Abstract
Elongation factor Tu (EF-Tu) from bacterial plant pathogens acts as a pathogen-associated molecular pattern (PAMP), which elicits the first line of defence of innate immunity. Ralstonia solanacearum is the causal agent of bacterial wilt in many Solanaceous plants, including Nicotiana tabacum and N. benthamiana. Here, we report that R. solanacearum EF-Tu can promote the resistance of Nicotiana plants to wilt disease. A synthetic elf26 epitope of R. solanacearum EF-Tu induced a clear accumulation of callose and hydrogen peroxide in Arabidopsis thaliana. It was very striking that elf26 induced the expression of PTI marker genes as well as a low dose of callose and hydrogen peroxide accumulation in N. tabacum as EF-Tu triggering innate immunity was previously considered to be Brassicaceae specific. Although innate immunity was not found in N. benthamiana, the defence-related PR1a gene was induced. Thus, elf26 treatments enhanced the resistance of N. tabacum and N. benthamiana plants to R. solanacearum. Together, our findings demonstrated that the R. solanacearum elf26 epitope functions as a PAMP and can promote resistance to bacterial wilt disease in Nicotiana spp.
Résumé
Le facteur d’élongation Tu (EF-Tu) des agents pathogènes bactériens des plantes agit à titre de motif moléculaire associé aux agents pathogènes (PAMP) en sollicitant la première ligne de défense de l’immunité innée. Ralstonia solanacearum est l’agent causal de la flétrissure bactérienne chez plusieurs plantes de la famille des solanacées, y compris Nicotiana tabacum et N. benthamiana. Dans cet article, nous mentionnons que l’EF-Tu de R. solanacearum peut promouvoir la résistance des plants de Nicotiana à la flétrissure. Un épitope synthétique elf26 de l’EF-Tu de R. solanacearum a provoqué une accumulation notable de callosités et de peroxyde d’hydrogène chez Arabidopsis thaliana. C’était très étonnant de constater qu’elf26 avait provoqué l’expression des gènes marqueurs de la PTI de même qu’une faible accumulation de callosités et de peroxyde d’hydrogène chez N. tabacum, d’autant plus que l’amorce de l’immunité innée provoquée par elf26 était considérée auparavant comme propre aux brassicacées. Bien que l’immunité innée n’ait pas été observée chez N. benthamiana, le gène PR1a associé aux mécanismes de défense a été activé. Par conséquent, les traitements avec elf26 ont amélioré la résistance des plants de N. tabacum et de N. benthamiana à R. solanacearum. En somme, nos résultats ont démontré que l’épitope elf26 de R. solanacearum fonctionne comme un PAMP et qu’il peut promouvoir la résistance à la flétrissure bactérienne chez plusieurs espèces de Nicotiana.
Introduction
Plants perceive several conserved pathogen-associated molecular patterns (PAMPs) through a set of pattern recognition receptors (PRRs), thereby triggering innate immunity (PAMP triggered immunity, PTI; Jones & Dangl, Citation2006; Zipfel et al., Citation2006). EF-Tu, flagellin, lipopolysaccharides and bacterial cold-shock protein are well-known PAMPs from plant pathogenic bacteria (Felix & Boller, Citation2003; Zipfel & Felix, Citation2005). Substantial attention has been placed on PTI as a result of the recognition of bacterial flagellin and EF-Tu by host plants. Flg22, a 22-amino acid peptide derived from a conserved domain at the N-terminus of flagellin, is sufficient for eliciting an oxidative burst in tomato, tobacco, potato and Arabidopsis plants (Meindl et al., Citation2000; Daudi et al., Citation2012; Hao et al., Citation2014). The recognition of flagellin or flg22 depends on FLS2 (flagellin sensing 2), which is a receptor-like kinase (RLK) with an extracellular leucine-rich repeat (LRR) domain, a cytoplasmic serine/threonine kinase domain, and a single membrane spanning domain (Chinchilla et al., Citation2006). EF-Tu, an abundant and highly conserved bacterial protein, is another proteinaceous PAMP (Kunze et al., Citation2004) that is perceived by a LRR receptor kinase named EFR (Zipfel et al., Citation2006). Like flg22, the acetylated N-terminus amino acid elf26 acts as a potent elicitor that induces defence responses in Arabidopsis (Kunze et al., Citation2004). However, more emerging studies have inferred that the specific domains perceived by plants are varied in proteinaceous flagellin and ET-Tu. For example, a 15-amino-acid flagellin peptide from Escherichia coli is recognized by tomato, but not Arabidopsis (Felix et al., Citation1999; Meindl et al., Citation2000). Rice plants are able to recognize the middle region EFa50 of Acidovorax avenae EF-Tu, rather than the N-terminus of elf26 (Furukawa et al., Citation2014).
