Abstract
Heterobasidion irregulare (Underw.) Garbel. & Otrosina is an important fungal parasitic basidiomycete of coniferous trees throughout temperate regions of the world. In Canada, the disease was first reported on red pine in Ontario in 1955, then in Quebec in 1989. As infection probability is proportional to the density of its aerial basidiospores infecting freshly cut pine stumps, the purpose of this study was to quantify the H. irregulare aerial basidiospore deposition along a southern Quebec transect. Spore counts from automated rotary arm collectors were determined by using a ribosomal ITS TaqMan real-time PCR detection assay. Total count was performed on rods at each location, with the highest score of 706.3 spores in Harrington, then 459.6 spores in Cowansville, rapidly decreasing to 52.3 spores in Compton and reaching lows in St-Aubert and Rimouski, with an eastward direction on the transect. Clearly, plantations in southwestern Quebec are at a higher risk of infection than anywhere else in the province. With few exceptions, weekly spore depositions at all sites other than Harrington were greatly below 0.2 per m−2 h−1. Despite this very low spore deposition rate observed in one season, the large number of trees thinned annually warrants that stump treatment with Rotstop® C is still the best strategy, especially in valuable plantations, to prevent against infection by Heterobasidion.
Résumé
Heterobasidion irregulare (Underw.) Garbel. & Otrosina est un important basidiomycète parasite des conifères des régions tempérées de la planète. Au Canada, la maladie a été détectée pour la première fois en Ontario, en 1955, sur le pin rouge, puis au Québec en 1989. Étant donné que la probabilité d’infection est proportionnelle à la densité des basidiospores dispersées par voie aérienne infectant les souches des arbres qui viennent d’être coupés, l’objectif de cette étude était de quantifier le dépôt des basidiospores de H. irregulare dispersées par voie aérienne le long d’un transect traversant le sud du Québec. Les décomptes de spores résultant des collecteurs automatiques à bras rotatifs ont été déterminés par analyse de détection basée sur une PCR en temps réel de type TaqMan de l’ITS ribosomique. Le décompte total a été effectué sur des tiges à chaque location, le plus haut compte étant de 706.3 spores, provenant de Harrington, puis de 459.6 spores, de Cowansville, décroissant rapidement à 52.3 spores à Compton pour atteindre les plus bas comptes à Saint-Aubert et à Rimouski, en direction est en suivant le transect. De toute évidence, les plantations du Sud-Ouest québécois sont plus susceptibles d’être infectées que celles des autres régions de la province. À quelques exceptions près, les dépôts hebdomadaires de spores à tous les sites, sauf à Harrington, étaient bien sous les 0.2/m−2 h−1. Malgré ce très faible taux de dépôt de spores observé en une saison, le grand nombre d’arbres prélevés chaque année garantit que le traitement des souches avec Rotstop C est encore la meilleure stratégie, spécialement dans les plantations de grande valeur, pour prévenir l’infection causée par Heterobasidion.
Introduction
Heterobasidion irregulare (Underw.) Garbel. & Otrosina is an important basidiomycete pathogen of coniferous trees throughout the temperate regions of the world, and it is particularly damaging in plantations. In Canada, the disease was first reported on red pines (Pinus resinosa Ait.) in south-central Ontario (Jorgensen, Citation1956), and then further north later in the 1960s near Ottawa (Sippell et al., Citation1968; Punter, Citation1970). It was first observed in Quebec in 1989 (Laflamme & Blais, Citation1995), and a few new infection centres have thereafter been found only in plantations, never in natural stands in southern Quebec (MFFP, Citation2016). In eastern Canada, the pathogen is found mainly in red pine plantations of southern Ontario and Quebec and is progressing northward and eastward.
Red pines planted since the 1970s occupy about 10 000 ha in southern Quebec (MFFP, Citation2015), typically in plantations ranging from 1–3 ha in size, almost randomly dispersed, with some local patches. Periodic thinning of the stand is a silvicultural treatment recommended in order to increase stand quality and volume, but thinning makes these plantations very vulnerable to H. irregulare infection as it exposes fresh stumps in valuable plantations. Freshly cut pine stump surfaces serve as an entry for inoculation by airborne basidiospores. After germinating, the spores grow through the stump into the roots, and then, by crossing natural root grafts or root contacts, infect nearby valuable trees and spread within the plantation.
