicity and progress of disease symptoms on highbush blueberry cultivars ‘Draper’, ‘Duke’ and ‘Liberty’ inoculated with three isolates of P. vaccinii.
To generate mature, viable conidia from the isolates for inoculation, each isolate was first grown on ¼PDA at 20°C in the dark for 14 days, and then placed at 16°C and 14 h UV-A light/10 h dark for 3–4 weeks until oozing of conidia from pycnidia was observed. Conidia from each isolate were harvested by flooding the cultures with 10 mL of 0.002% Tween 20 in cold sterile distilled water, and dislodging with a glass spatula. The resulting conidial suspension of each isolate was filtered through a layer of sterile glass wool to remove mycelial fragments and transferred to a sterile 50 mL centrifuge vial. The suspension was centrifuged at 3200 rpm for 15 min, the supernatant was pipetted out and the resulting pellet of conidia was re-suspended in 20 mL of 0.002% Tween 20 in cold, sterile distilled water. The concentration of conidia from each isolate was adjusted to 1 × 106 conidia mL−1 with the aid of a hemocytometer (Bright Line, Hausser Scientific).
In October 2013, 2-year-old, disease-free blueberry plants of ‘Draper’, ‘Duke’ and ‘Liberty’ were obtained from a local propagation nursery in Abbotsford, British Columbia. Plants were replanted in a bark-based blueberry-specific planting medium in 18-litre pots and maintained under direct sunlight in a secure outdoor facility at the Abbotsford Agriculture Centre, Ministry of Agriculture, in Abbotsford. Plants were fertilized with 15-10-11 fertilizer (Evergro Crop Protection Services, BC) twice, on 14 April 2014 and 9 April 2015, and watered regularly over the course of the study period. For the pathogenicity test, 20 plants of each cultivar were divided into four sets of five plants, and, on 16 May 2014, each set was inoculated with each of the three isolates of P. vaccinii. For inoculation, two healthy stems were chosen from each plant, and inoculation was carried out using two different methods when plants were at 50% bloom. In method one, flowers on one of the two stems were spray-inoculated with a conidial suspension of 1 × 106 conidia mL−1, as prepared previously, until wetness using a hand-held atomizer. In method two, 50 µL of conidial suspension was applied onto each of four leaf scars made on the second stem by defoliating the leaves; a wet cotton plug was placed on each leaf scar, and secured with a tape to provide adequate moisture for infection to occur. The control plant sets were inoculated similarly with sterile distilled water. Soon after inoculation, the plants were kept in a humidity chamber at ambient temperature for 18 h to facilitate the infection process, and then removed from the moist chamber and maintained as described previously. The plants were observed weekly for the onset and progress of twig and blossom blight, stem dieback or stem canker during the growing seasons of 2014 and 2015; symptoms were compared with the healthy control plants. Representative tissue samples were collected from symptomatic plants and the pathogen was re-isolated and confirmed as described previously. A sub-set of samples was also incubated in a moist chamber to induce sporulation and conidia were examined microscopically to confirm the presence of P. vaccinii.
Production of pycnidia and sporulation by P. vaccinii
Estimation of pycnidia containing conidia.
Two commercial blueberry fields in the Fraser Valley, designated as Field 1 and Field 2 in Abbotsford and Mission districts, respectively, were chosen as the study sites. These fields had 4- and 5-year-old ‘Draper’, respectively, and a history of Phomopsis incidence of 20–30% as confirmed by field inspections and laboratory analysis of symptomatic plant tissues collected from the fields. In 2014 and 2015, the fields were closely monitored at 7- to 10-day intervals, from early March to December, and plants with visible symptoms of twig and blossom blight, stem dieback, or stem canker were tagged and assessed for disease progress. From each field, 10–20 symptomatic tissue samples were randomly collected at 7- to 10-day intervals, placed in a cooler box and brought to the laboratory for the analyses of pycnidia and contents of conidia. Sampling was not conducted in the winter months of January and February due to dormancy of blueberry plants and inactivity of the pathogen. The samples were processed immediately or placed at 4°C and processed within 24–72 h. Plant tissue samples were first examined under a stereo dissection microscope for the presence of pycnidia. From each of 10 plant tissue samples, about 20 pycnidia were randomly extracted using a sterile surgical scalpel and individually placed in lactophenol-cotton blue stain on a microscope glass slide with a coverslip, gently pressed to release conidia, and examined with a compound microscope (200×). Each pycnidium was scored for the presence or absence of alpha or beta conidia, and the percentage of pycnidia containing conidia was estimated from a total of 200 pycnidia at each sampling time from each field.
Quantification of conidia.
In the 2015 growing season, symptomatic tissue samples were collected from blueberry plants in Field 1 and Field 2, according to the methodology described above. Tissue samples were examined under a stereo dissection microscope, and eight randomly selected pycnidia from each sample were extracted using a sterile surgical scalpel. The extracted pycnidia were individually placed in a cold 50 µL aliquot of 20% glycerol in a sterile 1.2 mL micro-centrifuge tube (Corning Inc., NY) with a 3-mm tungsten bead (QIAGEN, Valencia, CA). The tubes were sealed securely and placed in an ice-bucket to prevent conidia from germinating. The procedure was repeated for a set of 12 tissue samples taken at each sampling time; thus a total of 96 pycnidia were selected for each field at each sampling time. The micro-centrifuge tubes with pycnidia were arranged in a 96 micro-centrifuge tube rack and securely placed in a tissue-lyser apparatus (TissueLyser II, QIAGEN) at 4°C in a cold room. Tissue-lyser was operated at a frequency of 40 Hz for 25 s, adequate to cause rupture and release conidia from pycnidia without damaging the structural integrity of conidia. The micro-centrifuge tubes with conidial suspensions were then centrifuged (Sorvall LegendRT Centrifuge, Thermo-Fisher Scientific, Waltham, MA) at 800 rpm for 4 min, and the tubes with 50 µL aliquots of spore suspensions were stored immediately at −20°C for further analysis. Each tube was thawed over ice and mixed thoroughly, using a vortex mixer, to obtain a homogeneous suspension of conidia for quantification. The number of conidia in two replicate samples of 10 µL aliquots of the conidial suspension withdrawn from each tube was quantified using a hemocytometer (Bright Line, Hausser Scientific, Horsham, PA). The average number of conidia/10 µL volume was calculated from the two counts and the total number of conidia present in a 50 µL sample, i.e. in a single pycnidium, was calculated. The procedure was repeated for a total of 96 pycnidia collected at each sampling time from each field, and the mean number of conidia present in a pycnidium at each sampling time was calculated.