Ralstonia solanacearum is the causal agent of bacterial wilt, which affects over 50 families of plants worldwide, including tobacco, Arabidopsis and tomato by invading host plants through root wounds to cause vascular disease (Vasse et al., Citation1995; Mansfield et al., Citation2012; Peeters et al., Citation2013). This soilborne pathogen causes substantial crop losses in the tropics and subtropics, resulting in great economic hardship (Hayward, Citation1991; Huet, Citation2014). The analysis of interactions between R. solanacearum and host plants has shown that PAMP-triggered PTI plays specific roles during R. solanacearum infection (Esposito et al., Citation2008; Lacombe et al., Citation2010). Purified lipopolysaccharides extracted from the R. solanacearum cell wall inhibited the hypersensitivity reaction in non-host tobacco plants and induced localized resistance in host potato plants (Esposito et al., Citation2008). However, R. solanacearum flagellin does not act as a PAMP and therefore does not elicit defence responses in Arabidopsis. Boiled extracts from wild-type bacteria and fliC or flhDC mutants, which are defective in flagellin production, all elicited similar plant responses (Pfund et al., Citation2004). A more recent study reported that Arabidopsis showed no response to flg22 treatment from R. solanacearum strain K60 (Helft et al., Citation2016). The PAMP function of EF-Tu is revealed by the induced pathogen resistance of Arabidopsis plants pre-treated with boiled R. solanacearum extract. The active molecule in boiled extract is sensitive to pronase; meanwhile, the populations of R. solanacearum slightly increase in Arabidopsis efr mutants relative to wild-type plants (Takabatake & Mukaihara, Citation2011). Therefore, the EF-Tu from R. solanacearum is presumed to be involved in basal defence induction (Takabatake & Mukaihara, Citation2011).
The aim of this research was to study the PAMP function of R. solanacearum EF-Tu by using a synthetic elf26 epitope. Tobacco is the natural host of R. solanacearum. Detailed data were generated to infer the responses of Nicotiana tabacum and N. benthamiana plants to elf26. We found that the elf26 treatment increased the resistance of tobacco plants to R. solanacearum and induced a low dose PTI response in N. tabacum. The results enhance our understanding of interaction mechanisms between R. solanacearum and its hosts, which may help to develop an efficient management strategy.
Materials and methods
elf26 infiltration, callose deposition and H2O2 accumulation
The elf26 oligopeptide (AKEKFERTKPHVNVGTIGHVDHGKTT) was synthesized by ChinaPeptides (Suzhou, Jiangsu, China), yielding a 2887.14-Da peptide. The synthetic peptide was diluted with double-distilled water to final concentrations of 1.0, 10.0 and 100.0 nM. The dilutions were then infiltrated into 5-week-old Arabidopsis and Nicotiana plant leaves using needleless syringes. Double-distilled water was used as a negative control. Twelve hours after the infiltration of the peptides into the plant leaves, callose and H2O2 depositions were assayed. All infiltration experiments were repeated three times.
To visualize callose deposition in situ, plant leaf tissues were cleared and dehydrated with 100% ethanol, washed with 67 mM K2HPO4 (pH 12.0) to remove excess ethanol, and stained with 0.01% aniline blue in 67 mM K2HPO4 (pH 12.0) for 1 h at room temperature. The stained materials were equilibrated in 50% glycerol and examined using ultraviolet epifluorescence (Zou et al., Citation2012). To visualize H2O2 accumulation, 3,3′-diaminobenzidine (DAB) staining was performed as previously described (Yakushiji et al., Citation2009). Leaves were placed in 1 mg mL−1 DAB and incubated under a vacuum for 3 h. H2O2 accumulation was scored after boiling leaf tissues in 100% ethanol for 15 min. Images were captured under bright light. All of the assays were performed with three independent biological replications.
RNA extraction and qRT-PCR
Leaves from 5-week-old A. thaliana (ecotype Col-0) and tobacco plants (N. tabacum ‘NC89‘ and N. benthamiana) were infiltrated with 100 nM elf26 peptides. Double-distilled water was used as a negative control. At 0, 4, 8 and 12 h after infiltration, whole leaves were harvested and frozen in liquid nitrogen. Total RNA was then extracted from the samples using Trizol Reagent (Invitrogen, CA). One μg of RNA was reverse-transcribed in a 10-μL reaction system using the AMV RNA PCR Kit (TaKaRa, Shiga, Japan) according to the manufacturer’s protocol. Each qPCR reaction included 2 μL of cDNA template (10-fold dilution), 0.4 μL of each primer (10 μM), 0.4 μL of Rox Reference Dye, and 10 μL of SYBR Green PCR Master Mix kit (Promega, USA) in a total volume of 20 μL. The PCR procedure consisted of one cycle at 95°C for 30 s followed by 40 cycles of 95°C for 5 s and 60°C for 30 s. The primers used to evaluate expression levels of PR1a, EFR and the homologues of EFR in Nicotiana plants were designed using Primer 5.0 based on the sequences deposited in the National Center for Biotechnology Information database and Sol Genomics Network (; Zipfel et al., Citation2006; Shen et al., Citation2010). The primers for PTI marker genes in Nicotiana and Arabidopsis were validated previously (Nguyen et al., Citation2010; Singh et al., Citation2012). Three replicate assays were performed with independently isolated RNA samples. The expression of the β-actin gene was used as an internal standard to verify the absence of any significant variation in overall cDNA levels.
