Abstract
Hot pepper is an exceedingly popular vegetable crop in China and is cultivated on 737 hectares in Beijing. A severe leaf disease with typical symptoms of spots with grey centres and dark brown borders was observed on hot pepper plants in Beijing in the winter of 2016. More than 50% of all plants in the region were infected. Fungal cultures were isolated from naturally infected leaf tissue, and identified as Stemphylium lycopersici based on morphological features, cultural characteristics and molecular identification by sequencing the ITS, gpd and cmdA genes. Pathogenicity was determined by inoculating healthy pepper plants with hyphal suspensions, and the fungus was re-isolated from developing lesions on the inoculated plants, thus fulfilling Koch’s postulates. This is the first report of natural infection by S. lycopersici causing leaf spot on hot pepper in China.
Résumé
Le piment fort est un légume extrêmement populaire en Chine et, à Beijing, on consacre 737 ha à sa culture. Une grave maladie foliaire, affichant les symptômes typiques de taches aux centres gris et aux contours bruns, a été observée sur les plants de piments forts à Beijing durant l’hiver de 2016. Plus de 50% de tous les plants de la région étaient infectés. Des cultures fongiques ont été isolées à partir de tissus foliaires infectés naturellement et, en se basant sur les traits morphologiques, les caractéristiques culturales et l’identification moléculaire en séquençant l’ITS ainsi que les gènes gpd et cmdA, l’agent a été identifié en tant que Stemphylium lycopersici. La pathogénicité a été déterminée en inoculant des plants de piments sains avec des hyphes en suspension, puis le champignon a été de nouveau isolé à partir des lésions qui s’étaient développées sur ces derniers, confirmant ainsi les postulats de Koch. Il s’agit de la première mention d’une infection naturelle provoquée par S. lycopersici, causant des taches foliaires sur le piment fort en Chine.
Introduction
Hot pepper (Capsicum annuum L.), a member of the Solanaceae family, is the most widely grown spice in the world and is a major ingredient in most global cuisines (Bosland & Votava, Citation2012). It provides a number of vitamins and nutrients that are beneficial to human health (Kim et al., Citation2014). Hot pepper is planted on ~1.3 million hectares every year in China, producing 27 million tons of hot pepper (Xu et al., Citation2008). It provides the second highest economic return among all vegetable crops (Xin & Huang, Citation2014). In Beijing, the hot pepper cultivation area is 737 hectares and Beijing’s annual hot pepper yield is 27,500 tons (Liu et al., Citation2016).
A severe disease on the leaves of hot pepper was observed in Beijing (116°33′E, 39°73′N) in 2016, with disease incidence of more than 50% of all plants in the area. The symptoms on leaves of the naturally affected hot pepper plants were necrotic lesions with grey centres and dark brown borders, and curled leaves at late developmental stages (). The purpose of the study was to isolate the causal agent and identify and describe the pathogen using morphological and molecular methods, as well as to affirm its pathogenicity.
Fig. 1 (Colour online) Symptoms and morphological characteristics of Stemphylium lycopersici infecting hot pepper plants. (A) Necrotic lesions with grey centres and dark brown borders; (B) Curled leaves of naturally affected hot pepper in the field; (C) Colony of S. lycopersici on PDA (7-day-old), surface view; (D) Colony of S. lycopersici on PDA (7-day-old), bottom view; (E) Microscopic structures of conidiophores (×400); (F) conidia (×400). (scale bars: E, F = 20 μm). Symptoms observed in pathogenicity test 8 days post-inoculation; (G) necrotic lesions and (H) curled leaves.

Materials and methods
Sample collection
Diseased leaves of hot pepper ‘Zhongjiao 0812' with typical leaf spot symptoms were sampled in Beijing in 2016. Two randomly selected plants from each of five independent locations were sampled by collecting three diseased leaves per plant. The detached leaflets were cut into ~3-mm2 pieces, surface-disinfected in 70% ethanol for 30 s, rinsed in sterile distilled water, placed on potato dextrose agar (PDA) and incubated at 26°C for 5–7 days. Mycelia arising from the leaf pieces were transferred onto fresh PDA dishes or slant tubes for successive growth and identification. A total of 25 isolates were recovered, which were purified by transferring 6-mm diameter mycelium plugs from the margin of growing colonies onto fresh PDA dishes. The shape, length and width of a total of 50 conidia and 50 conidiophores on the infected leaves were examined and recorded at 400× magnification using a light microscope (Nikon Eclipse 80i, 220V; Nikon Corp., Tokyo, Japan).
