Abstract
Black spot is a common disease occurring on Dendrobium officinale, which significantly reduces the quality and yield of this medicinal plant (family Orchidaceae). The disease manifests itself as small, black spots on leaves which can develop into circular streaks that become necrotic tan or brownish black, with the leaves eventually turning yellow or brown-yellow. Fungal pathogens such as Alternaria tenuissima and Phyllosticta spp. are known to cause black spot of D. officinale. A fungus which was atypical of A. tenuissima or Phyllosticta spp. was isolated from leaves of D. officinale showing symptoms of black spot from Zhejiang and Guangxi provinces, China. Pathogenicity tests in vitro as well as on tissue-cultured seedlings using fungal isolate GXDF24 showed that it was able to cause typical black spot symptoms. Molecular identification based on the ITS sequence revealed that isolate GXDF24 shared 99% similarity with Cladosporium oxysporum. This is the first report of C. oxysporum as a pathogen causing black spot of D. officinale in China.
Résumé
La tache noire est une maladie courante chez Dendrobium officinale, ce qui réduit substantiellement la qualité et le rendement de cette plante médicinale (famille des orchidacées). La maladie se manifeste par l’apparition de petites taches noires sur les feuilles, qui peuvent se transformer en stries circulaires nécrotiques brun clair ou brun-noir. Par la suite, les feuilles jaunissent ou deviennent brun-jaune. Les agents pathogènes fongiques tels qu’Alternaria tenuissima et Phyllosticta spp. sont reconnus pour causer la tache noire chez D. officinale. Une forme atypique d’A. tenuissima ou de Phyllosticta spp. a été isolée à partir de feuilles de D. officinale collectées dans les provinces du Zhejiang et du Guangxi, en Chine, affichant les symptômes de la tache noire. Des tests de pathogénicité effectués in vitro et sur des semis issus de la culture tissulaire, réalisés avec l’isolat fongique GXDF24, ont montré qu’il pouvait provoquer les symptômes typiques de la tache noire. L’identification moléculaire, basée sur la séquence de l’ITS, a révélé que l’isolat GXDF24 partageait 99% de similitude avec Cladosporium oxysporum. Il s’agit de la première mention de C. oxysporum en tant qu’agent pathogène causant la tache noire chez D. officinale en Chine.
Introduction
Dendrobium officinale Kimura & Migo, a member of the family Orchidaceae, is a popular epiphytic perennial herb in South and South-east Asia. In China, it grows naturally in Anhui, Zhejiang, Fujian, Guangxi, Sichuan and Yunnan provinces at an elevation of about 1600 m (Zhang et al., Citation2012). The stem of D. officinale contains polysaccharides and other volatile compounds (Ma et al., Citation2017), which makes it valuable for use in relieving stomach upsets, promoting body fluid production, nourishing ‘yin’ antipyresis (Liang et al., Citation2015), preventing the development of cataracts, relieving throat inflammation and fatigue, reducing peripheral vascular obstruction (Wang et al., Citation2014), and enhancing immunity. However, it usually requires 3–5 years of growth to reach the state of maturity at which it can be effectively utilized as a drug (Yang et al., Citation2014). The supply of wild D. officinale has fallen far short of demand, and the vast majority of D. officinale-based products are now sourced from cultivated plants.
Cultivated D. officinale plants are prone to pests and diseases in their typical warm, wet, and shady natural environment, which normally has a relative humidity of 70–80%, which facilitates infection by numerous phytopathogens (Song, Citation2012). Black spot is one of the most common diseases occurring in almost all the D. officinale fields (Zhang et al., Citation2005), which significantly reduces the quality and yield. Several microbes have been reported to cause black spot of D. officinale, such as Alternaria tenuissima (Zhang & Zheng, Citation2004) and Phyllosticta spp. (Song et al., Citation2011). While conducting isolations from symptomatic leaves of D. officinale to determine which pathogen(s) were causing black spot in production areas of China, we isolated a fungus atypical of A. tenuissma or Phyllosticta spp. The aim of the study was to determine if this atypical fungus was causing black spot on D. officinale.
