Abstract
Potato cyst nematodes (PCN) – Globodera rostochiensis and G. pallida – cause significant yield losses on potato worldwide. One of the main challenges to PCN management is the ability of PCN to remain dormant in the soil for several decades. For that reason, many countries have strict quarantine regulations for PCN. These regulations, although expensive and restrictive for growers, are necessary to prevent further spread of PCN but should be lifted when no more viable cysts are found. Here, we report a promising qRT-PCR method for the quantification of viable eggs and propose that this method be included in routine testing. The method was successful for quantifying G. ellingtonae, G. rostochiensis and G. pallida and was found to be very sensitive with the systematic detection of a single larva. Intron-flanking probes were used to eliminate the possibility of false positives due to genomic DNA, and an internal control was added to detect failure in PCR due to inhibitors. No amplification occurred during the testing of eggs that had previously received heat treatments or fumigation with methyl bromide. This qRT-PCR assay was used to evaluate the viability of field populations of G. rostochiensis 10 years after the establishment of a quarantine area in Saint-Amable, Quebec, Canada. The number of viable eggs after a decade of regulation was found to be very low and confirmed the effectiveness of the measures put in place. Egg viability was also monitored in microplots following five continuous years of planting resistant potatoes, and no signs of resistance-breaking genotypes were observed.
Résumé
Les nématodes à kyste de la pomme de terre (NKPT), Globodera rostochiensis et G. pallida, causent des pertes économiques significatives à la culture de pomme de terre à travers le monde. Un des principaux défis dans la gestion des NKPT est leur capacité à survivre durant plusieurs décennies dans le sol. Pour cette raison, les NKPT sont justiciables de quarantaine dans plusieurs pays. Même si les coûts et l’impact sur les producteurs sont élevés, cette réglementation est nécessaire afin de prévenir la dispersion des NKPT. Par contre, ces mesures devraient être levées dès que les NKPT viables ne sont plus détectés. Nous proposons ici une méthode prometteuse de qRT-PCR pour la quantification de la viabilité des œufs et suggérons qu’elle soit incluse dans les tests de routine pour les NKPT. La méthode s’est avérée être efficace pour G. ellingtonae, G. rostochiensis et G. pallida en plus de démontrer une très bonne sensibilité avec la détection systématique de larves uniques. Une sonde flanquant un intron a été utilisée afin d’éliminer les risques de faux positifs causés par la présence d’ADNg. De plus, un contrôle interne a été ajouté afin de détecter les faux négatifs causés par la présence d’inhibiteurs. Aucune amplification n’a eu lieu lorsque les œufs avaient préalablement été tués par la chaleur ou fumigés avec du bromure de méthyle. Cette méthode a également été utilisée afin d’évaluer la viabilité de populations de G. rostochiensis dans des champs sous quarantaine depuis 10 ans dans la municipalité de Saint-Amable, Québec, Canada. Le nombre d’œufs viables retrouvé était très faible ce qui valide l’efficacité des méthodes de lutte mises en place. Une évaluation d’échantillons en provenance de micro-parcelles ayant été cultivées durant cinq années consécutives avec des cultivars de pomme de terre résistants à G. rostochiensis n’a démontrée aucun signe de développement de génotypes ayant contournés cette résistance.
Introduction
Potato cyst nematodes (PCN) – Globodera rostochiensis and G. pallida – are obligate parasites of solanaceous plants. These species are considered the most important nematode threat to potato production worldwide (Turner & Evans, Citation1998), causing estimated annual losses of 9% (Turner & Rowe, Citation2006). In North America, the golden nematode, G. rostochiensis, was first discovered in New York State, USA in 1932 (Brodie, Citation1998), while the pale potato cyst nematode, G. pallida, was discovered in Idaho, USA, in 2006 (Hafez et al., Citation2007). In 2008, atypical cyst nematodes were found in a potato trial in Oregon, USA, and were identified as a new species of Globodera, which would be named G. ellingtonae (Fraley et al., Citation2009; Handoo et al., Citation2012). In Canada, G. rostochiensis was first discovered in Newfoundland in 1962 (Olsen & Mulvey, Citation1962) and then in British Columbia in 1965 (Orchard, Citation1965). More recently, G. rostochiensis was detected in the province of Quebec, in the municipality of Saint-Amable, in 2006 (Sun et al., Citation2007; Mahran et al., Citation2010). Globodera pallida was also detected in Newfoundland in 1977 (Stone et al., Citation1977). Worldwide, PCN have been confirmed in 75 countries ([EPPO] European and Mediterranean Plant Protection Organization, Citation2017). Because of their economic impact, G. rostochiensis and G. pallida are listed as quarantine pests and subject to strict regulation in many countries, including the USA and Canada (Seehofer, Citation2007).
One of the main challenges to PCN management is the capability of cysts to remain dormant in soil for several years if a suitable host is not available (Grainger, Citation1964). The decline in populations due to spontaneous hatching under North American conditions is estimated to be around 30% annually (Bélair et al., Citation2016). However, it was shown that fields that did not have susceptible hosts for 20 years still contained PCN able to infest potato (Turner, Citation1996). The cyst shell is even more durable, and some cysts have been retrieved after more than four decades without potato cultivation (Turner, Citation1998). Sustainable management practices against PCN include rotations with non-host crops in conjunction with the use of resistant cultivars. For G. rostochiensis, all the commercially available resistant cultivars are currently derived from Solanum tuberosum subsp. andigena containing the H1 gene (Ellenby, Citation1954; Gebhardt et al., Citation1993; Bakker et al., Citation2004). This resistance gene is, however, active only against the pathotypes Ro1 (Huijsman, Citation1955) and Ro4 (Ross, Citation1986). Also, there is some evidence that the repeated use of these cultivars may promote the emergence of populations that have overcome the H1 resistance (Bakker et al., Citation1993, Citation2004). This was illustrated by the development of a Ro2 population in the state of New York, USA, in fields where resistant cultivars had been used repeatedly (Evans & Brodie, Citation1980; Brodie, Citation1995, Citation1996). The genetic similarities between the Ro1 and Ro2 populations in these fields support the hypothesis of an adaptation to the resistance gene rather than a new introduction (Mimee et al., Citation2015a).
