Abstract
Rice blast, caused by Magnaporthe oryzae, is one of the most devastating diseases of rice worldwide. The aim of the present study was to elucidate the virulence structure of M. oryzae populations in Fujian Province, China, over the period 2006–2015. For this purpose, 456 M. oryzae isolates collected from diverse cultivars from eight rice cropping regions in Fujian were screened for the presence of 11 known avirulence genes: Avr-Pik, Avr-Pita1, Avr-Pita2, Avr-Pita3, PWL2, Avr1-CO39, ACE1, Avr-Piz-t, Avr-Pia, Avr-Pii and Avr-Pikm, with gene-specific molecular markers. The results showed that Avr-Pik and Avr-Pita3 occurred at the highest frequency (94.5% and 91.7%, respectively), while Avr1-CO39 and Avr-Pii were not detected. Further, the remaining avirulence genes occurred at frequencies ranging from 5.9% to 89.0%. Temporal population dynamics revealed that Avr-Pik was uniformly distributed in all 10 years, at frequencies of more than 87.5%. Spatial distribution analysis showed avirulence genes were present at different frequencies among the geographic regions. In addition, 24 rice monogenic lines of IRRI-Japan with known blast resistance genes were inoculated to assess the virulence of 60 isolates. The results revealed that the resistance gene Pik showed the broadest resistance spectrum to the isolates tested and therefore would be the most useful in rice blast resistance breeding. The resistance genes Pi-z5, Pi-1(1), Pi-kp, Pi-9(t), Pi-ta(1) and Pi-kh also were effective and would therefore also be of value to resistance breeding programmes. The present study provides information that should be useful for the development and deployment of rice blast resistant cultivars in Fujian Province.
Résumé
La pyriculariose du riz, causée par Magnaporthe oryzae, est une des maladies les plus dévastatrices du riz, et ce, à l’échelle mondiale. Le but de cette étude était d’élucider la structure de virulence des populations de M. oryzae dans la province du Fujian, en Chine, de 2006 à 2015. À cette fin, 456 isolats de M. oryzae, collectés sur divers cultivars de 8 régions productrices de riz du Fujian, ont été criblés avec des marqueurs moléculaires génospécifiques afin de détecter 11 gènes d’avirulence connus: Avr-Pik, Avr-Pita1, Avr-Pita2, Avr-Pita3, PWL2, Avr1-CO39, ACE1, Avr-Piz-t, Avr-Pia, Avr-Pii et Avr-Pikm. Les résultats ont montré qu’Avr-Pik et Avr-Pita3 affichaient la fréquence la plus élevée (94.5% et 91.7%, respectivement), tandis que Avr1-CO39 et Avr-Pii n’ont pas été détectés. De plus, les autres gènes d’avirulence se sont manifestés à des fréquences variant de 5.9% à 89.0%. La dynamique temporelle des populations a révélé que le gène Avr-Pik était uniformément distribué durant les 10 années à une fréquence supérieure à 87.5%. L’analyse de la distribution spatiale a montré que les gènes d’avirulence apparaissaient à différentes fréquences dans les diverses régions. De plus, 24 lignées de riz monogéniques de l’Institut international pour la recherche sur le riz du Japon, dont les gènes de résistance à la pyriculariose sont connus, ont été inoculées pour évaluer la virulence de 60 isolats. Les résultats ont révélé que le gène de résistance Pik affichait le plus large spectre de résistance aux isolats testés et, par conséquent, serait le plus utile pour la sélection de riz résistant à la pyriculariose. Les gènes de résistance Pi-z5, Pi-1(1), Pi-kp, Pi-9(t), Pi-ta(1) et Pi-kh étaient aussi efficaces et pourraient donc être utiles dans le cadre des programmes de sélection en vue de développer la résistance. Cette étude fournit de l’information qui pourrait servir à développer et à commercialiser éventuellement des cultivars résistants à la pyriculariose dans la province du Fujian.
Introduction
Rice blast disease, caused by the ascomycete Magnaporthe oryzae (Hebert) Barr. (Couch & Kohn, Citation2002), is one of the most devastating diseases of rice (Rossman et al., Citation1990). Yield losses can be as high as 10–30% annually (Talbot, Citation2003). The pathogen infects rice plants at all developmental stages, causing leaf, collar, neck and panicle blast symptoms. In addition to rice, M. oryzae can also infect a broad range of grass species, including wheat (Maciel et al., Citation2014), graminaceous weeds (Sweigard et al., Citation1995; Yan, Citation2015) and millet (Singh & Kumar, Citation2010).