Table 1. Primers for quantitative real-time PCR used in this study.
Plant resistance assay
The virulent R. solanacearum strain FJ1003 was used for testing the resistance of N. tabacum and N. benthamiana plants (Zhang et al., Citation2017), and R. solanacearum GMI1000 was used to examine the resistance of A. thaliana accession Col-0 (Solé et al., Citation2012). The fully expanded leaves of 7-week-old tobacco plants and 5-week-old Arabidopsis plants were infiltrated with 100 nM elf26 peptide solution using a needleless syringe. Control plants were inoculated with distilled water. Then, 24 h after elf26 treatment, the bacterial suspensions of the cultured FJ1003 and GMI1000 strains (at 108 CFU (colony forming unit) mL−1) were inoculated into the infiltration zone by leaflet cutting (i.e. a perpendicular cut into the midrib of the leaflet, to a depth of 2/3 into the midrib). Two to three leaflets were inoculated per plant. For one parallel experiment, eight plants were used for each treatment. The tobacco plant resistance experiments were repeated four times. The typical symptoms of bacterial wilt were recorded daily for each plant according to a wilting scale of 0 to 4 (0 = no wilting; 1 = 1–25%, 2 = 26–50%, 3 = 51–75% and 4 = 76–100% leaves wilted). For the Arabidopsis plants, three treated leaves were taken at 3 and 6 days from different seedlings after inoculation, weighed and ground with sterilized water. Then the bacterial cells in leaf extracts were serially 10-fold diluted and plated onto BG medium plates (Boucher et al., Citation1985). CFU counts were assessed from three individual replicates according to Fan et al. (Citation2016). Three replicate assays were conducted.
Statistical analyses
All data were analysed with SPSS, version 17.0 (SPSS Inc., Chicago, IL, USA). Statistically significant differences were assessed by using a two-tailed Student’s t-test or one-way ANOVA followed by Tukey’s honest significant difference (HSD) test.
Results
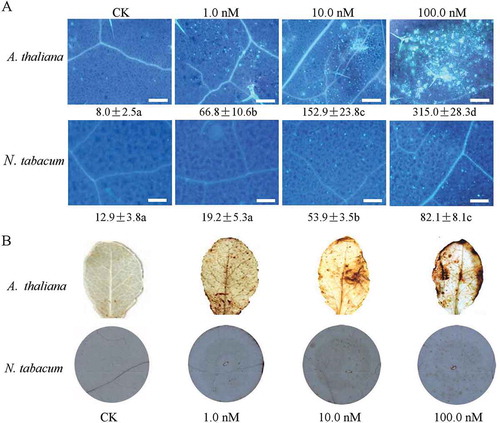
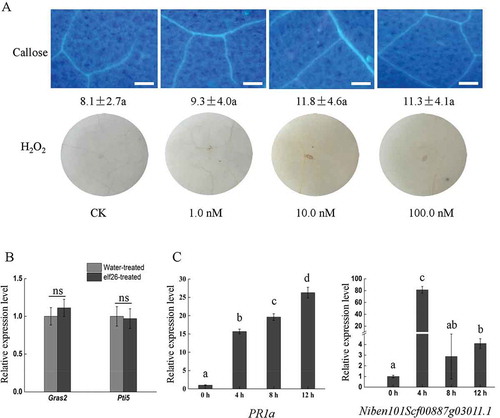
R. solanacearum elf26 induces callose and H2O2 accumulation in A. thaliana and N. tabacum
In Arabidopsis and other brassicaceous species, the acetylated N-terminus of EF-Tu comprising the first 26 amino acid residues (elf26) is fully active as an inducer of defence responses, and it is strongly conserved across a wide range of bacteria (Kunze et al., Citation2004). Alignments of the elf26 amino acid sequences of R. solanacearum GMI1000 with the elf26 peptide sequence from E. coli revealed there is only one amino acid substitution. It has four residues that differ from those of Pseudomonas syringae pv. tomato DC3000 (Lacombe et al., Citation2010). To investigate the biological function of elf26 from R. solanacearum GMI1000, we used the N-acetylated synthetic peptide elf26 to test whether it was sufficient to induce the immune responses in tobacco plants. Arabidopsis thaliana was used as a control. At 12 h after inoculation, both callose and H2O2 generation were induced by elf26 at inoculum concentrations of 1.0, 10.0 and 100.0 nM in A. thaliana leaves (). Callose and H2O2 accumulation were also observed in N. tabacum leaves under the same concentrations. However, the quantity was much less than that observed in A. thaliana under the same concentrations (). In each experimental replicate, we did not observe clear cell death in either A. thaliana or N. tabacum plants treated with elf26.