Developmental stages of pycnidia produced by P. vaccinii
Pycnidial production by P. vaccinii on symptomatic blueberry plants was studied by analysing the developmental stages of pycnidia in relation to different phenological status of blueberry plants during the growing season. A subset of symptomatic tissue samples collected previously during the growing seasons of 2014 and 2015 from Field 1 and Field 2 were used in this study. Samples were examined under a stereo dissection microscope, and 5–8 mm long tissue segments with pycnidia were excised. At each sampling time, a total of 5–6 tissue segments were chosen from a set of four symptomatic tissue samples, and the tissue segments were prepared for microtome sectioning according to the procedures described by Ruzin (Citation1999). The tissue segments were fixed in FAA solution (40% formalin:5% acetic acid:47.5% ethanol, v/v) for 7 days. After that, tissue segments were washed in distilled water, dehydrated in a gradient of 50% to 100% ethanol, and placed sequentially in a series of xylene-ethanol solutions, with an increase in xylene concentration. The tissues were then fixed in paraffin, embedded in beeswax and subjected to microtome sectioning. The tissues were transversely sectioned at 50 µm thickness with a rotary microtome, and stained in hematoxylin and eosin. Pycnidia at different developmental stages found on the tissue sections were examined, and the images were photographed at 400× under a compound microscope. The images of pycnidia at different developmental stages were analysed in relation to phenological status of blueberry plants during the growing season.
Weather data for 2014 and 2015 growing seasons
To further understand the disease epidemiology, weather data consisting of weekly average maximum and minimum temperatures and weekly average precipitation (rainfall) for the years 2014 and 2015 for Abbotsford/Mission district were obtained from the Provincial weather forecast, Environment Canada database (https://weather.gc.ca/canada_e.html).
Effect of temperature on mycelial growth rate of P. vaccinii
Six representative isolates of P. vaccinii isolated from blueberry plants with twig and blossom blight, and stem dieback symptoms, and, as a comparison, two isolates of P. vaccinii isolated from cranberry (V. macrocarpon Aiton) plants with dieback symptoms, were used in this experiment (). The mycelial growth rate of the isolates was assessed on three growth media – PDA (Difco Laboratories, USA), corn meal agar (Sigma, USA) and clarified V8 juice agar (V8). PDA and CMA media were prepared according to the manufacturers’ label instructions. For V8 medium, 5 g of CaCO3 was suspended in 355 mL of V8™ juice (Campbell, USA). The suspension was centrifuged at 3200 rpm for 30 min in an IEC Clinical Centrifuge (Damon/IEC Division) and the supernatant was mixed with distilled water at a ratio of 1:4 to a final volume of 500 mL, to which 9 g of agar (Difco Laboratories, USA) was added, and autoclaved at 121°C and 15 psi for 20 min. For the experiment, 9 cm Petri plates containing 20 mL of PDA, CMA and V8 were prepared and used after 48 h. The isolates of P. vaccinii were first grown on PDA for 5–7 days at 22°C in the dark, and 5-mm-diameter mycelial plugs were taken from the actively growing margins of the cultures and placed in the centre of the Petri plates containing PDA, CMA or V8 media. For each isolate, sets of two similarly prepared Petri plates of each medium were used as replicates. The isolates were incubated in the dark at 4, 8, 12, 16, 20, 24, 28 or 32°C in a climate/growth chamber (Sanyo, MLR-350H). After 48 h of incubation, the mycelial growth of each isolate was measured at 24-h intervals for a period of 192 h or until the mycelium almost reached the edge of the Petri plates. From each replicate plate, two measurements of the fungal colony diameter were recorded along two perpendicular axes through the centre of the mycelial plug. Mycelial growth rate was recorded as the growth of mycelium (mm) h−1, calculated from the difference between two consecutive measurements of the fungal colony diameters divided by 24 h. At every 24-h interval, the average growth rate of each isolate was calculated from the four mycelial growth rates recorded from the two replicate plates. At each temperature, the mean optimum growth rate for the isolates of P. vaccinii was calculated from the four highest average growth rates. Statistical analysis was conducted for the combined data from the two separate experiments using SAS Version 9.3. Multiple mean comparison was conducted using one-way ANOVA, Tukey’s test at P = 0.05.
Table 2. Isolates of P. vaccinii obtained from symptomatic tissues of blueberry and cranberry used in the experiments.
