Abstract
An increase in the cultivation of Cannabis sativa (cannabis or marijuana) plants in Canada is becoming associated with increased incidence and severity of various diseases, many of which have not been previously reported. In this study, hydroponically grown C. sativa plants were sampled over a 3-year period (2014–2017) to determine the prevalence of root pathogens. Following isolation, pathogenicity studies were conducted to establish the extent of disease symptoms caused by the recovered microbes. Root rot was found to be caused by two Pythium species – Pythium dissotocum Drechsler and P. myriotylum Drechsler. As well, two Fusarium species were recovered from diseased plants – Fusarium oxysporum Schlecht. emend. Snyder & Hansen and F. solani (Mart.) Sacc. Upon inoculation onto healthy plants, all isolates of Pythium spp. caused browning and a reduction in root mass, accompanied by stunting. Inoculation of plants with F. oxysporum caused browning of roots and crown rot infection, accompanied by pith and vascular discolouration, and in some cases wilting of plants, while root and crown infection was observed with F. solani. Phylogenetic analysis of internal transcribed spacer (ITS) and elongation factor 1α (EF-1α) sequences revealed that the Fusarium species affecting cannabis plants shared 99–100% sequence homology with isolates causing stem rot and wilt in other hosts, including cumin and tomato, suggesting they were not uniquely adapted to cannabis. The potential for spread of F. oxysporum through the hydroponic system was confirmed by its detection in the recirculating nutrient solution. Furthermore, rooted cuttings obtained from commercial propagators were found to harbour Fusarium root infection that resulted in subsequent stunting, yellowing and occasional death of plants. This demonstrates the potential for long-distance spread of the pathogen. The two Pythium species recovered from cannabis plants have an extremely broad host range and are not unique to this host. An additional species, P. aphanidermatum (Edson) Fitzp., was recovered from diseased plants grown under greenhouse conditions in 2018. The management of these root pathogens on C. sativa will require the evaluation and implementation of sanitization methods, biological control agents, and chemical products adapted from greenhouse vegetable production practices. The use of pathogen-free propagation materials and identification of potential sources of disease resistance should also become a priority.
Résumé
L’expansion de la culture de Cannabis sativa (cannabis or marijuana) au Canada est de plus en plus associée à une incidence et à une gravité accrues de différentes maladies, dont plusieurs n’ont pas encore été rapportées. Dans cette étude, des plants de C. Sativa issus de la culture hydroponique ont été échantillonnés durant trois ans (de 2014 à 2017) afin de déterminer la prévalence d’agents pathogènes racinaires. Après isolement, des études de pathogénicité ont été menées pour établir l’ampleur des symptômes des maladies causées par les microbes récupérés. Il a été établi que la pourriture racinaire avait été causée par deux espèces de Pythium — Pythium dissotocum Drechsler et P. myriotylum Drechsler. Deux espèces de Fusarium ont aussi été récupérées sur les plants morts — Fusarium oxysporum Schlecht. emend. Snyder & Hansen et F. solani (Mart.) Sacc. Lors de l’inoculation de plants sains, tous les isolats de Pythium spp. ont causé du brunissement et une réduction de la masse racinaire ainsi que du rabougrissement. L’inoculation de plants avec F. oxysporum a engendré le brunissement des racines et la pourriture du collet ainsi qu’une altération de la couleur de la moelle et des vaisseaux, tandis que F. solani a provoqué la pourriture des racines et du collet. L’analyse phylogénétique des séquences de l’espaceur interne transcrit et du facteur d’élongation a révélé que les espèces de Fusarium infectant les plants de cannabis partageaient de 99 à 100% de l’homologie de séquence avec les isolats causant la pourriture de la tige et la flétrissure chez d’autres hôtes, y compris chez le cumin et la tomate, suggérant qu’ils n’étaient pas uniquement adaptés au cannabis. La probabilité de diffusion de F. oxysporum dans le système hydroponique a été confirmée par sa détection dans la solution nutritive qui circule constamment dans le système. De plus, des boutures enracinées, obtenues de propagateurs commerciaux, abritaient de l’infection racinaire causée par Fusarium, ce qui a entraîné éventuellement du rabougrissement, du jaunissement et la mort occasionnelle de plants. Cela démontre la capacité de l’agent pathogène à se disséminer sur de grandes distances. Les deux espèces de Pythium récupérées sur les plants de cannabis possèdent, elles aussi, une gamme d’hôtes extrêmement vaste et ne sont pas spécifiques de celui-ci. La gestion de ces agents pathogènes racinaires sur C. sativa requerra l’évaluation et l’utilisation de méthodes de désinfection, d’agents de lutte biologique et de produits de synthèse adaptés de la culture des légumes en serre. L’utilisation de matériel de propagation exempt d’agents pathogènes et l’identification de sources possibles résistantes à la maladie devraient également faire partie des priorités.
Introduction
Cannabis sativa L., a member of the family Cannabeaceae, is one of the oldest cultivated plants in the world, and the species includes both hemp and marijuana (the latter is also referred to here as cannabis). One of the major characteristics that distinguishes industrial hemp, which is grown extensively in Canada (Laate, Citation2012), from cannabis plants is the level of the psychoactive compound delta-9 tetrahydrocannabinol (Δ9-THC) (Van Bakel et al., Citation2011). Hemp is legally required to contain 0.3% or lower levels of THC (Laate, Citation2012) whereas cannabis inflorescences (flower buds) may contain THC levels ranging from under 10% to over 23% on a dry weight basis (Potter et al., Citation2008; Mehmedic et al., Citation2010), as well as a range of other cannabinoids, terpenoids and phenolic compounds (Elsohly & Slade, Citation2005; Andre et al., Citation2016). In addition, hemp plants are grown outdoors in Canadian provinces such as Alberta, Manitoba and Saskatchewan (Laate, Citation2012), whereas cannabis cultivation occurs mostly in greenhouses or indoor controlled environment facilities, although outdoor cultivation is permitted. Both soil and hydroponic (soil-free) methods are used during cannabis cultivation indoors.
The pathogens affecting production of hemp under field conditions have been described previously, and include fungal, bacterial, viral and nematode species (McPartland, Citation1991, Citation1996). In contrast, the pathogens affecting cannabis have not been extensively studied, in part due to restrictions placed on the cultivation of the psychoactive THC-containing plants in the USA and Canada. It is likely that a number of the pathogens previously reported to infect hemp would also occur on cannabis, given that they are closely related genetically (Sawler et al., Citation2015). However, the different growing environments (indoor vs. outdoor) and cultivation methods, as well as genetic differences (strain or cultivar) between hemp and cannabis (Van Bakel et al., Citation2011), require that studies on the pathogens potentially affecting cannabis plants be conducted so that methods to manage emerging diseases (Punja, Citation2018a; Punja, Citation2018b) can be developed. Since the species C. sativa is dioecious (the male and female flowers are produced on different plants) (Moliterni et al., Citation2004), only female plants that bear the inflorescences are cultivated commercially. In addition, while hemp plants are grown from seeds, cannabis plants are primarily propagated vegetatively from cuttings made from an originally seed-derived strain. This can allow pathogen inoculum to be transmitted between cropping cycles.