Materials and methods
Sample collection and fungal isolation
Plants of D. officinale were randomly collected from two fields in Zhejiang (ZJ) and Guangxi (GX) Provinces, China during 2015 (Fig. 1a). Ten 2-year-old plants were transported to the laboratory and stored at 4°C. Symptomatic leaves were rinsed under running water for 30 min, immersed in 70% ethanol for 30 s, soaked in 1% sodium hypochlorite for 3 min, and washed with sterilized ddH2O. The surface-sterilized leaves were cut into fragments, transferred to an axenic mortar, ground with a sterile pestle, and diluted with approximately 2 mL of sterilized ddH2O. A series of 100 μL sample dilutions (10−1, 10−2, 10−3, 10−4 and 10−5) were spread on PDA amended with 100 mg L−1 streptomycin sulphate (Bioshrap, China), with three replications per dilution, and then incubated at 25°C in the dark. After incubation for 5–10 days, morphologically different colonies were selected as candidate fungi to be purified as well as preserved for further experiments.
Pathogenicity tests
In vitro and in vivo tests were carried out on leaves and tissue-cultured plants of D. officinale to determine the pathogenicity of a fungal isolate, GXDF24, which had a colony morphology that was atypical to known black spot pathogens A. tenuissima or Phyllosticta spp. The spores of the fungus produced on PDA were washed with sterilized ddH2O and used to prepare a 106 CFU mL−1 suspension. The healthy leaves of D. officinale were sterilized as above, and placed on sterilized filter papers in Petri dishes, with three leaves per Petri dish. Each leaf was inoculated with 20 μL of spore suspension, and also sterilized ddH2O as the negative control. There were three replications. Petri dishes were kept in a 25°C incubator until assessment.
The pathogenicity of the isolate GXDF24 was further evaluated on 3-month-old tissue-cultured plants of D. officinale. A 106 CFU mL−1 spore suspension was used to inoculate 10 plants. The same number of plants, inoculated with sterilized ddH2O, was used as negative controls. Seedlings were incubated in an incubator at 25 ± 2°C with a photoperiod of 12 h per day for 4 weeks. Sterile water was added every 3 days to keep the incubator at a humidity over 80%. All the plants were observed daily, and symptomatic leaves were used for fungal isolation to confirm the presence of the original isolate.
Characterization of pathogen
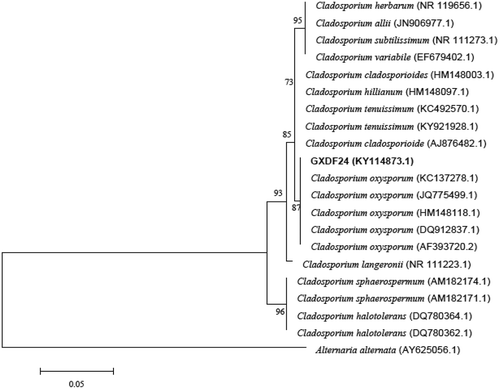
Isolate GXDF24 was characterized for phenotype and ITS sequence. Colony and spore morphology was examined from PDA cultures grown at 25°C using a light microscope. Genomic DNA was extracted using the modified cetyltrimethyl ammonium bromide (CTAB) method (Doyle, Citation1990). The ITS-rDNA region was amplified using primers ITS1 (5ʹ-TCCGTAGGTGAACCTGCGG-3ʹ) and ITS4 (5ʹ-TCCTCCGCTTATTGATATGC-3ʹ) as described previously (White et al., Citation1990). A 50 μL polymerase chain reaction (PCR) mixture was prepared, which contained 2 μL of 10 μmol L−1 of each primer, 0.4 μL of 5 U μL−1 Taq DNA Polymerase (Vazyme, China), 5 μL 10×Taq Buffer (Tris, KCl, MgCl2 and H2O) (Vazyme, China), 1.6 μL of 10 mM dNTPs (dATP, dCTP, dGTP, dTTP), 1 μL DNA template, and ddH2O to 50 μL. The PCR conditions for amplification of the ITS-rDNA region were initial denaturing at 94°C for 4 min, followed by 35 cycles of denaturing at 94°C for 1 min, annealing at 55°C for 1 min, and extension at 72°C for 2 min, and then a final extension at 72°C for 10 min. The PCR product was sequenced at Sangon Biotech Engineering Co., Ltd (Shanghai, China). The sequence was aligned with 19 sequences from species of Cladosporium that had previously been deposited in GenBank. Alternaria alternata was used as the out-group taxon. The phylogenetic tree was constructed by the Neighbour-joining method (MEGA version 5.0) with 1000 repetitions (Saitou & Nei, Citation1987).