Fig. 1 (Colour online) Innate immunity induction of Ralstonia solanacearum elf26 in Arabidopsis thaliana and Nicotiana tabacum. (a) elf26-treated leaves were stained with aniline blue, and callose deposition was observed under a UV epifluorescence microscope. All scale bars are 100 µm in length. The mean number of callose deposits per 0.4 mm2 area is shown with the standard error (SE) of values from three independent experiments. Means followed by different lowercase letters are significantly different (ANOVA and Tukey’s HSD test, P < 0.05). (b) H2O2 accumulation visualized by 3,3′-diaminobenzidine (DAB) staining of leaves at 12 h after infiltration.
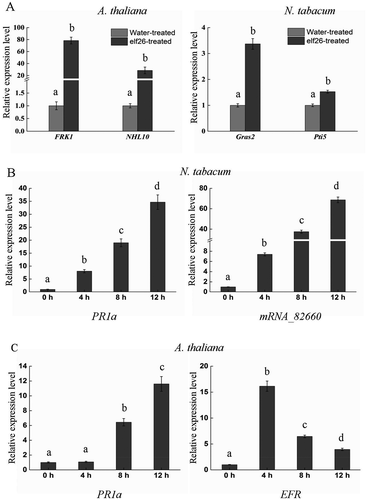
R. solanacearum elf26 induces PTI- and defence-associated gene expression in A. thaliana and N. tabacum
The accumulation of callose and H2O2 in N. tabacum plants prompted us to determine whether PTI-associated genes were actually induced by elf26 peptide. The Pto-interacting 5 (Pti5) and Gras (GAI, RGA, SCR) 2 (Gras2) genes are two marker genes for PTI response in tobacco (Nguyen et al., Citation2010). The expression levels of PTI marker genes were analysed in plants treated with elf26 relative to a water treatment control. The FLG22-INDUCED RECEPTOR KINASE1 (FRK1) and NDR1/HIN1-LIKE10 (NHL10) are two marker genes for PTI response in A. thaliana (Singh et al., Citation2012). FRK1 and NHL10 were therefore studied to confirm the PAMP function of the elf26 peptide in A. thaliana. At 8 h post-treatment, Gras2, FRK1 and NHL10 were significantly induced in response to elf26 compared with the water control, whereas the expression of Pti5 was slightly induced (Fig. 2a). The considerable induction of FRK1 and NHL10 were in agreement with the high accumulation of callose and H2O2 in A. thaliana. In contrast, the relatively low expression levels of Gras2 and Pti5 in N. tabacum were in agreement with the weak accumulation of callose and H2O2.
From genome databases, 19 EFR homologues were discovered in N. tabacum, and the receptor-like kinase gene mRNA_82660 showed the highest identity with the ET-Tu receptor EFR of A. thaliana. The mRNA_82660 and systemic acquired resistance marker gene PR1a were selected to study the expression of defence-related genes after elf26 treatment in N. tabacum. shows that mRNA_82660 and PR1a were all induced by elf26, but the expression pattern of mRNA_82660 in N. tabacum differed from that of EFR in A. thaliana. The expression level of Arabidopsis EFR increased 16-fold in A. thaliana at 4 h after inoculation and declined to 6.5- and 3.9-fold at 8 and 12 h after inoculation, respectively (). In contrast, the transcription level of mRNA_82660 was gradually increased at 4, 8 and 12 h after inoculation (). The expression level of PR1a was gradually enhanced at each time point in both plants. Thus, elf26 induced the expression of PTI marker genes as well as plant defence-related genes.
Fig. 2 Induction of PTI-responsive genes and defence-related genes by Ralstonia solanacearum elf26 (100 nM) in Nicotiana tabacum and Arabidopsis thaliana. (a) Relative expression level of PTI reporter genes FRK1 and NHL10 in A. thaliana leaves or Gras2 and Pti5 in N. tabacum leaves at 8 h post-infiltration with elf26, respectively. Fold-induction levels compared with water-treated samples (defined as 1) are shown. Values are means ± SE fold change relative to the transcript level of water-treated plants from three independent experiments. Significant differences were assessed using a Student’s t-test (P < 0.05). (b, c) Transcript abundance levels of the defence-related genes under elf26 treatment. Real-time RT-PCR was carried out to analyse expressional levels of PR1a and mRNA_82660 in N. tabacum, as well as PR1a and EFR in A. thaliana. Values are relative gene expression ratios of elf26- to water-treated samples. Each histogram bar represents the mean ± SE of three replicates. Means followed by different lowercase letters are significantly different (ANOVA and Tukey’s HSD test, P < 0.05).