Arms-race coevolution (Kanzaki et al., Citation2012) dramatically impacts the genome of pathogens and plants. Rice blast has become one of the model pathosystems of host–pathogen interactions (Liu et al., Citation2010). The interaction of rice and M. oryzae follows the ‘gene-for-gene’ hypothesis, which indicates that for every resistance gene (R) in the host plant, there is a complementary virulence gene (Avr) in the pathogen (Flor, Citation1971; Heath, Citation2000). Further, their interaction, directly or indirectly, leads to an activation of the host defence response, resulting in resistance and the suppression of infection (Ellis et al., Citation2007). To date, 22 R genes effective against different races of M. oryzae have been cloned from rice (Wang et al., Citation1999; Bryan et al., Citation2000; Qu et al., Citation2006). In addition, more than 40 Avr genes have been identified in M. oryzae, of which 12 have been cloned and characterized (Kang et al., Citation1995; Sweigard et al., Citation1995; Farman & Leong, Citation1998; Orbach et al., Citation2000; Bohnert et al., Citation2004; Ma et al., Citation2006; Feng et al., Citation2007; Li et al., Citation2009; Yoshida et al., Citation2009; Zhang et al., Citation2015; Ray et al., Citation2016). Owing to the large number of rice R genes against M. oryzae, there are still many Avr genes that need to be characterized. The cloned Avr genes can be classified into two classes based on their functional characteristics. One subclass of Avr genes determines host species specificity of the pathogen, mainly the PWL gene family consisting of PWL2, PWL1, PWL3 and PWL4 (Kang et al., Citation1995; Sweigard et al., Citation1995). The other subclass encodes cultivar-specific elicitors, including Avr-Pita, Avr1-CO39, Avr-Piz-t, ACE1, Avr-Pia, Avr-Pii, Avr-Pik/km/kp and Avr-Pi54.
At present, the most economical and effective strategy to control rice blast has been the deployment of R genes in rice cultivars and multi-lines. However, many R genes often become ineffective after three to five years due to shifts in M. oryzae populations from avirulence to virulence (Zhou et al., Citation2007; Chuma et al., Citation2011) and the development of new races of M. oryzae (Singh et al., Citation2014). The structure of a population reflects its evolutionary history and its potential to evolve (McDonald & Linde, Citation2002). Therefore, a clear understanding of the structure of M. oryzae populations is needed for the rapid and effective deployment of R genes in rice cultivars and to improve the durability of R genes in rice breeding programmes.
The objectives of this study were to: (i) evaluate the presence/absence of Avr genes in 456 M. oryzae isolates collected from diseased field plots of rice in Fujian Province over different years using a molecular assay, and (ii) survey the temporal and spatial distribution of these Avr genes and to assess the effectiveness of the main rice blast R genes known. The general aim was to provide information for the development of resistant varieties and for the rational deployment of resistant cultivars in Fujian Province of China.
Materials and methods
M. oryzae collections, near-isogenic lines and rice cultivars
Eight different M. oryzae populations were sampled from various rice cropping zones in Fujian Province, China, from 2006 to 2015. A population was defined by the common field origin of a group of isolates across 10 years. Cultivars used in these fields included ‘Yongyou 9’, ‘Yongyou 15’, ‘Yiyou 673’, ‘Liangyou 7216’, ‘Zhongzheyou 86’, ‘Tianyou 3301’, ‘Taifengyou 3301’, ‘Chuangyou P30’, ‘Minghui 86’. The geographical origin of the isolates and years of collection are described in Supplementary Table 1. Further, a total of 456 single-spore isolates were obtained from sporulating lesions on rice panicles according to the method described by Pei & Ling (Citation1986).
Table 1. Details of PCR based markers for 11 avirulence genes used in this study.
Twenty-four rice monogenic lines of the International Rice Research Institute (IRRI)-Japan with known blast resistance genes (R genes) (), provided by the China Agricultural University, were used for virulence assays of M. oryzae isolates. The susceptible check cultivar ‘Lijiangxintuanheigu’ was kept in the Institute of Plant Protection, Fujian Academy of Agricultural Sciences, Fuzhou, Fujian, China.
Table 2. Virulence frequencies of 60 M. oryzae isolates to 24 monogenic lines with known resistance genes.
