Abstract
Current agricultural output is challenged by considerable losses in crop yield and post-harvest storage due to fungal infection. Traditional chemical fungicides used to treat these fungi can be ineffective and harmful to the environment if not used properly. With fungicide resistance increasing in fungal pathogens, new environmentally friendly and sustainable technologies are required to manage diseases on the world’s most important crops. RNA interference (RNAi) is an intrinsic cellular mechanism, mediated by double-stranded RNA (dsRNA), which can suppress protein expression through targeted destruction of mRNAs. With recent advances in dsRNA delivery or expression in plants, this mechanism has the potential to provide alternative disease management strategies. Examples of RNAi-based control to manage pathogenic fungal species are steadily increasing, and the technology offers new options to increase species-specificity and/or potency against fungi for which existing fungicides have been ineffective. RNAi technology can be adapted to provide either robust and multi-crop plant protection using topical sprays or can provide more durable resistance through transgene expression of dsRNAs within susceptible plant tissues. Using RNA sequencing to identify fungal gene targets, RNAi-based control technology continues to show promise as an alternative to traditional agrochemicals for crop protection.
Résumé
Les infections fongiques menacent la production agricole actuelle, et ce, tant à cause des immenses pertes de rendement que de celles survenant durant l’entreposage des récoltes. Lorsqu’ils ne sont pas utilisés correctement, les fongicides de synthèse traditionnels utilisés pour combattre les champignons peuvent être inefficaces, en plus d’être nocifs pour l’environnement. Avec la résistance accrue des agents pathogènes fongiques aux fongicides, nous avons besoin de nouvelles technologies respectueuses de l’environnement et durables pour lutter contre les maladies des cultures les plus importantes sur la planète. L’interférence ARN (iARN) est un mécanisme cellulaire intrinsèque médié par un ARN à double brin (ARNdb) qui peut supprimer l’expression d’une protéine par la destruction ciblée des ARNm. Avec les récents progrès réalisés dans le domaine de la délivrance des ARNdb ou de l’expression dans les plants, ce mécanisme peut fournir des stratégies de rechange pour lutter contre les maladies. Les exemples de lutte contre des espèces d’agents pathogènes fongiques, basée sur l’iARN, sont de plus en plus nombreux, et la technologie offre de nouveaux choix permettant d’accroître la spécificité relative aux espèces ou la puissance à l’égard de champignons là où des fongicides traditionnels s’avèrent inefficaces. La technologie découlant de l’iARN peut être adaptée pour fournir soit une protection énergique s’appliquant à plusieurs cultures à l’aide de pulvérisations topiques, soit une résistance plus durable par l’expression de transgènes des ARNdb dans les tissus végétaux sensibles. En utilisant le séquençage de l’ARN pour identifier les cibles génétiques fongiques, la technologie basée sur l’iARN continue d’être prometteuse en tant que solution de rechange aux produits agrochimiques utilisés à ce jour pour protéger les cultures.
Introduction
Access to safe, healthy and sustainable food sources is one of the defining challenges of our time. Given the rapid increase of the human population, it is estimated that we will require an additional production of 200 billion calories, equating to a 100–110% increase in crop production to meet nutritional needs by the year 2050 (Tilman et al., Citation2011; Bebber & Gurr, Citation2015; United Nations, Citation2017). Currently, 40% of ice-free land is used to grow crops; however, global climatic changes challenge our current production systems by decreasing yield potentials and shrinking arable land resources (Pugh et al., Citation2016; Myers et al., Citation2017). Moreover, biotic agents, such as necrotrophic and biotrophic fungi, further complicate agronomic production, causing global losses of up to 30–40% in crop yield in-field and post-harvest (Bebber & Gurr, Citation2015; Myers et al., Citation2017). With rapid globalization and migration across the globe, fungal pathogens are predicted to spread rapidly to virgin lands, presenting new challenges for crop production globally (Bebber et al., Citation2014).
Table 1. Summary of publications utilizing RNA interference technology to target plant pathogens.
Traditional chemical treatments used to combat fungal disease epidemics and to control necrotrophic pathogens such as Sclerotinia sclerotiorum have achieved only mixed success (Huzar-Novakowiski et al., Citation2017). When used at sub-lethal levels, common fungicides like boscalid, iprodine, thiophanate methyl, azoxystrobin and pyracostrobin, were associated with increased mutation rates of up to 60-fold in S. sclerotiorum (Amaradasa & Everhart, Citation2016). Subsequent treatments of fungicides resulted in reduced fungal sensitivity due to the accumulated genetic mutations, suggesting that inadequate dispersal of fungicides on crops could promote the development of fungicide resistance in pathogens (Amaradasa & Everhart, Citation2016). Development of resistance can lead to greater or more frequent applications of fungicide, and potentially stronger selection for further resistance, attributing to the rise of fungicide-resistant S. sclerotiorum, Botrytis cinerea and Magnaporthe oryzae (Castroagudín et al., Citation2015; Penaud & Walker, Citation2015; Rupp et al., Citation2017). To break this cycle of developing resistance to our current fungicides, new methods to control disease outbreaks using sustainable technologies are needed.
Despite the suggestion that precise chemical applications could reduce environmental impacts, fungicide use can have deleterious effects on the surrounding agro-ecological landscape due to biocidal non-target effects, as well as its dispersal and persistence within the environment (Smalling et al., Citation2013; Sabatier et al., Citation2014; Le Cointe et al., Citation2016). The use of fungicidal compounds was noted to alter the structure and function of aquatic communities, culminating in severe physiological pathologies, such as increased mortality, reduced reproductive rates and decreased enzyme activity, for zooplankton, gastropods, amphibians and earthworms (Zubrod et al., Citation2011; McMahon et al., Citation2012; Rico et al., Citation2016). Furthermore, fungicidal compounds have been implicated in reduced bee health and abnormal behaviours, such as reduced nest recognition, decreased colony initiation and uncoupled mitochondrial respiration, all of which may contribute to the decline of bee populations (Elston et al., Citation2013; Simon-Delso et al., Citation2014; Syromyatnikov et al., Citation2017). The development of any new species-specific fungicides should provide both environmentally safer control strategies, but also provide effective control of the fungus to ensure improved crop yields.
Recently, a new generation of species-specific control methods, taking advantage of a cellular defence mechanism called RNA interference (RNAi), demonstrated successful control of insects, nematodes, viruses and parasitic plants (Whyard et al., Citation2009; Alakonya et al., Citation2012; Papolu et al., Citation2013; Schmitt-Engel et al., Citation2015). Pioneering studies utilizing RNA interference to control plant pathogens are summarized in . The first commercially approved, transgenic plants carrying RNAi constructs against corn rootworm (Diabrotica virgifera virgifera) and Bean golden mosaic virus were approved for cultivation in the USA and Brazil, respectively (Tollefson, Citation2011; United States Environmental Protection Agency, Citation2017). Despite the successes of RNAi approaches, few studies have applied this revolutionary technique for the management of fungal phytopathogens, likely due to the lack of reasonable target identification tools and poor fungal genomic annotation. Here, we describe how RNAi technology exploits intrinsic cellular pathways in eukaryotes for the development of novel fungal control strategies. A brief summary of aspects relating to safety of this new technology will be explored, as well as how integrating modern genomics techniques could help guide the development of next-generation RNAi-based control of fungal pathogens.