elf26 treatment promotes resistance to R. solanacearum in N. tabacum and A. thaliana
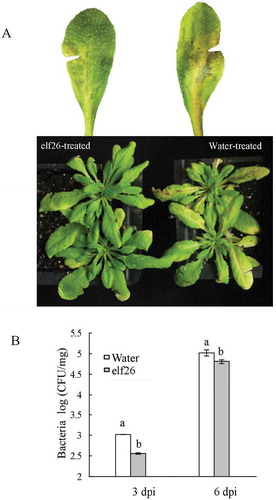
To determine whether defence gene induction contributes to resistance, tobacco and Arabidopsis plants receiving elf26 treatment were inoculated with R. solanacearum or distilled water as a control. At 4 days after inoculation, bacterial wilt symptoms appeared on N. tabacum control plants (Fig. 3a). At the inoculation sites, N. tabacum leaves became dehydrated and shrunken. Meanwhile, leaves that had received elf26 treatments did not show any wilt symptoms. As the disease developed, the symptoms intensified and the wilt symptoms gradually appeared on stems. Ultimately, entire plants collapsed by 10 days after inoculation (). However, the plants pre-treated with elf26 did not show bacterial wilt symptom. Sometimes, slight dehydration and shrinking were observed in the area surrounding the inoculation sites, but the plants remained healthy. The disease index of the plants receiving the elf26 treatment was extremely low relative to control plants (). A similar result was obtained from Arabidopsis plants treated with elf26. Ralstonia solanacearum GMI1000 caused less tissue collapse in leaves relative to the water control (Fig. 4a). Furthermore, elf26 treatment showed a suppressive effect on R. solanacearum GMI1000 replication in leaf tissue (). These results demonstrated that defence induction by elf26 inhibited bacterial growth and disease development in treated plants (Takabatake & Mukaihara, Citation2011).
Fig. 3 (Colour online) Resistance to Ralstonia solanacearum FJ1003 of Nicotiana tabacum plants after receiving elf26 treatment. (a) The leaves in the upper panels show representative wilt disease development at 4 days after inoculation. In the lower panels, the disease symptoms 6 days after inoculation are shown. (b) Average disease index of N. tabacum plants pre-treated with elf26 and water as a control before infection with R. solanacearum FJ1003. Means ± SE from four biological replicates are shown. Statistical significance was determined by a Student’s t-test at each time point versus the control (water), *P < 0.05.
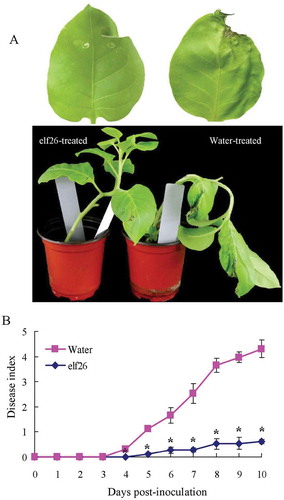
Fig. 4 (Colour online) The elf26 epitope from Ralstonia solanacearum conferred resistance to the pathogen in Arabidopsis thaliana. (a) Disease symptoms on Arabidopsis leaves pre-treated with elf26 or distilled water as a control 24 h before inoculation with GMI1000. This representative image was captured 8 days after inoculation. (b) Bacterial growth in Arabidopsis leaves pre-treated with elf26 or distilled water as a control before inoculation with GMI1000 was measured at 3 and 6 days after inoculation. Values represent the mean of three biological replicates. Significant differences were assessed by Student’s t-test (P < 0.05).
elf26 does not elicit PTI response in N. benthamiana
We also examined whether elf26 from R. solanacearum induces innate immune responses in N. benthamiana. Figure 5a shows that neither callose nor H2O2 accumulation was observed in leaves treated with elf26. The elf26 treatment had no effect on expression of PTI marker genes either (). EFR homologues Niben101Scf00887g03011.1 and PR1a were chosen to study the expression profile of defence-related genes in N. benthamiana. The expression of PR1a gradually increased after elf26 treatment, showing a similar expression pattern as that in A. thaliana and N. tabacum. The expression pattern of Niben101Scf00887g03011.1 was similar with EFR in A. thaliana. It was induced at 4 h after elf26 treatment and declined at 8 h ().
Fig. 5 (Colour online) Defence responses after treatment with elf26 in Nicotiana benthamiana. (a) Callose and H2O2 were stained with aniline blue and 3,3′-diaminobenzidine (DAB), respectively. Mean number of callose deposits per 0.4 mm2 area is shown with SE of values from three independent experiments. Means followed by the same lowercase letter are not significantly different at P < 0.05 (ANOVA and Tukey’s HSD test). Scale bars are 100 µm in length. (b) Relative expression level of PTI reporter genes Gras2 and Pti5 in N. benthamiana leaves at 8 h post-infiltration with elf26 (100 nM). Fold-induction levels compared with water-treated samples (defined as 1) are shown. Values are means ± SE fold change relative to the transcript level of water-treated plants from three independent experiments. Significant differences were assessed by Student’s t-test at P < 0.05 (ns indicates non-significance). (c) Relative transcript levels of PR1a and Niben101Scf00887g03011.1 in N. benthamiana leaves were evaluated by qRT-PCR after treatment with elf26 (100 nM). Values are relative gene expression ratios of elf26- to water-treated samples. Means ± SE from three biological replicates are shown. Means followed by different lowercase letters are significantly different (ANOVA and Tukey’s HSD test, P < 0.05).