In the province of Quebec, Canada, and the state of New York, USA, regulatory agencies restrict the crops cultivated in regulated fields to golden-nematode-resistant potato cultivars or to approved non-host crops. If resistant cultivars are grown, fields will automatically be surveyed (USDA-APHIS, Citation2008). Because of the long-lasting viability of cysts and the heterogeneity in their content, a cyst count does not provide a reliable estimate of a field’s population density. Thus, an assessment of the number of viable eggs is needed to provide a proper estimate of the population. However, these surveys are very labour-intensive when conventional viability testing is used. The assessment of egg viability is also a crucial step in the deregulation process. According to the Guidelines on Surveillance and Phytosanitary Actions for the Potato Cyst Nematodes Globodera rostochiensis and G. pallida, infested fields are eligible to have some restrictions lifted when intensive surveys, viability assays and bioassays are completed with no live PCN detections (Canada & United States, Citation2014). However, it takes only two cysts from two different soil samples, with one of the cysts containing viable PCN eggs or juveniles, for the field to be considered infested (Canada & United States, Citation2014). This demonstrates the importance of an accurate determination of viability.
Many techniques have been used to determine the viability of PCN. Visual examination of the inner structures of the eggs and larvae is an accepted method in Europe (Den Nijs, Citation2008). Other techniques described in the literature are staining (Ogiga & Estey, Citation1974; Shepherd, Citation1986), hatching tests (Devine et al., Citation2001) and trehalose-based quantification (van den Elsen et al., Citation2012; Ebrahimi et al., Citation2015). There are major drawbacks to these techniques: they are subjective, imprecise or time-consuming (Back et al., Citation2004). Christoforou et al. (Citation2014) recently proposed a method for the selective detection of viable PCN eggs using propidium monoazide as a dye. However, other studies showed that propidium monoazide did not completely inhibit the detection of dead cells (Pacholewicz et al., Citation2013; Seinige et al., Citation2014). A new approach for measuring PCN viability based on RNA and reverse transcription PCR (RT-PCR) was developed initially by Back et al. (Citation2004) and then modified slightly by Kaemmerer (Citation2012). After cell death, gene expression stops and messenger RNAs (mRNAs) are rapidly degraded. This mechanism shows interesting potential for the development of an assay using qRT-PCR for the quantitative evaluation of living PCN eggs. However, this method is not currently optimized to a point that it can be implemented for routine evaluation of PCN viability and replace visual enumeration. Although primers were developed in a region containing multiple introns and amplicons from DNA, and mRNA could be differentiated by their size on gel (Kaemmerer, Citation2012), this is not practical and incompatible with automation. Quantification is also important to obtain an estimate of how many eggs are still viable in a sample and not only the overall status of viability. Finally, sensitivity and reproducibility of qRT-PCR can be highly affected by the presence of PCR inhibitors, especially in soil samples. It is therefore crucial to control this aspect.
We hypothesize that the design of a TaqMan probe spanning an intron (in gDNA) would greatly improve specificity and reproducibility for detection of PCN. We also think that the inclusion of an exogenous internal positive control is necessary to detect failure due to PCR inhibitors. Used with a standard curve, we believe that this tool could be amenable to routine quantification of PCN viability. Thus, the aims of the present study were to: (i) develop a TaqMan-based qRT-PCR technique for detection and quantification of viable eggs of G. rostochiensis, G. pallida and G. ellingtonae; (ii) determine the effectiveness of the management programmes enforced by regulation agencies against G. rostochiensis in Quebec; and (iii) evaluate the effect of the long-term selection pressure of H1-resistant potatoes on G. rostochiensis Ro1 populations.
Materials and methods
Potato cyst nematodes
Populations of G. rostochiensis from Saint-Amable, Quebec, Canada, and G. pallida from Saint-Malo, France, were reared on the susceptible potato cv. ‘Snowden’ in 15-cm plastic pots filled with a pasteurized mixed soil composed of sand and muck (3:1). The pots were incubated in a greenhouse under the following conditions: a daytime temperature range of 21–25°C, a night-time temperature range of 18–20°C, and a 16:8 h photoperiod. Three months after inoculation, the foliage was cut off, pots were allowed to dry, and the entire soil contents of each pot were processed using the Fenwick can method for extracting newly formed cysts (Fenwick, Citation1940). Cysts of G. ellingtonae were obtained from collaborators in Oregon, USA. All cysts had achieved a minimum vernalization time of 3 months before they were used.
DNA isolation
Globodera rostochiensis, G. ellingtonae and G. pallida cysts were washed twice in 400 µL of sterile TE buffer (10 mM Tris, 1 mM EDTA, pH 8.0) using a vortex to eliminate any adhering soil. The cysts were then suspended in buffer (Qiagen Inc., Mississauga, ON) and ground with 0.4 g of zirconium beads (1.0–1.2 mm) and another 6.35-mm bead for 1 min using a PowerLyzer 24 homogenizer (Mo Bio Laboratories Inc., Carlsbad, CA) set at 3000 rpm. Extraction of DNA was performed using a DNeasy Blood & Tissue Kit (Qiagen Inc.) according to the manufacturer’s instructions.