With only 14 infested plantations detected in eight locations in a territory ranging over 80 000 km2, Quebec plantations are still at the beginning of a possible provincial epidemic and the probability of new infections is proportional to the density of aerial basidiospores (Stambaugh et al., Citation1962) where thinning and partial cuttings are conducted. The probability of infection on a freshly cut stump may be as high as 100% in many countries in Europe (Vollbrecht et al., Citation1995), which is fortunately not the case in eastern Canada. Data about H. irregulare spore deposition are scarce in Canada and only available from one study in Ontario (Punter, Citation1970). In a recent study, Bérubé et al. (Citation2017) measured the weekly spore deposition along a transect from an infected plantation in central southern Quebec and found it to be highest at 0.314 spore per m−2 h−1 at a distance of 1.5 km from the infected plantation.
The purpose of this study was to quantify the H. irregulare aerial spore deposition along a southern Quebec transect from the Ontario to New Brunswick borders.
Materials and methods
Sticky rods were prepared as described in Fall et al. (Citation2015). Airborne spores were collected on silicone-coated (Navagard, G697) rods prepared by dipping them into a silicone-hexane solution (7%, w/v) (Lamarche et al., Citation2016). Rods were on a rotating-arm impaction spore sampler (Phytodata Inc, Sherrington, QC, Canada). They consisted of two vertical rods (sampling surface of 1.65 mm × 20 mm) separated by 83 mm. Airborne particles are impacted onto the leading edges of the rotation rods coated with silicone grease. After exposure, rods were stored at −20°C.
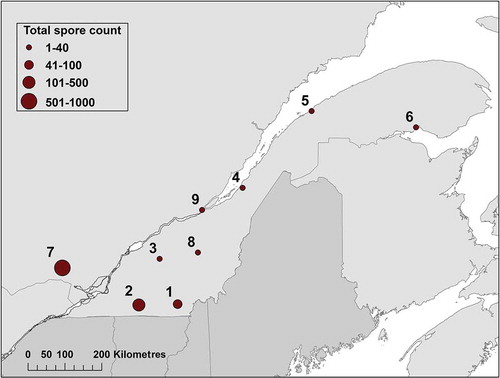
Nine automated rotary arm spore collectors were deployed in the fields, during late September to November 2015, from Harrington in south-western Quebec to Maria on the Gaspé Peninsula (). Due to the small number of infected plantations and the large distances between plantations minimizing the effect of local spores that are deposited at 99.9% within 100 m from basidiocarp fruiting bodies (Kallio, Citation1970; Gonthier et al., Citation2012), we chose to locate our spore collectors in open lands with no spatial relationships to forests. In doing so, we aimed to measure the background spore load from long distance dispersal (Rishbeth, Citation1959) most likely responsible for most past infections in the province. They were programmed to spin their rotary arms for 5 min every 90 min and the sticky rods were collected weekly from 28 September to 23 November, for a total of 560 min of exposure for each rod/week (Bérubé et al., Citation2017). Each sample was placed in a separate tube, then DNA extraction and spore count by quantitative PCR were performed using the protocols developed by Lamarche et al. (Citation2016) and used in Bérubé et al. (Citation2017). As conidia can be multinucleate, a selected mono-basidiosporal isolate of H. irregulare (#98–008 confirmed with 1–2 nuclei/conidia), was used to generate standard curves with a correlation coefficient (r2) of 0.997 (Lamarche et al., Citation2016).
Total amount of basidiospores sampled per m3 was calculated using the following formula: (number of basidiospores per rod × 1000 L−1 m−3 h−1)/(21.65 L min−1 per rod × 60 min h−1 × 9.33h) (Fall et al. Citation2015). Amount of basidiospores m−2 h−1 for each week was calculated by dividing the weekly amount of basidiospores per m3 by 9.33 hours, assuming total basidiospores falling on the m2 cross-section was obtained by summing all spores in the m−3 h−1 above (Pfender et al., Citation2007).
Results
Basidiospore counts from all locations reached a peak during the third week of October 2015 (), with a total of 566 spores collected on the rods that week and rapidly decreasing afterwards. The location with the highest cumulated spore count was 706.3 in Harrington (site 7), followed by 459.6 in Cowansville (site 2), dropping quickly to 52.3 in Compton (site 1) and reaching lows in St-Aubert (site 4) and Rimouski (site 5) with an eastward direction on the transect (). When transformed into spore deposition per m−2 h−1, it briefly reached 3.48 m−2 h−1 on site 2 (), but was more consistent with weekly values between 0.3 and 1.78 m−2 h−1 in Harrington (site 7). With three exceptions, weekly spore depositions at all sites other than Harrington were greatly below 0.2 m−2 h−1.