The mechanism of RNA interference
RNAi is a conserved pathway in eukaryotes that protects cells from viruses and controls transposon activity. The mechanism utilizes short interfering RNAs (siRNAs) to guide the targeted degradation of transcripts using sequence homology (Torres-Martínez & Ruiz-Vázquez, Citation2017). Prior to the discovery of RNAi, Rothstein et al. (Citation1987) originally described an ‘antisense effect’ in tobacco plants through the silencing of a nopaline synthase transgene using the expression of antisense nopaline synthase. Subsequently, the description of RNAi in Caenorhabditis elegans by Andrew Fire and Craig Mello earned them the Nobel Prize for Medicine in 2006 (Fire et al., Citation1998; Nobel Media AB, Citation2017). However, similar phenomena had been noted in other organisms. Romano & Macino (Citation1992) earlier had described a post-transcriptional silencing phenomenon, which they termed quelling, in the ascomycete Neurospora crassa. The core proteins in the quelling pathway are the same proteins implicated in the RNAi pathway: ARGONAUTE (AGO), QUELLING DEPENDENT-2 (QDE-2), DICER-like (DCL) and RNA-dependent RNA Polymerase (RdRP) (Torres-Martínez & Ruiz-Vázquez, Citation2017).
Since RNAi is an intrinsic cellular defence process against invading double stranded RNA (dsRNA) viruses, introducing in vitro synthesized dsRNAs or producing the molecules in planta exploits this cellular reaction as a crop management strategy naturally (Wang et al., Citation2016) (Fig. 1a). Transport of long dsRNA and shorter, small interfering RNA (siRNA) into B. cinerea spores was observed using fluorescein-labelled nucleotides (nt) (Wang et al., Citation2016); however, the mechanism of transport remains undefined in fungi. In both humans and C. elegans, dsRNA diffuses passively through a dsRNA-specific channel, SID1 (Duxbury et al., Citation2005; Whangbo et al., Citation2017). Homologues of the SID1 channel do not exist in fungi, and thus, dsRNA transport must occur via an alternative mechanism (Wang et al., Citation2016). In various insects, exogenous dsRNA is transported using receptor-mediated clathrin endocytosis (reviewed in Huvenne & Smagghe, Citation2010). An ‘RNAi of RNAi’ approach provided evidence for endocytic involvement in dsRNA transport. Initially, cells were treated with dsRNA targeting a specific component implicated in clathrin-mediated endocytosis, before a following treatment of GFP-dsRNA was applied. Using a GFP-reporter system, components involved in uptake of the molecules could be elucidated by observing a diminished fluorescent signal. Thus, the components of receptor mediated endocytosis were inferred to be clathrin heavy chain, clathrin adaptor protein 50, vacuolar H+ ATPase and ADP ribosylation factor ARF72A (Saleh et al., Citation2006). Similarly, application of chemical inhibitors confirmed endocytosis as a secondary pathway of dsRNA uptake in C. elegans (Xiao et al., Citation2015). Without SID1, clathrin-mediated endocytic pathways may play an integral function in the transport of dsRNA in fungi.
Once in the cytoplasm, the presence of a dsRNA molecule is recognized by the dsRNA binding domain of DCL1 or DCL2 (Lee et al., Citation2010; Li et al., Citation2010) (). Upon recognition of dsRNA, DCL recruits the SAGA complex with histone acetyltransferase activity to increase transcription from the DCL and AGO promoters, and mobilize RNAi machinery within the fungus (Andika et al., Citation2017). To cleave dsRNA molecules, the 5ʹ end of the dsRNA anchors in the PAZ (Piwi-Argonaute-Zwille/Pinhead) domain within DCL, allowing two consecutive RNase III domains to cleave the ribose-phosphate backbone, resulting in siRNAs of 21–25 nt in length with a 5ʹ monophosphate and a 3ʹ 2 nt overhang (Kandasamy & Fukunaga, Citation2016) (). Once the double stranded siRNA is generated, AGO complexes with the siRNA to recruit QIP (QDE-2-INTERACTING PROTEIN) and form the RNA-induced silencing complex (RISC) (Dang et al., Citation2011) (). Once bound, AGO nicks the siRNA duplex; QIP recognizes and degrades the nicked passenger strand with exonuclease activity (Maiti & Lee H-C, Citation2007; Cheng et al., Citation2016) (). The RISC complex then becomes activated to seek transcripts with complementary sequences to the remaining siRNA strand, termed the guide strand (). When a messenger RNA (mRNA) base pairs to the guide strand in RISC, exonuclease activity is activated to degrade complementary RNA, resulting in a reduction of mRNA accumulation within the fungal hyphae (Dang et al., Citation2011) ().
Fig. 1 (Colour online) Overview of the mechanism of RNAi within fungal hyphae. (Colour) Upon encountering double stranded RNA (dsRNA), the molecules are transported into the cytoplasm through an undefined mechanism (a). Once in the cytoplasm, the molecules are recognized by DICER-LIKE (DCL) (b), which cleaves the molecules into small interfering RNA (siRNA) 21–25 nucleotides in length (c). The siRNA molecules then complex with ARGONAUTE (AGO) (d), which nicks the siRNA and recruits QUELLING DEFICIENT-2-INTERACTING PROTEIN (QIP) to degrade the passenger strand (e). With the removal of the passenger strand, RNA induced silencing complex (RISC) becomes activated to seek messenger RNA (mRNA) transcripts with complementary sequences (f) for degradation (g). The degraded mRNA and the siRNA can function as primers in secondary dsRNA synthesis using RNA-dependent RNA Polymerase (RdRP) (h) to further amplify gene silencing.
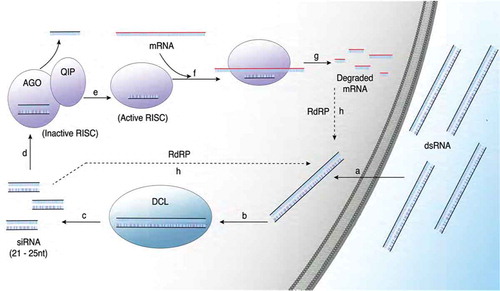
In some organisms, siRNAs produced during long dsRNA processing can act as primers to initiate RdRP activity and temporarily sustain silencing (). siRNAs anneal to complementary mRNA transcripts to act as a primer for second strand synthesis through recruited RdRPs. The secondary dsRNAs produced by RdRPs invoke further DCL activity and RISC complex formation to amplify RNAi-mediated silencing (Ghildiyal & Zamore, Citation2009; Villalobos-Escobedo et al., Citation2016). The number of RdRPs vary amongst fungi, with Fusarium graminearum having five, while N. crassa has two (Zong et al., Citation2009; Chen et al., Citation2015). RdRP can be essential for potent silencing, as in Mucor circinelloides, but for many fungi, RdRPs may not be essential for RNAi signal amplification, but are involved in miRNA (microRNA) biogenesis and transposon silencing (Dang et al., Citation2011; Calo et al., Citation2012).