Temperature and photoperiod requirements for pycnidia production by P. vaccinii
Temperature and photoperiod requirements for the production of pycnidia by six isolates of P. vaccinii () was investigated on full-, half- and quarter-strength PDA (Difco Laboratories, USA), designated as PDA, ½PDA and ¼PDA, respectively. The isolates were first grown on PDA for 14 days at 22°C in the dark, and 5-mm-diameter mycelial plugs taken from the actively growing margins of the cultures were placed in the centre of the Petri plates containing PDA, ½PDA or ¼PDA. For each treatment, three similarly prepared Petri plates of each medium were used as replicates for each isolate. The plates were first incubated in the dark for 14 days at 22°C to attain sufficient mycelial growth and then subjected to the following treatments for 30 days: 12°C and 24 h dark, 14°C and 24 h dark, 16°C and 24 h dark, 16°C and 14 h light/10 h dark, 16°C and 14 h UV-A (320–400 nm)/10 h dark, and 18°C and 24 h dark a climate/growth chamber (Sanyo, MLR-350H). Exposure of fungal mycelium to electromagnetic radiation of 350–500 nm and near-ultraviolet light (i.e. UV-A or black light) has been shown to induce sporulation of many fungi (Leach Citation1962; Dahlberg & Etten Citation1982). Where applicable, the intensity of light was used at 15 000 lux, similar to intermittent daylight intensity encountered during bud break to early bloom stages of blueberry in the Fraser Valley. The isolates were examined every 24-h, and the time taken for the first appearance of pycnidia by each isolate was recorded. For each treatment, the number of pycnidia produced by each isolate at 15- and 30-day incubation was counted from the three replicate plates and the mean number of pycnidia formed by each isolate was calculated. The experiment was repeated twice. Statistical analysis was conducted for the combined data from the two separate experiments using SAS Version 9.3. Multiple mean comparison was conducted using one-way ANOVA, Tukey’s test at P = 0.05.
Time and temperature requirements for the germination of conidia of P. vaccinii
As a component of the disease cycle, time and temperature requirements for the initiation of germination of conidia in vitro by P. vaccinii was investigated. Isolate 11 BB 14, previously used in the assessment of pathogenicity, was selected for this study. The isolate was first grown on ¼PDA at 22°C in the dark for 14 days and then incubated at 16°C and 14 h UV-A/10 h dark in a climate/growth chamber (Sanyo, MLR-350H) for 3–4 weeks until off-white to cream coloured conidial masses on pycnidia were visible; these conidia were considered matured and capable of germinating, and therefore used in the experiment. Using a sterile loop, conidia were collected from pycnidia, suspended in cold, sterile distilled water, and the concentration was adjusted to 1 × 106 conidia mL−1 and kept in an ice bath to prevent germination. A thin layer of sterile, 2% agar medium (WA) coated on microscope glass slides was used as a base-substrate for the germination of conidia. The conidial suspension was mixed on a vortex mixer and two 15 µL aliquots of the suspension were placed apart from each other on each of three similarly prepared microscope slides coated with WA and secured with coverslips. The slides were incubated at 8, 12, 16, 20, or 24°C in a climate/growth chamber (Sanyo, MLR-350H). At 2-h intervals, the number of germinated conidia from a total of 200 randomly selected conidia on each of the three slides was counted using a compound microscope (200×), and the mean percentage of germinated conidia, with germ tubes of ≥6–8 µm length, was estimated. From the graph of percentage germination vs. duration of germination, the time taken for 50% germination of conidia at each temperature was determined.
Results
Phomopsis-associated symptoms on blueberry
Blighting of young twigs and flowers, followed by necrosis and dieback of stems, were the most commonly observed symptoms on blueberry cultivars grown in the Fraser Valley of British Columbia (). These symptoms generally originated as necrosis of buds or blighting of young twigs as early as from bud-break (in early March) to flowering (until May) of blueberry plants. As the disease progressed, infected twigs turned brown to dark-brown in colour, and symptoms appeared to spread downwards to the rest of the stem over time, resulting in necrosis and dieback of stems, and weakening of plants. Infected flowers showed early symptoms of browning, and became blighted and shrivelled. In some cases, particularly on ‘Aurora’, premature colouring and dropping of young fruit were noticeable (). Regardless of cultivar, infected stems often had browning of pith, and discolouration of internal tissues just beneath the bark; a mottled pattern of green, healthy tissue was interspersed with brown discolouration (). Although the symptoms were seen on all cultivars grown in the Fraser Valley, i.e. ‘Bluecrop’, ‘Duke’, ‘Elliot’, ‘Reka’, ‘Draper’, ‘Liberty’ and ‘Aurora’, the degree of disease severity varied amongst cultivars. As a general observation, young, non-fruit bearing plants, particularly ‘Draper’, appeared to be more susceptible to twig blight, stem necrosis and dieback than mature, fruit-bearing plants. The observed symptoms on blueberry plants could also be mistaken for symptoms of bacterial blight, caused by Pseudomonas syringae pv. syringae, blueberry scorch, caused by Blueberry scorch virus, or blueberry shock, caused by Blueberry shock virus. In addition to these commonly observed symptoms, a few plants (less than 1%) with twig and blossom blight or stem dieback symptoms also produced young stems that had stunted growth and shiny, silvery leaves ().
Fig. 1 (Colour online) Symptoms expressed by blueberry plants infected with P. vaccinii. (a) Twig blight on ‘Aurora’; (b) blossom blight; (c) stem necrosis and dieback; (d) stem canker; (e) silvery leaf; (f) internal tissue discolouration on ‘Draper’.

Over 98% of the fungal isolates obtained from the representative symptomatic tissues collected from blueberry fields were identified as Phomopsis, based on colony characteristics and morphology of alpha and beta conidia (Shear et al. Citation1931; Farr et al. Citation2002; Polashock et al. Citation2017). These isolates were further confirmed as P. vaccinii that had 98–100% nucleotide identity and 99% query coverage with P. vaccinii (teleomorph Diaporthe vaccinii) strains in the BLAST search. Besides P. vaccinii, fungal isolates recovered from tissue samples collected from plants that had silvery leaf symptom were identified as Chondrostereum purpureum Pouzar.