Pathogenicity test
The 25 isolates were tested for pathogenicity on hot pepper plants grown in a greenhouse at the Institute of Vegetables and Flowers of CAAS in Beijing. Mycelia from each isolate were grown in 50 mL potato dextrose broth (PDB) for 7 days at 27°C to prepare a hyphal suspension (the fungus did not sporulate in culture), according to the method described by Guidry & Trelles (Citation1962). Three healthy hot pepper plants (‘Zhongjiao 0812'; China Vegetable Seed Technology Co., Ltd, Beijing, China) at the 4-true-leaf stage per isolate were inoculated by spraying with a hyphal suspension of 1 × 106 CFU mL−1. Another three hot pepper plants were sprayed with sterilized water as controls. The experiment was repeated three times. All plants were kept on a mist bench at 25 ± 5°C and relative humidity of 90% for 48 h, and then transferred to a greenhouse at 18°C (night)/25°C (day) under natural daylight conditions. The development of symptoms was observed and recorded each day. To fullfill Koch’s postulates, fungal isolations were made from developing lesions on inoculated hot pepper plants, and the cultural characteristics of the isolated fungi were compared with those of the original strains. In addition, the morphological characteristics of the isolated fungi were observed on the diseased leaves, and compared with those of the original strains.
Molecular identification
DNA of each of the 25 isolates was extracted from mycelia grown in PDB using the CTAB method (Lee, Citation1990). The internal transcribed spacer region (ITS) of ribosomal DNA (rDNA) was amplified using the universal primers ITS1 (5ʹ-TCCGTAGGTGAACCTGCGC-3ʹ) and ITS4 (5ʹ-TCCTCCGCTTATTGATATGC-3ʹ) (White et al., Citation1990). The amplification of the glyceraldehyde-3-phosphate dehydrogenase (gpd) fragment was performed using the primers gpdF (5ʹ-GCACCGACCACAAAAATC-3ʹ) and gpdR (5ʹ-GGGCCGTCAACGACCTTC-3ʹ) (Huang & Tsai, Citation2017). The calmodulin region (cmdA) was amplified with the primers CALDF1 (5ʹ-AGCAAGTCTCCGAGTTCAAGG-3ʹ) and CALDR2 (5ʹ-CTTCTGCATCATCAYCTGGACG3ʹ) (Lawrence et al., Citation2013). PCR was performed in a 25-μL reaction mixture containing 50 ng DNA, 2 mM dNTPs (Biomed, Beijing, China), 1 unit of Taq DNA polymerase (Biomed, Beijing, China), and 1× PCR buffer (15 mM MgCl2, 200 mM Tris-HCl (pH 8.4), and 100 mM (NH4)2SO4) (Biomed, Beijing, China). The PCR amplification procedure contained the following steps: 94°C for 5 min, 35 cycles of denaturation at 94°C for 40 s, annealing at 55°C for 45 s, and extension at 72°C for 45 s, with a final extension at 72°C for 5 min. PCR products were detected by 1% agarose (Biowest, Spain) gel electrophoresis and sequenced by Biomed (Beijing, China). The resulting ITS, gpd and cmdA gene sequences were blasted in the GenBank database of NCBI (http://www.ncbi.nlm.nih.gov). With reference to the latest revision of the Stemphylium genus (Woudenberg et al., Citation2017), sequences of 13 strains representing nine species of Stemphylium were obtained, and these sequences along with those obtained in this study were aligned using MEGA version 6.0 (Tamura et al., Citation2013). Phylogenetic analysis of the combined dataset of the ITS-5.8S rDNA, gpd and cmdA regions using the Maximum Likelihood method was performed with the Tamura three-parameter model in MEGA 6.0. Alternaria alternata (Woudenberg et al., Citation2017) was selected as an outgroup.