Results
Isolation and pathogenicity of fungi
A fungus, named GXDF24, was isolated from the symptomatic leaves of D. officinale in Zhejiang (ZJ) and Guangxi (GX) provinces. The symptoms on detached leaves after inoculation with GXDF24 manifested as small black spots on the third day following inoculation (). Some of the spots developed into circular streaks after 3 weeks or longer and gradually became necrotic tan or brownish black. The whole leaves eventually became yellow or brown yellow. No symptom was observed on the control leaves.
Fig. 1 (Colour online) Black spot on leaves of Dendrobium officinale. a, Symptomatic leaves from fields in Zhejiang (ZJ) and Guangxi (GX) provinces, China. b, Pathogenicity tests in vitro. Leaves showing black spot symptoms after inoculation with isolate GXDF24, and CK: healthy leaves inoculated with sterile water as negative control. c, Pathogenicity tests in vivo. Disease symptoms associated with inoculation of isolate GXDF24 in the illumination incubator (12 hours light, 12 hours dark; 28°C) at 2–3 months post-inoculation.

Isolate GXDF24 caused the same symptoms on tissue-cultured seedlings () as those observed under natural conditions. No symptom was observed in the control. Fungi similar to GXDF24 were obtained from the inoculated plants, but nothing was isolated from the control plants.
Identification of pathogenic isolate
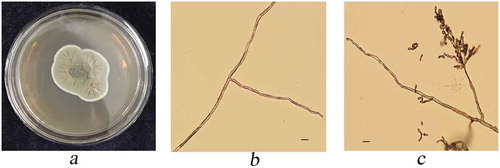
The isolate GXDF24 cultured on PDA at 25°C developed flat to low convex colonies at 5 to 7 days, with the surface glabrous and wrinkled. The colony was initially olivaceous (), and then turned olivaceous-grey after 2 weeks. The reverse of the colony was leaden grey. The hyphae were 2–4 μm wide with septa (). Conidiophores were 3–5 μm in width, 50–150 μm or longer in length and arose from the hyphae terminally or laterally. The conidia were ellipsoidal, aseptate, catenate, branching in all directions, and 2–4 × 3–7 μm in size ().
Fig. 2 a, (Colour online) Colony growth on PDA. b, hypha. c, conidiophore of Cladosporium oxysporum. Scale bar = 10 μm.
The ITS sequence of isolate GXDF24 was submitted to NCBI GenBank, and given an accession no. KY114873. The sequence shared 99% similarity with that of C. oxysporum (JQ775499.1). In the phylogenetic tree, the pathogenic isolate clustered with C. oxysporum (). Therefore, the pathogen GXDF24 was identified as C. oxysporum based on morphological characteristics and rDNA-ITS sequence analysis.
Discussion
Black spot is a common problem on D. officinale, and has been reported to be associated with A. tenuissma or Phyllosticta spp. (Zhang & Zheng, Citation2004; Song et al., 2011). In this work, C. oxysporum was demonstrated to be pathogenic and cause black spot symptoms. Cladosporium constitutes one of the largest genera of hyphomycetes, comprising more than 772 species (Dugan et al., Citation2004). Many species are plant pathogens, such as C. dracaenatum, which is associated with leaf spots of the endemic and now nearly extinct Dracaena ombet in Sudan (Baka & Krzywinski, Citation1996). Cladosporium haplophylli is a leaf-spotting phytopathogenic fungus of Heliotropium peruvianum and is a causal agent of leaf spots and other lesions on plants in Sweden (Heuchert et al., Citation2005).