The objective of this study was to determine the prevalence of root pathogens affecting hydroponically grown cannabis plants sampled over a 3-year period (2014–2017). Following isolation and identification, pathogenicity studies were conducted to establish the extent to which the recovered microbes could induce disease symptoms. Preliminary results have been previously published (Rodriguez et al., Citation2015).
Materials and methods
Sampling and isolation of pathogens from diseased plants
Over the period from May 2014 to May 2017, cannabis plants of several strains (defined as morphologically and genetically distinct plants identified by a unique name e.g. ‘Pennywise’, ‘Moby Dick’) (Punja et al., Citation2017) that exhibited symptoms of disease were sampled. These symptoms included stunted growth, minor leaf chlorosis, and brown root lesions that developed into root rot. Wilting symptoms were not observed. Tissues were sampled at various times during growth of the crop, depending on when symptoms were apparent, and ranged from plants in propagation (1–3 weeks old) to vegetative growth (3–6 weeks of age). A total of around 50 plants were sampled in the study.
Root samples were placed under running water for 15 min, sectioned into ~1 cm long segments, dipped into a 0.5% NaOCl solution for 30 s followed by 20 s in 70% EtOH, rinsed thrice in sterile water, blotted dry on sterile paper towels and then plated on agar media. Initially, roots were plated on water agar (WA), Komada’s semi-selective medium (Komada, Citation1975), P5ARP (Jeffers & Martin, Citation1986), and potato dextrose agar (Sigma Chemicals, St Louis, MO) amended with 100 mg L−1 streptomycin sulphate (PDA). All dishes were incubated at room temperature (23 ± 2°C) for 5–7 days. Emerging colonies were transferred to fresh PDA for subsequent identification to the genus level using morphological criteria (Van der Plaats-Niterink, Citation1981; Leslie & Summerell, Citation2006). Hyphal tip sub-cultures were made of representative isolates (total of 20) and stored on PDA slants at 4°C.
In 2018, rooted cannabis cuttings that had been initiated in rockwool plugs (5 cm2) were received from two commercial propagators in Ontario and were transferred into 15 cm2 rockwool blocks and grown under hydroponic conditions. The plants showed initial symptoms of yellowing and necrosis of the lower leaves and general stunting within 1–2 weeks after transfer (); no wilting was observed. In addition, leaves developed downward curling and necrosis of the leaf edges, and pronounced stunting (). Isolations were made from brown root lesions () as described above. In total, 40 plants were sampled. In addition, swabs of the surface of symptomatic roots growing on the underside of rockwool blocks were conducted using a cotton swab and streaked onto PDA containing 100 mg L−1 streptomycin sulphate. To assess the potential for airborne dispersal of spores within the growing environment, Petri dishes containing PDA with streptomycin sulphate were placed with the lids removed on benches in the areas between rows containing plants, ~30 cm away from the nearest plant. The dishes were left for 30 min and then covered and brought back to the laboratory. Control dishes were placed in similar locations with the Petri dish lids left on. A total of 10 Petri dishes was placed in the production facility used in the study at three different times during the production cycle. Fungal colonies that developed were subcultured and identified as described below.
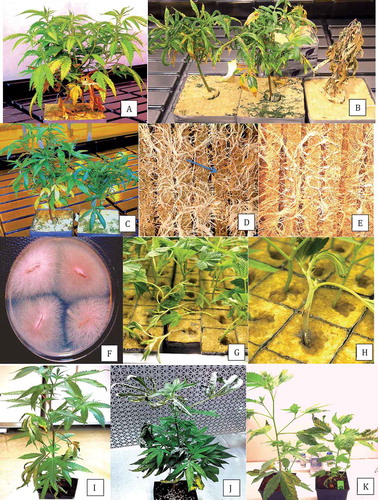
Fig. 1 (Colour online) Hydroponic production of Cannabis sativa. (a) Source (mother) plant for vegetative propagation from which cuttings were obtained; (b) Rooted cuttings in pots filled with clay pellets placed on a propagation bench suspended over a recirculating hydroponic nutrient solution; (c) Plants growing in tubs containing hydroponic nutrient solution. (d) Close-up of healthy plant; (e) Symptoms of browning indicative of root infection; (f) Healthy root system; (g) Colonies of Fusarium oxysporum growing from infected root pieces on PDA; (h) Colony of Pythium sp. on PDA originating from infected roots; (i) Colonies of F. oxysporum recovered from hydroponic nutrient solution.

Fig. 2 (Colour online) Symptoms of Fusarium infection on commercially propagated cannabis plants grown in rockwool blocks. (a) General yellowing of leaves, stunted growth, and necrosis of lower leaves of diseased plant; (b) Stunted growth, curled and necrotic leaves, and death of plant (extreme right); (c) Varying degrees of stunting of two infected plants, with curled and yellowing leaves; (d) The underside of the rockwool block of a diseased plant showing necrotic roots (arrow); (e) Root system of a healthy plant with white roots; (f) Colonies of F. oxysporum emerging from diseased roots plated on PDA; (g) Damping off symptoms on cuttings caused by F. oxysporum; (h) Close-up of stem lesion leading to girdling of rooted cutting in a rockwool block; (i–k) Symptoms on plants artificially inoculated with F. oxysporum, showing yellowing and necrosis of leaves (i), curling and drying of leaves and stunted growth (j) and varying degrees of stunting of plants, curling and necrosis of leaves (k).