The safety of RNA interference technology
The sequence-specific mechanism of RNAi provides unparalleled opportunities for RNAi-based technologies to offer safe and environmentally friendly alternatives to more traditional agrochemicals. DsRNA can be designed to avoid sequences of other organisms within the environment. Recently, transgenic corn expressing dsRNA targeting corn rootworm was approved for commercial use (United States Environmental Protection Agency, Citation2017). The transgenic plant material and in vitro synthesized dsRNA molecules were used to assess cross-reactivity amongst a variety of invertebrates (e.g. Apis mellifera, Eisenia adrei, Coleomegilla maculata), vertebrates (e.g. Gallus domesticus, Ictalurus punctatus) and soil microorganisms. No observable changes in physiology, nutrient assimilation or reproduction in the tested organisms was observed, regardless of the dsRNA source (Bachman et al., Citation2016). The presence of the corn rootworm-specific dsRNA in the pollen did not adversely affect honeybees (A. mellifera) consuming and distributing the pollen within the hives (Bachman et al., Citation2016). Larval or adult honeybees fed the dsRNA at doses exceeding 10 times the environmentally relevant exposure levels showed no adverse effects on their growth, development or longevity (Tan et al., Citation2016). The findings from these studies suggest that exogenously delivered dsRNAs, whether derived from either transgenic plants or foliar applications, may not pose serious threats to these important pollinators. Further studies will likely be required to satisfy government regulators that any dsRNA applied to a crop does not affect honeybees or any other species within the surrounding environment.
One somewhat surprising advantage of RNAi-based control is the relative stability of the dsRNA molecule within the phyllosphere (Miguel & Scott, Citation2015). Synthetic dsRNA molecules can be readily made in the laboratory and are more thermodynamically stable than single stranded RNA (Nicholson, Citation2014; Wang & Jin, Citation2017). Due to the double stranded structure of the molecules, dsRNAs are also more resistant to nuclease degradation than mRNA (Hoerter et al., Citation2011; Aryani & Denecke, Citation2015). As a topical application, dsRNA molecules were bioactive against Colorado potato beetle (Leptinotarsa decemlineata) on the surface of potato (Solanum tuberosum L.) leaves for over 28 days under greenhouse conditions (Miguel & Scott, Citation2015). Using natural chemistries, such as clay nanosheets (BioClay; Mitter et al., Citation2017), dsRNA efficacy was improved against Cucumber mosaic virus and Pepper mild mottle virus under adverse environmental conditions. The clay nanosheets shielded dsRNAs from environmental RNase III degradation and improved adhesion to the leaf surface (Ladewig et al., Citation2009; Mitter et al., Citation2017). With suitable formulations, it is entirely possible that dsRNAs could be used in topical applications against a variety of foliar pathogens.
Another advantage of RNAi-based pathogen management is the lack of persistence of dsRNAs within the pedosphere (Dubelman et al., Citation2014). Regardless of dsRNA length, these molecules rapidly degrade within 24 hours in all soil types examined thus far (Dubelman et al., Citation2014; Fischer et al., Citation2016). Similarly, dsRNAs are almost fully degraded within 96 hours upon entering natural water systems (Albright et al., Citation2017). Currently, there is a lack of evidence describing the timing of dsRNA degradation in the phyllosphere versus the pedosphere, although differences may exist due to distinct microbial communities (Bodenhausen et al., Citation2013). Bacterial nucleases, and in particular, RNase III enzymes are most likely responsible for much of the dsRNA degradation in the soil and aquatic environments (Urich et al., Citation2008; Cho, Citation2017). Therefore, dsRNA derived from plant material or from foliar sprays are unlikely to spread far from the point of application through the soil. Coupled with its sequence specificity, RNAi-based approaches are unlikely to have environmentally adverse effects on non-target species and therefore represent an attractive alternative to chemical fungicides.
With respect to food production, RNAi technology should not pose any additional risks to our food supply. All of our food already contains a diversity of small RNAs (sRNAs) including siRNAs, miRNAs, piRNAs and endogenous dsRNAs, produced from a variety of sources, including naturally occurring viruses, transposons or the host genome itself (Ivashuta et al., Citation2009; Frizzi et al., Citation2014). In silico analyses predict endogenous siRNAs with 100% complementarity to human mRNA transcripts are present in many non-GM crops, yet no pathological effects have been associated with daily consumption of the siRNAs derived from these dsRNAs (Jensen et al., Citation2013). Higher animals have evolved many barriers that would prevent or limit the transport of siRNAs, such as nucleases in the saliva and gastrointestinal tract, acid in the stomach, and the unfavourable transport of large, polar molecules across a hydrophobic membrane (Juliano et al., Citation2009; O’Neill et al., Citation2011). The lack of dietary siRNA efficacy has been noted in mouse studies; for example, mice dosed daily with either siRNA or long dsRNA targeting an essential vacuolar ATPase over a 28-day period showed no evidence of RNAi-mediated knockdown of the target transcripts and no adverse cellular pathologies (Petrick et al., Citation2015). The lack of any observable RNAi following ingestion of dsRNA in mammals could be attributed to instability of the molecules passing through the gastrointestinal tract or rapid metabolism in the bloodstream (Christensen et al., Citation2013; Dickinson et al., Citation2013). It therefore seems unlikely that any consumed dsRNA or siRNA molecules will elicit adverse effects on higher organisms.
Furthermore, many food products undergo many processing techniques before consumption (Chemat et al., Citation2017; Misra et al., Citation2017). The majority of processing techniques (baking, microwaving, solvent extraction, thermal treatment, fermentation, acidification, alkalization and bleaching) result in effective nucleic acid destabilization and degradation prior to the final food product (Vijayakumar et al., Citation2009; Gryson, Citation2010). For example, edible oils undergo multiple steps involving heat, pressure and solvent treatments, which exclude polar molecules, such as nucleic acid, and/or result in molecule fragmentation (Mba & Dumont M-J, Citation2015; Belur et al., Citation2017). Similarly, heating and purification in sugar production eliminates DNA by a factor of 1014 (Klein et al., Citation1998). Based on the chemical and physical similarities, dsRNA would likely have a similar fate to DNA during food processing (Forbes & Peppas, Citation2012; Lipfert et al., Citation2014). Thus, any nucleic acid introduced from RNAi technology would not resist food processing. Taken together, RNAi technology, due to both the chemistry of the RNA molecules and the sequence-specificity of the molecule, could be considered a safe, green technology, which can be expressed as a novel trait (transgene) or through topical formulations.