Amplification, cloning and sequencing of the gpd1 gene
Primers were designed based on the consensus sequence of G. rostochiensis glyceraldehyde-3-phosphate dehydrogenase (gpd1) mRNA (EMBL: AF004522). This gene codes for a vital enzyme in the glycolysis component of cellular respiration. The resulting oligonucleotides gpd1-F1 (5′-CGTTCATCGAACTCGACTACATG-3′) and gpd1-R1 (5′-ACGGAAYGCCATGCCGGTYA-3′) were synthesized (Integrated DNA Technologies, Coralville, IA) and used to amplify the gpd1 gene from G. rostochiensis, G. ellingtonae and G. pallida. Terra PCR Direct Polymerase Mix (Clontech Laboratories, Mountain View, CA) was used on genomic DNA (gDNA) extracts for 35 cycles under the following conditions: denaturing at 94°C for 35 s, annealing at 54°C for 35 s, and extension at 68°C for 1 min 15 s. The resulting amplicons were cloned into pCR2.1-TOPO cloning vector using the TOPO TA Cloning Kit (Invitrogen Canada) following the manufacturer’s instructions. Transformants were selected on LB (Lysogeny Broth) agar plates supplemented with kanamycin (50 µg mL−1) and X-gal (5-bromo-4-chloro-3-indolyl-beta-d-galactopyranoside; 40 µg mL−1). Recombinant plasmids were isolated using a QIAprep Spin Miniprep Kit (Qiagen Inc.), digested with EcoRI and visualized on agarose gels to confirm the presence of an inserted fragment. The nucleotide sequences were determined by the dideoxynucleotide chain-termination method (Sanger et al., Citation1977) using a capillary-array automated DNA sequencer (ABI3730xl DNA Analyzer; Applied Biosystems, Foster City, CA). The sequences were determined for both strands and submitted to GenBank.
qRT-PCR primers, TaqMan probe design and exogenous internal positive control
The primer pair of the gpd1 gene used for qRT-PCR was GPDN F4 (5′-GTGTTCACCACCATTGAGAAGG-3′) and GPDN R5 (5′-ACCTTGCCCACTGCTTTGG-3′). These primers were developed initially by Back et al. (Citation2004) and then modified by Kaemmerer (Citation2012). The gpd1-probe dye FAM (5′-AACGTGATTAGCAACGCTTCGTGC-3′) was designed to bind specifically on the complementary DNA (cDNA) of the gpd1 gene in G. rostochiensis, G. ellingtonae and G. pallida. An intron of 48 nucleotides divides the probe into two parts, preventing it from settling on gDNA (). Consequently, no DNase treatment was necessary. An exogenous internal positive control (EIPC) was included in the assay to detect the presence of inhibitors and prevent false negative results. The EIPC used was a 500-bp synthetic fragment of double-stranded DNA (dsDNA) with a known sequence. The EIPC was added directly in the master mix in order to obtain 2000 copies per reaction. Specific primers SEIPC-F (5′-CTGAGTAGCCACGTTATTATC-3′) and SEIPC-R (5′-CAATCAAGTTAGTTGTTCGC-3′) and a probe using the HEX dye (5′-CGCACTTGTCCTACACCCTTCATAC-3′) were also added. The difference (delay) in the quantification cycle (Cq) values obtained for a sample and for the negative control (sterile distilled water) were indicative of the presence of inhibitors.
Total RNA extraction and cDNA synthesis
Cysts were suspended in 350 µL of RLT buffer (Qiagen Inc.) and ground with 0.4 g of zirconium beads (1.0–1.2 mm) and another 6.35-mm bead for 1 min using a PowerLyzer 24 homogenizer (Mo Bio Laboratories Inc.) set at 3000 rpm. Total RNA of each sample was extracted using an RNeasy Plus Mini Kit (Qiagen Inc.) according to the manufacturer’s instructions. The RNA was eluted in 40 µL and stored at −80°C. No treatment with DNase was needed. The single-stranded cDNAs were synthesized by reverse transcription from 2 µL of mRNA and amplified using the PrimeScript One Step RT-PCR Kit (Clontech Laboratories) in a total volume of 20 µL according to the manufacturer’s instructions in a Stratagene Mx3000P qPCR System (Agilent Technologies Canada Inc., Mississauga, ON). The parameters of the reaction programme were: (1) reverse transcription of the Globodera RNA at 42°C for 5 min, followed by (2) hot-start Taq polymerase enzyme activation at 95°C for 10 s and (3) 45 cycles of denaturation at 95°C for 5 s and annealing/extension at 58°C for 1 min. In order to confirm the specificity of the gpd1 probe to gpd1 cDNA during the qRT-PCR assay, RNA samples were treated with RNase A (100 µg mL−1) (9 µL of sample + 1 µL of RNase A) and incubated at 37°C for 1 h. The Cq values corresponding to the number of cycles required to produce a constant emission of fluorescence were calculated with MxPro software, version 4.10 (Agilent Technologies Canada Inc.).
Standard curves and variability
A dsDNA ‘genomic block’ (gBlock) containing the cDNA sequence of the gpd1 gene was synthesized by Integrated DNA Technologies. This DNA fragment was suspended in 20 µL of sterile TE buffer (10 mM Tris, 1 mM EDTA, pH 8.0), and the concentration was determined with a Bioanalyzer system (Agilent Technologies Canada Inc.) and confirmed with a Qubit Fluorometer (Life Technologies, Grand Island, NY). The amount of gBlock solution was converted to the number of copies of the gpd1 gene using converters on the websites http://www.bioinformatics.org/sms2/about.html and http://www.webconversiononline.com/default.aspx (Stothard, Citation2000). A set of serial dilutions (1/10) was prepared and used as a standard curve. A second standard curve was generated using RNA extracted from three biological replicates for each number of viable eggs of G. rostochiensis: 1, 5, 10, 50, 100 and 500. Viability was determined by a visual assessment, under a microscope, based on the internal morphology of both living and dead eggs and juveniles (Den Nijs, Citation2008; EPPO, Citation2013). After confirming that the quantification of these egg numbers was linear, we were able to replace gBlock concentrations by the corresponding numbers of viable eggs in the synthetic standard curve, which was used routinely thereafter. Sensitivity and variability were evaluated further by comparing the amplification of five replicates each of one, two or three viable eggs.