Pathogen identification
Four cultures of Pythium and six of Fusarium from diseased roots were sent to the University of Guelph Laboratory Services, Agriculture and Food Laboratory, Guelph, ON (www.guelphlabservices.com) for species identification by PCR using the primers ITS1–ITS4 (ITS1-F CTTGGTCATTTAGAGGAAGTAA and ITS4 TCCTCCGCTTATTGATATGC). The elongation factor 1α (EF-1α) primer set EF-1 (5ʹATG GGT AAG GAG GAC AAG AC 3ʹ) and EF-2 (5ʹ GGA GGT ACC AGT GAT CAT GTT 3ʹ) (O’Donnell et al., Citation1998) was also used for identification of Fusarium spp. Cultures of representative isolates from cannabis were grown in potato dextrose broth at room temperature for 7 days and DNA was extracted from harvested mycelium using the QIAGEN DNeasy Plant Mini Kit. Aliquots of 1 uL containing 5–20 ng DNA were used for PCR in a 25 uL reaction volume consisting of 2.5 uL 10× buffer (containing 15 mM MgCl2), 0.5 uL 10 mM dNTP, 0.25 uL Taq DNA Polymerase (QIAGEN), 0.25 uL 10 mM forward and reverse primers, as well as 20.25 uL DNAse- and RNAse-free water (Invitrogen). All PCR amplifications were performed in a MyCycler thermocycler (BIORAD) with the following programme: 3 min at 94°C; 30 s at 94°C, 30 s at 60°C, 3 min at 72°C (35 cycles); and 7 min at 72°C. PCR products were separated on 1% agarose gels and bands of the expected size (~700 bp) were purified with QIAquick Gel Extraction Kit and sent to Eurofins Genomics (Eurofins MWG Operon LLC 2016, Louisville, KY) for sequencing. The resulting sequences were compared to the corresponding ITS1-5.8S-ITS2 or EF-1α sequences from the National Center for Biotechnology Information (NCBI) GenBank database. Multiple sequence alignment of the respective isolates of each species was done using the CLUSTAL W program (http://www.genome.jp/tools/clustalw). The sequences of Pythium and Fusarium species were subsequently included in a phylogenetic analysis using the neighbour-joining (NJ) method (Saitou & Nei, Citation1987; Tamura et al., Citation2004) in the software MEGA v. 5 (Tamura et al., Citation2011). A bootstrap consensus tree was inferred from 1000 replicates. Sclerotinia sclerotiorum was used as an outgroup. Branches corresponding to partitions reproduced in less than 50% bootstrap replicates were collapsed. The percentages of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) were shown next to the branches as described by Felsenstein (Citation1985).
Pathogenicity tests
Stem cuttings (~15 cm in height) of strain ‘Pennywise’ were rooted by inserting them into Jiffy-7® peat pellets (http://www.jiffypot.com/) or 5 cm2 rockwool cubes that had been pre-soaked in Current Culture H2O® hydroponic nutrient solution (https://www.cch2o.com/). The peat pellets and blocks were placed in a plastic tray containing water, the cuttings were misted, and covered with a plastic dome to maintain high humidity and placed in a Conviron incubator set at 25°C with a 12-h photoperiod. Misting was repeated every other day. Plants were fertilized with All-Purpose nutrient solution (N-P-K, 20-20-20) (J.R. Peters, Inc.). After 2 weeks, the exterior netting of the peat pellet was removed and the peat was gently rinsed off and the rooted cuttings were inoculated.
Pythium inoculation. The rooted cuttings were suspended in 750 mL plastic containers, 2 plants per container, with 8 containers per treatment. A hydroponic nutrient solution was added and the containers were continuously aerated through a plastic tube inserted into the solution. Pythium cultures were grown on PDA for 5 days and six 5 mm plugs were added to the nutrient solution at the time the plants were set up. Control plants received no inoculum. Plants were grown at 25°C in a Conviron growth chamber with a 16 h photoperiod. Roots were visually rated for extent of root browning and rot after 10–14 days and compared with the control. Root segments ~1 cm long were surface-sterilized for 30 s in a 0.5% NaOCl solution, rinsed three times for 30 s in sterile water, blotted dry on sterilized paper towel and then plated on PDA. Plates were incubated at room temperature (22 ± 3°C) for three days. The experiment was conducted twice for each of two Pythium species.
Fusarium inoculation. For F. oxysporum and F. solani, the same procedure described above was used, except cultures were grown for 2 weeks on PDA, 4 mycelial plugs were used as inoculum and plants were rated at 21 days after inoculation. In addition, cuttings grown in potting medium/coconut fibre and in rockwool cubes were also inoculated with F. oxysporum and F. solani as follows. The root tips were trimmed with scissors and cuttings were suspended in a spore suspension of either pathogen (at 106 spores per mL, prepared from PDA cultures) for 10 min. The cuttings were then transferred into pots containing either Miracle Grow™ potting medium (Home Depot Canada) or coconut fibre (Canna Coco brick) (http://www.cannagardening.ca/coco_brick) and the pots were placed in a tray containing nutrient solution. The plants were grown under a high-pressure sodium lamp (16 h photoperiod, light intensity of 240 umol m−2 s−1), and a temperature range of 23–25° C. After 2–3 weeks, all plants were visually rated for foliar symptoms, removed from the pots, and the extent of root browning, root length and root volume on 10 replicate plants was compared with the non-inoculated control plants. Root, crown and lower stem segments ~1 cm long were surface-sterilized for 30 s in a 0.5% NaOCl solution, rinsed three times for 30 s in sterile water, blotted dry on sterilized paper towel and then plated on PDA. Plates were incubated at room temperature (22 ± 3°C) for 3 days. The experiment was conducted twice for each of two Fusarium species.
Scanning electron microscopy
Crown tissues (1 cm in length) with symptoms of crown rot following inoculation with F. oxysporum were incubated in a Petri dish lined with moistened filter paper for 48 h and then prepared for scanning electron microscopy (SEM). Samples were adhered to a stub using a graphite-water colloidal mixture (G303 Colloidal Graphite, Agar Scientific, UK) and Tissue-Tek (O.C.T. Compound, Sakura Finetek, NL). The sample was submerged in a nitrogen slush for 10–20 s to rapidly freeze it. After freezing, the sample was placed in the preparation chamber of a Quorum PP3010T cryosystem attached to a FEI Helios NanoLab 650 scanning electron microscope (Dept. of Chemistry, 4D Labs, Simon Fraser University). The frozen sample was sublimed for 5 min at −80°C, after which a thin layer of platinum (10 nm thickness) was sputter-coated onto the sample for 30 s at a current of 10 mA. The sample was moved into the SEM chamber and the electron beam was set to a current of 50 pA at 3 kV. Images were captured at a working distance of 4 mm, at a scanning resolution of 3072 × 2207 collected over 128 low-dose scanning passes with drift correction.