Table 1. Number of H. irregulare spores collected weekly at nine stations in southern Quebec.
Table 2. Weekly H. irregulare spore deposition per m−2 h−1 for the 7-day period.
Discussion
The basidiospore deposition observed during our 8-week study, ranging from 0 to the highest weekly value of 3.48 spores m−2 h−1 (), is similar to Punter’s (Citation1970) measurements on fresh wood discs in Ontario, who reported values between 0.1 to 2.1 spores m−2 h−1. In infected forests in Washington state, deposition rates ranging from 3 to 70 spores m−2 h−1 were observed (Edmonds & Driver, Citation1974). In the Italian Alps forests, where disease incidence ranges from 30–50%, deposition rates of 169 to 15 550 spores m−2 h−1 were reported (Gonthier et al., Citation2005). Most of the weekly values we observed are greatly under 0.2 spores m−2 h−1, indicating that the Heterobasidion disease is at the very early stages of the epidemic in the province of Quebec.
Data from the southern Quebec transect are similar to those obtained from a previous 2014 study (Bérubé et al., Citation2017) that aimed at determining the importance of the local spore load originating from a nearby infested plantation. The authors reported a peak during the fourth week of October, which also featured a sharp decrease in the spore count from 405 spores in the affected plantation, to 88 spores at a location 500 m away and 79 spores at 1.5 km. The latter site was used again for a measurement in 2015 (site 2), during the current study, which resulted in a cumulated count of 21.2 spores (). Such variation is expected as deposition rates are greatly dependent on where spore collectors are placed. Close to an infected plantation would overestimate general risk in the province. Hence, we chose to locate spore collectors in open lands with no known infected plantation in the vicinity, hoping to measure background spore load responsible for past infections in the province and most likely for new infections in the near future.
Although some spores of Heterobasidion can travel as far as 300 km (Rishbeth, Citation1959), Kallio (Citation1970) reported that 99.9% of them are deposited within 100 m from fruiting bodies. While they can increase infection risk locally from a nearby infested plantation, spore deposition at a distance of 5 km is not different from the background spore load produced by long-distance dispersal (Bérubé et al., Citation2017). Garbelotto et al. (Citation2013) reported a migration threshold when fragmentation exceeds 10–15 km. However, multiple infected plantations in a locality can increase spore deposition regionally with overlapping 5 km zones. This is probably the case for spore counts in site 7 as five infected plantations were reported in that locality and region (MFFP, Citation2016).
The only three sites with cumulative spores count above 50 are all located in south-western Quebec (), closest to the known H. irregulare infected plantations in Ontario and Vermont, USA (Chase & Ullrich, Citation1990). Eastward along the transect, the counts show a low background spore load, probably indicating plantations in south-western Quebec are at a higher infection risk than those located in south central and eastern parts of the province. In northern Quebec where jack pine (Pinus bansksiana) occupies large areas and is a known host for H. irregulare, infections have not been reported but the potential for future infection has to be monitored.
Gonthier et al. (Citation2005) data suggested that a deposition rate of 5 spores m−2 h−1 is needed to initiate infection on stumps, a value far beyond those we observed. In southern Quebec, the low spore deposition rate observed was, however, still sufficient to be responsible for past infections in the province’s plantations. Despite the very low risk level associated with the estimated spore deposition rate observed in this study, the large number of trees thinned annually warrants that stump treatment (Dumas & Laflamme, Citation2013) using the biological control agent Rotstop® C containing Phlebiopsis gigantea (BioForest Technologies Inc., Sault Ste. Marie, ON) remains the best strategy, especially in valuable plantations. In future experiments, we will deploy new rotary arm spore collectors in south-western Quebec to achieve better monitoring in that higher risk area and develop a spatial risk hazard model.
Acknowledgements
The authors would like to thank our field collaborators who accepted to host and operate the rotating-arm spore collectors: Normand Bérubé, Jean-Francois Pépin, Jacques Turcotte, Réjean Ouellet, Martin Lepage, Pascale St-Laurent, Robert Morisset and Alexander Bates.
