Abstract
India is one of the leading litchi (Litchi chinensis Sonn.) producing countries in the world. A leaf blight on litchi was observed in April 2012 on nursery plants at Muzaffarpur, Bihar, India. Light to dark brown necrosis starting from leaf tips resulted in complete drying of leaves. Blighting of panicles and fruits were also noticed during April–June, 2014. Panicles dried out as a result of necrosis, while necrosis of pedicels led to complete drying of the rind of developing fruits. Infected tissues collected from different sites in diseased fields were cultured on potato dextrose agar (PDA), and the pathogen was identified as Alternaria alternata (Fr.) Keissler on the basis of morphological and cultural characteristics. The fungus produced greyish-black colonies on PDA with obclavate or obpyriform conidia in chains. Conidia had 1–4 transverse and 0–3 longitudinal septa, and measured 16–40 × 4–13 μm. Morphological identification was confirmed by sequencing the internal transcribed spacer (ITS) region of rDNA using ITS1 and ITS4 primers. Pathogenicity tests indicated that A. alternata infected and caused blights of the leaves, panicles and fruit of litchi. Alternaria alternata has been reported to cause post-harvest decay of litchi fruit, but this is the first report of A. alternata causing blights of leaves, panicles and fruit in the field.
Résumé
: L’Inde est une des plus grosses productrices de litchis (Litchi chinensis Sonn.) au monde. En avril 2012, on a observé de la brûlure des feuilles sur les plants de litchis d’une pépinière à Muzaffarpur, Bihar, en Inde. Une nécrose brun pâle à brun foncé, apparaissant à la pointe des feuilles, provoquait le dessèchement complet de celles-ci. D’avril à juin 2014, on a également remarqué la brûlure des panicules et des fruits. À la suite de cette nécrose, les panicules se sont desséchés, tandis que la nécrose des pédicelles a engendré le dessèchement complet de la peau des fruits en cours de développement. Des tissus infectés collectés dans des champs atteints à différents sites ont été mis en culture sur de la gélose dextrosée à la pomme de terre (GDPT) et, en se basant sur les caractéristiques morphologiques et culturales, l’agent pathogène a été identifié en tant qu’Alternaria alternata (Fr.) Keissler. Sur la GDPT, le champignon produisait des colonies gris-noir avec des chaînes de conidies obclavées ou obpiriformes. Les conidies possédaient de 1 à 4 cloisons transversales et de 0 à 3 cloisons longitudinales, et mesuraient 16 à 40 × 4 à 13 μm. L’identification morphologique a été confirmée par le séquençage de la région de l’espaceur transcrit interne (ITS) de l’ADNr en utilisant les amorces ITS1 et ITS4. Les tests de pathogénicité ont indiqué qu’A. alternata avait infecté les plants de litchis et avait causé la brûlure des feuilles, des panicules et des fruits. On a déjà rapporté qu’Alternaria alternata a causé du pourrissement chez les fruits après la récolte, mais il s’agit de la première mention d’A. alternata causant la brûlure des feuilles, des panicules et des fruits dans les champs.
Introduction
Litchi or lychee (Litchi chinensis Sonn.) is an evergreen subtropical fruit tree of the family Sapindaceae. World production of litchi is estimated to be around 2.1 million tonnes, with more than 95% of the world cultivation occurring in Asia. The top five litchi producing countries are China, India, Taiwan, Thailand and Vietnam (FAO, Citation2002). India and China account for 91% of the world litchi production. The acreage under litchi cultivation in India was 84 000 ha with a production of 585 000 tonnes during 2013–14 (NHB, Citation2016). Major litchi producing states in India are Bihar, West Bengal, Assam and Jharkhand. Bihar contributes 45% of total litchi production and has 40% of the acreage (Kumar et al., Citation2014).
Litchi is less affected by diseases than many other fruit trees in India. Some of the economically important diseases of litchi are leaf spots (Colletotrichum gloeosporioides (Penz.) Penz. & Sacc. and Botryodiplodia theobromae Pat.), anthracnose (C. gloeosporioides) and twig blight (C. gloeosporioides and Gloeosporium sp.) at the pre-harvest stage (Kumar et al., Citation2011, Citation2014). Diseases are more of a postharvest issue, although many of the fruit are infected before harvest. Postharvest fruit rots are caused by several pathogens, including Alternaria alternata (Fr.) Keissl., Aspergillus flavus Link, Cylindrocarpon tonkinense Bugn., Botryodiplodia theobromae and C. gloeosporioides (Prasad & Bilgrami, Citation1974; Awasthi et al., Citation2005; Kumar et al., Citation2016a, Citation2016b).