Results
Hydroponic production of cannabis
All plants used in this study for pathogen isolation were grown hydroponically. During commercial production, specific cannabis strains to be propagated vegetatively were started from mother plants (moms) which were grown in 25 L pots for 18–24 months (). Cuttings up to 15 cm in length dipped in rooting gel (Clonex, containing indole-acetic acid) were rooted either in rockwool blocks (Grodan) (http://www.grodan.com/) or in peat-based medium under high-humidity conditions. After 2 weeks, cuttings with roots initiated were transferred to pots filled with clay pellets () and placed on a propagation bench suspended over a recirculating hydroponic nutrient solution (Current Culture H2O®) (https://www.cch2o.com/). After 2–3 weeks, these pots were transferred into tubs filled with hydroponic nutrient solution (), each provided with 2–3 air delivery intake pumps. After 4–6 weeks of vegetative growth (), plants were transferred to ‘flowering rooms’ with specific temperature settings and lighting requirements, depending on the strain.
Isolation of root pathogens
Symptoms of root rot were generally observed during the early phases of vegetative growth in the propagation stage as browning and rotting of the roots (), compared with white healthy roots of unaffected plants (). Few above-ground symptoms were apparent, except in severe cases where a majority of the root system was destroyed; these plants appeared stunted and displayed mild symptoms of yellowing and leaf curl but no wilting was observed. On average, ~1% of the plants in the growing facility were affected by root rot symptoms over each of the 3 years of study. From these symptomatic plants, Fusarium colonies () as well as Pythium colonies () were obtained from root segments plated onto PDA. When P5ARP medium and Komada’s medium were used, either Pythium spp. or Fusarium spp. were isolated from symptomatic tissue on the respective media, but rarely from the same root samples (data not shown). The roots yielding Pythium were usually more severely rotted compared with those yielding Fusarium. A sample of hydroponic solution from a tub containing a diseased plant yielded colonies of Fusarium on PDA ().
Symptomatic rooted cuttings received from two commercial propagators displayed stunted growth and yellowing (). Upon further vegetative growth for 2–3 weeks under conditions designed to promote plant growth using hydroponic culture, a high proportion (30%) of the plants developed advanced symptoms of downward curling, dark green leaves, drying of leaf edges, stunted growth, and in some instance complete collapse and death of the plants (). Examination of the exposed root system on the underside of the rockwool block showed brown necrotic roots, especially at the tips, compared with healthy plants (). Colonies of Fusarium were recovered from plated root tips at a frequency of 100% () as well as from swabs of root surfaces streaked onto PDA. In addition, cuttings placed in rockwool blocks for propagation developed damping off symptoms and stem lesions that girdled the plant and from which Fusarium was recovered. Petri dishes placed with the lids removed on benches in the areas between rows containing plants, ~30 cm away from the nearest plant and left exposed for 30 min, revealed the presence of colonies of Fusarium (data not shown).
Pathogen identification
Two morphologically distinct colonies of Pythium spp. were obtained and were identified following PCR of the ITS region as P. dissotocum Drechsler (GenBank accession no. MH782047) and P. myriotylum Drechsler (GenBank accession no. MH782048) (). The predominant species was P. dissotocum, representing about 70% of the isolates recovered. The Fusarium colonies were identified as F. oxysporum () and F. solani () using ITS sequence analysis, with less than 10% of the isolates belonging to F. solani. Phylogenetic analyses grouped the isolates from cannabis with a high degree of sequence identity (99–100%) with isolates from other hosts. For example, the isolates of F. oxysporum from cannabis showed 100% sequence identity in the ITS region to KJ653447.1 and LT841208.1 (causing wilt on cumin) (). In the EF-1α region, the isolates of F. oxysporum showed 100% identity to KY379852.1 and KT183483.1 (causing stem and root rot on members of the Cactaceae and identified as f. sp. opuntiarum) (Lops et al., Citation2013; Garibaldi et al., Citation2016; Bertetti et al., Citation2017; Quezada-Salinas et al., Citation2017), 100% identity to KX253985.1 (causing wilt of Dendrobium orchids in China) and 99% identity to KM886236.1 identified as F. oxysporum f. sp. lycopersici (causing fruit rot of tomato) ().
Fig. 3 Phylogenetic analysis of ITS1-5.8S-ITS2 sequences using the neighbour-joining (NJ) method for (a) Pythium dissotocum and (b) Pythium myriotylum originating from cannabis plants compared with isolates from a range of other hosts (GenBank numbers are shown). A bootstrap consensus tree was inferred from 1000 replicates to represent the distance. Branches corresponding to partitions reproduced in less than 50% bootstrap replicates were collapsed.
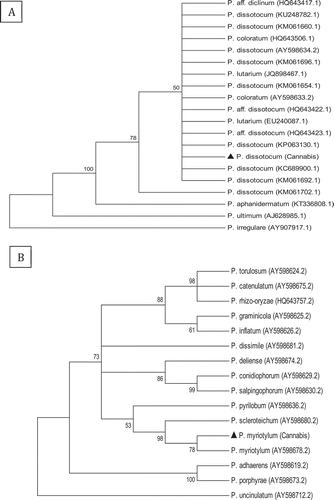
Fig. 4 Phylogenetic analysis of Fusarium oxysporum isolates originating from cannabis plants using (a) ITS1-5.8S-ITS2 sequences and (b) EF-1 sequences compared with isolates from a range of other hosts (GenBank numbers are shown). A bootstrap consensus tree was inferred from 1000 replicates to represent the distance using the neighbour-joining (NJ) method. Branches corresponding to partitions reproduced in less than 50% bootstrap replicates were collapsed. The scale bar indicates the expected number of nucleotide substitutions.
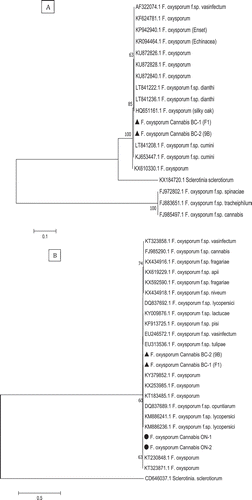
Fig. 5 Phylogenetic analysis of Fusarium solani isolates originating from cannabis plants using ITS1-5.8S-ITS2 sequences compared with isolates from a range of other hosts (GenBank numbers are shown). A bootstrap consensus tree was inferred from 1000 replicates to represent the distance using the neighbour-joining (NJ) method. Branches corresponding to partitions reproduced in less than 50% bootstrap replicates were collapsed. The scale bar indicates the expected number of nucleotide substitutions.