Heat, autoclaving and methyl bromide treatments
In order to evaluate, by qRT-PCR, the expression of the gpd1 gene in dead nematodes, eggs were killed using heat, autoclaving and methyl bromide treatments. Ten cysts taken from the same batch and containing viable eggs of G. rostochiensis were used for each treatment and for an untreated control. For all treatments, the cysts were placed beforehand in tubes with 100 µL of distilled water. The heat treatment was carried out at 65°C for 1 h using a heating block. The autoclaving was carried out at 121°C (liquid cycle) for 30 min. The methyl bromide treatment used 1 mL of methyl bromide 98% from ULTRA Scientific, Inc. (North Kingstown, RI, USA) (methyl bromide 98% and chloropicrin 2% in methanol matrix) at a concentration of 100 µg mL−1. After all treatments, the glass tubes were then hermetically closed for 24 h. For the untreated control, the cysts were soaked in 100 µL of distilled water only. For each treatment, three replicates of 10 eggs were prepared and analysed by qRT-PCR.
Comparison between species and influence of cyst shells on qRT-PCR results
The efficiency of the qRT-PCR assay was tested on the other Globodera species by using 25 and 100 eggs of G. rostochiensis, G. pallida and G. ellingtonae. Three replicates were tested for each species at both concentrations. The influence of cyst shells, which are sometime hypothesized to interfere negatively with PCR, was tested using the contents of 25 G. rostochiensis eggs with or without the cyst shells, and these tests were replicated three times.
Microplot samples and H1 selection pressure
Microplots (1 × 2 m) were established in a field (loamy sand) in Saint-Amable, Quebec, Canada. The field was naturally infested with G. rostochiensis and is under quarantine regulations. The H1-resistant potato ‘Andover’ and the susceptible potato ‘Snowden’ were grown repeatedly in the same five replicate microplots for five consecutive years. Potatoes were planted at a density of six plants per microplot and fertilized according to the recommendations of the CRAAQ (2011). The soil was tilled before planting, and weed control was performed manually three or four times during the growing season. An insecticide treatment was applied once or twice each year for the management of Colorado potato beetle on appearance of actively feeding larvae. The populations of PCN were recorded each year before planting and after harvesting. In each microplot, 16 soil cores were collected at a depth of 0–20 cm using a trowel. A 300 cm3 subsample of air-dried soil was used for cyst extraction with a Fenwick can (Fenwick, Citation1940). The number of cysts and egg viability were determined by visual examination as described by Den Nijs (Citation2008) and EPPO (2013). Egg viability was also assessed using qRT-PCR at the end of the fifth year. Species identity was confirmed by qPCR (Madani et al., Citation2008) on randomly picked cysts from several samples from each year. These assays were performed on samples from five replicate microplots of each cultivar.
Field samples
In total, 24 fields located in the PCN quarantine area of Saint-Amable, Quebec, were sampled in 2015 or 2016 (10 years after the quarantine had been established). These fields had all been cultivated with resistant potatoes (H1 gene) during the sampling year and 3 years previously and with non-host crops (corn or soybean) in the remaining years since PCN was discovered and quarantine was initiated in 2007. One-third of the fields had high numbers of cysts (>300 cysts kg−1) at the establishment of quarantine, while the other two-thirds of the fields had low numbers (<30 cysts kg−1). In each field, an area of 1 acre (0.4 ha) was independently sampled three times, resulting in three replicate samples from each 0.4 ha area. Each 0.4 ha area was divided into sections measuring 6 × 4 m and a trowel was used to remove a 12 cm3 sample from each section. This method (Canadian Food Inspection Agency [CFIA] method ‘B’) was designed to collect a total volume of 2000 cm3 of soil per acre (5000 cm3 ha−1) in a regular grid pattern. Soil samples were air-dried and cysts were extracted from dry soil using the Fenwick can procedure (Fenwick, Citation1940) and then counted. The RNA was extracted directly from the cysts as described above and immediately used in qRT-PCR assays.
Results
Amplification of the gpd1 gene
The primer pair gpd1-F1/-R1 successfully amplified the gpd1 gene in Globodera rostochiensis, G. ellingtonae and G. pallida. The three nematode species yielded single amplicons of 952, 972 and 983 bp in length for G. rostochiensis, G. ellingtonae and G. pallida, respectively. These nucleotide sequences were aligned with the published sequence of G. rostochiensis mRNA (EMBL: AF004522) (Suppl. Figure 1). These new sequences of the gpd1 gene were deposited in GenBank under accession nos. KR072509 to KR072511. An intron was present in the three species. The gpd1 probe was designed in the flanking regions of the intron to prevent the annealing of the probe with gDNA and eliminate the need for a DNase treatment (Suppl. Figure 1). This probe was used with the primer pair GPDN F4/R5 and successfully detected gpd1 cDNA. No detection was observed when the RNA samples were treated with RNase or when gDNA was used.
Standard curves
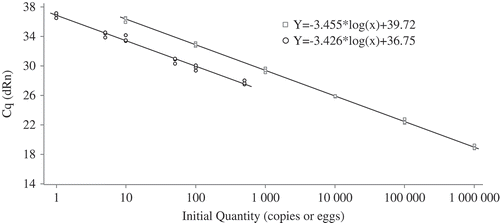
The first standard curve was generated by plotting the Cq values against the logarithm of each dilution of synthetic gpd1 (gBlock). The relationship between the Cq values and the gpd1 gene copy was highly significant, with a regression coefficient (R2) of 0.999 (). The second standard curve was generated by plotting the Cq values against the quantity of viable G. rostochiensis eggs. A linear correlation was observed between the Cq values and the quantity of eggs, with a regression coefficient (R2) of 0.987 ().