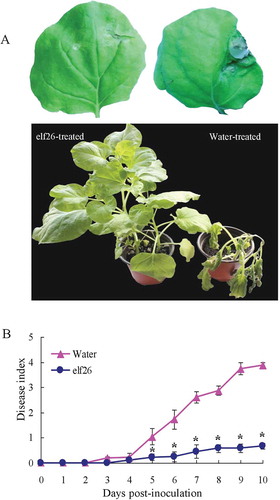
As the expression of defence genes was induced by elf26 in N. benthamiana plants, we subsequently examined plant resistance to bacterial wilt. As expected, a significant reduction of wilting symptoms was observed (). The disease index was lower than 1 until 10 days post-inoculation (). In contrast, bacterial wilt symptoms appeared on the control plants at 3 days post-inoculation. By 10 days post-inoculation, all the control plants had collapsed, indicating that elf26 peptide treatment could increase N. benthamiana resistance to bacterial wilt.
Fig. 6 (Colour online) Resistance to Ralstonia solanacearum FJ1003 of Nicotiana benthamiana plants after elf26 treatment. (a) Representative images of leaves and plants were captured at 4 and 10 days post-inoculation, respectively. (b) Disease index of N. benthamiana plants pre-treated with elf26 before infection with R. solanacearum FJ1003, with water pre-treatment as a control. Means ± SE from four biological replicates are shown. The statistical significance shown is relative to the control (water), *P < 0.05.
Discussion
PAMP-triggered immunity in plants is characterized by production of reactive oxygen species, callose deposition, activation of mitogen-activated protein kinase cascades, and the expression of defence-related genes (Boller & Felix, Citation2009). Pti5 and Gras2 genes are rapidly induced in response to PAMPs, and they have been used as PTI marker genes in Nicotiana (Nguyen et al., Citation2010). We found that N. tabacum plants exhibited an innate immune response to R. solanacearum elf26 peptide. Callose and H2O2 generation were observed 12 h after infiltration with elf26. PTI marker genes Pti5 and Gras2 were also induced. Our data showed that the induction of Gras2 was higher than that of Pti5 at 8 h after infiltration with elf26. A previous study reported that the induction level of Pti5 was higher than that of Gras2 in N. benthamiana (Nguyen et al., Citation2010). We speculated that Pti5 might be induced earlier than Gras2 or that the expression pattern of marker genes may stem from the application of different PAMPs.
PTI induction relies on the recognition of bacterial PAMPs by plant cell-surface PRRs (Jones & Dangl, Citation2006), which are plasma membrane-localized receptor-like kinases (RLKs). By searching Nicotiana plant genome databases, 19 EFR homologues were discovered in N. tabacum. The mRNA_82660 product shows the highest similarity (45.56%) with EFR from A. thaliana, and thus was chosen for further study in this research. At 12 h after treatment with elf26, mRNA_82660 was induced over 60-fold. Two previous studies have inferred that the perception of EF-Tu or its oligopeptide elf18 is Brassicaceae specific (Kunze et al., Citation2004; Zipfel et al., Citation2006) and that solanaceous plants lack the endogenous EF-Tu recognition system (Kunze et al., Citation2004). Our results indicated that the EFR homologue in N. tabacum might function as a receptor recognizing elf26. By comparison with the strong response in A. thaliana, low sequence identity between mRNA_82660 and EFR probably explains the weak PTI response in N. tabacum. Currently, the biological function of mRNA_82660 has not been experimentally validated. More studies are required to confirm the recognition of R. solanacearum elf26 by the mRNA_82660 product at the protein–protein interaction level.
Upon PAMP recognition, several signalling pathways are induced in host plants (Bigeard et al., Citation2015), including those associated with defence-related genes (Navarro et al., Citation2004; Pfund et al., Citation2004). In A. thaliana, applications of flagellin, epitope flg22 or lipopolysaccharide increased the expression levels of SA and PR genes in treated or distant leaves (Gómez-Gómez et al., Citation1999; Mishina & Zeier, Citation2007). The enhanced expression of PR1a in this study suggested that the SA-mediated signalling pathway was evoked by elf26 in A. thaliana and N. tabacum leaves. Together with the PTI response, elf26 pretreatment remarkably promoted N. tabacum and A. thaliana plant resistance to R. solanacearum. As PR1a is a marker gene for the SA signalling pathway, a detailed investigation is required to determine salicylic acid accumulation in plants after elf26 treatment.