DNA preparation and molecular markers
The isolates were grown in 100 mL potato sucrose broth (PSB) at 28°C for 5 days at 200 rpm on an orbital shaker. Mycelia were harvested by passing the liquid culture through Whatman No. 1 filter paper, then ground in liquid nitrogen into a powder in a mortar and pestle. Fungal genomic DNA was extracted using the HP Plant DNA Kit (Omega Biotech, Guangzhou, China) according to the manufacturer’s instructions. Magnaporthe oryzae isolates were screened for the presence of the avirulence genes (Avr), Avr-Pik, Avr-Pita1, Avr-Pita2, Avr-Pita3, PWL2, Avr1-CO39, ACE1, Avr-Piz-t, Avr-Pia, Avr-Pii and Avr-Pikm, using a set of 11 PCR primer pairs (). Each primer pair could amplify a partial fragment of each of the 11 Avr genes, respectively (). The primers of the first 10 genes, from Avr-Pik to Avr-Pii, were designed using Primer 5.0 based on the sequences deposited in the GenBank databases (), and the primers for Avr-Pikm gene were validated previously (Zhang et al., Citation2004). The primers were synthesized by Thermo Fisher Scientific (Guangzhou, China).
PCR amplification
PCR amplifications were performed in a 25 µL reaction volume containing 1 unit of Taq DNA polymerase (TaKaRa, Dalian, China), 10× PCR buffer (Mg2+ plus), 0.2 mM of dNTP mix, 0.2 µM each primer, and 50 ng of template DNA. Amplification was carried out in a C1000TM thermal cycler (Bio-Rad, Hercules, CA, USA) with the following program: 3 min initial denaturation at 94°C, 35 cycles of 45 s denaturation (for Avr-Pik, Avr-Pita1, Avr-Pita2, Avr-Pita3, PWL2 and Avr1-CO39) or 30 cycles of 60 s denaturation (for ACE1, Avr-Piz-t, Avr-Pia, Avr-Pii and Avr-Pikm) at 94°C, 60 s primer annealing at different Tm (), and 2 min extension at 72°C, and a final extension for 5 min at 72°C. The PCR products were separated in 2% agarose gels in 1× TAE buffer, stained in ethidium bromide and visualized with a UV transilluminator (UVP, LLC Upland, CA, USA). All PCR assays were repeated three times for confirmation of results. The amplified fragments were scored as present (1) or absent (0) of the amplicon for each assay.
Virulence assay
Sixty monoconidial isolates of M. oryzae were selected randomly for virulence analysis on 24 monogenic lines. The rice cultivar ‘Lijiangxintuanheigu’ was used as a susceptible check. All rice cultivars and lines were sown in plastic seedling trays (54 cm × 28 cm × 5 cm with 50 holes) filled with soil and maintained in a greenhouse (28 ± 1°C and RH 70–80%). Ten to 15 seeds of each cultivar (line) were sown together with three replicates.
Inoculation of the M. oryzae isolates was as per the method of Hayashi & Yoshida (Citation2009) with slight modifications. All fungal cultures were grown in starch medium (Du et al., Citation2011) and stored on dry filter paper at −20°C, prior to use. The stored cultures of each isolate were revived by placing the filter paper on starch medium again at 28°C. Agar pieces (6-mm diameter) from 7-day-old colonies were transferred to fresh rice bran culture medium (rice bran 20 g, agar 20 g, H2O 1000 mL, pH 6.5) and placed at four locations on the Petri dishes. After 10 days of incubation at 28 ± 1°C, the aerial hyphae were scraped off with a sterilized brush. The dishes were incubated under black light at 28 ± 1°C (RH 85%–90%) for sporulation. Three days later, a conidial suspension of each isolate was prepared by flooding the top surface of the colonies with distilled water, and the concentration was adjusted to 1.0 × 105 spores mL−1. Tween-20 (polyethylene glycol sorbitan monolaurate) (0.01%) was added to the spore suspension (Du et al., Citation2011). Next, 40 mL of conidial suspension per tray was applied to the seedlings at 3-to-4-leaf stage using an air-compressor sprayer. After inoculation, the plants were kept in the dark for 24 h and later in 12 h light/12 h dark in the greenhouse at 25 ± 1°C and 95% relative humidity (Lan et al., Citation2014).