Sensitivity and variability
Eggs killed by heat, autoclaving or exposure to methyl bromide did not generate any signals when tested with the proposed qRT-PCR assay (). However, mRNA from a single viable egg was systematically amplified, yielding Cq values of 36.77 ± 0.30 (). There was no significant difference in Cq values between G. rostochiensis, G. pallida and G. ellingtonae when the number of viable eggs used was 25 (P = 0.214, F = 2.131) or 100 (P = 0.122, F = 3.042) (). The presence of cyst shells in the reaction mix did not affect the efficacy of RNA amplification (P = 0.800, F = 0.073) (). Overall, the coefficient of variation in the Cq values between biological replicates was 1.3%.
Table 1. Effect of different treatments and different numbers of eggs of three Globodera species on the quantification cycle (Cq) of gpd1 messenger RNA by qRT-PCR.
Microplot samples
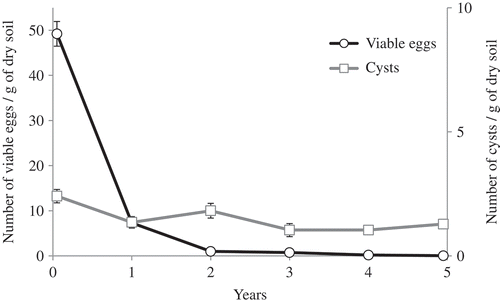
The development of G. rostochiensis on H1-resistant potato cultivars grown continuously over a period of 5 years was monitored in the experimental microplots. Over this period, the average number of viable eggs of G. rostochiensis per gram of soil fell by 99.9%, while the number of cysts per gram of soil remained stable (). No signs of the development of a resistance-breaking strain were observed. Egg viability was also evaluated after 5 continuous years of cultivation with susceptible potato cultivars. The mean number of viable eggs or cysts was surprisingly low and was influenced by the evaluation method: viability was lower when estimated by visual examination (21 ± 17) than when evaluated by qRT-PCR (39.4 ± 22.8) (). However, the results obtained by these methods showed a good correlation (R2 = 0.73).
Table 2. Estimation, by visual evaluation and qRT-PCR, of Globodera rostochiensis egg viability from 50 cysts that developed on the susceptible potato cultivar ‘Snowden’ in microplots.
Field samples
The surveys in the commercial fields indicated that cysts were present in all the samples analysed. The concentration of cysts ranged from 1 to 713 cysts kg−1 of soil tested, with a mean of 108 cysts kg−1 (). The initial level of infestation in the soil 10 years ago (average cyst densities) were 4.1 cysts kg−1 in low-initial-density fields and 315.7 cysts kg−1 in high-initial-density fields (CFIA, unpublished data). In addition, egg viability was very poor. Overall, only two fields yielded positive results for viable eggs when analysed by qRT-PCR, with average estimates of 2.8 and 11.8 viable eggs kg−1 of soil tested. The Cq values for the internal control (EIPC) were in the expected range for all the samples, thus eliminating the possibility of false negatives in the other fields.
Table 3. Evaluation of Globodera rostochiensis population densities (cysts and viable eggs) 10 years after the establishment of quarantine procedures (including two crops of resistant potatoes) in 24 fields located in Saint-Amable, Quebec, Canada.
Discussion
The discovery of PCN in a new area and the establishment of quarantine measures always raise numerous concerns among potato growers and stakeholders of the potato industry, and will negatively affect trade. Also, the threat of becoming contaminated and the ordeal of being prohibited from growing potatoes generate a great deal of fear and anxiety amongst growers. However, regulatory measures are required to prevent the spread of PCN and to minimize the long-term impact on the industry. Some fields in the state of New York, USA, have been regulated for golden nematode since the 1940s. After decades of strict procedures, including crop rotations using resistant cultivars and sanitation of equipment, the United States Animal and Plant Health Inspection Service began running intensive soil testing and re-evaluating the status of all those fields in 2010. This effort has resulted in the deregulation of 76% of the acreage that was initially under quarantine (USDA-APHIS, Citation2014). In Canada, the golden nematode was found in 1965 in Central Saanich on Vancouver Island, British Columbia (Orchard, Citation1965). After quarantine procedures were established in the early 1980s, those fields were fumigated, and a complete ban on the cultivation of Solanaceae plants was imposed (Government of Canada, Citation1980). Thirty years later, intensive surveys and bioassays – with an estimated 95% probability of detecting only one viable cyst per 5 L of soil – did not find any viable nematodes in fields without quarantine infractions (Rott et al., Citation2010). The results from this extensive sampling are currently being analysed by the Canadian Food Inspection Agency to review the quarantine programme and update the status of PCN in Central Saanich. Thus, this land could be released from regulatory control in the near future.
According to the Canada–United States guidelines for PCN (Canada and United States, Citation2014), infested fields could be released from most regulatory controls after an intensive sampling with a negative outcome in viability assay, a negative bioassay and three additional full-field surveys using viability assays after the harvest of a susceptible host crop. Currently, in the quarantine area of Saint-Amable, Quebec, the CFIA systematically evaluates the number of cysts after each crop of H1-resistant potatoes. However, we have shown here that the number of viable eggs can decrease drastically (>99.9% after 5 continuous years of resistant potatoes) without any change in the number of cysts recovered. This finding again confirms that cyst concentration is not a good estimator of population density in the short term. Unfortunately, the large number of samples required to be processed causes a burden on regulatory agencies and the systematic quantification of viability using visual evaluation is not realistic. Therefore, we propose to systematically evaluate egg viability using molecular techniques. The adoption of this technique would, in addition to reducing labour time, generate more pertinent information. For example, the effect on cyst counts of the introduction of a different pathotype or the development of a virulent population following mutation would go unnoticed for several years but would be rapidly highlighted by viability testing.