PAMPs produced by microbes and the PAMP perception systems in plants constitute a dynamic evolutionary system (Boller & Felix, Citation2009). For instance, Physcomitrella patens possesses a number of RLKs but does not show responses to flg22 (Boller & Felix, Citation2009). The perception of flg22 occurs in most plant species, but some pathogens alter flg22 structures to evade recognition by FLS2 (Pfund et al., Citation2004; Sun et al., Citation2006). Nicotiana benthamiana plants did not respond to elf26. We did not observe the accumulation of callose or H2O2; meanwhile, PTI marker genes were not induced. This result was consistent with the previous conclusion that N. benthamiana plants lack an endogenous EF-Tu perception system (Kunze et al., Citation2004). We also searched the EFR homologues in N. benthamiana, and found that Niben101Scf00887g03011.1 showed the highest similarity with EFR (46.01%). However, the amino acid sequence of Niben101Scf00887g03011.1 shared only 52.9% identity with mRNA_82660 in N. tabacum, suggesting that although N. tabacum may possess RLK that specifically recognize elf26, the RLK might not be conserved in tobacco. Thus, only N. tabacum can generate the PAMP triggered immunity response. In addition, Niben101Scf00887g03011.1 in N. benthamiana was also induced at 4 h after treatment with elf26; after 4 h, the expression was reduced sharply. Further investigation is necessary to elucidate the role of Niben101Scf00887g03011.1 in plant immunity.
Although there is no PTI response in N. benthamiana, elf26 treatment still confers resistance to R. solanacearum. The qRT-PCR analysis revealed that the expression pattern of PR1a was similar to that in N. tabacum. This indicated that a SA-dependent signalling pathway was induced by R. solanacearum elf26. As there was no effect on PTI marker gene expression, elf26 obviously activates various independent defence signalling pathways. This may explain why N. benthamiana showed a similar level of resistance to R. solanacearum relative to N. tabacum plants when pre-treated with elf26.
In conclusion, our data demonstrated that R. solanacearum elf26 functions as a PAMP, inducing PTI responses in A. thaliana. Notably, a low-dose PTI response was observed in N. tabacum, illustrating the diversity of EF-Tu perception systems in host plants. Furthermore, the elf26 treatment increased the resistance of N. tabacum and N. benthamiana against R. solanacearum. This is the first report to elucidate the induction of innate immunity in N. tabacum plants by elf26.
Additional information
Funding
References
- Bigeard J, Colcombet J, Hirt H. 2015. Signaling mechanisms in pattern-triggered immunity (PTI). Mol Plant. 8:521–539.
- Boller T, Felix G. 2009. A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol. 60:379–406.
- Boucher CA, Barberis PA, Demery DA. 1985. Transposon mutagenesis of Pseudomonas solanacearum: isolation of Tn5-induced avirulent mutants. Microbiology. 131:2449–2457.
- Chinchilla D, Bauer Z, Regenass M, Boller T, Felix G. 2006. The Arabidopsis receptor kinase FLS2 binds flg22 and determines the specificity of flagellin perception. Plant Cell. 18:465–476.
- Daudi A, Cheng Z, O’Brien JA, Mammarella N, Khan S, Ausubel FM, Bolwell GP. 2012. The apoplastic oxidative burst peroxidase in Arabidopsis is a major component of pattern-triggered immunity. Plant Cell. 24:275–287.
- Esposito N, Ovchinnikova OG, Barone A, Zoina A, Holst O, Evidente A. 2008. Host and non-host plant response to bacterial wilt in potato: role of the lipopolysaccharide isolated from Ralstonia solanacearum and molecular analysis of plant-pathogen interaction. Chem Biodiver. 5:2662–2675.
- Fan X, Yang R, Qiu S, Cai X, Zou H, Hu F. 2016. The endo-β-1,4-glucanase of Bacillus amyloliquefaciens is required for optimum endophytic colonization of plants. J Microbiol Biotech. 26:946–952.
- Felix G, Boller T. 2003. Molecular sensing of bacteria in plants. The highly conserved RNA-binding motif RNP-1 of bacterial cold shock proteins is recognized as an elicitor signal in tobacco. J Biol Chem. 278:6201–6208.
- Felix G, Duran JD, Volko S, Boller T. 1999. Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 18:265–276.
- Furukawa T, Inaqaki H, Takai R, Hirai H, Che FS. 2014. Two distinct EF-Tu epitopes induce immune responses in rice and Arabidopsis. Mol Plant-Microbe Interact. 27:113–124.
- Gómez-Gómez L, Felix G, Boller T. 1999. A single locus determines sensitivity to bacterial flagellin in Arabidopsis thaliana. Plant J. 18:277–284.
- Hao GX, Pitino M, Ding F, Lin H, Stover E, Duan YP. 2014. Induction of innate immune responses by flagellin from the intracellular bacterium, ‘Candidatus Liberibacter solanacearum’. BMC Plant Biol. 14:211.
- Hayward HC. 1991. Biology and epidemiology of bacterial wilt caused by Pseudomonas solanacearum. Annu Rev of Phytopathol. 29:65–87.
- Helft L, Thompson M, Bent AF. 2016. Directed evolution of FLS2 towards novel flagellin peptide recognition. PLoS One. 11:e0157155.
- Huet G. 2014. Breeding for resistances to Ralstonia solanacearum. Front Plant Sci. 5:715.
- Jones JDG, Dangl JL. 2006. The plant immune system. Nature. 444:323–329.