Development of novel traits through host-induced gene silencing
Host-induced gene silencing (HIGS) is an emerging biotechnology in which plants are engineered to produce siRNAs capable of silencing target genes of a target organism. HIGS utilizes the RNAi pathway by equipping the host plant with hairpin RNAs (hpRNAs) containing sequence homology to target genes. Upon transcription, these hpRNA molecules mimic dsRNA and initiate the inherent cellular RNAi pathway. Export of hpRNA from the plant nucleus to the cytoplasm is hypothesized to be facilitated by the binding of an exportin protein HASTY to guide successful nucleocytoplasmic transport (Bollman, Citation2003). Once in the cytoplasm, hpRNA initiates the host RNAi pathway leading to the generation of approximately 21 nt siRNA molecules. For siRNAs to function in targeted gene silencing for plant protection, they must undergo successful transfer from host plant cell to the pest or pathogen. While evidence suggests target gene silencing in pathogenic fungal species may operate through host derived siRNAs (Panwar et al., Citation2013), the mechanism of siRNA transfer from host to pathogen remains unclear. Studies in animal systems show that secreted miRNAs may be associated with host-derived exosomes and lead to successful transfer between organisms (Valadi et al., Citation2007). Exosomal uptake by the receiving cell is hypothesized to utilize exosome-mediated endocytosis, where the vesicular membrane of the sRNA-containing exosome fuses with the receiving plasma membrane, leading to the release of sRNA into the pathogen’s cytoplasm (Valadi et al., Citation2007). Alternatively, sRNA transfer may involve transmembrane transporter-mediated uptake without utilization of host-derived vesicles. Membrane-free sRNAs have been found within the extracellular space and associated with high density lipoproteins. These lipoproteins may facilitate successful transfer of extracellular sRNA to recipient cells (Vickers et al. Citation2011). With a more complete understanding of dsRNA transport in and between cells, it may be possible to enhance both HIGS technology and topical dsRNA delivery formulations to maximize the degree of RNAi-mediated protection of crop plants to a broad range of pathogens and pests.
HIGS technology for crop protection has been approved in two RNAi crops for commercial production in the USA and Brazil. The Brazilian National Technical Commission approved RNAi pinto beans (Phaseolus vulgaris) for commercial production in 2011 (Tollefson, Citation2011). The plants were engineered to disrupt early viral replication of the Bean golden mosaic virus by targeting the viral gene AC1 (Bonfim et al., Citation2007). Similarly, the Environmental Protection Agency approved maize plants expressing dsRNA targeting DvSnf4, a component of the ESCRT-III complex involved in endosomal sorting and lysosomal degradation in corn rootworms, Diabrotica virgifera virgifera, for production in the USA (Bolognesi et al., Citation2012; United States Environmental Protection Agency, Citation2017). DvSnf4 dsRNA was potent at low doses, leading to accumulation of ubiquinated proteins in the midgut cells of larvae. Since autophagy was impaired, the cells malfunctioned, the gut’s digestive processes ceased, and the insects failed to grow and eventually died (Baum et al., Citation2007; Ramaseshadri et al., Citation2013). While targeted genes are commonly selected due to their essential role in pathogenicity, an alternative strategy that has demonstrated success is the targeting of host susceptibility genes. In a study by Sun et al. (Citation2016), the silencing of six previously identified susceptibility genes in potato ‘Desiree’ led to significant reductions in susceptibility against potato late blight (Phytophthora infestans). Given the flexibility and utility of the technology, more RNAi-HIGS crops will undoubtedly be developed and commercialized in the near future, including plants with RNAi-mediated protection from fungal pathogens.
In planta expression of hpRNA overcomes fungal pressure
The development of stably transformed plants expressing dsRNA molecules could impart full plant protection from fungal infections. HIGS has been implemented to reduce fungal pressure and toxin biosynthesis for a variety of phytopathogenic fungi (Koch et al., Citation2013; Zhang et al., Citation2016; Thakare et al., Citation2017). Engineering novel traits like fungal resistance using genetic techniques is an efficient strategy to build resistance in crop plants. While traditional germplasm screening and breeding strategies have achieved limited success in providing resistance against S. sclerotiorum (Disi et al., Citation2014), the use of HIGS in Nicotiana tabacum expressing hpRNA targeting fungal chitin synthase (SSCHS) proved effective in controlling S. sclerotiorum. Expression of hpRNA reduced the level of SSCHS mRNA within the fungus, indicating S. sclerotiorum could readily take up dsRNA from the host (Andrade et al., Citation2016). Since other phytopathogens are likely capable of taking up environmental dsRNA from host cells, HIGS could provide a functional strategy to reduce fungal pressure on the plant.
The root–pathogen interface could also be protected from many fungal pathogens that initiate root infections from the soil, such as Verticillium sp., Fusarium sp. and Rhizoctonia solani (Tedersoo et al., Citation2014; De Coninck et al., Citation2015). While topical formulations of dsRNA would likely degrade rapidly within the soil (Dubelman et al., Citation2014), GM plants could deliver dsRNAs to the pathogen at the root–pathogen interface. Roots of cotton (Gossypium sp.) expressing hpRNA targeting V. dahliae hygrophobins1 gene were able to resist severe root infection (Zhang et al., Citation2016). Additionally, plants engineered with RNAi constructs expressing hpRNA could convey resistance throughout the plant life cycle. Transgenic banana plants (Musa spp.) expressing hpRNA directed at either F. oxysporum genes VELVET or FUSARIUM TRANSCRIPTION FACTOR 1 resisted infection for 8 months post-inoculation (Ghag et al., Citation2014). Thus, engineered plants expressing novel RNAi traits conferring resistance against economically important plant pathogens represents an additional level of durability that would benefit growers interested in sustainable crop protection technologies.
Foliar applications of long dsRNA reduce fungal disease
Foliar dsRNA applications offer shorter-term protection from fungal infections, relative to transgene mediated resistance, but nevertheless, they could be particularly useful to protect agri-food products during post-harvest storage and protecting plants without defined or efficient transformation protocols for HIGS (Wang & Jin, Citation2017). Despite recent advances to control insect pests and viral pathogens, few studies have implemented in vitro synthesized molecules for fungal control. In a pioneering study by Mumbanza et al. (Citation2013), 14 different genes involved in processes such as transcription, RNA modification, DNA replication and intracellular transport were tested in vitro, using nutrient plates rather than plants, for spore germination inhibition in F. oxysporum f. sp. cubense and Mycosphaerella fijiensis. Treatment of fungal spores with dsRNA molecules inhibited germination of the two banana pathogens by up to 95.9% and 65.8%, respectively. In vitro testing provided compelling evidence for the potential for fungal suppression, despite not having tested these molecules on their plant hosts.
Recently, topical application of dsRNA to the surface of treated barley (Hordeum vulgare L.) leaves reduced F. graminearum growth. By targeting three fungal cytochrome P450 lanosterol C-14α demethylases, required for fungal ergosterol synthesis and a common fungicide target, reductions over 50% of both target transcript and fungal DNA accumulation were achieved (Koch et al., Citation2016). Similarly, using long dsRNA targeting DCL1 and DCL2 simultaneously in B. cinerea, Wang et al. (Citation2016) demonstrated remarkable levels of protection in Arabidopsis thaliana, tomato, grape, strawberry, onion, and rose petals from infection, with average lesion sizes, transcript levels and fungal DNA all being reduced by more than 80%. The observed protection in a wide-range of host tissues suggests broad utility of the technology in the protection of food and ornamental species. The protection of barley leaves and tomato leaves demonstrates the potential utility for field management of diseases, while reduced fungal presence on various fruits and vegetables could be useful after harvest, protecting food in transport, storage, or on store shelves (Koch et al., Citation2016; Wang et al., Citation2016). Taken together, these studies provide evidence of the flexibility of RNAi-based molecular sprays that could confer protection against diseases in food production.