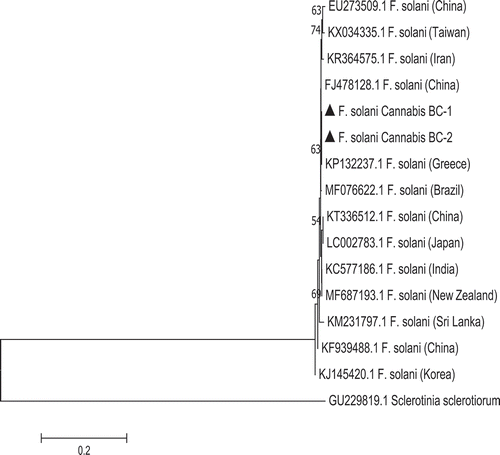
There was 100% sequence identity in the ITS region for the isolates of F. solani from cannabis to FJ478128.1 (China) and KP132237.1 (Greece) (). In the EF-1α region, the isolates of F. solani showed 100% sequence identity to MF040645.1 (causing fruit rot of kiwi), KY128333.1 (associated with roots of leguminous species) and KF939496.1 (causing root rot on alfalfa) (data not shown). Sequences of the ITS and EF-1 regions of two representative isolates of each Fusarium species from cannabis plants have been submitted to GenBank (accession nos. MH782043 and MH782044 for ITS for F. oxysporum isolates F1 and 9B and MH82045 and MH782046 for F. solani isolates F5 and Fs). For the EF-1 region, accession nos. are MH844828 and MH844829 for F. oxysporum and MH844836 and MH844837 for F. solani, respectively.
Pathogenicity tests
Pythium inoculation
Rooted cuttings that had been inoculated with either P. dissotocum or P. myriotylum and placed in plastic containers with hydroponic solution were stunted compared with the controls and the root volume was visibly reduced (). The roots exhibited browning and root rot, similar to symptoms on plants from which the isolates were initially obtained. The corresponding Pythium spp. were successfully reisolated from the inoculated plants.
Fig. 6 (Colour online) Pathogenicity studies conducted on rooted cannabis cuttings using isolates of (a) Pythium dissotocum and (b) Fusarium oxysporum conducted in hydroponic solution. In (a), uninoculated control plant is on the right and photo was taken 2 weeks after inoculation; in (b), uninoculated control plant is on the left and photo was taken 3 weeks after inoculation; (c–e) Pathogenicity studies conducted in potting soil comparing control plant (c) to F. oxysporum-inoculated (d) and F. solani-inoculated (e) plants. Photos were taken 21 days after inoculation and show yellowing and necrosis of leaves, and wilting symptoms; (f) Root systems of plants grown in coco fibre showing uninoculated control (left), F. oxysporum-inoculated (middle) and F. solani-inoculated (right); photo was taken 3 weeks after inoculation and shows a significant reduction in root growth compared with the uninoculated control plant; (g) Internal pith and vascular discolouration progressing upward from the crown region in three F. oxysporum-inoculated plants; photo was taken 3 weeks after inoculation.

Fusarium inoculaion
Rooted cuttings that had been inoculated with F. oxysporum and placed in plastic containers with hydroponic solution were stunted compared with the controls and the root volume was visibly reduced (). The roots exhibited browning and root rot, similar to symptoms on plants from which the isolates were initially obtained. When inoculations were conducted on rooted cuttings and grown in potting soil, wilting symptoms became apparent after 16–18 days on plants inoculated with F. oxysporum () and F. solani (); uninoculated control plants showed no symptoms (). Root rot symptoms were not readily observed due to the difficulty of separating soil from the roots.
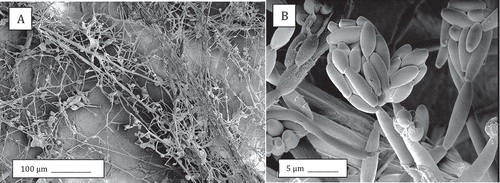
When the same experiment was repeated in coconut fibre as a substrate, a significant reduction in root volume was observed following inoculation with both Fusarium species, with F. solani showing more severe symptoms of root rot (). In plants inoculated with F. oxysporum, internal stem discolouration of the pith and vascular tissues was seen extending about 5–8 cm upwards from the crown region (), while plants inoculated with F. solani exhibited a discolouration mainly at the crown region (0–2 cm). Recovery of F. oxysporum was confirmed from the roots, crown and stem tissues (90–100% frequency), while F. solani was reisolated mainly from the roots and crown region (100% frequency). There were no wilting symptoms seen on these plants unlike those grown in soil. Scanning electron microscopic observations of crown tissues infected with F. oxysporum showed prolific growth of mycelium on the tissue surface () and production of microconidia ().
Fig. 7 Scanning electron microscopy images of crown tissues inoculated with F. oxysporum. (a) Mycelium and spore clusters; (b) Close-up of microconidia in a cluster on a phialide.
A series of inoculation experiments were conducted with the isolates of F. oxysporum recovered from rooted cuttings originating from two commercial propagators using coconut fibre as described above. The results showed the development of dry curled leaves (, ), yellowing of leaves and stunting of plants (), and reduced root length and mass within 2 weeks following inoculation. These isolates (ON-1, ON-2) grouped within the F. oxysporum clade () (GenBank accession nos. MH844830 and MH844831 for the EF-1 region).
In addition, in 2018, isolates of P. aphanidermatum (Edson) Fitzp. were recovered from diseased cannabis rooted cuttings, where they caused damping-off, and from five flowering plants grown using cocofibre as a substrate under hydroponic conditions in a commercial greenhouse, where they were associated with yellowing of plants and death (). The isolate BC-1 (VF) (accession no. MH782049) showed 100% ITS sequence identity with isolates reported to cause root rot on soybean seedlings (GenBank accession no. KU211462.1) (Rojas et al. Citation2017), damping off on cucumber seedlings (GenBank accession no. JF775593.1) and basal rot of tomato plants (GenBank accession no. KF561235).
Discussion
Cannabis plants grown under indoor conditions in a hydroponic system were found to be infected with either Pythium or Fusarium spp. and showed stunted growth, but no obvious symptoms of chlorosis or wilting were seen. Occasional chlorosis associated with Pythium infection was observed on plants at the vegetative growth stage (authors, unpublished observations). However, under greenhouse hydroponic conditions, P. aphanidermatum was observed to cause plant death. In regards to F. oxysporum, rooted cuttings initiated in rockwool blocks received from commercial propagators and maintained under hydroponic cultivation showed the early development of characteristic symptoms of damping-off and root root, i.e. stunted plants with dark green or yellow curled leaves, and root lesions especially at root tips. These symptoms were apparent on 1-week-old cuttings and 3–6-week-old plants. This suggests that early infection can cause above-ground symptoms on younger plants but may be difficult to detect when plants reach a larger size, either in vegetative growth or in the flowering period. However, visual inspection of the root systems should reveal root browning, which is relatively easy to do in the hydroponic system but more difficult for plants grown in soil.