Pathogenicity of Phomopsis on blueberry
Pathogenicity of P. vaccinii on blueberry cultivars ‘Draper’, ‘Duke’ and ‘Liberty’ are shown in . Initial symptoms on blueberry plants inoculated with the isolates of P. vaccinii began as twig blight in the early summer of 2014, and later progressed as necrosis and dieback of stems over a period of 12 months. These symptoms were mainly observed on ‘Draper’ and ‘Duke’, but no visible symptoms were observed on ‘Liberty’. In 2015, symptoms of severe dieback and flagging of stems were observed on ‘Draper’ and ‘Duke’ inoculated with each of the three isolates of P. vaccinii; whereas, only one out of five plants of ‘Liberty’ inoculated with either P. vaccinii isolate 12 BB 10 or BB GP 2.2 resulted in stem dieback. Symptoms of twig blight followed by stem necrosis and dieback observed on the inoculated plants over a 12-month period resembled the symptoms on blueberry plants naturally infected with P. vaccinii in the field. Although the observed symptoms were expressed by all three cultivars, symptoms were more pronounced on ‘Draper’ and ‘Duke’ than on ‘Liberty’. On all three cultivars, plants inoculated with P. vaccinii isolates through leaf scars failed to develop visible canker lesions on stems during the course of the study. Pathogenicity was confirmed by re-isolating P. vaccinii isolates from the symptomatic plant tissues, including twig, stem and crown of all three cultivars. Plants from the non-inoculated control treatment remained healthy during the duration of the study; however, one of the five plants from ‘Draper’ and ‘Liberty’ had blighted stems, but no pathogen was isolated from the blighted tissues.
Production of pycnidia and sporulation by P. vaccinii
Production of pycnidia and conidia by P. vaccinii on symptomatic tissues collected from Field 1 and Field 2 showed a similar trend during the growing seasons of 2014 and 2015 (, ). Regardless of the time of sample collection during the growing season, pycnidia were continually present on symptomatic tissues throughout the sampling period. In 2014 and 2015, the percentage of pycnidia with conidia on symptomatic tissues collected from bud-break to early fruit-set in both fields remained over 70 and 80%, respectively, and, thereafter, declined to nearly 50%. Regardless of sampling time, at least 50% of the total number of pycnidia analysed contained conidia at varying densities. The mean number of conidia pycnidium−1 on symptomatic tissues collected from Field 1 and Field 2 in 2015 varied during the growing season (). The highest number of conidia of 1.0–2.0 × 104 pycnidium−1 was estimated on symptomatic tissues collected from bud-break to early bloom. The mean number of conidia pycnidium−1 declined to 4.0–8.0 × 103 during fruit set and green fruit stages and, thereafter, declined to as low as <1.0 × 102 conidia pycnidium−1 during bud-set and leaf-fall stages.
Developmental stages of pycnidia produced by P. vaccinii
The photographic images of the microtome sections of pycnidia of P. vaccinii found on symptomatic blueberry plant tissues collected during the growing season showed various developmental stages of pycnidia (). Based on the histological analysis of the photographic images, pycnidia were classified into five distinct developmental stages: pre-conidial stage, early-conidial stage, conidial stage, conidial-release stage and post-conidial stage. Pycnidia at the pre-conidial stage () were predominantly found on symptomatic tissues collected late in the crop season during bud-set and leaf-fall. These pycnidia were elliptical to oblong in shape and often immersed in the epidermal and upper parenchymatous layers of infected plant tissues. They had a thin layer of hymenium, and no conidiophore initials or conidia were present. These immature pycnidia appeared to be arrested from developing further. Pycnidia at the early-conidial stage () were mostly found on symptomatic tissues collected early in the crop season, from bud-swell to bud-break, and less abundantly during the rest of the crop season. Pycnidia at the early-conidial stage appeared to be darker and more swollen than those at the pre-conidial stage. They had a dense layer of hymenium and no ostiole was present. In some cases, differentiation of cells into conidiophores and initiation of conidia on the proximal layer of the hymenium tissue was evident. Pycnidia at the conidial () and conidial release stages () were found in abundance on symptomatic tissues collected early in the growing season, from bud-break to bloom, and had varying densities of conidia. Pycnidia at the conidial stage had nearly one-third to half of the proximal hymenium tissue with conidiogenous cells and conidia and, in some cases, conidia were directed towards the apical neck of pycnidia. These pycnidia were dark-coloured, conically shaped and found mostly on the upper parenchymatous and epidermal layers of the host plant. At the conidial-release stage, masses of conidia were seen in the centre and towards the ostiole of pycnidia. Pycnidia at the post-conidial stage () had ruptured conidiomata with none or very few conidia present; these pycnidia were mostly observed on symptomatic tissues collected late in the summer and early autumn, during bud-set and leaf-fall.
Fig. 2 Percentage of pycnidia of P. vaccinii containing conidia as estimated from 200 randomly selected pycnidia from symptomatic tissue samples of blueberry plants ‘Draper’. Symptomatic tissue samples were collected from two blueberry fields at different sampling times during the growing season, from March to December of 2014 (a) and 2015 (b).
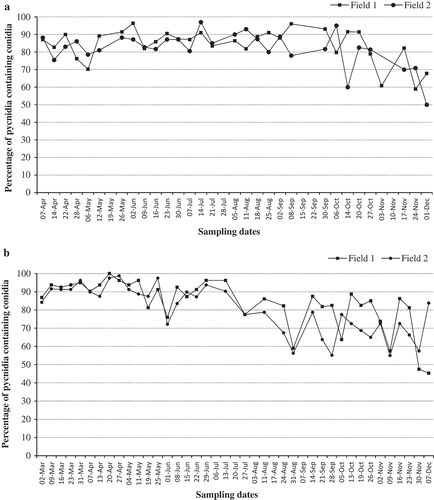
Fig. 3 Mean number of conidia per pycnidium produced by P. vaccinii, estimated from 96 randomly collected pycnidia from symptomatic tissue samples of ‘Draper’ collected from two blueberry fields at different sampling times during the growing season, from March to December, of 2015. Vertical capped lines = Standard error (n = 96).