Results
Fungal isolation and morphological characteristics
The 25 isolates of the fungus obtained in the study had similar cultural characteristics and were deposited at the Institute of Vegetables and Flowers, Chinese Academy of Agricultural Sciences (CAAS) for future reference. The colonies were grey and cottony, and produced yellow to dark red pigments on PDA (). The conidiophores on lesions of inoculated leaves were solitary, straight or slightly curved, smooth, with 3–5 septa, light brown, 78.6–99.7 μm in length, and 3.8–4.7 μm in width, with an apical cell distinctly swollen (). The conidia were solitary, oblong, rounded, or sometimes pointed at the apex, blunt rounded at the base and constricted at the main transverse septum. The dimensions of the conidia were 23.8–47.6 × 13.9–20.5 μm, with a mean conidial length/width ratio of 2.3, and 1–7 transverse and 1–2 longitudinal septa ().
Pathogenicity test
The disease incidence on the inoculated hot pepper plants was 100%, with symptoms similar to those observed in the field. Small spots developed on the leaves (, h). The cultural characteristics of the isolates obtained from the inoculated symptomatic tissues were similar to the original isolates, and the morphological characteristics of the isolates observed on the diseased leaves were similar to the original isolates observed on the original diseased leaves. The plants sprayed with sterile water remained asymptomatic, and the fungus was not isolated from their leaves. The results were the same in all three replicates of the pathogenicity tests.
Molecular identification
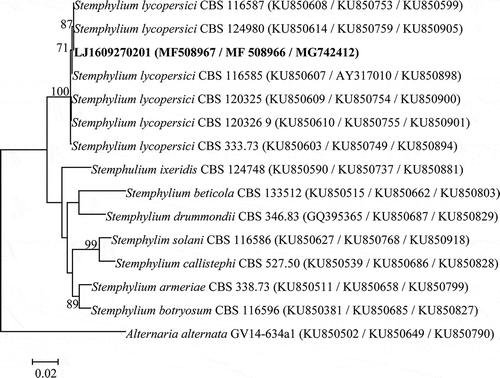
Fragments of approximately 546, 834 and 700 bp were obtained from the ITS, gpd and cmdA genes, respectively, in all 25 isolates. The sequence alignment indicated that all of ITS, gpd and cmdA sequences from the 25 isolates were identical. Thus, one representative isolate LJ1609270201 was chosen for further analysis. The ITS, gpd and cmdA sequences of this isolate were submitted to the NCBI database (accession nos. MF508967, MF508966 and MG742412, respectively). The BLAST analysis indicated our sequences showed 100% similarity to the ITS sequence of the strain HZ2111 of S. lycopersici (KR633052), 100% similarity to the gpd sequence of the HZ2115 S. lycopersici strain (KT957743) and 100% similarity to the cmdA sequence of the CBS124980 S. lycopersici strain (KU85095). In the phylogenetic tree established on the basis of the combined data, isolate LJ1609270201 and other S. lycopersici strains were clustered in a clade with a robust bootstrap (), and these strains were separated from S. solani and other Stemphylium species.
Fig. 2 Phylogenetic tree generated from the maximum likelihood analysis of combined dataset of ITS-5.8S rDNA, gpd and cmdA sequences of Stemphylium lycopersici isolates and related species, including the isolate obtained in the present study (LJ1609270201 in bold), and 13 isolates retrieved from GenBank. The tree was rooted with Alternaria alternata. Numbers of bootstrap support values ≥70% based on 1000 replicates are indicated. The bar indicates nucleotide substitutions per site. The GenBank accession numbers of ITS, gpd and cmdA genes are adjacent to each strain.
Discussion
A comparison of our isolates to those reported previously indicated they were similar to S. lycopersici in cultural features, particularly the secretion of a yellow to dark red pigment on PDA (Ellis & Gibson, Citation1975a). Moreover, the conidiophores of our isolates were similar to those of S. solani or S. lycopersici, and conidia were similar in length, width and length/width ratio to S. solani (Ellis & Gibson, Citation1975b). Based on the cultural and morphological characteristics, the isolates could be classified as S. lycopersici or S. solani, as is consistent with other previous reports (Hong et al., Citation2012). However, based on the multiple loci phylogenetic analysis, the isolates were distinguished from S. solani and other Stemphylium species, and clustered in a clade with S. lycopersici.