This is the first report that C. oxysporum is a pathogen causing black spot of D. officinale in China. Cladosporium grows very slowly in culture compared with other pathogens (Sautour et al., Citation2002) and it was difficult to obtain the isolate of Cladosporium from diseased leaves since other fast-growing colonies grew on plates.
In conclusion, C. oxysporum GXDF24 was identified as a causal organism leading to black spot disease of D. officinale, and this is the first report in China.
Acknowledgements
We are grateful to Dr Greg Duns for his valuable comments on the manuscript.
Additional information
Funding
References
- Baka ZAM, Krzywinski K. 1996. Fungi associated with leaf spots of Dracaena ombet (Kotschy and Peyr). Microbiol Res. 151:49–56.
- Doyle JJ. 1990. Isolation of plant DNA from fresh tissue. Focus. 12:13–15.
- Dugan FM, Schubert K, Braun U, Dugan FM, Schubert K. 2004. Check-list of Cladosporium names. Schlechtendalia. 11:1–103.
- Heuchert B, Braun U, Schubert K. 2005. Morphotaxonomic revision of fungicolous Cladosporium species (hyphomycetes). Schlechtendalia. 13:1–78.
- Liang Y, Xiao W, Hui L, Yang T, Lian J, Yang R, Hao S. 2015. The genome of Dendrobium officinale illuminates the biology of the important traditional Chinese orchid herb. Mol Plant. 8:922–934.
- Ma Y, Hou Z, Liang Z, Liu L. 2017. Quality evaluation of the rare medicinal plant Dendrobium officinale based on volatile constituents, methanol extracts and polysaccharides. Curr Pharm Anal. doi:10.2174/1573412913666161201122530
- Saitou N, Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 4:406–425.
- Sautour M, Mansur CS, Divies C, Bensoussan M, Dantigny P. 2002. Comparison of the effects of temperature and water activity on growth rate of food spoilage moulds. J Ind Microbiol Biotechnol. 28:311–315.
- Song H, Zhang Y, Xing B, Xie Y, Liu F, Deng S, Shi X. 2011. Identification on causal agent of Dendrobium black spot and indoor determination of fungicide toxicity. Chin Agric Sci Bull. 27:110–114. Chinese.
- Song XM. 2012. Prevention and control of main diseases and pests for artificial cultivation of Dendrobium officinale. J Anhui Agri Sci. 40:15697–15714. Chinese.
- Wang Q, Sun P, Li G, Zhu K, Wang C, Zhao X. 2014. Inhibitory effects of Dendrobium candidum Wall ex Lindl. on azoxymethane- and dextran sulfate sodium-induced colon carcinogenesis in C57BL/6 mice. Oncol Lett. 7:493–498.
- White TJ, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: A guide to methods and applications. New York (NY): Academic Press; p. 315–322.
- Yang S, Zhang X, Cao Z, Zhao K, Wang S, Chen M, Hu X. 2014. Growth-promoting Sphingomonas paucimobilis ZJSH1 associated with Dendrobium officinale through phytohormone production and nitrogen fixation. Microb Biotechnol. 7:611–620.
- Zhang J, Fang Y, Zhang H, Cheng F. 2005. Efficacy tests on fungicides of Alternaria tenuissima in the laboratory. J Plant Prot. 31:44–47. Chinese.
- Zhang JZ, Zheng XJ. 2004. Identification of an Alternaria species associated with Dendrobium candidium and cytological study on its infection process. Acta Phytopathol Sin. 34:92–94. Chinese.
- Zhang L, Chen J, Lv Y, Gao C, Guo S. 2012. Mycena sp., a mycorrhizal fungus of the orchid Dendrobium officinale. Mycol Prog. 11:395–401.