A leaf blight disease was observed during May 2012 on potted litchi plants in nurseries at Muzaffarpur, Bihar, and subsequently, leaf blight was observed on adult trees in litchi orchards. The occurrence of panicle/inflorescence and fruit blights was first noticed during April to June 2014 at the National Research Centre on Litchi (NRCL), Muzaffarpur (26°05ʹ87′′N, 85°26ʹ39′′E, 47 m asl), Bihar Experimental Farm. In subsequent years, blights of leaves, panicle/inflorescence and fruits were commonly observed on orchard trees in Bihar and adjoining states of India. No similar disease on litchi has been previously reported. The objective of the present study was to identify the causal agent of the disease at different phenophases of litchi growth in Bihar, India.
Materials and methods
Isolation and morphological identification of the pathogen
Symptomatic leaves were collected from 10 infected plants from six nurseries, and from two orchard trees of litchi at the NRCL Experimental Farm, Bihar in May 2012. Similarly, six diseased panicle and six diseased fruit specimens were collected in April and June 2014, respectively, from trees in orchards located at Mushahari, Muzaffarpur. Fungal isolations were made by surface-disinfesting small fragments of symptomatic leaf, panicle and fruit tissues in 0.5% NaOCl, double-rinsing in sterile water, and plating onto potato dextrose agar (PDA) amended with 0.05 g L−1 streptomycin sulphate. Dishes were incubated at 28 ± 1°C for 6 days and pure cultures were obtained using the hyphal tip method. The frequency of recovery of isolates from plated tissues was 100%. Microscopic examinations were conducted by mounting fungal tissues in water and lactophenol, and dimensions of 30 conidia per culture were measured from 6-day-old cultures. Initial identification of the fungi isolated from diseased tissues collected in the field was made on the basis of morphology and growth characteristics on PDA. Dimensions of the conidia and conidiophores were measured using ocular and stage micrometer mounted on a light microscope (Nikon Eclipse 50i). The isolates were maintained on PDA slants.
Molecular identification
Molecular identification was done by sequencing the internal transcribed spacer (ITS) region of rDNA using primers ITS-1 and ITS-4 (White et al. Citation1990; Naik et al. Citation2017) using the dideoxynucleotide chain termination method. Four isolates were grown in potato dextrose broth at 28 ± 1ºC for 7 days. After incubation, the mycelial mats were collected and dried completely on pre-autoclaved filter paper. The dried mycelial mats were ground with liquid nitrogen and DNA was extracted using the CTAB (cetyltrimethyl ammonium bromide) extraction method (Aradhya et al. Citation2001). The DNA was amplified with ITS-1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS-4 (5′-TCCTCCGCTTATTGATATGC-3′) primers. Amplified DNA from PCR was purified using the QIAquick PCR Purification Kit (Qiagen Inc., CA) as specified by the manufacturer and directly cycle sequenced in both directions using the GenomeLab™ Dye Terminator Cycle Sequencing Quick Start Kit on a GenomeLab™ GeXP Genetic Analysis System (Beckman Coulter Inc, CA). The sequence obtained was submitted to NCBI GenBank and an accession number was obtained. The sequence analysis was carried out using the BLAST bioinformatics tool of NCBI. Consensus sequences of the ITS1 region of four isolates and reference sequences downloaded from GenBank were aligned using the multiple sequence alignment program Clustal W and a phylogenetic analysis was performed using the program MEGA version 5 (Tamura et al., Citation2011). Bipolaris tetramera was used as the out-group taxon.