On pear trees in Europe, levels of Stemphylium leaf spot leading to 5–10% infected fruit in one year may be followed by up to 90% infected fruit in the next year, even though control measures were applied (Llorente & Montesinos, Citation2006). If Stemphylium leaf spot of hot pepper in China follows a similar pattern, the incidence of this disease will need to be monitored, and the necessary prevention and control practices applied.
Production of hot pepper in China is often severely limited by one or more disease problems, for example, bacterial wilt caused by Ralstonia solanacearum (Qiu et al., Citation2014), anthracnose caused by Colletotrichum capsici (Lu et al., Citation2016), phytophthora blight caused by Phytophthora capsici (Jiang et al., Citation2017), southern blight caused by Sclerotium rolfsii (Nie et al., Citation2016), pepper blight caused by Rhizoctonia solani (Li et al., Citation2016), and pepper virus diseases caused by Cucumber mosaic virus or Pepper mild mottle virus (Wang et al., Citation2017).
Previously, S. lycopersici has been reported to Solanum lycopersicum, Solanum melongena and Lactuca sativa in Malaysia (Nasehi et al., Citation2014a,Citation2014b), Chrysanthemum morifolium and Juncus roemerianus in Florida, USA (Fell & Hunter, Citation1979; Câmara et al., Citation2002), Chrysanthemum sp. and Solanum lycopersicum in Russia (Gannibal, Citation2012), and Centella asiatica and Capsicum annuum in Japan (Kurose et al., Citation2015). In China, S. lycopersici has been reported infecting Solanum melongena and Solanum lycopersicum (Sun et al., Citation2016; Yang et al., Citation2017). To our knowledge, this is the first report of leaf spot caused by S. lycopersici on hot pepper in China.
Additional information
Funding
References
- Bosland PW, Votava EJ, Bosland PW, Votava EJ. 2012. Peppers: vegetable and spice capsicums. CABI Bookshop. 2:14–39.
- Câmara MP, O’Neill NR, Van BP. 2002. Phylogeny of Stemphylium spp. based on ITS and glyceraldehyde-3-phosphate dehydrogenase gene sequences. Mycologia. 94:660.
- Ellis MB, Gibson IA 1975a. CMI descriptions of pathogenic fungi and bacteria No. 471. Stemphylium lycopersici. Kew (Surrey): Commonwealth Mycological Institute.
- Ellis MB, Gibson IA 1975b. CMI descriptions of pathogenic fungi and bacteria No. 472. Stemphylium solani. Kew (Surrey): Commonwealth Mycological Institute.
- Fell JW, Hunter IL. 1979. Fungi associated with the decomposition of the black rush, Juncus roemerianus, in South Florida. Mycologia. 71:322–342.
- Gannibal PB. 2012. First report of Stemphylium lycopersici from far East Russia: a new record and new host. Mycotaxon. 121:371–374.
- Guidry DJ, Trelles GH. 1962. Evaluation of a new method for the preparation of homogeneous mycelial suspensions. J Bacteriol. 83:53–60.
- Hong SK, Won CH, Kee LY, Sik SH, Yeob LS. 2012. Leaf spot and stem rot on wilford swallowwort caused by Stemphylium lycopersici in Korea. Mycobiology. 40:268–271.
- Huang CJ, Tsai WS. 2017. Occurrence and identification of Stemphylium lycopersici causing Stemphylium leaf spot disease on tomato in Taiwan. Eur J Plant Pathol. 148:35–44.
- Jiang B, Zhang Y, Guo C, Yang C, Zhu S, Yang M. 2017. Control effects and allelopathic mechanism of pepper and Chinese chives intercropping on pepper phytophthora blight. J Plant Prot. 44:145–151.
- Kim S, Park M, Yeom SI, Kim YM, Lee JM, Lee HA, Seo E, Choi J, Cheong K, Kim KT. 2014. Genome sequence of the hot pepper provides insights into the evolution of pungency in Capsicum species. Nat Gen. 46:270–278.