RNA sequencing as an informative guide for RNAi
Despite the advances in the development of RNAi-based phytopathogen control, the selection of target genes still represents a significant challenge in the design and effectiveness of fungal suppression. Previously, RNAi targets were selected using gene deletions or chemical inhibition. For example, Koch et al. (Citation2016) chose a common fungicidal target, while Wang et al. (Citation2016) developed foliar applications based on previous genetic deletions of B. cinerea strains (Weiberg et al., Citation2013). Fungal transformation protocols are lengthy and at times inefficient, thereby limiting the effective size of target gene identification screens (Meyer, Citation2008). However, dual RNA-sequencing of both fungal pathogen and host plant now provides unprecedented opportunities to identify novel RNAi targets based on the transcriptome atlas of the pathogen life cycle, infection state, tissue type, host defence response or treatment conditions (Westermann & Barquist, Citation2017). Genes implicated in plant resistance response provide useful information for plant breeding and manipulation, while specific fungal responses essential for pathogenesis could eventually be used as targets for RNAi-based fungal control (Girard et al., Citation2016). For example, McLoughlin et al. (Citation2018) utilized RNA-seq to develop foliar antifungal dsRNAs that inhibit disease pressure on two plant species. By comparing global transcriptomic changes of S. sclerotiorum infection on susceptible and tolerant B. napus cultivars, as well as growth in vitro, hundreds of genes involved in fungal pathogenesis were uncovered. Subsequently, topical dsRNAs were developed to target genes implicated in various processes such as respiration, toxin biosynthesis, protein modification, translation and transcription, which reduced fungal infection on both B. napus and A. thaliana significantly. This suggests that dsRNAs could impart protection for many host species, and shows potential in controlling devastating crop pathogens. Furthermore, homologous dsRNAs were also successful in supressing the related pathogen, B. cinerea (McLoughlin et al., Citation2018). Consequently, homologous targets could be exploited to provide broad levels of control. Taken together, RNA-seq identification of host–pathogen interfaces could provide useful insight for the development of future RNAi target genes for fungal control.
The lack of development of RNAi-based fungal strategies is likely due to poor genomic annotation of many fungi and a lack of accessible bio-computational platforms. User-friendly programs, such as FungiFun2 (Priebe et al., Citation2015; elbe.hki-jena.de/fungifun/fungifun.php) and SeqEnrich (Becker et al., Citation2017; belmontelab.com), have overcome many of the problems associated with both a lack of functional annotation and complicated bioinformatics analyses. Once gene ontology (GO) is resolved using FungiFun2, researchers could use SeqEnrich, to identify significantly upregulated GO terms and genes. Melding both programs together would provide a solid basis for the execution of RNAi-based technologies. Upregulated transcripts and processes could then be selected for the development of either foliar implementations or HIGS technology. RNA-seq provides greater depth and efficiency for the rapid identification of hundreds of critical, upregulated transcripts and processes within infecting pathogens. Moreover, RNA-seq offers a marked improvement over previous searches reliant on labour-intensive fungal transformation studies. The use of RNA sequencing technology has the potential to revolutionize and expedite the next generation of species-specific fungal management strategies.
Outlook
RNAi technology provides a flexible and environmentally friendly solution to combat an array of devastating pathogens. Both HIGS and foliar dsRNAs represent potent and sustainable next-generation strategies capable of controlling pathogens that affect global food production. While the advent of RNAi technologies holds significant promise, concerns over pathogen resistance will continue to exist. Furthermore, while RNAi machinery is known to be conserved in eukaryotic organisms, those that lack the RNAi machinery like Ustilago maydis, the causal agent of corn smut (Billmyre et al., Citation2013) would not be a candidate for host resistance through RNAi. Although further research exploring the interaction of dsRNA within food should be explored, RNAi strategies have proven to be safe through its species-specificity and molecule degradation during food processing. Fundamental aspects of small molecule transport have yet to uncover the underlying molecular mechanism of RNAi-based control of fungal species. The identification of new targets for pathogen control may provide additional clues into conserved pathogenesis genes or common targets for broad levels of efficacious fungal control. Furthermore, RNA sequencing could provide an effective and efficient method for recognizing targets for RNAi, accelerating the establishment of additional novel, species-specific fungicides. The field of RNAi technology is developing for fungi and RNAi is a sustainable solution to deal with fungal diseases and food security challenges.
Acknowledgements
We would like to thank the Canadian Phytopathological Society for the invitation to present this review.
Additional information
Funding
References
- Alakonya A, Kumar R, Koenig D, Kimura S, Townsley B, Runo S, Garces HM, Kang J, Yanez A, David-Schwartz R, et al. 2012. Interspecific RNA interference of SHOOT MERISTEMLESS-like disrupts Cuscuta pentagona plant parasitism. Plant Cell. 24:3153–3166.
- Albright VC, Wong CR, Hellmich RL, Coats JR. 2017. Dissipation of double-stranded RNA in aquatic microcosms. Environ Toxicol Chem. 36:1249–1253.
- Amaradasa BS, Everhart SE. 2016. Effects of sublethal fungicides on mutation rates and genomic variation in the fungal plant pathogen, Sclerotinia sclerotiorum. PLoS One. 11:e0168079.
- Andika IB, Jamal A, Kondo H, Suzuki N. 2017. SAGA complex mediates the transcriptional up-regulation of antiviral RNA silencing. Proc Natl Acad Sci USA. 114:E3499–E3506.
- Andrade CM, Tinoco MLP, Rieth AF, Maia FCO, Aragão FJL. 2016. Host-induced gene silencing in the necrotrophic fungal pathogen Sclerotinia sclerotiorum. Plant Pathol. 65:626–632.
- Aryani A, Denecke B. 2015. In vitro application of ribonucleases: comparison of the effects on mRNA and miRNA stability. BMC Res Notes. 8:164.
- Bachman PM, Huizinga KM, Jensen PD, Mueller G, Tan J, Uffman JP, Levine SL. 2016. Ecological risk assessment for DvSnf7 RNA: a plant-incorporated protectant with targeted activity against western corn rootworm. Regul Toxicol Pharmacol. 81:77–88.
- Bebber DP, Gurr SJ. 2015. Crop-destroying fungal and oomycete pathogens challenge food security. Fungal Genet Biol. 74:62–64.
- Bebber DP, Holmes T, Gurr SJ. 2014. The global spread of crop pests and pathogens. Glob Ecol Biogeogr. 23:1398–1407.
- Becker MG, Walker PL, Pulgar-Vidal NC, Belmonte MF. 2017. SeqEnrich: a tool to predict transcription factor networks from co-expressed Arabidopsis and Brassica napus gene sets. PLoS One. 12:1–13.
- Belur PD, Iyyasami R, Sampath C, Chandrasekhar V. 2017. Refining technologies for edible oils. In: Chemat S, editor. Edible oils estraction, processing and applications. Boca Raton (FL, USA): Taylor & Francis Group; p. 99–128.
- Billmyre RB, Calo S, Feretzaki M, Wang X, Heitman J. 2013. RNAi function, diversity, and loss in the fungal kingdom. Chromosome Res. 21:561–572. doi:10.1007/s10577-013-9388-2.
- Bodenhausen N, Horton MW, Bergelson J. 2013. Bacterial communities associated with the leaves and the roots of Arabidopsis thaliana. PLoS One. 8:e56329.