Pathogenicity test
Three leaf blight isolates were used separately for leaf inoculation, one panicle blight isolate for panicle inoculation and one fruit blight isolate for fruit inoculation tests. Leaf inoculation tests were conducted by spraying about 20 mL of a 106 conidia mL−1 suspension per plant onto the upper and lower surfaces of leaves of 2-year-old potted litchi plants of cv. ‘Shahi’. Conidia were harvested from 6-day-old PDA cultures. For each isolate, five plants were inoculated, and five control plants were sprayed with sterile water. After spraying, the foliage of plants was covered with clear plastic bags. These plants were then kept in a greenhouse at 32 ± 2°C, with 68 ± 4% relative humidity and a 12-h photoperiod. The greenhouse was made up of polycarbonate twin wall glazing (6 mm sheet), equipped with a proportional-integral-derivative controller for precise climate control. Panicle inoculation tests were conducted by spraying four bunches of panicles on orchard trees with 20 mL of a 106 conidia mL−1 suspension per bunch under natural daylight conditions. The panicles were then covered with thin plastic bags that had several small holes for aeration. Six developing fruit (~45 days after fruit set) were inoculated with 20 mL of a conidial suspension following similar methods as for the panicle inoculation tests. Symptom development was monitored daily. Fungi from lesions which formed on the inoculated plant tissues were re-isolated on PDA as described above. The morphological and cultural characteristics of the re-isolated organisms were compared with the original isolates.
Results and discussion
Symptoms and occurrence of disease
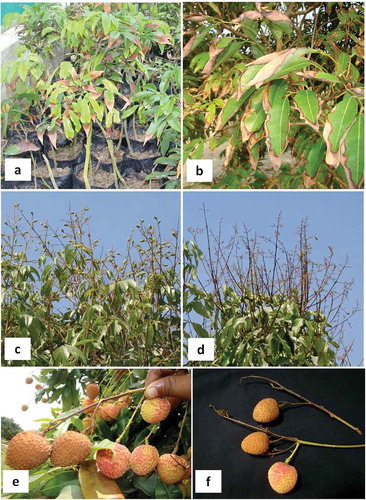
A leaf blight disease affected a significant number of litchi plants in nurseries at the NRCL Experimental Farm, Bihar, India in May 2012 (, b) and caused heavy losses. Initial symptoms resembled potassium deficiency and started from the leaf tips as a light to dark brown necrosis that advanced towards the margins, leading to complete necrosis and drying of the affected leaves. On orchard trees, leaf blight similar to that on nursery plants was observed. The leaf blight was more damaging to nursery plants and new orchard plantings in the early establishment phase where it drastically hampered growth of plants as compared to mature orchard trees. Initially, senescing leaves were infected but with the progression of the disease, all the leaves became blighted except a few upper leaves. The leaf blight was not economically important in mature orchards as the pathogen was mostly limited to old senescing leaves. During flowering and fruit development, blighting of panicles and fruits occurred. Panicles shrivelled and dried up as a result of necrosis (, d), while necrosis of the pedicel led to complete drying of the rind of developing fruits (, f). Fruit blight developed only when the pedicel was infected by the pathogen, and if fruit were infected, small black lesions developed on the rinds which later caused post-harvest fruit decay. Major losses were the result of infection of panicles leading to partial blighting of panicles on portions of trees. Most of the trees surveyed in orchards had less than 20% diseased panicles, but there was, nevertheless, heavy loss to the crop. Fruit blight incidence was sporadic in orchards and only 5–10% of the fruit were affected.
Isolation and morphological identification of the pathogen
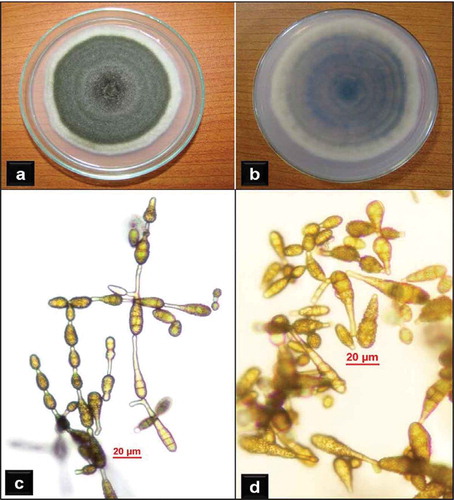
Typical fungal colonies isolated from the diseased litchi tissues on PDA were olive green in colour, having a prominent 2–6 mm white margin (, b). A total of 24 isolates (12 from leaves, 6 from panicles and 6 from fruits) were obtained from diseased tissues. They grew to 43.5–81.5 mm in diameter after 6 days. The branched, brownish, septate mycelia produced dark brown, obclavate to obpyriform, catenulate conidia on short conidiophores (, d). The number of conidia in chains varied from 6 to 14 with secondary chains having 3–8 conidia. Conidia (n = 30) were 16–40 μm long (avg. 29.8; SD ± 4.3 µm), 4–13 μm wide in the broadest part (avg. 7.6; SD ± 2.6 µm) with a beak 0–10 μm long (avg. 4.4; SD ± 2.7 μm), and 1–4 transverse and 0–3 longitudinal or oblique septa. These morphological characteristics and measurements match those of A. alternata (Ellis, Citation1971; Simmons, Citation2007) and hence the isolated fungi were identified as Alternaria alternata (Fr.) Keissler. The conidial surface of all the isolates was delicately pitted rather than smooth-walled, unlike typical A. alternata strains. Among the leaf blight isolates, three distinct strains designated as AA-L1, AA-L2, AA-L3 were identified based on variation in cultural characteristics on PDA. The morpho-cultural characteristics of all the panicle blight and the fruit blight isolates of the fungus resembled the strain AA-L3.