Disease evaluation
The reaction type of each line was evaluated 7 days post-inoculation on a 0–5 scale according to the ISSR standard evaluation system (IRRI, Citation1996), where: ‘0’ = no evidence of infection; ‘1’ = brown specks about 0.5 mm in diameter; ‘2’ = brown specks about 0.5–1 mm in diameter; ‘3’ = roundish to elliptical lesion about 1–3 mm in diameter with grey centre and brown margins; ‘4’ = typical spindle-shaped lesion, 3 mm or longer with little or no coalescence of lesions; and ‘5’ = lesion similar to that of grade 4, but 1 or 2 leaf blades half-killed owing to coalescence of lesions. Rice lines with scores of 0–3 were considered resistant and those with scores of 4–5 were rated as susceptible (Zhou et al., Citation2003).
Data analysis
Pathogenicity frequency (PF) and virulence frequency (VF) were calculated according to the following formulas:
Pathogenicity or virulence was categorized into four classes based on the PF or VF values, i.e. strong (≥ 70%), relatively strong (50%–70%), moderate (20–50%) and weak (< 20%).
Results
Avr genes and their frequencies in M. oryzae populations
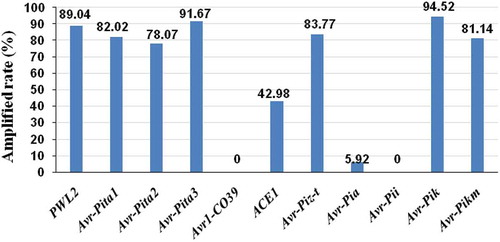
A total of 456 M. oryzae isolates were characterized with 11 pairs of primers. The results showed that 431, 418, 406, 382, 374, 370, 356, 196, 27, 0 and 0 isolates had amplified positive bands in the Avr genes Avr-Pik, Avr-Pita3, PWL2, Avr-Piz-t, Avr-Pita1, Avr-Pikm, Avr-Pita2, ACE1, Avr-Pia, Avr1-CO39 and Avr-Pii, respectively (). The frequencies of all 11 Avr genes differed significantly among the 456 isolates tested. Avr-Pik and Avr-Pita3 occurred at the highest frequency (94.5% and 91.7%, respectively), while Avr1-CO39 and Avr-Pii occurred at the lowest frequency (0.0%). Further, the remaining Avr genes occurred with frequencies between 5.9 and 89% ().
Fig. 1. Patterns amplified by PCR of avirulence genes of Magnaporthe oryzae. A – Avr-Pik; B – Avr-Pita1; C – Avr-Pita2; D – Avr-Pita3; E – Avr-Piz-t; F – PWL2; G – ACE1; H –Avr1-CO39, P1 – positive control; I – Avr-Pii, P2 – positive control; J – Avr-Pia; K – Avr-Pikm; M – 5000 bp marker; 1–23 for isolates from Fujian Province, 24 for negative control.
Fig. 2. (Colour online) The avirulence genes and amplification rate in 456 Magnaporthe oryzae isolates from Fujian Province, China. Avr-Pik and Avr-Pita3 had the highest frequencies (94.5% and 91.7%, respectively), while Avr1-CO39 and Avr-Pii had the lowest (0%). Frequencies of other Avr genes varied from 5.9 to 89%.
Temporal and spatial distribution of Avr genes
The distribution frequencies of the Avr genes in the 456 M. oryzae isolates were analysed in different years (). Across all isolates, Avr-Pik was found in > 87.5% of isolates collected from 2006 to 2015. Avr-Pita3, Avr-Piz-t, Avr-Pita1 and PWL2 were observed in > 67.4% of isolates. In contrast, Avr-Pia was detected in < 15.6% of isolates collected from 2006 to 2015. Further, Avr1-CO39 and Avr-Pii were not detected during the survey. The Avr genes Avr-Pik and Avr-Pita3 were at relatively stable frequencies over the period examined. The highest fluctuations in frequency were observed with Avr-Pita2 and ranged from 40–100%. Similarly, the frequency of ACE1 was also variable (22.9–77.1%).
Table 3. The frequency of occurrence of avirulence genes in 456 M. oryzae isolates from 2006 to 2015 in Fujian Province, China.
The distribution frequencies of the Avr genes in 456 M. oryzae isolates pooled from different locations were also analysed (). Among the 11 Avr genes, Avr-Pik, PWL2 and Avr-Pita3 were found with the highest frequency among locations, ranging from 83–100%, 73.6–98.4% and 81.1–100%, respectively. In contrast, with the exception of Avr-Pii and Avr1-CO39, Avr-Pia had the lowest frequency, ranging from 0–9.1%. In this survey, all isolates from Putian City harboured Avr-Pik and all isolates from Zhangzhou City carried Avr-Pita3. However, no isolates from eight populations possessed Avr-Pii or Avr1-CO39.