References
- Bérubé JA, Potvin A, Stewart D. 2017. Importance of local and long-distance Heterobasidion irregulare aerial spore dispersal for future infection centres in thinned red pine plantation in Quebec. For Chron. 93:1–3.
- Chase TE, Ullrich RC. 1990. Five genes determining intersterility in Heterobasidion annosum. Mycologia. 82:73–81.
- Dumas MT, Laflamme G. 2013. Efficacy of two Phlebiopsis gigantea formulations in preventing Heterobasidion irregulare colonization of red pine stumps in eastern Canada. Phytoprotection. 93:25–31.
- Edmonds RL, Driver CH. 1974. Dispersion and deposition of spores of Fomes annosus and fluorescent particles. Phytopathology. 64:1313–1321.
- Fall ML, Tremblay DM, Gobeil-Richard M, Couillard J, Rocheleau H, Van der Heyden H, Lévesque CA, Beaulieu C, Carisse O, Wilson RA. 2015. Infection efficiency of four Phytophthora infestans clonal lineages and DNA-based quantification of sporangia. PloS One. 10:e0136312.
- Garbelotto M, Guglielmo F, Mascheretti S, Croucher PJP, Gonthier P. 2013. Population genetic analyses provide insights on the introduction pathway and spread patterns of the North American forest pathogen Heterobasidion irregulare in Italy. Mol Ecol. 22:4855–4869.
- Gonthier P, Garbelotto MM, Nicolotti G. 2005. Seasonal patterns of spore deposition of Heterobasidion species in four forests of the western Alps. Phytopathology. 95:759–767.
- Gonthier P, Lione G, Giordano L, Garbelotto M. 2012. The American forest pathogen Heterobasidion irregulare colonizes unexpected habitats after its introduction in Italy. Ecol Applic. 22:2135–2143.
- Jorgensen E. 1956. Fomes annosus (Fr.) Cke. on red pine in Ontario. For Chron. 32:86–88.
- Kallio T. 1970. Aerial distribution of the root rot fungus Fomes annosus in Finland. Acta Forest Fenn. 107:1–55.
- Laflamme G, Blais R. 1995. Détection du Heterobasidion annosum au Québec. Phytoprotection. 76:39–43.
- Lamarche J, Potvin A, Stewart D, Blais M, Pelletier G, Shamoun SF, Hamelin RC, Tanguay P. 2016. Real-time PCR assays for the detection of Heterobasidion irregulare, H. occidentale, H. annosum sensu stricto and the Heterobasidion annosum complex. For Path. doi:10.1111/efp.12321
- MFFP. 2015. Provincial insect and disease plantation surveillance database. Québec, QC: Ministère des Forêts, de la Faune et des Parcs du Québec.
- MFFP. 2016. La maladie du rond. Direction de la protection des forêts, Ministère des Forêts, de la Faune et des Parcs, Québec, QC, Canada. [ Accessed 2017 Jan 24]. https://mffp.gouv.qc.ca/forets/fimaq/insectes/fimaq-insectes-maladies-rond.jsp
- Pfender W, Graw R, Bradley W, Carney M, Maxwell L. 2007. Emission rates, survival, and modeled dispersal of viable pollen of creeping bentgrass. Crop Sci. 47:2529–2539.
- Punter D. 1970. Fomes annosus in eastern Canada. In: Toussoun TA, Bega RV, Nelson PE, editors. Root Diseases and Soil-Borne Pathogens. Berkeley: University of California Press; p. 156–170.
- Rishbeth J. 1959. Dispersal of Fomes annosus and Peniophora gigantea. Trans Br Mycol Soc. 42:243–260.
- Sippell WL, Gross HL, Rose AH. 1968. Ontario region: important forest diseases, p.66-76, in Annual report of the forest insect and disease survey. Ottawa: Dept. of Fisheries and Forestry.
- Stambaugh WJ, Cobb FW Jr, Schmidt RA, Krieger FC. 1962. Seasonal inoculum dispersal and white pine stump invasion by Fomes annosus. Plant Dis Rep. 46:194–198.
- Vollbrecht G, Johansson U, Eriksson H, Stenlid J. 1995. Butt rot incidence, yield and growth pattern in a tree species experiment in southwestern Sweden. For Ecol Manag. 76:87–93.