Molecular identification
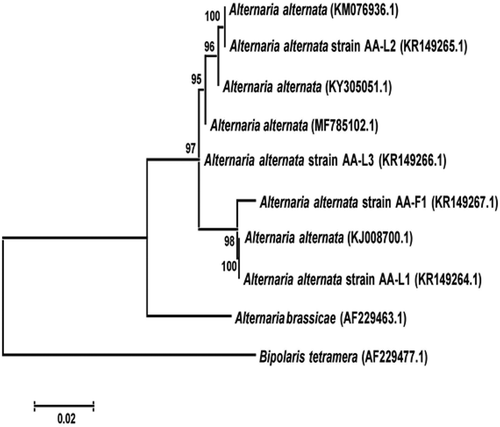
BLAST analysis of sequences showed 100% homology with several A. alternata strains (e.g. KJ008700.1, KY305051.1, KM076936.1, MF785102.1, KR149267.1). The generated sequences of the three strains of the pathogen (AA-L1 – 527 bp, AA-L2 – 540 bp, AA-L3 – 567 bp) were submitted to GenBank (accession nos. KR149264, KR149265 and KR149266, respectively). Additionally, a sequence of one fruit decay-causing strain (AA-F1 – 556 bp) of A. alternata from litchi was also submitted to GenBank (accession no. KR149267). The isolates were clustered in a distinct clade with those of A. alternata isolates retrieved from GenBank with 100% bootstrap value (). These results confirmed the identity of the fungus as A. alternata.
Fig. 3 Phylogenetic tree constructed with the ITS-5.8S rDNA sequences of the three strains of Alternaria alternata isolated from leaves of litchi (AA-L1, AA-L2, AA-L3), one strain isolated from litchi fruit (AA-F1), some other strains of A. alternata, and a strain of A. brassicae retrieved from GenBank. Bipolaris tetramera was used as the out-group taxon. The scale bar indicates the number of base changes per 1000 nucleotide positions in the neighbour-joining analysis.
Pathogenicity test
Leaf blight symptoms appeared 2 weeks after inoculation, while panicle blight and fruit blight were apparent after 10 days. No symptoms were observed on control leaves, panicles or fruit. Re-isolation of fungi with the same morphological characters was achieved from symptomatic plants/tissue, but not healthy control plant tissue, confirming the causal agent as A. alternata. Cross infectivity of the three leaf blight strains for panicles and fruit infection was observed with varying severity, and conversely panicle and fruit blight isolates caused leaf infection.
Alternaria alternata is an opportunistic pathogen reported to occur on over 380 host species (Ellis, Citation1971; Farr et al., Citation1989; Murthy et al., Citation2003) causing leaf spots, rots and blights on many plant parts. Among fruit crops, it has previously been reported on apple (Johnson et al., Citation2000), citrus (Peever et al., Citation2002) and pomegranate (Ezra et al., Citation2010). It has also been reported to cause a post-harvest fruit decay of litchi fruits in Australia (Johnson et al., Citation2002), India (Kumar et al., Citation2016a) and Pakistan (Alam et al., Citation2017). Since 2014, Alternaria alternata has been an important pathogen of litchi in India causing blights of leaf, panicle and fruits.
Kumar et al. (Citation2016a) reported A. alternata as the most common pathogen causing litchi fruit post-harvest decay in India. This paper reports blights of leaf, panicle and fruit caused by this pathogen. The cultivation of litchi over a large area in Bihar and adjoining states of India provides the pathogen with the opportunity to spread and cause heavy crop loss. A post-harvest loss of 35–44% of litchi fruits has been reported by Kumar et al. (Citation2016a), and much of this is due to decay caused by A. alternata. Thus, considering cumulative loss incurred at different stages of the crop, this pathogen is a serious problem on litchi. Further research is warranted to investigate the epidemiology and develop strategies for management of this economically important disease. To our knowledge, this is the first report of A. alternata causing blight of leaves, panicles and pre-harvest fruit of litchi in India.