Table 4. Frequency and spatial distribution of avirulence genes in 456 M. oryzae isolates collected from eight geographic locations in Fujian Province, China.
Pathogenicity of M. oryzae isolates to R genes
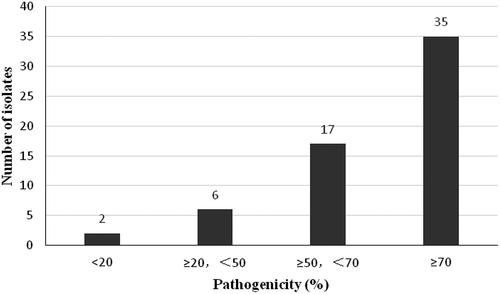
Sixty of the 456 isolates were tested for their pathogenicity on 24 rice monogenic lines with the Pi-gene. PFs ranged from 8.3–91.7%. Thirty-five isolates (58.3%) were strongly pathogenic, with PFs ranging from 70.8–91.7%, 17 isolates (28.3%) were relatively strongly pathogenic, with PFs ranging from 50–66.7%, six isolates (10%) were moderately pathogenic, with PFs ranging from 20.8–45.8%, and two isolates were weakly pathogenic with PFs of 8.3% and 16.7% (). Strong or relatively strong pathogenicity was observed in 86.7% of the isolates tested, which indicated that such isolates possessed an advantage in M. oryzae populations in Fujian Province.
Virulence spectrum of M. oryzae isolates to R genes
Sixty of the 456 isolates were tested for their virulence to 24 rice monogenic lines carrying the Pi-gene. Marked differences in virulence were observed among isolates. Generally, the virulence frequencies of 60 isolates against R genes ranged from 10–93.3% (). The virulence frequency against Pik and Pikm was lowest, with values of 10% and 13.3%, respectively, suggesting that these two R genes could be useful in rice breeding in Fujian Province. In contrast, virulence frequency on the R genes Pia and Pit was highest, at 93.3%. The virulence frequency on the R genes PiZ5, Pi1(1), Pikp, Pi9(t), Pita(1) and Pikh ranged from 23.3–43.3%. Virulence frequency on the genes Pita(2), Pizt, Piz, and Pi7(t) ranged from 51.7–63.3%. Finally, virulence frequency on the remaining 10 genes ranged from 76.7–91.7%.
Discussion
Understanding the temporal and spatial dynamics of Avr genes, as well as the mechanisms underlying their emergence and evolution, is important for the effective use of major R genes in sustainable disease management. In this study, 456 M. oryzae isolates were sampled from eight locations across the main rice producing regions of Fujian Province from 2006–2015. These isolates were tested using 11 pairs of primers to identify the predominant Avr genes and their temporal and spatial distribution. The results indicate high and relatively stable frequencies of Avr-Pik and Avr-Pita3. We propose that cultivars carrying a corresponding R gene (Pik and Pita3) are useful for rice breeding programmes in Fujian Province. In contrast, the Avr genes Avr1-CO39 and Avr-Pii are not used in breeding rice in Fujian Province. Research conducted by Mu (2013) reported that the Avr1-CO39 and PWL1 loci of all strains were absent in Zhejiang Province, China.
To investigate gene-for-gene interactions, the current study also used 24 rice monogenic lines with the Pi-gene to assess the virulence of 60 M. oryzae isolates. The isolates had strong or relatively strong pathogenicity, accounting for 86.6% of the isolates tested, which implies that M. oryzae populations are virulent on most of the R genes tested, consistent with the findings of Yang et al. (Citation2007). The virulence frequency of the analysed M. oryzae isolates to Pik and Pikm was very low which indicates the high level of resistance conditioned by these two genes and the great potential of these genes for cultivar improvement, consistent with the results of Ruan et al. (Citation2017). Wang et al. (Citation2009) reported that Pikm had a broader resistance spectrum than Pik in four populations (FJ, YN, JS and HLJ), but this was not the case for the other six populations tested, where some resistance frequencies of Pik were greater than those of Pikm. In our study, the level of virulence of the analysed isolates to Piz5, Pi1(1), Pikp, Pi9(t), Pita(1) and Pikh was moderate, implying that these R genes could be combined in elite cultivars through a marker-assisted selection approach in Fujian Province.