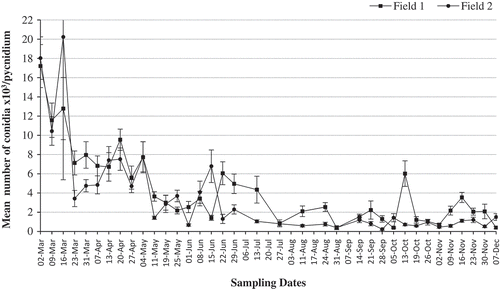
Fig. 4 (Colour online) Pycnidia of P. vaccinii at different developmental stages (magnification 400×) observed on symptomatic tissues of ‘Draper’, collected from two blueberry fields at different sampling times during the growing season, from March to December of 2014 and 2015. (a, b) pre-conidial stage; (c, d) early-conidial stage; (e, f) conidial stage; (g, h) conidial-release stage, and (i, j) post-conidial stage. Scale bar = 50 µm.
Weather data for 2014 and 2015 growing seasons
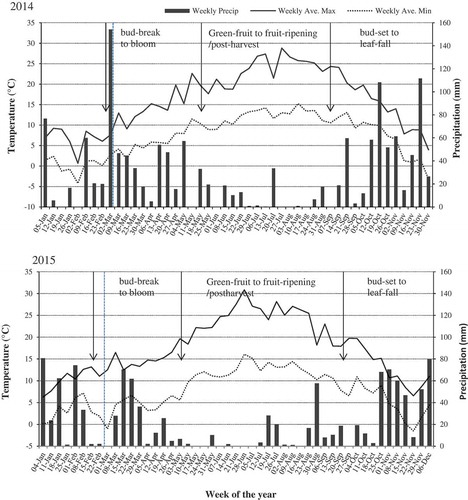
Weekly average daily maximum and minimum temperatures and weekly average precipitation (rainfall) for 2014 and 2015 growing seasons are shown in . With reference to blueberry growing season in the Fraser Valley, approximate phenological stages of blueberry plants in relation to field climatic conditions are depicted in . In general, the south-western region of the Fraser Valley, where blueberry crops are grown, experiences cool and wet weather conditions in spring and autumn, and dry and moderate temperatures in summer.
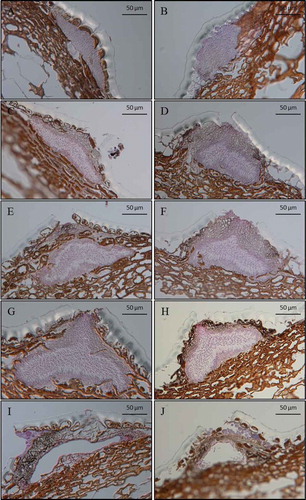
Fig. 5 Mean growth rate of mycelium of P. vaccinii isolates 11 BB 14, 12 BB 01, 12 BB 10, 12 BB 11, 12 BB 12 and BB GP 4.3 from blueberry, and 07 CB 04 and 07 CB 10 from cranberry, as determined on PDA in the dark at 4, 8, 12, 16, 20, 24, 28 or 32°C. Bars representing the same P. vaccinii isolate with the same letter do not differ at P = 0.05, based on Tukey’s multiple means comparison test. Vertical capped lines = Standard deviation (n = 8).
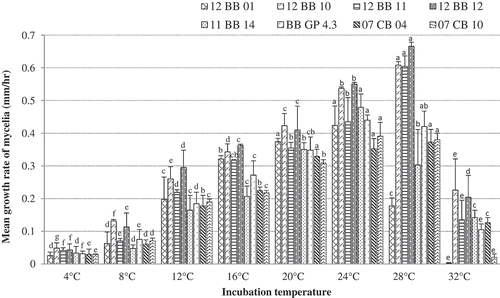
Fig. 6 Mean number of pycnidia of P. vaccinii isolates 11 BB 14, 12 BB 10, 12 BB 12 and BB GP 4.3 from blueberry, and 07 CB 04 and 07 CB 10 from cranberry, produced on ¼PDA incubated for 30 days at (a) 12°C and 24 h dark; (b) 14°C and 24 h dark; (c) 16°C and 24 h dark; (d) 16°C and 14 h light/10 h dark; (e) 16°C and 14 UV-A/10 h dark; and (f) 18°C and 24 h dark. Bars representing the same P. vaccinii isolate with the same letter do not differ at P = 0.05, based on Tukey’s multiple means comparison test. Vertical capped lines = Standard deviation (n = 6).
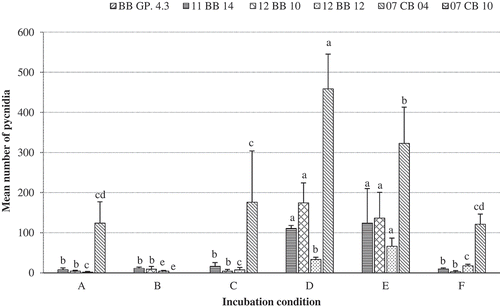
Fig. 7 Mean percentage germination of conidia of P. vaccinii isolate 11 BB 14 from blueberry as determined at 8, 12, 16, 20 and 24°C on a thin layer of WA medium on microscope glass slides in a moist chamber for a period of 104 h. Vertical capped lines = Standard deviation (n = 3).
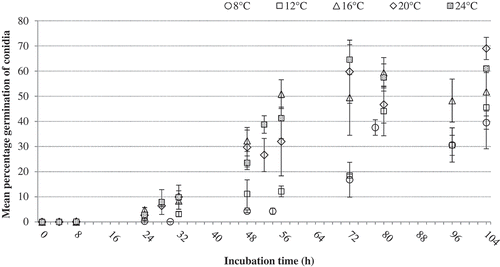
Fig. 8 Weekly average day maximum (horizontal line) and minimum (horizontal dotted line) temperatures and weekly average precipitation (solid vertical bar) recorded for 2014 and 2015 growing seasons in the Fraser Valley, British Columbia. With reference to blueberry growing season in the Fraser Valley, approximate timing of sequential phenological stages of blueberry plants in relation to climatic conditions are indicated within vertical arrows. Vertical broken lines indicate the beginning of sampling of symptomatic plant tissues from blueberry fields. Weather data were obtained from Environment Canada database, Provincial weather forecast (https://weather.gc.ca/canada_e.html).