We report a promising method for the quantification of viable eggs based on the detection of the expression of gpd1, an essential gene coding for glyceraldehyde-3-phosphate dehydrogenase (GAPDH). This glycolytic enzyme is expressed in all prokaryotic and eukaryotic organisms. In most cases, the reported half-life of mRNA is relatively short. For example, in Escherichia coli and Cryptosporidium parvum, GAPDH mRNA was not detectable 16 h after heat treatment (Sheridan et al., Citation1998). It was reported that the mRNA of the gpd1 gene in G. rostochiensis cysts could be detected for a maximum of 7 days after heat treatment and for up to 28 days after fumigation treatment (Back et al., Citation2004; Kaemmerer, Citation2012).
Our technique differs from the methods of Back et al. (Citation2004) and Kaemmerer (Citation2012) because the expression of the gpd1 gene was found to be a good indicator of egg viability for G. ellingtonae, G. rostochiensis and G. pallida. Also, no amplification occurred when eggs that had previously received heat treatment, autoclaving or fumigation with methyl bromide were tested. Care was also taken to eliminate the risk of errors for both false positives and false negatives. The target site of the TaqMan probe was chosen in a region where mRNA is split in two by introns in gDNA. This prevents the binding of the probe on remnant DNA and reduces the possibility of false positives. The inclusion of an internal control also increases confidence in the analysis by eliminating the chances of false negatives due to PCR inhibitors. This TaqMan-based qRT-PCR upgrade of the initial RT-PCR method proposed by Back et al. (Citation2004) and modified by Kaemmerer (Citation2012) is therefore more robust and better suited to the high standards required by regulatory agencies monitoring for the presence of PCN.
The assay was also found to be very sensitive, with the systematic detection of a single viable egg. This is a great improvement over other molecular methods. For example, the limit of detection reported by Beniers et al. (Citation2014) was 30 eggs in a RT-PCR assay targeting a different gene. The same authors also reported a method based on the detection of trehalose released by viable eggs. That method exhibited good sensitivity but was affected by factors interfering with absorbance measurement, including the presence of cyst shells (Beniers et al., Citation2014). This was not the case with our proposed method, which can be used on cysts without additional preparation steps.
Our proposed method was used to evaluate the viability of G. rostochiensis eggs 10 years after the establishment of a quarantine area in Saint-Amable, Quebec. During that period, resistant potatoes were grown on two occasions by the participating growers. Viable eggs were found only in two adjacent fields and at very low concentrations. These findings confirm the effectiveness of the measures put in place to reduce nematode population densities. The effectiveness of H1-resistant cultivars in controlling PCN in this climatic zone was already confirmed in a previous study (Bélair et al., Citation2016). We further demonstrate here that the reduction in population density was following a general assumption for H1-resistant cultivars. No signs of a breakdown in resistance were observed after 5 continuous years of cropping with these cultivars. We are therefore able to state with great confidence that the use of cultivars harbouring the H1 gene is, and should remain, an effective tool to control G. rostochiensis in these fields. Currently, potato growers are allowed to grow an H1-resistant crop once every 3 years. Based on the microplot results, the probability of a failure of the H1 gene and the build-up of a variant population appears to be quite low for the ensuing years. This general statement does not take into account several other factors, such as the possibility of a new introduction, a quarantine infraction or the incorrect application of the rotation programme. The effects of climate change could also contribute to the adaptation of PCN to resistant cultivars by increasing its rate of development or by affecting molecular interactions with its host (Gendron St-Marseille et al., Citation2015). A second generation of larvae in soil was already observed, and only a short extension of the growing season could result in the completion of a second life cycle (Mimee et al., Citation2015b). Alongside tomato and eggplant, which are entirely prohibited in the quarantine area, other plants can support PCN development in the absence of potato. Some solanaceous weeds are good hosts, and it was shown that some weed species were favoured by the establishment of the quarantine area (Mimee et al., Citation2014).
Many viable eggs were found in experimental microplots grown with susceptible potato cultivars, although viability was much lower than expected. This finding is probably explained by the high initial population level, which limited the development of new cysts, combined with the presence of empty cysts from previous years, which reduced the average number of viable eggs per cyst. When comparing the number of viable eggs obtained by qRT-PCR with the number obtained by visual evaluation, we observed higher values with the qRT-PCR method. It is known that the visual estimation of viability could be subjective and that eggs that are apparently dead could actually still be alive. However, the variation observed at high nematode concentrations in the qRT-PCR results indicates that the quantification is probably not linear over a wide range. Still, the method was able to detect a single viable larva 100% of the time, whereas visual enumeration seemed to misclassify viable eggs as dead. For the purposes of regulation, variability at high concentrations is not of great concern, but effectiveness in detecting a rare occurrence of viable eggs is crucial. A misinterpretation of egg viability could make all the difference between a new outbreak and the spread of this important pest on one hand, and the unnecessary application of costly and frustrating regulations on the other.
In conclusion, the qRT-PCR approach proposed here is very sensitive and reliable and does not require microscopic examination, which is potentially subjective and labour-intensive for the assessment of PCN egg viability.
Supplemental Figure 1
Download MS Word (30.1 KB)Supplemental material
Supplemental data for this article can be accessed online here: https://doi.org/10.1080/07060661.2017.1382574.
Additional information
Funding
References
- [CRAAQ] Centre de référence en agriculture et agroalimentaire du Québec. 2011. Guide de référence en fertilisation [Fertilization reference guide]. 2nd ed. Quebec City (QC): CRAAQ.
- [EPPO] European and Mediterranean Plant Protection Organization. 2013. PM 7/40 (3) Globodera rostochiensis and Globodera pallida. Bulletin OEPP/EPPO Bulletin. 43:119–138.
- [EPPO] European and Mediterranean Plant Protection Organization. 2017. EPPO Global Database: Globodera rostochiensis. [ updated 2017 Apr 04]. https://gd.eppo.int/taxon/HETDRO/distribution.