Previous reports demonstrated that Piz-t, Piz, Pi9 and Pi2 conferred broad-spectrum resistance to different sets of M. grisea strains (Liu et al., Citation2002; Hayashi et al., Citation2004). Similarly, Mutiga et al. (Citation2017) reported that Pi9 was resistant to 86% of the isolates from nine African countries. Lei et al. (Citation2011) reported Pi9 showed the broadest resistance spectrum (on average 94.8%) to all the blast isolates tested in Heilongjiang Province, China. The Pita gene has been used widely to control rice blast disease in the southern USA and is found commonly in rice germplasm used throughout the world (Wang et al., Citation2007). The Pi-sh, Pi-11, Pi-b and nine other R genes conferred a low level of resistance and would be less valuable in rice breeding in Fujian Province. Nonetheless, Pi-b, introduced from the IRRI in the early 1960s, was used extensively in indica rice breeding programmes in southern China, especially in Guangdong and Hainan provinces (Zhang et al., Citation2015). Additionally, the discovery of additional Avr genes to enhance monitoring of the virulence spectrum of M. oryzae populations and to minimize the risk of breakdown in rice resistance in Fujian Province is critical for effective management of this disease.
The general tendency of M. oryzae Avr genes to be located on unstable chromosome regions (Orbach et al., Citation2000; Yoshida et al., Citation2009) enhances the possibility of mutations in those genes, which could result in a M. oryzae isolate becoming virulent on a formerly resistant host (Bryan et al., Citation2000). It is therefore vital to develop a series of new rice cultivars with broad-spectrum or durable blast resistance. For now, to reduce the chances of R gene breakdown, these should be pyramided into adapted crop cultivars (Miah et al., Citation2013). Resistance gene pyramids have been thought to be advantageous over traditional approaches of deploying single resistance gene for sustainable disease management (Mundt, Citation2014).
Supplemental Table 1
Download MS Word (14 KB)Supplementary material
Supplemental data for this article can be accessed online here: https://doi.org/10.1080/07060661.2018.1504821
Additional information
Funding
References
- Bohnert HU, Fudal I, Dioh W, Tharreau D, Notteghem JL, Lebrun MH. 2004. A putative polyketide synthase/peptide synthetase from Magnaporthe grisea signals pathogen attack to resistant rice. Plant Cell. 16:2499–2513.
- Bryan GT, Wu K, Farrall L, Jia Y, Hershey HP, McAdams SA, Faulk KN, Donaldson GK, Tarchini R, Valent B. 2000. A single amino acid difference distinguishes resistant and susceptible alleles of rice blast resistance gene Pi-ta. Plant Cell. 12:2033–2045.
- Chuma I, Isobe C, Hotta Y, Ibaragi K, Futamata N, Kusaba M, Yoshida K, Terauchi R, Fujita Y, Nakayashiki H, et al. 2011. Multiple translocation of the AVR-Pita effector gene among chromosomes of the rice blast fungus Magnaporthe oryzae and related species. PLoS Pathog. 7:e1002147.
- Couch BC, Kohn LM. 2002. A multilocus gene genealogy concordant with host preference indicates segregation of a new species, Magnaporthe oryzae, from M. grisea. Mycologia. 94:683–693.
- Du YX, Li K, Ruan HC, Yang XJ, Gan L, Chen FR. 2011. Sensitivities of Magnaporthe grises to isprothiolane, iprobenfos and tricyclazole. J Plant Protec. 38:455–460. in Chinese with English abstract.
- Ellis J, Dodds P, Lawrence G. 2007. Flax rust resistance gene specificity is based on direct resistance-avirulence protein interactions. Annu Rev Phytopathol. 45:289–306.
- Farman ML, Leong SA. 1998. Chromosome walking to the AVR1-CO39 avirulence gene of Magnaporthe grisea: discrepancy between the physical and genetic maps. Genetics. 150:1049–1058.
- Feng SJ, Wang L, Ma JH, Lin F, Pan QH. 2007. Genetic and physical mapping of AvrPi7, a novel avirulence gene of Magnaporthe oryzae using physical position-ready makers. Chin Sci Bull. 52:903–911.
- Flor HH. 1971. Current status of the gene-for-gene concept. Annu Rev Phytopathol. 9:275–296.
- Hayashi K, Hashimoto N, Daigen M, Ashikawa I. 2004. Development of PCR-based SNP markers for rice blast resistance genes at the Piz locus. Theor Appl Genet. 108:1212–1220.
- Hayashi K, Yoshida H. 2009. Refunctionalization of the ancient rice blast disease resistance gene Pit by the recruitment of a retrotransposon as a promoter. Plant J. 57:413–425.