Inoculation experiments confirmed the ability of the tested isolates of Pythium and Fusarium species to reduce root growth and cause root rot. Wilting symptoms on plants inoculated with F. oxysporum were only observed in soil, where uptake of the nutrient solution by diseased roots would be more difficult compared with infected plants grown in a hydroponic system. Therefore, symptoms on soil-grown plants are likely to be more visible than on hydroponically grown plants. However, when the infection levels were high in vegetatively propagated plants grown in rockwool substrate, the plants showed visible symptoms of stunting, yellowing and curling of leaves, and root browning and damping-off during hydroponic cultivation at an early age. Artificial inoculation with F. solani resulted in infection of the crown tissues in soil-grown plants, and only root rot symptoms were observed on hydroponically grown plants. This species was isolated from diseased roots with <10% frequency, suggesting that it is not as prevalent as F. oxysporum, although it appears to be comparatively more damaging to roots.
Phylogenetic analysis using the ITS and EF-1α regions revealed that the Fusarium species affecting cannabis plants were grouped with other isolates originating from a range of hosts and were not unique to cannabis. Previously, F. oxysporum and F. solani were reported to cause damping-off on hemp seedlings, while F. solani was reported to cause root rot on older plants and F. oxysporum caused a wilt disease (McPartland, Citation1996). The wilt pathogens were reported to be F. oxysporum f. sp. cannabis Noviello & W.C. Snyder and F. oxysporum f. sp. vasinfectum W.C. Snyder & N.H. Hans. (McPartland & Hillig, Citation2004). The former formae specialis was reported to have a restricted host range limited to Cannabis sativa while the latter infected a broad range of hosts, including cotton, soybeans, tobacco (McPartland & Hillig, Citation2004) and is a widespread pathogen of cotton in the USA (Cianchetta et al., Citation2015). The F. oxysporum isolates recovered in the present study were not grouped with either of the formae speciales previously reported to affect hemp, and based on the symptoms and relatedness to other F. oxysporum isolates, they are probably generalized root and crown rot pathogens.
In the phylogenetic analysis of the ITS region, F. oxysporum f. sp. cannabis was grouped with two other f. sp. (spinaceae and tracheiphilum), supporting a close similarity that was also previously reported in a study involving the nuclear ribosomal intergeneric spacer (IGS) region (O’Donnell et al., Citation2009). In the 1980s, F. oxysporum f. sp. cannabis was considered as a potential biocontrol pathogen to eliminate illicit growing of cannabis plants for drug purposes (Hildebrand & McCain, Citation1978; McCain & Noviello, Citation1985); however, a detailed analysis of its use as a bioherbicide concluded that it was not sufficiently aggressive (Charudattan, Citation2011). The extent of distribution of F. oxysporum f. sp. cannabis and its host range are presently unknown due to the limited number of isolates available for study. Fusarium oxysporum contains a large number of formae speciales (Michielse & Rep, Citation2009), which were originally described to reflect host range specialization (Snyder & Hansen, Citation1940). While the f. sp. designations describe the pathogenic specialization within the species, they are not part of the formal taxonomic hierarchy (Kistler, Citation1997). Although comparisons of the EF-1α gene sequences distinguished among some formae speciales in this study, further studies of the host range of the isolates from cannabis in this study are needed. O’Donnell et al. (Citation2009) reported that formae speciales of Fusarium are not always restricted to a specific range of hosts, and cross-infection between hosts can occur. Therefore, strains from cannabis are likely able to infect other hosts, and vice versa.
The three species of Pythium recovered from cannabis plants in this study have a wide host range, including many horticultural and field crops worldwide, e.g. beans, cilantro, opium, spinach, strawberry, soybean and tobacco (McCarter & Littrell, Citation1970; Watanabe, Citation1977; Kageyama & Ui, Citation1983; Bates & Stanghellini, Citation1984; Alam et al., Citation1996; Fortnum et al., Citation2000; Watanabe & Tojo, Citation2006; Corrêa et al., Citation2011; Romero et al., Citation2012; Tomioka et al., Citation2013; Rojas et al., Citation2017). Pythium root rot is also destructive on various crops grown in hydroponic systems, including lettuce, cucumber, tomato, sweet pepper and roses (Stanghellini & Kronland, Citation1986; Sutton et al., Citation2006). Previously, P. aphanidermatum and P. ultimum were reported to cause damping-off on hemp seedlings (McPartland, Citation1996), while P. aphanidermatum was recently reported to cause crown and root rot on field-grown hemp (Beckerman et al., Citation2017) and cannabis plants (Punja et al., Citation2018). Neither of P. dissotocum or P. myriotylum isolated in this study have been previously reported to infect cannabis plants. Pythium dissotocum Drechsler is capable of rapid growth at temperatures of 24–32°C and can infect via zoospores or mycelium (Van der Plaats-Niterink, Citation1981; Sutton et al., Citation2006). Similarly, P. myriotylum grows best at 25–30°C and can produce zoospores within this temperature range. Cannabis plants are generally grown at 24–27°C, which is within the range for growth of both Pythium species. Additionally, hydroponic cultivation may promote spread of Fusarium and Pythium pathogens, which are commonly encountered on other hydroponically grown horticultural crops (Sutton et al., Citation2006). While the overall infection level was around 1% of plants at later stages of production, once established, these pathogens can persist and spread in the recirculated hydroponic solution (Menzies & Bélanger, Citation1996). The most important initial sources of inoculum include infected rooted cuttings and propagation media, as well as potential hydroponic pipes and tubing, tools and equipment, and water (Sutton et al., Citation2006). Movement of infected rooted cuttings by propagators from one region of Canada to another can be an important source of Fusarium spread. Currently, there are no restrictions on the movement of propagation materials of cannabis as long as they are between licensed producers. The severity of the symptoms observed on 2–3-week-old plants suggested that a large proportion of the plants were infected early during propagation. The sporulation of F. oxysporum observed on crown tissues in this study suggests that airborne spread is likely to occur, as has been observed with F. oxysporum causing wilt and crown rot of tomato, basil and cucumber plants (Rowe et al., Citation1977; Gamliel et al., Citation1996; Katan et al., Citation1997; Rekah et al., Citation2000; Scarlett et al., Citation2015). Colonies of F. oxysporum were detected on Petri dishes left exposed in the growing environment, confirming the possibility of airborne spread of inoculum (authors, unpublished). Rooted plants produced by commercial propagators that are infected with Fusarium are a potentially important means of pathogen spread and should be monitored.