Temperature dependent mycelial growth of P. vaccinii
The isolates of P. vaccinii from blueberry and cranberry displayed similar trends in their mycelial growth between 4 and 32°C on all three media; however, all the isolates had much higher growth rates on PDA () than on either CMA or V8 (data not presented). Although mycelial growth occurred between 4 and 32°C, greater mycelial growth rates were recorded between 12 and 28°C than at 4, 8 or 32°C. Isolates 12 BB 10, 12 BB 11 and 12 BB 12 from blueberry had the highest growth rate at 28°C, whereas isolates 11 BB 14, 12 BB 01 and BB Gp 4.3 from blueberry and 07 CB 04 and 07 CB 10 from cranberry had the highest growth rate at 24°C. Although the temperature requirements for mycelial growth of cranberry isolates 07 CB 04 and 07 CB 10 were similar to the blueberry isolates, they had relatively lower growth rates than the blueberry isolates.
Temperature and photoperiod requirements for pycnidia production by P. vaccinii
The mean number of pycnidia produced by isolates of P. vaccinii on ¼PDA exposed to various conditions for 30 days is depicted in . In general, isolates of P. vaccinii produced more pycnidia on ¼PDA than on ½PDA or PDA (data not presented). Regardless of the isolate, initiation of pycnidia was observed at 5–7 days of being exposed to various incubation conditions, and the number of pycnidia produced increased over the 30 days of incubation. Isolate BB GP 4.3 from blueberry and 07 CB 10 from cranberry did not produce any pycnidia under the experimental conditions tested. Isolates 11 BB 14, 12 BB 10 and 12 BB 12 from blueberry produced pycnidia in high numbers when incubated at 16°C, but low numbers of pycnidia (≤20) were produced at 12, 14, or 18°C. In contrast, production of pycnidia by isolate 07 CB 04 was high at temperatures between 12 and 18°C; however, the highest number of pycnidia was produced at 16°C. In general, P. vaccinii isolates exposed to 16°C and 14 h light/10 h dark or 16°C and 14 h UV-A/10 h dark produced significantly higher numbers of pycnidia than when they were exposed to 16°C and 24 h dark.
Time and temperature requirements for the germination of conidia of P. vaccinii
The mean percentage germination of conidia of P. vaccinii isolate 11 BB 14, as determined on a thin layer of WA on microscope glass slides at different temperatures over a period of 104 h, is shown in . In general, the mean percentage germination of conidia at all temperatures increased with the increase in the length of incubation; germination of <10% was observed at 24 h but the percentage germination increased thereafter. The mean percentage germination at 16, 20 or 24°C was comparatively higher than the mean percentage germination at 8 or 12°C. Conidia when incubated at 16°C reached 50% germination at ≥56 h, whereas conidia at 20 and 24°C achieved 50% germination at ≥72 h. At 8 and 12°C, the mean percentage germination of conidia remained below 50% over the period of 104 h incubation.
Discussion
The primary symptoms of twig and blossom blight, and stem dieback, observed on highbush blueberry grown in the south-western region (Fraser Valley) of British Columbia were primarily caused by the fungal pathogen P. vaccinii. More than 98% of the isolates of Phomopsis recovered from the symptomatic tissues were confirmed as P. vaccinii, based on their colony characteristics on culture media, conidial morphology and pathogenicity assessment on blueberry cultivars. Inoculation of blueberry cultivars at flowering with a conidial suspension of P. vaccinii isolates resulted in twig and blossom blight and dieback of stems, indicating that blueberry plants at the bloom stage are more vulnerable to infection by P. vaccinii and, thus, flowers serve as an entry point for the pathogen. Our results complement the studies conducted in North Carolina where blighting of twigs was primarily the result of infection of blueberry plants via flowers by P. vaccinii (Weingartner & Klos Citation1975; Milholland Citation1982). In contrast, in our study, inoculation of 3-year-old stems with P. vaccinii isolates through fresh leaf scars failed to produce canker lesions on the inoculated stems. Although the failure to develop canker lesions at the wounded, inoculated sites cannot be fully explained, Baker et al. (Citation1995) demonstrated that inoculation of young shoots with P. vaccinii led to the mortality of shoots without producing any visible cankers on stems. Nevertheless, canker lesions were often observed on mature, woody stems of blueberry plants in the Fraser Valley, supported by the studies where woody stems inoculated with P. vaccinii through open wounds resulted in stem canker development (Wilcox Citation1939; Weingartner & Klos Citation1975). It was also reported that P. vaccinii tended to cause symptoms of twig blight more than stem canker in the south-western regions while stem canker was more pronounced in the north-eastern USA (Schilder et al. Citation2006). In Massachusetts and Michigan, symptom of dieback was the direct result of stem canker caused by P. vaccinii (Wilcox Citation1939; Parker & Ramsdell Citation1977), whereas in North Carolina and New York, twig blight and stem dieback were more prevalent than stem canker (Milholland Citation1982; Carrol et al. Citation2009). The primary symptoms of blighting of twigs and blossoms followed by necrosis and dieback of stems observed on blueberry plants subsequently resulted in discolouration of internal stem tissues and infection of crown tissue, where stems and roots originate. In our study, isolation of P. vaccinii from symptomatic stem and crown tissues over the course of disease development confirmed the pathogen’s ability to colonize blueberry plants systemically, as described previously (Weingartner & Klos Citation1975; Milholland Citation1982). Amongst blueberry plants infected with P. vaccinii that had symptoms of stunting and silvering of leaves, the fungus Chondrostereum purpureum Pouzar was also isolated from the symptomatic tissues. Chondrostereum purpureum has been reported to cause silver leaf on blueberry in the USA and Chile (France et al. Citation2009). In our observation, infection of P. vaccinii-infected plants by C. purpureum could be considered secondary, since wounds created by pruning of P. vaccinii-infected stems, as a standard management practice, perhaps aided as entry sites for C. purpureum.