- Bollman KM. 2003. HASTY, the Arabidopsis ortholog of exportin 5/MSN5, regulates phase change and morphogenesis. Development. 130:1493–1504.
- Bolognesi R, Ramaseshadri P, Anderson J, Bachman P, Clinton W, Flannagan R, Ilagan O, Lawrence C, Levine S, Moar W, et al. 2012. Characterizing the mechanism of action of double- stranded RNA activity against western corn rootworm (Diabrotica virgifera virgifera LeConte). PLoS One. 7(10):e7534.
- Bonfim K, Faria JC, Nogueira EOPL, Éa M, Fjl A, Recursos E, Norte PW, De BU. 2007. RNAi-mediated resistance to bean golden mosaic virus in genetically engineered common bean (Phaseolus vulgaris). Mol Plant Microbe Interact. 20:717–726.
- Baum JA, Bogaert T, Clinton W, Heck GR, Feldmann P, Ilagan O, Johnson S, Plaetinck G, Munyikwa T, Pleau M, Vaughn T, Roberts J. 2007. Control of coleopteran insect pests through RNA interference. Nat Biotechnol. 25:1322–1326.
- Calo S, Nicolás FE, Vila A, Torres-Martínez S, Ruiz-Vázquez RM. 2012. Two distinct RNA-dependent RNA polymerases are required for initiation and amplification of RNA silencing in the basal fungus Mucor circinelloides. Mol Microbiol. 83:379–394.
- Castroagudín VL, Ceresini PC, de Oliveira SC, Reges JTA, Maciel JLN, Al V B, Dorigan AF, McDonald BA. 2015. Resistance to QoI fungicides is widespread in Brazilian populations of the wheat blast pathogen Magnaporthe oryzae. Phytopathology. 105:284–294.
- Chemat F, Rombaut N, Meullemiestre A, Turk M, Perino S, Fabiano-Tixier AS, Abert-Vian M. 2017. Review of green food processing techniques. Preservation, transformation, and extraction. Innov Food Sci Emerg Technol. 41:357–377.
- Chen Y, Gao Q, Huang M, Liu Y, Liu Z, Liu X, Ma Z. 2015. Characterization of RNA silencing components in the plant pathogenic fungus Fusarium graminearum. Sci Rep. 5:12500.
- Cheng L, Ling J, Liang L, Luo Z, Zhang J, Xie B. 2016. Qip gene in Fusarium oxysporum is required for normal hyphae morphology and virulence. Mycology. 6(2):130–137.
- Cho KH. 2017. The structure and function of the gram-positive bacterial RNA degradosome. Front Microbiol. 8:1–10.
- Christensen J, Litherland K, Faller T, Van De Kerkhof E, Natt F, Hunziker J, Krauser J, Swart P. 2013. Metabolism studies of unformulated internally [3H] -labeled short interfering RNAs in mice. Drug Metab Dispos. 41:1211–1219.
- Dang Y, Yang Q, Xue Z, Liu Y. 2011. RNA interference in fungi: pathways, functions, and applications. Eukaryot Cell. 10:1148–1155.
- De Coninck B, Timmermans P, Vos C, Cammue BPA, Kazan K. 2015. What lies beneath: belowground defense strategies in plants. Trends Plant Sci. 20:91–101.
- Dickinson B, Zhang Y, Petrick J, Heck G, Ivashuta S, Marshall WS. 2013. Lack of detectable oral bioavailability of plant microRNAs after feeding in mice. Nat Biotechnol. 31:965–967.
- Disi JO, Mei J, Wei D, Ding Y, Qian W. 2014. Inheritance of leaf and stem resistance to Sclerotinia sclerotiorum in a cross between Brassica incana and Brassica oleracea var. alboglabra. J Agric Sci. 152:146–152.
- Dubelman S, Fischer J, Zapata F, Huizinga K, Jiang C, Uffman J, Levine S, Carson D. 2014. Environmental fate of double-stranded RNA in agricultural soils. PLoS One. 9:1–7.
- Duxbury MS, Ashley SW, Whang EE. 2005. RNA interference: a mammalian SID-1 homologue enhances siRNA uptake and gene silencing efficacy in human cells. Biochem Biophys Res Commun. 331:459–463.
- Elston C, Thompson HM, Walters KFA. 2013. Sub-lethal effects of thiamethoxam, a neonicotinoid pesticide, and propiconazole, a DMI fungicide, on colony initiation in bumblebee (Bombus terrestris) micro-colonies. Apidologie. 44:563–574.
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. 1998. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 391:806–811.
- Fischer JR, Zapata F, Dubelman S, Mueller GM, Jensen PD, Levine SL. 2016. Characterizing a novel and sensitive method to measure dsRNA in soil. Chemosphere. 161:319–324.
- Forbes DC, Peppas NA. 2012. Oral delivery of small RNA and DNA. J Control Release. 162:438–445.
- Frizzi A, Zhang Y, Kao J, Hagen C, Huang S. 2014. Small RNA profiles from virus-infected fresh market vegetables. J Agric Food Chem. 62:12067–12074.
- Ghag SB, Shekhawat UKS, Ganapathi TR. 2014. Host-induced post-transcriptional hairpin RNA-mediated gene silencing of vital fungal genes confers efficient resistance against Fusarium wilt in banana. Plant Biotechnol J. 12:541–553.
- Ghildiyal M, Zamore PD. 2009. Small silencing RNAs: an expanding universe. Nat Rev Genet. 10:94–108.
- Girard IJ, McLoughlin AG, De Kievit TR, Fernando DWG, Belmonte MF. 2016. Integrating large-scale data and RNA technology to protect crops from fungal pathogens. Front Plant Sci. 7:631.
- Gryson N. 2010. Effect of food processing on plant DNA degradation and PCR-based GMO analysis: a review. Anal Bioanal Chem. 396:2003–2022.
- Hoerter JAH, Krishnan V, Lionberger TA, Walter NG. 2011. SiRNA-like double-stranded RNAs are specifically protected against degradation in human cell extract. PLoS One. 6:1–9.
- Huvenne H, Smagghe G. 2010. Mechanisms of dsRNA uptake in insects and potential of RNAi for pest control: a review. J Insect Physiol. 56:227–235.
- Huzar-Novakowiski J, Paul PA, Dorrance AE. 2017. Host resistance and chemical control for management of Sclerotinia stem rot of soybean in Ohio. Phytopathology. 107:937–949.
- Ivashuta SI, Petrick JS, Heisel SE, Zhang Y, Guo L, Reynolds TL, Rice JF, Allen E, Roberts JK. 2009. Endogenous small RNAs in grain: semi-quantification and sequence homology to human and animal genes. Food Chem Toxicol. 47:353–360.
- Jensen PD, Zhang Y, Wiggins BE, Petrick JS, Zhu J, Kerstetter RA, Heck GR, Ivashuta SI. 2013. Computational sequence analysis of predicted long dsRNA transcriptomes of major crops reveals sequence complementarity with human genes. GM Crops Food. 4:90–97.
- Juliano R, Bauman J, Kang H, Ming X. 2009. Biological barriers to therapy with antisense and siRNA. Mol Pharm. 6:686–695.