- Heath MC. 2000. Nonhost resistance and nonspecific plant defenses. Curr Opin Plant Biol. 3:315–319.
- IRRI. 1996. Standard evaluation system for rice. 4th ed. Manila: IRRI; p. 52.
- Kang S, Sweigard JA, Valent B. 1995. The PWL host specificity gene family in the blast fungus Magnaporthe grisea. Mol Plant-Microbe Inter. 8:939–948.
- Kanzaki H, Yoshida K, Saitoh H, Fujisaki K, Hirabuchi A, Alaux L, Fournie E, Tharreau D, Terauchi R. 2012. Arms race co-evolution of Magnaporthe oryzae AVR-Pik and rice Pik genes driven by their physical interactions. Plant J. 72:894–907.
- Lan B, Yang YQ, Xu PD, Li XM, He LG. 2014. Analysis of the resistance of rice major Pi-genes to the isolates in Jiangxi Province. J Plant Protec. 41:163–168. in Chinese with English abstract.
- Lei CL, Zhang GM, Cheng ZJ, Jun-Tao MA, Wang JL, Xin AH, Chen P, Xiao JL, Zhang X, Liu YX, et al. 2011. Pathogenic races and virulence gene structure of Magnaporthe oryzae population and breeding strategy for blast resistance in Heilongjiang Province. Acta Agron Sin. 37:18–27.
- Li W, Wang B, Wu J, Lu G, Hu Y, Zhang X, Zhang Z, Zhao Q, Feng Q, Zhang H, et al. 2009. The Magnaporthe oryzae avirulence gene Avr-Piz-t encodes a predicted secreted protein that triggers the immunity in rice mediated by the blast resistance gene Piz-t. Mol Plant-Microbe Inter. 22:411–420.
- Liu G, Lu G, Zeng L, Wang GL. 2002. Two broad-spectrum blast resistance genes, Pi9(t) and Pi2(t), are physically linked on rice chromosome 6. Mol Genet Genom. 267:472–480.
- Liu J, Wang X, Mitchell T, Hu Y, Liu X, Dai L, Wang G-L. 2010. Recent progress and understanding of the molecular mechanisms of the rice-Magnaporthe oryzae interaction. Mol Plant Pathol. 11:419–427.
- Ma JH, Wang L, Feng SJ, Lin F, Xiao Y, Pan QH. 2006. Identification and fine mapping of AvrPi15, a novel avirulence gene of Magnaporthe grisea. Theor Appl Genet. 113:875–883.
- Maciel JL, Ceresini PC, Castroagudin VL, Zala M, Kema GH, McDonald BA. 2014. Population structure and pathotype diversity of the wheat blast pathogen Magnaporthe oryzae 25 years after its emergence in Brazil. Phytopathol. 104:95–107.
- McDonald BA, Linde C. 2002. Pathogen population genetics, evolutionary potential, and durable resistance. Ann Rev Phytopathol. 40:349–379.
- Miah G, Rafii MY, Ismail MR, Puteh AB, Rahim HA, Asfaliza R, Latif MA. 2013. Blast resistance in rice: a review of conventional breeding to molecular approaches. Mol Biol Rep. 40:2369–2388.
- Mu HM. 2013. Analysis of genetic diversity of Magnaporthe oryzae isolates from Zhejiang Province, over the past thirty years based on avirulence gene [dissertation]. Nanjing, China: Nanjing Agricultural University.
- Mundt CC. 2014. Durable resistance: A key to sustainable management of pathogens and pests. Infect Genet Evol. 27:446–455.
- Mutiga SK, Rotich F, Devi Ganeshan V, Mwongera DT, Mgonja EM, Were VM, Harvey JW, Zhou B, Wasilwa L, Chunda F, et al. 2017. Assessment of the virulence spectrum and its association with genetic diversity in Magnaporthe oryzae populations from sub-Saharan Africa. Phytopathol. 107:852–863.
- Orbach MJ, Farrall L, Sweigard JA, Chumley FG, Valent B. 2000. A telomeric avirulence gene determines efficacy for the rice blast resistance gene Pi-ta. Plant Cell. 12:2019–2032.
- Pei H, Ling ZZ. 1986. Study on pathologic races of blast fungus in Dandong, Liaoning. Acta Phytopathol Sin. 16:197–203.
- Qu S, Liu G, Zhou B, Bellizzi M, Zeng L, Dai L, Han B, Wang G-L. 2006. The Broad-spectrum blast resistance gene Pi9 encodes a nucleotide-binding site–leucine-rich repeat protein and is a member of multigene family in rice. Genetics. 172:1901–1914.