- Kunze G, Zipfel C, Robatzek S, Niehaus K, Boller T, Felix G. 2004. The N terminus of bacterial elongation factor Tu elicits innate immunity in Arabidopsis plants. Plant Cell. 16:3496–3507.
- Lacombe S, Rougon-Cardoso A, Sherwood E, Peeters N, Dahlbeck D, Van Esse HP, Smoker M, Rallapalli G, Thomma BP, Staskawicz B, et al. 2010. Interfamily transfer of a plant pattern-recognition receptor confers broad-spectrum bacterial resistance. Nat Biotechnol. 28:365–369.
- Mansfield J, Genin S, Magori S, Citovsky V, Sriariyanum M, Ronald P, Dow M, Verdier V, Beer SV, Machado MA, et al. 2012. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol Plant Pathol. 13:614–629.
- Meindl T, Boller T, Felix G. 2000. The bacterial elicitor flagellin activates its receptor in tomato cells according to the address-message concept. Plant Cell. 12:1783–1794.
- Mishina TE, Zeier J. 2007. Pathogen-associated molecular pattern recognition rather than development of tissue necrosis contributes to bacterial induction of systemic acquired resistance in Arabidopsis. Plant J. 50:500–513.
- Navarro L, Zipfel C, Rowland O, Keller I, Robatzek S, Boller T, Jones JDG. 2004. The transcriptional innate immune response to flg22. Interplay and overlap with Avr gene-dependent defense responses and bacterial pathogenesis. Plant Physiol. 135:1113–1128.
- Nguyen HP, Chakravarthy S, Velásquez AC, McLane HL, Zeng L, Nakayashiki H, Park DH, Collmer A, Martin GB. 2010. Methods to study PAMP-Triggered immunity using tomato and Nicotiana benthamiana. Mol Plant-Microbe Interact. 23:991–999.
- Peeters N, Guidot A, Vailleau F, Valls M. 2013. Ralstonia solanacearum, a widespread bacterial plant pathogen in the post-genomic era. Mol Plant Pathol. 14:651–662.
- Pfund C, Tans-Kersten J, Dunning FM, Alonso JM, Ecker JR, Allen C, Bent AF. 2004. Flagellin is not a major defense elicitor in Ralstonia solanacearum cells or extracts applied to Arabidopsis thaliana. Mol Plant-Microbe Interact. 17:696–706.
- Shen X, Yuan B, Liu H, Li X, Xu C, Wang S. 2010. Opposite functions of a rice mitogen-activated protein kinase during the process of resistance against Xanthomonas oryzae. Plant J. 64:86–99.
- Singh P, Kuo YC, Mishra S, Tsai CH, Chien CC, Chen CW, Desclos-Theveniau M, Chu PW, Schulze B, Chinchilla D, et al. 2012. The lectin receptor kinase-VI.2 is required for priming and positively regulates Arabidopsis pattern-triggered immunity. Plant Cell. 24:1256–1270.
- Solé M, Popa C, Mith O, Sohn KH, Jones JD, Deslandes L, Valls M. 2012. The awr gene family encodes a novel class of Ralstonia solanacearum type iii effectors displaying virulence and avirulence activities. Mol Plant-Microbe Interact. 25:941–953.
- Sun WX, Dunning FM, Pfund C, Weingarten R, Bent AF. 2006. Within-species flagellin polymorphism in Xanthomonas campestris pv. campestris and its impact on elicitation of Arabidopsis FLAGELLIN SENSING2-dependent defenses. Plant Cell. 18:764–779.
- Takabatake R, Mukaihara T. 2011. Extracts from Ralstonia Solanacearum induce effective resistance to the pathogen in both Arabidopsis and solanaceous plants. J Gen Plant Pathol. 77:33–42.
- Vasse J, Frey P, Trigalet A. 1995. Microscopic studies of intercellular infection and protoxylem invasion of tomato roots by Pseudomonas solanacearum. Mol Plant-Microbe Interact. 8:241–251.
- Yakushiji S, Ishiga Y, Inagaki Y, Toyoda K, Shiraishi T, Ichinose Y. 2009. Bacterial DNA activates immunity in Arabidopsis thaliana. J Gen Plant Pathol. 75:227–234.
- Zhang C, Chen H, Cai T, Deng Y, Zhuang R, Zhang N, Zeng Y, Zheng Y, Tang R, Pan R, et al. 2017. Overexpression of a novel peanut NBS-LRR gene AhRRS5 enhances disease resistance to Ralstonia solanacearum in tobacco. Plant Biotechnol J. 15:39–55.
- Zipfel C, Felix G. 2005. Plants and animals: a different taste for microbes?. Curr Opin Plant Biol. 8:353–360.
- Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JD, Boller T, Felix G. 2006. Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell. 125:749–760.
- Zou HS, Gowda S, Zhou LJ, Hajeri S, Chen GY, Duan YP. 2012. The destructive citrus pathogen, ‘Candidatus Liberibacter asiaticus’ encodes a functional flagellin characteristic of a pathogen-associated molecular pattern. PLoS One. 7:e46447.