With the current expansion of the cannabis industry in Canada, additional research on the epidemiology and management of cannabis diseases is warranted. Furthermore, the efficacy of biological control products and reduced-risk chemicals for root disease management on cannabis needs to be evaluated. At present, there are two biocontrol products registered in Canada for management of root diseases on cannabis – Rootshield WP (Trichoderma harzianum Rifai strain RRL-AG2) and Prestop WP (Gliocladium catenulatum strain J1446) for root-infecting pathogens (https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/licensed-producers/policies-directives-guidance-information-bulletins/testing-cannabis-medical-purposes-unauthorized-pest-control-products.html), while Actinovate SP (Streptomyces lydicus strain WYEC 10–8), which was previously registered for powdery mildew and botrytis control, has been withdrawn. Evaluation of additional biocontrol products that have shown to be effective against root diseases on other horticultural crops, including Rhapsody (Bacillus subtilis strain QST 713) (Punja et al., Citation2016) and Mycostop (Streptomyces griseoviridis strain K61) (Punja & Yip, Citation2003; Rose et al., Citation2003) is needed to identify additional non-fungicide options for disease management by cannabis producers. In addition, the efficacy of sanitation methods, such as ultraviolet light, ozonation, chlorination, hydrogen peroxide, heat pasteurization and/or mechanical filtration for reducing inoculum presence in greenhouse production should be evaluated.
Acknowledgements
We thank Sarah Chen for providing assistance with the molecular and phylogenetic analyses and Cameron Scott for conducting some of the pathogenicity tests.
Additional information
Funding
References
- Alam M, Sattar A, Chourasia HK, Janardhanan KK. 1996. Damping-off, a new disease of opium poppy caused by Pythium dissotocum. Indian Phytopath. 49:94–97.
- Andre CM, Hausman J-F, Guerriero G. 2016. Cannabis sativa: the plant of the thousand and one molecules. Front Plant Sci. 7:1–17.
- Bates M, Stanghellini M. 1984. Root rot of hydroponically-grown spinach caused by Pythium aphanidermatum and P. dissotocum. Plant Dis. 68:989–991.
- Beckerman J, Nisonson H, Albright N, Creswell T. 2017. First report of Pythium aphanidermatum causing crown and root rot of industrial hemp in the United States. Plant Dis. 101:1038.
- Bertetti D, Ortu G, Gullino ML, Garibaldi A. 2017. Identification of Fusarium oxysporum f. sp. opuntiarum on new hosts of the Cactaceae and Euphorbiaceae families. J. Plant Pathol. 99:347–354.
- Charudattan R. 2011. Fusarium oxysporum formae speciales as candidate biological control agents for cannabis and coca. In: Feasibility of Using Mycoherbicides for Controlling Illicit Drug Crops. Washington (DC): The National Academies Press; p. 61–100, 186. [accessed 2017 Sep 16] http://nap.edu/13278
- Cianchetta AN, Allen TW, Hutmacher RB, Kemerait RC, Kirkpatrick TL, Lawrence GW. 2015. Survey of Fusarium oxysporum f. sp. vasinfectum in the United States. J Cotton Sci. 19:328–336.
- Corrêa AS, Rocha AB, Willani SA, Dariva JM, Souza MV, Moraes MG. 2011. Yellow stunt, a tobacco disease caused by Pythium dissotocum in southern parts of Brazil. Plant Dis. 95:354.
- Elsohly MA, Slade D. 2005. Chemical constituents of marijuana: the complex mixture of natural cannabinoids. Life Sci. 78:539–548.
- Felsenstein J. 1985. Phylogenies and the comparative method. Amer Naturalist. 125:1–15.
- Fortnum BA, Rideout J, Martin SB, Gooden D. 2000. Nutrient solution temperature affects Pythium root rot of tobacco in greenhouse float systems. Plant Dis. 84:289–294.
- Gamliel A, Katan T, Yunis H, Katan J. 1996. Fusarium wilt and crown rot of sweet basil: involvement of soilborne and airborne inoculum. Phytopathology. 86:56–62.
- Garibaldi A, Bertetti D, Pensa P, Ortu G, Gullino ML. 2016. First report of Fusarium oxysporum causing wilt on Astrophytum myriostigma in Italy. Plant Dis. 100:215.
- Hildebrand DC, McCain AM. 1978. The use of various substrates for large scale production of Fusarium oxysporum f. sp. cannabis inoculum. Phytopathology. 68:1099–1101.
- Jeffers SN, Martin SB. 1986. Comparison of two media selective for Phytophthora and Pythium species. Plant Dis. 70:1038–1043.
- Kageyama K, Ui T. 1983. Host range and distribution of Pythium myriotylum and unidentified Pythium sp. contributed to the monoculture injury of bean and soybean plants. Japan J Phytopath. 49:148–152.
- Katan T, Shlevin E, Katan J. 1997. Sporulation of Fusarium oxysporum f. sp. lycopersici on stem surfaces of tomato plants and aerial dissemination of inoculum. Phytopathology. 87:712–719.
- Kistler HC. 1997. Genetic diversity in the plant-pathogenic fungus Fusarium oxysporum. Phytopathology. 87:474–479.
- Komada H. 1975. Development of a selective medium for quantitative isolation of Fusarium oxysporum from natural soil. Rev Plant Prot Res. 8:114–124.
- Laate EA 2012. Industrial hemp production in Canada. Alberta Agriculture and Rural Development Publication. accessed 2017 Sep 16. http://www1.agric.gov.ab.ca/$department/deptdocs.nsf/all/econ9631
- Leslie JF, Summerell BA. 2006. The Fusarium Laboratory Manual. 1st ed. Iowa (IA): Blackwell.
- Lops F, Cibelli F, Raimondo ML, Carlucci A. 2013. First report of stem wilt and root of Schlumbergera truncata caused by Fusarium oxysporum f. sp. opuntiarum in southern Italy. Plant Dis. 97:846.
- McCain AH, Noviello C. 1985. Biological control of Cannabis sativa. In: Delfosse ES, editor. Proceedings of the sixth international symposium on biological control of weeds; 1984 Aug 19–24; Vancouver (BC): Agriculture Canada Public. p. 635–642.
- McCarter SM, Littrell RH. 1970. Comparative pathogenicity of Pythium aphanidermatum and Pythium myriotylum to twelve plant species and intraspecific variation in virulence. Phytopathology. 60:264–268.
- McPartland JM. 1996. A review of Cannabis diseases. J Intern Hemp Assoc. 3:19–23.
- McPartland JM, Hillig KW. 2004. Cannabis Clinic – fusarium wilt. J Industr Hemp. 2:67–77.
- McPartland SM. 1991. Common names for diseases of Cannabis sativa L. Plant Dis. 75:226–227.