- [USDA-APHIS] United States Department of Agriculture, Animal and Plant Health Inspection Service. 2008. Golden nematode program manual. https://www.aphis.usda.gov/import_export/plants/manuals/domestic/downloads/gnpm.pdf.
- [USDA-APHIS] United States Department of Agriculture, Animal and Plant Health Inspection Service. 2014. Federal order. Domestic quarantine notice for revision of golden nematode (Globodera rostochiensis) regulated area. DA-2014-43. https://www.aphis.usda.gov/plant_health/plant_pest_info/nematode/downloads/DA-2014-43.pdf.
- Back M, Heath B, Edwards S, Haydock PPJ, Grove IGG, Minnis S. 2004. Development of a robust assay to assess the viability of potato cyst nematodes. Newport (UK): Harper Adams University. Final project report DEFRA project code HP0143.
- Bakker E, Achenbach U, Bakker J, van Vliet J, Peleman J, Segers B, van der Heijden S, van der Linde P, Graveland R, Hutten R, et al. 2004. A high-resolution map of the H1 locus harbouring resistance to the potato cyst nematode Globodera rostochiensis. Theor Appl Genet. 109:146–152.
- Bakker J, Folkertsma RT, Rouppe van der Voort JNAM, de Boer JM, Gommers FJ. 1993. Changing concepts and molecular approaches in the management of virulence genes in potato cyst nematodes. Annu Rev Phytopathol. 31:169–190.
- Bélair G, Dauphinais N, Mimee B. 2016. Evaluation of cultural methods for the management of the golden nematode (Globodera rostochiensis) in Quebec, Canada. Can J Plant Pathol. 38:209–217.
- Beniers JE, Been TH, Mendes O, van Gent-Pelzer MPE, van der Lee TAJ. 2014. Quantification of viable eggs of the potato cyst nematodes (Globodera spp.) using either trehalose or RNA-specific real-time PCR. Nematology. 16:1219–1232.
- Brodie BB. 1995. The occurrence of a second pathotype of potato cyst nematode in New York [abstract]. J Nematol. 27:493–494.
- Brodie BB. 1996. The identity and distribution of a second pathotype of potato cyst nematode in the United States [abstract]. Nematropica. 26:246.
- Brodie BB. 1998. Potato cyst nematodes (Globodera species) in Central and North America. In: Marks RJ, Brodie BB, editors. Potato cyst nematodes: biology, distribution and control. Wallingford (UK): CABI; p. 317–331.
- Canada and United States. 2014. Guidelines on surveillance and phytosanitary actions for the potato cyst nematodes Globodera rostochiensis and Globodera pallida. https://www.aphis.usda.gov/plant_health/plant_pest_info/nematode/downloads/potato_guidelines.pdf.
- Christoforou M, Pantelides IS, Kanetis L, Ioannou N, Tsaltas D. 2014. Rapid detection and quantification of viable potato cyst nematodes using qPCR in combination with propidium monoazide. Plant Pathol. 63:1185–1192.
- Den Nijs LJMF. 2008. PCN: dead or alive, that is the question [abstract]. 5th International Congress of Nematology; Jul 13–18; Brisbane, Australia: International Federation of Nematology Societies. p. 330
- Devine KJ, Byrne J, Jones PW. 2001. In vitro studies on the relative availability and mobility in soil of natural hatching factors for the potato cyst nematodes, Globodera rostochiensis and G. pallida. Nematology. 3:75–83.
- Ebrahimi N, Viaene N, Moens M. 2015. Optimizing trehalose-based quantification of live eggs in potato cyst nematodes (Globodera rostochiensis and G. pallida). Plant Dis. 99:947–953.
- Ellenby C. 1954. Tuber forming species and varieties of the genus Solanum tested for resistance to the potato root eelworm Heterodera rostochiensis Wollenweber. Euphytica. 3:195–202.
- Evans K, Brodie BB. 1980. The origin and distribution of the golden nematode and its potential in the U.S.A. Am Potato J. 57:79–89
- Fenwick DW. 1940. Methods for the recovery and counting of cysts of Heterodera schachtii from soil. J Helminthol. 18:155–172.
- Fraley CL, Meng SX, Osterbauer NK. 2009. Detection of a new Globodera species during surveys of Oregon seed potato fields for potato cyst nematodes. Poster session presented at: 2nd National Plant Diagnostic Network Meeting; Dec 6–10; Miami (FL). https://www.npdn.org/system/files/public/Meeting%20Information/2009_NationalMeeting/Fraley_Meng_Osterbauer_POSTER.pdf.
- Gebhardt C, Mugniery D, Ritter E, Salamini F, Bonnel E. 1993. Identification of RFLP markers closely linked to the H1 gene conferring resistance to Globodera rostochiensis in potato. Theor Appl Genet. 85:541–544.
- Gendron St-Marseille AF, Bélair G, Brodeur J, Bourgeois G, Mimee B. 2015. Impact des changements climatiques sur les interactions moléculaires entre le nématode à kyste du soya (Heterodera glycines) et son hôte principal, le soya (Glycine max). Phytoprotection. 95:41–47.
- Government of Canada. 1980. Plant protection act – Golden Nematode order SOR/80-260. Order prohibiting and restricting the transportation and movement of any plant or other matter that is likely to result in the spread of the Golden Nematode. Ottawa (ON): Minister of Justice. http://laws-lois.justice.gc.ca/eng/regulations/SOR-80-260/index.html
- Grainger J. 1964. Factors affecting the control of eelworm diseases 1). Nematologica. 10:5–20.
- Hafez SL, Sundararaj P, Handoo ZA, Skantar AM, Carta LK, Chitwood DJ. 2007. First report of the pale cyst nematode, Globodera pallida, in the United States. Plant Dis. 91:325.