- Kurose D, Misawa T, Suzui T, Ichikawa K, Gan K, Long HH, Furuya N, Tsuchiya K, Tsushima S, Sato T. 2015. Taxonomic re-examination of several Japanese Stemphylium strains based on morphological and molecular phylogenetic analyses. J Gen Plant Pathol. 81:358–367.
- Lawrence DP, Gannibal PB, Peever TL, Pryor BM. 2013. The sections of Alternaria: formalizing species-group concepts. Mycologia. 105:530.
- Lee SB. 1990. Isolation of DNA from fungal mycelia and single spore. In: Innis MA, Gelfand DH, Sninsky TJ, White TJ, editors. PCR protocols: a guide to methods and applications. New York (NY): Academic Press; p. 282–287.
- Li W, Zhao X, Xu Q, Yu H, Li Y. 2016. Study on antagonistic mechanism of antagonistic extracellular protein from Brevibacillus laterosporus B8 against damping off of sweet pepper. China Plant Prot. 36:5–9.
- Liu J, Wang LH, Zhang ZH, Cao YC, Zhang BX. 2016. Analysis of hot pepper virus diseases in Beijing. J Changjiang Veget. 14:48–50.
- Llorente I, Montesinos E. 2006. Brown spot of pear: an emerging disease of economic importance in Europe. Plant Dis. 90:1368.
- Lu HX, Zhang SX, Zheng FC, Zhao XM, Huang BH. 2016. Evaluation on controlling effect of oligo-chitosan on pepper anthracnose and chilling injury. China Plant Prot. 36:28–31.
- Nasehi A, Kadir JB, Esfahani MN, Mahmodi F, Golkhandan E, Akter S, Ghadirian H. 2014a. Cultural and physiological characteristics of Stemphylium lycopersici causing leaf blight disease on vegetable crops. Arch Phytopathol Plant Prot. 47:1658–1665.
- Nasehi A, Kadir JB, Nasr-Esfahani M, Abed-Ashtiani F, Wong MY, Rambe SK, Golkhandan E. 2014b. Analysis of genetic and virulence variability of Stemphylium lycopersici associated with leaf spot of vegetable crops. Eur J Plant Pathol. 140:261–273.
- Nie Z, Qiming L, Chen Z, Dai F, Wang W, Wang X. 2016. Efficacy of different fungicides on controlling pepper southern blight in field. J Changjiang Veget. 4:87–88.
- Qiu JP, Huang YX, Wang C, Yu YY, Ke HJ, Guo JH. 2014. Effects of bacterial consortium EG03 on control of pepper bacterial wilt and rhizosphere microbial community characteristics in fields. Chin J Appl Ecol. 25:1468–1474.
- Sun XT, Zhang L, Zhang JZ. 2016. First report of tomato gray leaf spot caused by Stemphylium lycopersici in Zhejiang province, China. Plant Dis. 100: 227.
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 30:2725–2729.
- Wang SL, Tan WP, Yang YY, Dai HJ, Sun XH, Qiao N, Zhu XP. 2017. Molecular detection and identification of main viruses on pepper in Shandong province. Sci Agr Sin. 50:2728–2738.
- White TJ, Bruns TD, Lee SB, Taylor JW. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. New York (NY): Academic Press; p. 315–322.
- Woudenberg JHC, Hanse B, Leeuwen GCMV, Groenewald JZ, Crous PW. 2017. Stemphylium revisited. Stud Mycol. 87:77–103.
- Xin J, Huang B. 2014. Subcellular distribution and chemical forms of cadmium in two hot pepper cultivars differing in cadmium accumulation. J Agr Food Chem. 62:508–515.
- Xu X, Li Y, Wang H. 2008. Present situation, development trend and countermeasures of pepper industry in China. Chin Agric Sci Bull. 24:332–338.
- Yang H, Xiangyang XU, Zhao T, Jiang J, Liu G, Jingfu LI. 2017. First report of Stemphylium lycopersici causing gray leaf spot on eggplant in China. Plant Dis. 101: 834.