- Kandasamy SK, Fukunaga R. 2016. Phosphate-binding pocket in Dicer-2 PAZ domain for high-fidelity siRNA production. Proc Natl Acad Sci USA. 113:14031–14036.
- Klein J, Altenbuchner J, Mattes R. 1998. Nucleic acid and protein elimination during the sugar manufacturing process of conventional and transgenic sugar beets. J Biotechnol. 60:145–153.
- Koch A, Biedenkopf D, Furch A, Weber L, Rossbach O, Abdellatef E, Linicus L, Johannsmeier J, Jelonek L, Goesmann A, et al. 2016. An RNAi-based control of Fusarium graminearum infections through spraying of long dsRNAs involves a plant passage and is controlled by the fungal silencing machinery. PLoS Pathog. 12:1–22.
- Koch A, Kumar N, Weber L, Keller H, Imani J, Kogel K. 2013. Host-induced gene silencing of cytochrome P450 lanosterol C14 α -demethylase – encoding genes confers strong resistance to Fusarium species. Proc Natl Acad Sci USA. 110:19324–19329.
- Ladewig K, Xu ZP, Lu GQ. 2009. Layered double hydroxide nanoparticles in gene and drug delivery. Expert Opin Drug Deliv. 6:907–922.
- Le Cointe R, Simon TE, Delarue P, Hervé M, Leclerc M, Poggi S. 2016. Reducing the use of pesticides with site-specific application: the chemical control of Rhizoctonia solani as a case of study for the management of soil-borne diseases. PLoS One. 11:1–18.
- Lee HC, Li L, Gu W, Xue Z, Crosthwaite SK, Pertsemlidis A, Lewis ZA, Freitag M, Selker EU, Mello CC, et al. 2010. Diverse pathways generate microRNA-like RNAs and Dicer-independent small interfering RNAs in fungi. Mol Cell. 38:803–814.
- Li L, Chang SS, Liu Y. 2010. RNA interference pathways in filamentous fungi. Cell Mol Life Sci. 67:3849–3863.
- Lipfert J, Skinner GM, Keegstra JM, Hensgens T, Jager T, Dulin D, Kober M, Yu Z, Donkers SP, Chou F-C, et al. 2014. Double-stranded RNA under force and torque: similarities to and striking differences from double-stranded DNA. Proc Natl Acad Sci USA. 111:15408–15413.
- Maiti M, Lee H-C LY. 2007. QIP, a putative exonuclease, interacts with the Neurospora Argonaute protein and facilitates conversion of duplex siRNA into single strands. Genes Dev. 21:590–600.
- Mba OI, Dumont M-J NM. 2015. Palm oil: processing, characterization and utilization in the food industry – A review. Food Biosci. 10:26–41.
- McLoughlin AG, Wytinck N, Walker PL, Girard IJ, Rashid KY, De Kievit T, Fernando WGD, Whyard S, Belmonte M. 2018. Identification and application of exogenous dsRNA confers plant protection against Sclerotinia sclerotiorum and Botrytis cinerea. Sci Rep. 8:7320.
- McMahon TA, Halstead NT, Johnson S, Raffel TR, Romansic JM, Crumrine PW, Rohr JR. 2012. Fungicide-induced declines of freshwater biodiversity modify ecosystem functions and services. Ecol Lett. 15:714–722.
- Meyer V. 2008. Genetic engineering of filamentous fungi - progress, obstacles and future trends. Biotechnol Adv. 26:177–185.
- Miguel KS, Scott JG. 2015. The next generation of insecticides: dsRNA is stable as a foliar-applied insecticide. Pest Manag Sci. 72:801–809.
- Misra NN, Koubaa M, Roohinejad S, Juliano P, Alpas H, Inácio RS, Saraiva JA, Barba FJ. 2017. Landmarks in the historical development of twenty-first century food processing technologies. Food Res Int. 97:318–339.
- Mitter N, Worrall EA, Robinson KE, Li P, Jain RG, Taochy C, Fletcher SJ, Carroll BJ, Lu GQ, Xu ZP. 2017. Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nat Plants. 3:16207.
- Mumbanza FM, Kiggundu A, Tusiime G, Tushemereirwe WK, Niblett C, Bailey A. 2013. In vitro antifungal activity of synthetic dsRNA molecules against two pathogens of banana, Fusarium oxysporum f. sp. cubense and Mycosphaerella fijiensis. Pest Manag Sci. 69:1155–1162.
- Myers SS, Smith MR, Guth S, Golden CD, Vaitla B, Mueller ND, Dangour AD, Huybers P. 2017. Climate change and global food systems: potential impacts on food security and undernutrition. Annu Rev Public Health. 38:259–277.
- Nations U. 2017. World population prospects: the 2017 revision, key findings and advance tables. Available from Department of Economic and Social Affairs - Population Division. UN (New York, NY).
- Nicholson AW. 2014. Ribonuclease III mechanisms of double-stranded RNA cleavage. Wiley Interdiscip Rev RNA. 5:31–48.
- Nobel Media AB. 2017. The nobel prize in physiology or medicine 2006 [Internet]. [ accessed 2017 Sep 14]. https://www.nobelprize.org/nobel_prizes/medicine/laureates/2006/
- O’Neill MJ, Bourre L, Melgar S, Driscoll CMO. 2011. Intestinal delivery of non-viral gene therapeutics : physiological barriers and preclinical models. Drug Discov Today. 16:203–218.
- Panwar V, McCallum B, Bakkeren G. 2013. Host-induced gene silencing of wheat leaf rust fungus Puccinia triticina pathogenicity genes mediated by the barley stripe mosaic virus. Plant Mol Biol. 81:595–608.
- Papolu PK, Gantasala NP, Kamaraju D, Banakar P, Sreevathsa R, Rao U. 2013. Utility of host delivered RNAi of two FMRF amide like peptides, flp-14 and flp-18, for the management of root knot nematode, Meloidogyne incognita. PLoS One. 8:1–16.
- Penaud A, Walker AS. 2015. Oilseed rape pathogens in France. In: Ishii H, Holloman DW, editors. Fungicide resistance in plant pathogens: principles and a guide to practical managagement. Minami Awaji (Japan): Springer. p. 389–399.
- Petrick JS, Moore WM, Heydens WF, Koch MS, Sherman JH, Lemke SL. 2015. A 28-day oral toxicity evaluation of small interfering RNAs and a long double-stranded RNA targeting vacuolar ATPase in mice. Regul Toxicol Pharmacol. 71:8–23.
- Priebe S, Kreisel C, Horn F, Guthke R. 2015. FungiFun2: a comprehensive online resource for systematic analysis of gene lists from fungal species. Bioinformatics. 31:445–446.
- Pugh TAM, Müller C, Elliott J, Deryng D, Folberth C, Olin S, Schmid E, Arneth A. 2016. Climate analogues suggest limited potential for intensification of production on current croplands under climate change. Nat Commun. 7:12608.
- Ramaseshadri P, Segers G, Flannagan R, Wiggins E, Clinton W, Ilagan O, McNulty B, Clark T, Bolognesi R. 2013. Physiological and cellular responses caused by RNAi- mediated suppression of Snf7 orthologue in Western corn rootworm (Diabrotica virgifera virgifera) Larvae. PLoS One. 8:1–10.