- Ray S, Singh PK, Gupta DK, Mahato AK, Chiranjib S, Rajeev R, Singh NK, Sharma TR. 2016. Analysis of Magnaporthe oryzae genome reveals a fungal effector, which is able to induce resistance response in transgenic rice line containing resistance gene, Pi54. Front Plant Sci. 7:1140.
- Rossman AY, Howard RJ, Valent B. 1990. Pyricularia grisea, the correct name for the rice blast fungus. Mycologia. 82:509–512.
- Ruan HC, Shi NN, Du YX, Gan L, Yang XJ, Dai YL, Chen FR. 2017. Analysis on resistance of Pi genes to predominant races of Mangnaporthe oryzae in Fujian Province, China. Chin J Rice Sci. 31:105–110. in Chinese with English abstract.
- Singh PK, Thakur S, Rathour R, Variar M, Prashanti SK, Singh AK, Singh UD, Sharma V, Singh NK, Sharma TR. 2014. Transposon-based high sequence diversity in Avr-Pita alleles increases the potential for pathogenicity of Magnaporthe oryzae populations. Funct Integr Genet. 14:419–429.
- Singh Y, Kumar J. 2010. Study of genomic fingerprints profile of Magnaporthe grisea from finger millet (Eleusine coracona) by random amplified polymorphic DNA-polymerase chain reaction. Afr J Biotechnol. 9:7798–7804.
- Sweigard JA, Carroll AM, Kang S, Farrall L, Chumley FG, Valent B. 1995. Identification, cloning, and characterization of PWL2, a gene for host species specificity in the rice blast fungus. Plant Cell. 7:1221–1233.
- Talbot NJ. 2003. On the trail of a cereal killer: exploring the biology of Magnaporthe oryzae. Annu Rev Microbiol. 57:177–202.
- Wang L, Xu XK, Lin F, Pan QH. 2009. Characterization of rice blast resistance genes in the Pik cluster and fine mapping of the Pik-p locus. Phytopathol. 99:900–905.
- Wang Z, Jia Y, Rutger JN, Xia Y. 2007. Rapid survey for presence of a blast resistance gene Pi-ta in rice cultivars using the dominant DNA markers derived from portions of the Pi-ta gene. Plant Breed. 126:36–42.
- Wang Z-X, Yano M, Yamanouchi U, Iwamoto M, Monna L, Hayasaka H, Katayose Y, Sasaki T. 1999. The Pib gene for rice blast resistance belongs to the nucleotide binding and leucine-rich repeat class of plant disease resistance genes. Plant J. 19:55–64.
- Yan XH 2015. Analysis of genetic diversity among populations and the preliminary forecasting of the virulence gene distribution frequency for the rice blast fungus Magnaporthe oryzae in southwest region of China [ dissertation]. Chengdu (China): Sichun Agriculture University.
- Yang XJ, Ruan HC, Du YX, Chen FR, Wang MM. 2007. Pathogenicity and avirulence genes analysis of Magnaporthe grises Barr. from rice in Fujian province of China. J Plant Protec. 34:337–342. in Chinese with English abstract.
- Yoshida K, Saitoh H, Fujisawa S, Kanzaki H, Matsumura H, Yoshida K, Tosa Y, Chuma I, Takano Y, Win J, et al. 2009. Association genetics reveals three novel avirulence genes from the rice blast fungal pathogen Magnaporthe oryzae. Plant Cell. 21:1573–1591.
- Zhang GZ, Liu JF, Ma QJ, Peng YL. 2004. A novel SCAR marker closely linked to the avirulence gene AVR-Pikm in rice blast fungus Magnaporthe oryzae. Acta Phytopathol Sin. 34:548–554.
- Zhang SL, Wang L, Wu WH, He LY, Yang XF, Pan QH. 2015. Function and evolution of Magnaporthe oryzae avirulence gene AvrPib responding to the rice blast resistance gene Pib. Sci Rep. 5:11642.
- Zhou EX, Jia YL, Singh P, Correll JC, Lee FN. 2007. Instability of the Magnaporthe oryzae avirulence gene Avr-Pita alters virulence. Fungal Genet Biol. 44:1024–1034.
- Zhou JH, Wang JL, Jiang WR, Lei CL, Ling ZZ. 2003. Virulence genes diversity and geographic distribution of Pyricularia grisea in China. Acta Agron Sin. 295:646–651.