The various developmental stages of pycnidia observed on P. vaccinii-infected blueberry plants apparently play a crucial role in the disease epidemiology. Although pycnidia were observed on symptomatic tissues throughout the growing seasons of 2014 and 2015, occurrence of different developmental stages of pycnidia and the number of conidia found within pycnidia varied depending on the phenological status of blueberry plants, mediated by climatic conditions during the growing season. In our study, pycnidia at the early-conidial, conidial and conidial-release stages were predominantly found on symptomatic plant tissues collected from bud-swell to bloom stages of blueberry, early in the growing season. During this time, weekly average temperatures were in the range of 8–16°C (day maximum) and 4–10°C (day minimum), and weekly average precipitation of 5 to ≥80 mm with frequent rainfall events, depending on the year (); these cool and wet weather conditions are typical for the south-western region of the Fraser Valley. During this period, blueberry plants undergo a sequence of phenological stages of bud-swell, bud-break, green-tip and bloom, and also become vulnerable to infection by pathogens, including P. vaccinii. It is apparent from our study that over 80% of pycnidia produced by P. vaccinii on blueberry from bud-break to bloom stages contained conidia in high numbers, 1.0–2.0 × 104 conidia pycnidium−1, when recurrent precipitation events and moisture were available for dispersal and germination of conidia. These findings correspond with the timing of sporulation of P. vaccinii on blueberries observed in Michigan (Parker & Ramsdell Citation1977) and North Carolina (Milholland Citation1982), where the highest number of conidia was encountered during bud break and bloom stages. While symptoms of twig and blossom blight observed early in the growing season progressed into necrosis and dieback of stems during mid-growing season, i.e. from green-fruit to fruit ripening, the percentage of pycnidia containing conidia was reduced to ≤50%, and the number of conidia within pycnidia declined to <1.0 × 102 conidia pycnidium−1. During this period, most of the pycnidia examined were at the post-conidial stage, while pycnidia at the pre- or early-conidial stages were seldom observed. Both 2014 and 2015 growing seasons had warm and dry weather conditions with weekly average temperatures of 20–28°C day maximum and 12–18°C day minimum, and weekly average precipitations of 0 to ≤20 mm with occasional rainfall events, depending on the year (). These observations indicated that, under warm and dry weather conditions, P. vaccinii was less active in producing conidia or initiating new infections on blueberry, particularly when rainfall events and moisture are limiting for the dispersal and germination of conidia. Pycnidia observed on symptomatic tissues collected from bud-set to leaf-fall stages of blueberry late in the growing season were predominantly at the pre-conidial stage. Further development of these pycnidia into early-conidial and conidial stages were not observed during the course of the study, despite the weather conditions (i.e. weekly average day maximum and minimum temperatures and weekly average precipitations) that were similar to those encountered early in the growing seasons of 2014 and 2015 (); these pycnidia apparently remained ‘dormant’ until the following growing season. This indicates that physiological changes take place in the host plant when entering dormancy that may trigger or signal the pathogen to prepare for overwintering. It is safe to predict that most of the actively sporulating pycnidia that were observed on symptomatic plant tissues from bud-break to bloom stages could be the result of overwintering pycnidia from the previous year and pycnidia produced by the pathogen in the current year. Milholland (Citation1982) observed that the total inoculum of P. vaccinii was the combination of conidia produced by pycnidia formed during the current-season and overwintered pycnidia from the previous season.
In our study, production of pycnidia by P. vaccinii isolates in vitro was observed at temperatures from 12 to 18°C with the highest number of pycnidia at 16°C, particularly when the isolates were exposed to 16°C and 14 h light/10 h dark or 14 h UV-A/10 h dark. Exposure of fungal mycelium to electromagnetic radiation of 350–500 nm and near-ultraviolet light (i.e. UV-A or black light) as being a part of the spectrum of sunlight, was shown to induce sporulation of many fungi (Leach Citation1962; Dahlberg & Etten Citation1982). Although the behaviour of the pathogen under laboratory conditions may not best predict the activity of the pathogen under natural environments, these results supported our findings of pre-conidial, early-conidial and conidial stages of pycnidia found on symptomatic plant tissues collected early in the growing season from bud-break to bloom, when conditions were cool and wet with intermittent rainfall and low light intensities. Therefore, it can be predicted that most of the infection of blueberry plants by P. vaccinii takes place early in the growing season. In our studies, germination of conidia of P. vaccinii isolate 11 BB 14 in vitro was recorded at temperatures between 8 and 24°C, where 50% germination was attained after 56 h incubation at 16, 20 or 24°C. In vitro mycelial growth of P. vaccinii isolates was measured at temperatures between 8 and 28°C with an optimum growth at 24 and 28°C. It has been shown previously that the onset of disease by P. vaccinii is dependent on the dispersal of conidia mediated by precipitation events, infection of host plants mediated by surface moisture (wetness), and host defence factors (Polashock & Kramer Citation2006). Results from the in vitro experiments suggest that although germinating of conidia may take many hours, infection and disease development by P. vaccinii can occur during most of the growing season; however, these events can be limited by lack of precipitation and moisture under dry weather conditions.