Acknowledgements
The authors thank Dr P.N. Chowdhry, mycologist and former head of the Indian Type Culture Collection, New Delhi, for confirming the identification of the pathogen.
References
- Alam MW, Gleason ML, Amin M, Ali S, Fiaz M, Rehman A, Ahmed R, Khan AS. 2017. First report of Alternaria alternata causing postharvest fruit rot of lychee in Pakistan. Plant Dis. 101:1041.
- Aradhya MK, Chan HM, Parfitt D. 2001. Genetic variability in the pistachio late blight fungus, Alternaria alternata. Mycol Res. 105:300–306.
- Awasthi DP, Sarkar S, Mishra NK, Kaiser SAKM. 2005. Disease situation of some major fruit crops in new alluvial plains of West Bengal. Environ Ecol. 23:497–499.
- Ellis MB. 1971. Dematiaceous hyphomycetes. Kew: CABI Publishing, Commonwealth Mycological Institute; p. 608.
- Ezra D, Gat T, Skovorodnikova Y, Vardi Y, Kosto I. 2010. First report of Alternaria black spot of pomegranate caused by Alternaria alternata in Israel. Australas Plant Dis Notes. 5:1–2.
- FAO. 2002. Lychee production in the Asian-Pacific Region. Bangkok (Thailand): RAP Publications.
- Farr DF, Bills GF, Chamuris PG, Rossman YA. 1989. Fungi on plants and plant products in the United States. St. Paul (MN): APS Press.
- Johnson GI, Cooke AW, Sardsud U. 2002. Postharvest disease control in lychee. Acta Hortic. 575:705–715.
- Johnson RD, Johnson L, Kohmoto K, Otani H, Lane CR, Kodama M. 2000. A polymerase chain reaction-based method to specifically detect Alternaria alternata apple pathotype (A. mali), the causal agent of Alternaria blotch of apple. Phytopathology. 90:973–976.
- Kumar V, Anal AKD, Nath V. 2014. Prevalence of some threatening pests and disease of litchi (Litchi chinensis Sonn.) in Bihar state of India. J Appl Hortic. 16:235–240.
- Kumar V, Kumar A, Nath V. 2011. Emerging pests and diseases of litchi (Litchi chinensis Sonn.). Pest Manag Hort Ecosyst. 17:11–13.
- Kumar V, Purbey SK, Anal AKD. 2016a. Losses in litchi at various stages of supply chain and changes in fruit quality parameters after harvest. Crop Prot. 79:97–104.
- Kumar V, Purbey SK, Pongener A, Anal AKD, Nath V. 2016b. Effect of some fructoplane antagonists and postharvest dip treatments on litchi fruit rots and shelf life. Int J Trop Agric. 64:333–343.
- Murthy KK, Shenoi MM, Sreenivas SS. 2003. Prediction of brown spot disease (Alternaria alternata) of tobacco (Nicotiana tabacum) as influenced by prevailing weather factors in Karnataka. Indian J Agric Sci. 73:459–461.
- Naik MK, Chennappa G, Amaresh YS, Sudha S, Chowdappa P, Patil S. 2017. Characterization of phytotoxin producing Alternaria species isolated from sesame. Indian J Exp Biol. 55:36–43.
- NHB. 2016. Horticultural statistics at a glance 2015. New Delhi (India): Ministry of Agriculture & Farmers Welfare, Government of India, Oxford University. p. 437. [ accessed 2017 Jul 01]. http://nhb.gov.in/area-pro/horst_galance_2016.pdf.
- Peever TL, Ibañez A, Akimitsu K, Timmer LW. 2002. Worldwide phylogeography of the citrus brown spot pathogen, Alternaria alternata. Phytopathology. 92:794–802.
- Prasad SS, Bilgrami RS. 1974. Investigations on diseases of litchi: VI. Post harvest diseases of fruit in India. Plant Dis Rep. 58:1134–1136.
- Simmons EG. 2007. Alternaria- an identification manual. 1st ed. Utrecht (The Netherlands): CBS Biodiversity Series.
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 28:2731–2739.
- White TJ, Bruns TD, Lee S, Taylor JW. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. San Diego (CA): Academic Press; p. 315–322.