- Handoo ZA, Carta LK, Skantar AM, Chitwood DJ. 2012. Description of Globodera ellingtonae n. sp. (Nematoda: Heteroderidae) from Oregon. J Nematol. 44:40–57.
- Huijsman CA. 1955. Breeding for resistance to the potato root eelworm. II. Data on the inheritance of resistance in andigenum-tuberosum crosses obtained in 1954. Euphytica. 4:133–140.
- Kaemmerer D. 2012. Detection of viable potato cyst nematodes (Globodera rostochiensis and G. pallida) by reverse transcription PCR. J Plant Dis Prot. 119:100–106.
- Madani M, Ward LJ, De Boer SH. 2008. Multiplex real-time polymerase chain reaction for identifying potato cyst nematodes, Globodera pallida and Globodera rostochiensis, and the tobacco cyst nematode, Globodera tabacum. Can J Plant Pathol. 30:554–564.
- Mahran A, Turner S, Martin T, Yu Q, Miller S, Sun F. 2010. The golden potato cyst nematode Globodera rostochiensis pathotype Ro1 in the Saint-Amable regulated area in Quebec, Canada. Plant Dis. 94:1510.
- Mimee B, Andersen R, Bélair G, Vanasse A, Rott M. 2014. Impact of quarantine procedures on weed biodiversity and abundance: implications for the management of the golden potato cyst nematode, Globodera rostochiensis. Crop Prot. 55:21–27.
- Mimee B, Dauphinais N, Bélair G. 2015b. Life cycle of the golden cyst nematode, Globodera rostochiensis, in Quebec, Canada. J Nematol. 47:290–295.
- Mimee B, Duceppe M-O, Véronneau P-Y, Lafond-Lapalme J, Jean M, Belzile F, Bélair G. 2015a. A new method for studying population genetics of cyst nematodes based on Pool-Seq and genomewide allele frequency analysis. Mol Ecol Resour. 15:1356–1365.
- Ogiga IR, Estey RH. 1974. The use of Meldola Blue and Nile Blue A, for distinguishing dead from living nematodes. Nematologica. 20:271–276.
- Olsen OA, Mulvey RH. 1962. The discovery of golden nematode in Newfoundland. Can Plant Dis Surv. 42:253.
- Orchard WR. 1965. Occurrence of the golden nematode on Vancouver Island, British Columbia. Can Plant Dis Surv. 45:89.
- Pacholewicz E, Swart E, Lipman LJA, Wagenaar JA, Havelaar AH, Duim B. 2013. Propidium monoazide does not fully inhibit the detection of dead Campylobacter on broiler chicken carcasses by qPCR. J Microbiol Meth. 95:32–38.
- Ross H. 1986. Potato breeding – problems and perspectives. In: Horn W, Robbelen G, editors. Advances in plant breeding vol. 13; Supplements to Journal of Plant Breeding. Berlin and Hamburg: Verlag Paul Parey; p. 75–82.
- Rott M, Lawrence T, Belton M, Sun F, Kyle D. 2010. Occurrence and detection of Globodera rostochiensis on Vancouver Island, British Columbia: an update. Plant Dis. 94:1367–1371.
- Sanger F, Nicklen S, Coulson AR. 1977. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 74:5463–5467.
- Seehofer H. 2007. Council Directive 2007/33/EC of 11 June 2007 on the control of potato cyst nematodes and repealing Directive 69/465/EEC. Off J Eur Union. 156:12–22.
- Seinige D, Krischek C, Klein G, Kehrenberg C. 2014. Comparative analysis and limitations of ethidium monoazide and propidium monoazide treatments for the differentiation of viable and nonviable Campylobacter cells. Appl Environ Microbiol. 80:2186–2192.
- Shepherd AM. 1986. Extraction and estimation of cyst nematodes. In: Southey JF, editor. Laboratory methods for work with plant and soil nematodes. Norwich (UK): HMSO books; p. 31–49.
- Sheridan GEC, Masters CI, Shallcross JA, Mackey BM. 1998. Detection of mRNA by reverse transcription-PCR as an indicator of viability in Escherichia coli cells. Appl Environ Microbiol. 64:1313–1318.
- Stone AR, Thompson PR, Hopper BE. 1977. Globodera pallida present in Newfoundland. Plant Dis Rept. 61:590–591.
- Stothard P. 2000. The sequence manipulation suite: JavaScript programs for analyzing and formatting protein and DNA sequences. BioTechniques. 28:1102–1104.
- Sun F, Miller S, Wood S, Côté M-J. 2007. Occurrence of potato cyst nematode, Globodera rostochiensis, on potato in the Saint-Amable region, Quebec, Canada. Plant Dis. 91:908.
- Turner SJ. 1996. Population decline of potato cyst nematodes (Globodera rostochiensis, G. pallida) in field soils in Northern Ireland. Ann Appl Biol. 129:315–322.
- Turner SJ. 1998. Sample preparation, soil extraction and laboratory facilities for the detection of potato cyst nematodes. In: Marks RJ, Brodie BB, editors. Potato cyst nematodes: biology, distribution and control. Wallingford (UK): CABI; p. 75–90.
- Turner SJ, Evans K. 1998. The origins, global distribution and biology of potato cyst nematodes (Globodera rostochiensis (Woll.) and Globodera pallida (Stone). In: Marks RJ, Brodie BB, editors. Potato cyst nematodes: biology, distribution and control. Wallingford (UK): CABI; p. 7–26.
- Turner SJ, Rowe JA. 2006. Cyst nematodes. In: Perry RN, Moens M, editors. Plant nematology. Wallingford (UK): CABI; p. 91–122.
- van den Elsen S, Ave M, Schoenmakers N, Landeweert R, Bakker J, Helder J. 2012. A rapid, sensitive, and cost-efficient assay to estimate viability of potato cyst nematodes. Phytopathology. 102:140–146.