- Mehmedic Z, Chandra S, Slade D, Denham H, Foster S, Patel AS, Ross SA, Khan IA, ElSohly MA. 2010. Potency trends of Δ9-THC and other cannabinoids in confiscated cannabis preparations from 1993 to 2008. J Forensic Sci. 55:1209–1710.
- Menzies JG, Bélanger RR. 1996. Recent advances in cultural management of diseases in greenhouse crops. Can J Plant Pathol. 18:186–193.
- Michielse CB, Rep M. 2009. Pathogen profile update: Fusarium oxysporum. Mol Plant Pathol. 10:311–324.
- Moliterni VMC, Cattivelli L, Ranalli P, Mandolino G. 2004. The sexual differentiation of Cannabis sativa L.: a morphological and molecular study. Euphytica. 140:95–106.
- O’Donnell, K., Gueidan, C., Sink, S., Johnston, P.R., Crous, P.W., Glenn, A., Riley, R., Zitomer, N.C., Colyer, P., Waalwijk, C., et al. 2009. A two-locus DNA sequence database for typing plant and human pathogens within the Fusarium oxysporum species complex. Fungal Genet Biol. 46:936–948.
- O’Donnell K, Kistler HC, Cigelnik E, Ploetz RC. 1998. Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. Proc Natl Acad Sci USA. 95:2044–2049.
- Potter D, Clark P, Brown M. 2008. Potency of Δ9-THC and other cannabinoids in cannabis in England in 2005: implications for psychoactivity and pharmacology. J Forensic Sci. 53:90–94.
- Punja ZK. 2018a. Flower and foliage-infecting pathogens of marijuana (Cannabis sativa L.) plants. Can J Plant Pathol. 40:(final acceptance pending revisions).
- Punja ZK. 2018b. Emerging diseases of Cannabis sativa L. Can J Plant Pathol. 40:158(abstr.).
- Punja ZK, Rodriguez G, Chen S. 2017. Assessing genetic diversity in Cannabis sativa using molecular approaches. In: Chandra S, Lata L, ElSohly MA, editors. Cannabis sativa L. Botany and Biotechnology. Berlin: Springer-Verlag; p. 395–418.
- Punja ZK, Rodriguez G, Tirajoh A. 2016. Evaluation of Bacillus subtilis strain QST 713 and post- harvest storage temperatures on disease development of greenhouse tomatoes. Crop Prot. 84:98–104.
- Punja ZK, Scott C, Chen S. 2018. Root and crown rot pathogens causing wilt symptoms on field-grown marijuana (Cannabis sativa L.) plants. Can J Plant Pathol. 40:(final acceptance pending revisions).
- Punja ZK, Yip R. 2003. Biological control of damping-off and root rot caused by Pythium aphanidermatum on greenhouse cucumber. Can J Plant Path. 25:411–417.
- Quezada-Salinas A, Moreno-Velazquez M, Cruz-Jaramillo JL, Garcia-Avila CJ, Lopez- Buenfil JA. 2017. First report of Fusarium oxysporum causing stem dry rot in Astrophytum ornatum in Mexico. Plant Dis. 101:1324.
- Rekah Y, Shtienberg D, Katan J. 2000. Disease development following infection of tomato and basil foliage by airborne conidia of the soilborne pathogens Fusarium oxysporum f. sp. radicis-lycopersici and F. oxysporum f. sp. basilici. Phytopathology. 90:1322–1329.
- Rodriguez G, Kibler A, Campbell P, Punja ZK 2015. Fungal diseases of Cannabis sativa in British Columbia (abstr.). www.apsnet.org/meetings/Documents/2015_meeting_abstracts/aps2015abP319.htm
- Rojas JA, Jacobs J, Napieralski S, Karaj B, Bradley CA, Chase T, Esker P, Giesler L, Jardine D, Malvick D, et al. 2017. Oomycete species associated with soybean seedlings in North America. Identification and pathogenicity characterization. Phytopathology. 107:280–292.
- Romero G, Estévez de Jensen C, Palmateer AJ. 2012. First report of Pythium dissotocum affecting cilantro in hydroponic systems in Puerto Rico. Plant Health Progress. 13:32.
- Rose S, Parker M, Punja ZK. 2003. Efficacy of biological and chemical treatments for control of Fusarium root and stem rot on greenhouse cucumber. Plant Dis. 87:1462–1470.
- Rowe RC, Farley JD, Coplin DL. 1977. Airborne spore dispersal and recolonization of steamed soil by Fusarium oxysporum in tomato greenhouses. Phytopathology. 67:1513–1517.
- Saitou N, Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 4:406–425.
- Sawler J, Stout JM, Gardner KM, Hudson D, Vidmar J, Butler L, Page JE, Myles S. 2015. The genetic structure of marijuana and hemp. PLOS One. 10:e0133292.
- Scarlett K, Tesoriero L, Daniel R, Maffi D, Faora F, Guest DI. 2015. Airborne inoculum of Fusarium oxysporum f. sp. cucumerinum. Eur J Plant Pathol. 141:779–787.
- Snyder WC, Hansen HN. 1940. The species concept in Fusarium. Am J Bot. 27:64–67.
- Stanghellini M, Kronland WC. 1986. Yield loss in hydroponically grown lettuce attributed to subclinical infection of feeder roots by Pythium dissotocum. Plant Dis. 70:1053–1056.
- Sutton JC, Sopher CR, Owen-Going TN, Liu W, Grodzinski B, Hall JC, Benchimol RA. 2006. Etiology and epidemiology of Pythium root rot in hydroponic crops: current knowledge and perspectives. Summa Phytopathol. 32:307–321.
- Tamura K, Nei M, Kumar S. 2004. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Nat Acad Sci. USA101:11030–11035.
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 28:2731–2739.
- Tomioka K, Takehara T, Osaki H, Sekiguchi H, Nomiyama K, Kageyama K. 2013. Damping-off of soybean caused by Pythium myriotylum in Japan. J Gen Plant Pathol. 79:162–164.
- Van Bakel H, Stout JM, Cote AG, Tallon CM, Sharpe AG, Hughes TR, Page JE. 2011. The draft genome and transcriptome of Cannabis sativa. Genome Biol. 12:R102.
- Van der Plaats-Niterink AJ. 1981. Monograph of the genus Pythium. Stud Mycol. No. 21. Baarn (The Netherlands): Centraalbureau Voor Schimmelcultures; p. 242.
- Watanabe T. 1977. Pathogenicity of Pythium myriotylum isolated from strawberry roots in Japan. Ann Phytopathol Soc Japan. 43:306–309.
- Watanabe T, Tojo M. 2006. Stem and root rot of scarlet runner bean (Phaseolus coccineus) caused by Pythium myriotylum. J Gen Plant Pathol. 72:126–128.