- Rico A, Sabater C, Castillo M-A. 2016. Lethal and sub-lethal effects of five pesticides used in rice farming on the earthworm Eisenia fetida. Ecotoxicol Environ Saf. 127:222–229.
- Romano N, Macino G. 1992. Quelling: transient inactivation of gene expression in Neurospora crassa by transformation with homologous sequences. Mol Microbiol. 6:3343–3353.
- Rothstein SJ, DiMaio J, Strand M, Rice D. 1987. Stable and heritable inhibition of the expression of nopaline synthase in tobacco expressing antisense RNA. Proc Natl Acad Sci USA. 84:8439–8443.
- Rupp S, Weber RWS, Rieger D, Detzel P, Hahn M. 2017. Spread of Botrytis cinerea strains with multiple fungicide resistance in german horticulture. Front Microbiol. 7:1–12.
- Sabatier P, Poulenard J, Fanget B, Reyss J-L, Develle A-L, Wilhelm B, Ployon E, Pignol C, Naffrechoux E, Dorioz J-M, et al. 2014. Long-term relationships among pesticide applications, mobility, and soil erosion in a vineyard watershed. Proc Natl Acad Sci USA. 111:15647–15652.
- Saleh M-C, Van Rij RP, Hekele A, Gillis A, Foley E, O’Farrell PH, Andino R. 2006. The endocytic pathway mediates cell entry of dsRNA to induce RNAi silencing. Nat Cell Biol. 8:793–802.
- Schmitt-Engel C, Schultheis D, Schwirz J, Ströhlein N, Troelenberg N, Majumdar U, Dao VA, Grossmann D, Richter T, Tech M, et al. 2015. The iBeetle large-scale RNAi screen reveals gene functions for insect development and physiology. Nat Commun. 6:7822.
- Simon-Delso N, Martin GS, Bruneau E, Minsart LA, Mouret C, Hautier L. 2014. Honeybee colony disorder in crop areas: the role of pesticides and viruses. PLoS One. 9:1–16.
- Smalling KL, Reilly TJ, Sandstrom MW, Kuivila KM. 2013. Occurrence and persistence of fungicides in bed sediments and suspended solids from three targeted use areas in the United States. Sci Total Environ. 447:179–185.
- Sun K, Wolters A-MA, Vossen JH, Rouwet ME, Loonen AEHM, Jacobson E, Visser RGF, Bai Y. 2016. Silencing of six susceptibility genes results in potato late blight resistance. Transgenic Res. 25:731–742.
- Syromyatnikov MY, Kokina AV, Lopatin AV, Starkov AA, Popov VN. 2017. Evaluation of the toxicity of fungicides to flight muscle mitochondria of bumblebee (Bombus terrestris L.). Pestic Biochem Physiol. 135:41–46.
- Tan J, Levine SL, Bachman PM, Jensen PD, Mueller GM, Uffman JP, Meng C, Song Z, Richards KB, Beevers MH. 2016. No impact of DvSnf7 RNA on honey bee (Apis mellifera L.) adults and larvae in dietary feeding tests. Environ Toxicol Chem. 35:287–294.
- Tedersoo L, Bahram M, Põlme S, Kõljalg U, Yorou NS, Wijesundera R, Ruiz LV, Vasco-Palacios AM, Thu PQ, Suija A, et al. 2014. Global diversity and geography of soil fungi. Science. 346:1052–1053.
- Thakare D, Zhang J, Wing RA, Cotty PJ, Schmidt MA. 2017. Aflatoxin-free transgenic maize using host-induced gene silencing. Sci Adv. 3:1–9.
- Tilman D, Balzer C, Hill J, Befort BL. 2011. Global food demand and the sustainable intensi fi cation of agriculture. Proc Natl Acad Sci USA. 108:20260–20264.
- Tollefson J. 2011. Brazil cooks up transgenic bean. Nature. 478:168.
- Torres-Martínez S, Ruiz-Vázquez RM. 2017. The RNAi universe in fungi: a varied landscape of small RNAs and biological functions. Annu Rev Microbiol. 71:371–391.
- United States Environmental Protection Agency. 2017. EPA registers innovative tool to control corn rootworm [Internet]. [ accessed 2017 Aug 29]. https://www.epa.gov/newsreleases/epa-registers-innovative-tool-control-corn-rootworm
- Urich T, Lanzén A, Qi J, Huson DH, Schleper C, Schuster SC. 2008. Simultaneous assessment of soil microbial community structure and function through analysis of the meta-transcriptome. PLoS One. 3:e2527.
- Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. 2007. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 9:654–659.
- Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. 2011. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 13:423–433.
- Vijayakumar KR, Martin A, Gowda LR, Prakash V. 2009. Detection of genetically modified soya and maize: impact of heat processing. Food Chem. 117:514–521.
- Villalobos-Escobedo JM, Carreras-Villaseñor N, Herrera-Estrella A. 2016. The interaction of fungi with the environment orchestrated by RNAi. Mycologia. 108:556–571.
- Wang M, Jin H. 2017. Spray-induced gene silencing: a powerful innovative strategy for crop protection. Trends Microbiol. 25:4–6.
- Wang M, Weiberg A, Lin F-M, Thomma BPHJ, Huang H-D JH. 2016. Bidirectional cross-kingdom RNAi and fungal uptake of external RNAs confer plant protection. Nat Plants. 2:16151.
- Weiberg A, Wang M, Lin F-M, Zhao H, Zhang Z, Kaloshian I, Huang H-D, Jin H, Ashida H, Ogawa M, et al. 2013. Fungal small RNAs suppress plant immunity by hijacking host RNA interference pathways. Science. 342:118–123.
- Westermann AJ, Barquist L. 2017. Resolving host – pathogen interactions by dual RNA-seq. PLoS Pathog. 13:1–19.
- Whangbo JS, Weisman AS, Lu J, Chae J, Hunter CP. 2017. SID-1 domains important for dsRNA import in C. elegans. Genes Genomes Genetics. 4:3887–3899.
- Whyard S, Singh AD, Wong S. 2009. Ingested double-stranded RNAs can act as species-specific insecticides. Insect Biochem Mol Biol. 39:824–832.
- Xiao D, Gao X, Xu J, Liang X, Li Q, Yao J, Zhu KY. 2015. Clathrin-dependent endocytosis plays a predominant role in cellular uptake of double-stranded RNA in the red flour beetle. Insect Biochem Mol Biol. 60:68–77.
- Zhang T, Jin Y, Zhao J-H, Gao F, Zhou B-J, Fang YY, Guo H-S. 2016. Host-induced gene silencing of the target gene in fungal cells confers effective resistance to the cotton wilt disease pathogen Verticillium dahliae. Mol Plant. 9:939–942.
- Zong J, Yao X, Yin J, Zhang D, Ma H. 2009. Evolution of the RNA-dependent RNA polymerase (RdRP) genes: duplications and possible losses before and after the divergence of major eukaryotic groups. Gene. 447:29–39.
- Zubrod JP, Bundschuh M, Feckler A, Englert D, Schulz R. 2011. Ecotoxicological impact of the fungicide tebuconazole on an aquatic decomposer-detritivore system. Environ Toxicol Chem. 30:2718–2724.