These findings provide an improved understanding of the role of pycnidia in the epidemiology of twig and blossom blight and stem dieback caused by P. vaccinii on highbush blueberry. Management of disease caused by P. vaccinii on blueberry warrants an integrated approach, including selection of resistant or tolerant varieties, plant canopy management to minimize surface wetness, and selection and application of effective fungicides at the right time. Although ‘Draper’ and ‘Duke’ appeared to be more susceptible to P. vaccinii than other cultivars grown in the Fraser Valley, a systematic approach for screening and selecting resistant varieties more suitable for the growing conditions in the south-western region of British Columbia is necessary. Our field observations also suggest that young, non-bearing plants are more susceptible to twig and blossom blight than mature plants. However, this could be due to the fact that unlike mature, fruit-bearing plants, non-bearing plants are generally not treated with fungicides during the growing season and, therefore, not protected from plant pathogens. Our studies also confirmed that P. vaccinii is most active in producing and dispersing conidia early in the growing season, when blueberry plants are at the phenological stages of bud-swell, bud-break, green-tip and bloom. Therefore, timing of application of fungicides needs to be adjusted from year to year, depending on weather conditions, to correspond with the crop developmental stages. It has been shown that conidia of P. vaccinii may require long hours of wetness, i.e. presence of plant surface moisture, for germination and, therefore, maintaining an open plant canopy to allow adequate airflow to reduce plant surface wetness would greatly reduce the probability of conidial germination and infection by P. vaccinii.
Acknowledgements
We sincerely thank Blueberry Junction Farm, Faulkner Farm, Gill’s Farm and South Alder Farm for their cooperation and allowing access for conducting observations and collecting plant tissue samples. We thank Sidhu and Growers Nursery for providing blueberry cultivars for the experiments. We gratefully acknowledge Mark Sweeney for his guidance and contribution to the project and Karina Sakalauskas for project administration. We also acknowledge Sandra Etheridge for providing technical assistance with the histology work. We extend our thanks to Gayle Jesperson for critical review of the manuscript. This work was primarily supported by the AgriInnovation Program, an industry-led research and development stream funded by Agriculture and Agri-Food Canada, and Growing Forward 2, a federal-provincial-territorial policy framework, by the government of British Columbia.
Additional information
Funding
References
- Baker JB, Hancock JF, Ramsdell DC. 1995. Screening highbush blueberry cultivars for resistance to Phomopsis canker. Hort Sci. 30:586–588.
- Berries Production Guide: Beneficial management practices for berry growers in British Columbia. British Columbia Ministry of Agriculture, Province of British Columbia; [accessed date: May 9, 2017]. http://productionguide.agrifoodbc.ca/.
- Carrol JE, Fuchs M, Cox K. 2009. Results of a New York blueberry survey. N Y Fruit Quart. 17:19–22.
- Dahlberg KR, Etten J. 1982. Physiology and biochemistry of fungal sporulation. Annu Rev Phytopathol. 20:281–301.
- Demaree JB, Wilcox MS. 1947. Fungi pathogenic to blueberries in the eastern United States. Phytopathology. 47:487–506.
- Farr DF, Castlebury LA, Rossman AY. 2002. Morphological and molecular characterization of Phomopsis vaccinii and additional isolates of Phomopsis from blueberry and cranberry in the eastern United States. Mycologia. 94:494–504.
- France A, Santelices C, Buddie A, Kirk P. 2009. Silver leaf: First worldwide report of a new and harmful disease on blueberry. Acta Hortic. 810:341–344.
- Glass NL, Donaldson G. 1995. Development of primer sets designed for use with PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol. 61:1322–1330.
- Leach CM. 1962. Sporulation of diverse species of fungi under near-ultraviolet radiation. Can J Bot. 40:151–161.
- Lee SB. 1990. Isolation of DNA from fungal mycelia and single spore. In: Innis MA, Gelfand DH, Sninsky TJ, White TJ, editors. PCR protocols: A guide to methods and applications. San Diego (CA): Academic Press; p. 282–287.
- Milholland RD. 1982. Blueberry twig blight caused by Phomopsis vaccinii. Plant Dis. 66:1034–1036.
- O’Donnell K, Cigelnik E. 1997. Two divergent intergenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol Phylogenet Evol. 7:103–116.
- Parker PE, Ramsdell DC. 1977. Epidemiology and chemical control of phomopsis canker of highbush blueberry. Phytopathology. 67:1481–1484.
- Polashock JJ, Caruso FL, Averill AL, Annemiek CS, editors. 2017. Compendium of blueberry, cranberry, and lingonberry diseases and pests. St. Paul (MN): APS Press.
- Polashock JJ, Kramer M. 2006. Resistance of blueberry cultivars to botryosphaeria stem blight and phomopsis twig blight. Hort Sci. 41:1457–1461.
- Ruzin SE. 1999. Plant microtechniques and microscopy. Oxford, New York: Oxford University Press; p. 322.
- Schilder AMC, Hancock JF, Hanson EJ. 2006. An integrated approach to disease control in blueberries in Michigan. Acta Hortic. 715:481–488.
- Shear CL, Steven NE, Bain HF. 1931. Fungal diseases of the cultivated cranberries. Technical Bulletin. United States Department of Agriculture. No. 254:7-8.
- Smith IM, Scott PR, Holderness M, McNamara DG, Burger BV, editors. 2005. Diaporthe vaccinii. Quarantine pests for Europe, 2nd ed. Data sheets on quarantine pests for the European Union and for the European and Mediterranean Plant Protection Organization. Wallingford (UK): CAB International.
- Uecker FA. 1988. A world list of Phomopsis names with notes on nomenclature, morphology, and biology. Mycol Mem. 13:1–323.
- Webber D. 2009. Endophytic fungi, occurrence and metabolites. In: Anke T, Webber D, editors. Physiology and genetics. The Mycota (Vol. 15). Berlin (DE): Springer-Verlag; p. 153–195.
- Weingartner DP, Klos EJ. 1975. Etiology and symptomatology of canker and dieback diseases on highbush blueberries caused by Godronia (Fusicoccum) cassandrae and Diaporthe (Phomopsis) vaccinii. Phytopathology. 65:105–110.
- White TJ, Bruns T, Lee J, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. San Diego (CA): Academic Press; p. 315–322.
- Wilcox M. 1939. Phomopsis twig blight of blueberry. Phytopathology. 29:136–142.
- Wilcox M. 1940. Diaporthe vaccinii, the ascigerous stage of Phomopsis, causing a twig blight of blueberry. Phytopathology. 30:441–443.
